Abstract
Attaching semantic meaning to sensory information received from both inside and outside our bodies is a fundamental function of the human brain. The theory of Controlled Semantic Cognition (CSC) proposes that the formation of semantic knowledge relies on connections between spatially distributed modality-specific spoke-nodes, and a modality-general hub in the anterior temporal lobes (ATLs). This theory can also be applied to social semantic knowledge, though certain domain-specific spoke-nodes may make a disproportionate contribution to the understanding of social concepts. The ATLs have strong connections with spoke-node structures such as the subgenual ACC (sgACC) and the orbitofrontal cortex (OFC) that play an important role in predicting the hedonic value of stimuli. We hypothesized that in addition to the ATL semantic hub, a social semantic task would also require input from hedonic evaluation structures. We used Voxel Based Morphometry (VBM) to examine structural brain-behavior relationships in 152 patients with neurodegeneration (Alzheimer’s disease [N=12], corticobasal syndrome (N=18], progressive supranuclear palsy [N=13], behavioral variant frontotemporal dementia [N=56], and primary progressive aphasia (PPA) [N=53]) using the Social Interaction Vocabulary Task (SIVT). This task measures the ability to correctly match a social term (e.g. “gossiping”) with a visual depiction of that social interaction. As predicted, VBM showed that worse SIVT scores corresponded with volume loss in the left ATL semantic hub region, but also in the sgACC, OFC, caudate and putamen (pFWE < 0.05). These results support the CSC model of a hub-and-spoke organization of social semantic knowledge with the ATL as a domain-general semantic hub, and ventromedial and striatal structures as domain specific spoke-nodes. Importantly, these results suggest that correct comprehension of social semantic concepts requires emotional ‘tagging’ of a concept by the evaluation system, and that the social deficits observed in some neurodegenerative disease syndromes may be caused by the break-down of this mechanism.
Keywords: Social semantics, evaluation system, anterior temporal lobe, frontotemporal dementia, voxel-based morphometry, semantic appraisal network
1. Introduction
Attaching meaning to the sensory information that our brain receives, both from around us and within us, is an elemental neural function. The mechanism that transforms this array of received information into meaningful representations is referred to as semantic cognition. It does not only support language production and comprehension but helps us infer the meaning of all types of stimuli, verbal and non-verbal. It is the foundational representation of concepts in the world, from concrete objects to abstract ideas. The theory of Controlled Semantic Cognition (CSC), proposed by Lambon Ralph and colleagues (2017), provides a model for how these semantic representations are produced in the brain. It suggests that semantics depend on two key neural systems that are highly interlinked with each other. First, the representation system encodes conceptual knowledge through integration of information from primary and association areas, and second, the control system modulates activation in this representation system based on contextual appropriateness.
The representation system has its foundations in the hub-and-spoke model of semantic cognition (Lambon Ralph et al., 2010b; Patterson et al., 2007; Rogers et al., 2004) This model represents two interacting systems. The first is a system of modality-specific neural regions distributed across the cortex that provide core sensory information necessary for creating concepts, such as audition, vision and olfaction (Kanwisher, 2010; Rice et al., 2015b) These modality-specific regions (i.e., “spoke nodes”) are reciprocally connected with a second system, located in the anterior temporal lobes (ATLs), which are bilateral modality-general hubs that mediate the formation of meaningful semantic representations from a stream of multimodal information (Lambon Ralph et al., 2010b).
Subregions of the ATL do not contribute equally to the construction of semantic concepts. Rather, variation exists in the connectivity of distinct subregions with modality-specific input nodes outside the ATL (Jackson et al., 2016; Papinutto et al., 2016; Pascual et al., 2015). For example, the ventrolateral portion of the ATL is considered the ‘true’ convergence zone where information from different modalities is accumulated through direct connections with other subregions of the ATL. However, moving away from the ventrolateral portion, semantic function gradually converges in additional locations with distinct functional specializations (Rice et al., 2015a), for which the nature of the inputs to a given ATL subregion largely determines the semantic function that subregion serves (Plaut, 2002). Besides functional specialization within the ATLs, it has also been proposed that each ATL demi hub (i.e., left and right) contributes asymmetrically to semantic concept representation (Rice et al., 2015a). To test this, Rice and colleagues (2015b) performed a meta-analysis with 97 functional neuroimaging studies to determine the role of the left versus right ATL in concept generation, and found that typically both hubs activate for all stimulus types; asymmetric hub activation was observed only for tasks that involved naming, reading words (word retrieval) and written word stimuli. Furthermore, bilateral ATL damage causes much more profound cognitive and behavioral deficits than unilateral damage (Lambon Ralph et al., 2010a; Schapiro et al., 2013). Combined, this suggests that rather than working independently, the left and right ATLs seem to contribute jointly to processing in all semantic domains (Schapiro et al., 2013).
A recent review paper by Binney and Ramsey (2020) proposed that similar to non-social concepts, social concepts are constructed following this CSC framework, with a domain-general representation system for social semantics in the ATL and domain-specific representations that provide the information necessary for social concept generation. Binney and colleagues (2016) compared temporal lobe activation for non-social and social concepts and revealed that the ventrolateral portion of the temporal lobe activates most strongly for all concepts, i.e., social and non-social. However, only the superior part of the temporal lobe was significantly activated for social concepts, which suggests that this region is selectively involved in social concept processing. The superior ATL is robustly connected via the uncinate fasciculus (UF) white matter tract (von der Heide et al., 2013) to frontal limbic regions such as the orbitofrontal cortex (OFC), subgenual anterior cingulate cortex (sgACC) and other structures involved in value-related evaluation processes (Cunningham and Brosch, 2012; Hiser and Koenigs, 2018; Rudebeck and Rich, 2018). As proposed in a review by Olson and colleagues (2013), these connections may support the superior ATL’s role in social concepts. By tagging concepts with an emotion, these frontal limbic structures potentially provide the category-specific information that is required to create meaningful social concepts. Furthermore, seed-based analysis inspecting functional connectivity of different subregions within the ATL with areas outside the ATL showed that differential connections exist; however, most of the superior part of the ATL is connected with frontal limbic structures (Pascual et al., 2015).
In neurodegenerative disease, neural systems are targeted differentially and in various degrees depending on the underlying pathology of the syndrome (Seeley et al., 2009). To advance our understanding of the semantic representation system, investigating how it is targeted in neurodegenerative disease, and how its functions break down in this patient population, can provide valuable insights. Lesions in the ATL are common for patients with semantic variant primary progressive aphasia (svPPA) and some patients with behavioral variant frontotemporal dementia (bvFTD). Early in disease progression, svPPA patients have primarily atrophy in the left temporal lobe, of which the left polar region is most prominently affected (Collins et al., 2017). The structures that are generally first targeted in bvFTD patients are the (right) anterior insula and anterior cingulate cortex, as well as paralimbic orbitofrontal structures (Seeley et al., 2008). However, subsets of these bvFTD patients also show gray matter atrophy in the temporal lobe, either bilaterally (frontotemporal variant) or more predominantly on the right (SAN variant; Ranasinghe et al., 2016). In these patients, the atrophy pattern typically extends beyond the orbitofrontal cortex into the temporal pole, though temporal lobe atrophy may not be as extensive as is observed in svPPA patients (Rosen et al., 2002). Behaviorally, the svPPA and bvFTD patient groups show deficits in social behavior, though there is variation across patients in intensity and characteristics of social behavior changes. For example, bvFTD patients demonstrate disinhibited or apathic behavior and loss of empathy (Rascovsky et al., 2011). whereas svPPA patients have difficulties recognizing emotions or social cues, and thinking about others’ mental states, i.e., theory of mind (Eldaief et al., 2021).
In this study we hypothesize that social concepts are processed similarly to non-social concepts, in accordance with the and hub-and-spoke architecture proposed in the CSC model. We expect that the superior subregion of the ATL, in combination with frontal limbic structures, form the hub-and-spoke network that supports the ability to make meaning of social concepts. We also expect that the integration of domain-specific information for social concepts occurs in both the left and right ATL (i.e., symmetrical contribution). To test this, we used Voxel-Based Morphometry (VBM) to examine structural brain-behavior relationships in 152 patients with neurodegenerative disease in relation to a test that measures the ability to correctly match a social term (e.g., “belittling”) with a visual depiction of that social interaction. To enable whole brain gray matter atrophy analysis, we used a transdiagnostic approach that, in addition to patients with bvFTD and svPPA, included patients with alternate patterns of atrophy covering a broad selection of cortical regions, including patients with Alzheimer’s disease, corticobasal syndrome, progressive supranuclear palsy, logopenic primary progressive aphasia (lvPPA) and non-fluent variant primary progressive aphasia (nfvPPA). We compared gray matter atrophy in this sample with the social interaction vocabulary test (SIVT), a performance metric for social semantic knowledge, and we used the Boston naming test (BNT) as a control for general semantic knowledge, to identify differences in brain correlation between social and non-social semantic processing.
2. Methods
2.1. Participants
In this study, we included 152 patients with neurodegenerative disease and 19 older normal controls (NC), which resulted in and 171 participants in total. Prior to analysis we determined our sample based on diagnostic evaluations that were made at the University of California San Francisco (UCSF) Memory and Aging Center (MAC) during consensus meetings with a multidisciplinary team. Evaluations were based on the patient’s clinical history, physical examination, neuropsychological assessment and structural imaging. Only NCs with no cognitive or functional deficits, and unremarkable neurological exam and MRI scan were included. Patients with CDR® plus NACC FTLD Sum of Boxes scores higher than 12 were excluded. Our study included 12 patients that met the Alzheimer’s disease syndrome (AD) probable clinical criteria (McKhann et al., 2011), 56 patients that met the behavioral variant frontotemporal dementia (bvFTD) criteria (Rascovsky et al., 2011), 13 that met criteria of the progressive supranuclear palsy syndrome (PSPS) (Höglinger et al., 2017), 18 that met criteria of cortical basal syndrome (CBS) (Armstrong et al., 2013), and patients that met primary progressive aphasia criteria of the semantic variant (N=22), the non-fluent variant (N=16) and the logopenic variant (N=15) (Gorno-Tempini et al., 2011). This study was approved by the UCSF institutional review board for human research, and all participants confirmed voluntary participation to data collection by signing informed consent prior to testing.
2.2. Behavioral measures
2.2.1. Social Interaction Vocabulary Task (SIVT)
The SIVT is a multiple-choice picture/word task that was developed to measure semantic knowledge of social interactions (Rankin et al., submitted). The test consists of four stimulus pictures and one target word that describes a social concept. The examinee is asked to pick from the four stimulus pictures the picture that best describes the target word (e.g., “scolding”). To perform well on this task, the participant needs to be able to scan through their semantic knowledge register to compare the mental picture they have of the word to the pictures displayed in the test, i.e., matching internal and visually represented social semantic concepts.
Items for the test were selected based on the familiarity and concreteness of the words. Additionally, only words that are frequently used in the English language were selected. The test has three subscales, Easy, Medium and Hard, each consisting of six items. The Easy subscale consists of items with highest familiarity and concreteness scores, the Medium subscale of items with average familiarity and concreteness, and the Hard subscales of items with low familiarity and concreteness. The SIVT total score is calculated based on all 18 test stimuli, and this total was the primary performance metric used in this study.
2.2.2. Boston Naming Test (BNT)
The BNT is a well-validated picture vocabulary test of general semantic concept knowledge such as ‘helicopter’ or ‘mushroom’. The test consists out of 15 black and white line drawn pictures of concepts that range from more common (average daily use) to less common (Mack et al., 1992). In the current study, this test is used to compare general semantic knowledge with specifically social semantic knowledge measured by the SIVT. Missing data was imputed using Multivariate Imputation by Chained Equations (MICE ) by specifying age, sex, education, SIVT (sub)total scores, MMSE total score and CDR® plus NACC FTLD Sum of Boxes as variables of reference. Based on visual inspection MICE yielded acceptable imputations, see supplementary figure 1 (van Buuren and Groothuis-Oudshoorn, 2011).
2.2.3. CDR® Dementia Staging Instrument PLUS National Alzheimer’s Coordinating Center (NACC) Behavior and Language Domains (FTLD)
The CDR plus NACC FTLD is a semi-structured interview that helps characterize the severity of FTLD-related dementia by rating eight domains of cognitive capacity, which are memory, orientation, judgment and problem solving, community affairs, home and hobbies, personal care, behavior and language (Miyagawa et al., 2020). These domains are rated from normal (score of 0), minimally impaired (score of 0.5), mildly impaired (score of 1), moderately impaired (score of 2) and severely impaired (score of 3). To assess disease severity, the global CDR® plus NACC FTLD score was calculated using published scoring rules that assign weights to domains to ensure score optimization (Knopman et al., 2011, 2008; Miyagawa et al., 2020). The CDR® plus NACC FTLD Sum of Boxes was calculated using the total sum of the domains to facilitate equal evaluation of all domains.
2.2.4. Mini Mental State Examination
The MMSE is a brief screening test that uses dimensions such as orientation, attention, recall and language to assess severity of cognitive impairment. It is a highly verbal screening tool and is mainly sensitive to picking up lower to moderate levels of impairment (Tombaugh and McIntyre, 1992).
2.3. Structural neuroimaging and preprocessing steps
All participants underwent 3-T structural magnetic resonance imaging (MRI) on average 8 days before or after completing the SIVT, MMSE and CDR (M=8, SD=17) using a Magnetom scanner (Siemens Inc., Iselin). All structural T1-weighted images were acquired on 3T-scanners (Siemens Trio and Siemens Prisma) at the University of California, San Francisco. T1-weighted 3D magnetization prepared rapid gradient echo (MPRAGE) sequence was used to obtain the structural images, with acquisition parameters as follows: 160 sagittal slices, 1-mm thick, skip=0 mm; repetition time=2300 ms; echo time=2.98 ms; flip angle=9°; field of view=240×256mm2; voxel size=1mm3; matrix size=256×256. The structural T-1 weighted images were first visually inspected for movement artifacts and underwent bias-correction, segmentation into tissue compartments, and special normalization using standard SPM12 parameters. We used the default tissue probability maps for grey matter, white matter, cerebrospinal fluid, and all other voxels from SPM12 (TPM.nii) (Ashburner et al., 2016). Then, to optimize inter-subject registration each participant’s image was warped to a template of 300 confirmed neurologically normal older controls (ages 44-86, M±SD: 67.2±7.3; 113 males, 186 females) by using the diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) method. Next, the total volume of gray matter, white matter and CSF tissue compartments was derived using the corresponding MWS probability map for each individual, and total intercranial volume (TIV) was calculated by summing all compartments. Finally, the spatially normalized, segmented, and modulated gray matter images were smoothed with an 8-mm FWHM isotropic Gaussian kernel for use in VBM analysis (Acosta-Cabronero et al., 2007; Ashburner et al., 2016).
2.4. Statistical analyses
2.4.1. Behavioral data
Regression diagnostics were used to identify potential influence of age and sex on performance on SIVT Total, which were used as covariates of no interest. Prior to analysis, the SIVT total score was inspected for outliers exerting undue leverage, but none were detected. Differences among diagnostic groups or diagnostic groups and normal controls were explored for the global CDR® plus NACC FTLD score, CDR® plus NACC FTLD Sum of Boxes, MMSE total score, BNT total score, and SIVT total score using the SAS PROC GLM method (see table 1). Significant differences between diagnostic groups and controls were identified for the BNT and SIVT score and demographics using Dunnett-Hsu post-hoc tests, and differences between diagnostic groups for disease severity scores were examined using Tukey-Kramer post-hoc tests.
Table 1.
Demographics and clinical characteristics
| NC (N=19) |
AD (N=12) |
bvFTD (N=56) |
CBS (N=18) |
lvPPA (N=15) |
nfvPPA (N=16) |
PSP (N=13) |
svPPA (N=22) |
Total (N=171) |
p value |
η2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 56.8 (18.1) | 69.7 (7.5)** | 63.9 (9.1)* | 70.1 (10.4) * | 65.9 (9.7)* | 69.3 (6.4) * | 67.5 (7.3)* | 66.2 (7.4)* | 65.5 (10.5) | .0013 | .13 |
| Sex (M/F) | 11/8 | 7/5 | 37/19 | 13/5 | 19/6 | 5/11 | 6/7 | 8/14 | 98/79 | .14 | .06 |
| Education ◊ | 17.5 (3.4) | 16.2 (3.0) | 16.2 (2.8) | 15.9 (3.4) | 16.2 (2.5) | 17.1 (2.7) | 16.3 (2.4) | 15.7 (2.4) | 16.3 (2.8) | .49 | .05 |
| Global CDR® plus NACC FTLD score ◊ | 0.00 (0.00) | 1.29 (0.54) | 1.41 (0.60)† | 0.88 (0.37)† | 0.97 (0.35) | 0.89 (0.53)† | 1.33 (0.62) | 1.10 (0.49) | 1.21 (0.58) | .0016 | .17 |
| CDR® plus NACC FTLD Sum of Boxes ◊ | 0.0 (0.0) | 7.0 (2.8) | 7.5 (3.4)† | 4.6 (2.5)† | 5.6 (2.2) | 3.9 (2.8)† | 6.8 (3.0) | 5.8 (3.1) | 6.4 (3.4) | < .001 | .20 |
| MMSE total | 27.0 (NA) | 21.0 (5.7) | 24.2 (4.8) | 23.5 (5.7) | 20.0 (5.5)† | 26.3 (4.0)† | 24.6 (4.2) | 21.6 (5.6) | 23.2 (5.2) | .013 | .13 |
| BNT total ◊◊ | 10 (4.6) | 9.3 (3.2) | 12.1 (2.8) | 12.2 (3.7) | 8.9 (3.6) | 13.5 (1.8)* | 14.1 (1.1)* | 3.4 (3.0)** | 10.6 (4.4) | < .001 | 0.52 |
| SIVT total | 17.1 (0.5) | 15.5 (2.3) | 15.2 (3.3) | 15.4 (3.7) | 15.6 (2.1) | 15.9 (2.3) | 15.0 (3.8) | 14.3 (3.3)* | 15.3 (3.2) | .28 | .06 |
Abbreviations: AD = Alzheimer’s disease; bvFTD = behavioral variant frontotemporal dementia; CBS = corticobasal syndrome; lvPPA = logopenic variant primary progressive aphasia; nfvPPA = non-fluent variant primary progressive aphasia; PSP = progressive supranuclear palsy; svPPA = semantic variant primary progressive aphasia; NACC = National Alzheimer’s Coordinating Center: CDR plus NACC FTLD = CDR Dementia Staging Instrument plus Behavior and Language domains from the NACC Frontotemporal Lobar Degeneration Module. Analyses are controlled for age and sex if appropriate.
Different from normal controls at p <.05 using Dunnett-Hsu tests.
Different from normal controls at p <.001 using Dunnett-Hsu tests.
Different from other disease group(s) at p <.05 using Tukey-Kramer tests.
Different from other disease group(s) at p <.001 using Tukey-Kramer tests.
N = 26 missing.
17% missing data, MICE imputed
2.4.2. Neuroimaging data
The correlation strength between test performance and whole-brain atrophy derived with VBM analysis is limited by the level of variance that exists in a group. This has important implications for studying groups with high atrophy regions such as dementia patients, since high levels of atrophy results in low levels of voxel-wise variance, which limits the emergence of this region in correlation analysis. To account for the influence of the level of variance, we generated voxel-wise variance maps based on the entire group.
Next, VBM was conducted over the entire sample to identify how the SIVT total score predicted gray matter volume. Voxel intensity was summed for the whole sample group, and linear regressions were used to model the predictive value of the SIVT total score on each voxel’s gray matter volume. In the main analysis, control variables sex, age and TIV were included as covariates of no interest. To compare the atrophy patterns correlated with social semantics to non-social semantics, we visually inspected the correlation between gray matter volume and performance on the BNT. The BNT total score was also included to the main analysis as an additional covariate. Next, we ran an error-check analysis using the CDR® plus NACC FTLD score to inspect influence of disease severity on the main effect. Family-wise error correction was used to identify the lower bound of the significance threshold for the VBM analysis. In this correction, the maximum t-values of the imaging data compared to behavioral data were re-sampled using the Monte Carlo approach, which includes a 1000 permutations of the error distribution. This was used to create a custom map of the error distribution based on the data set, i.e., distribution of maximum t-values when no true relationship exists. The t-value at the 95th percentile of this distribution was taken as the custom cut-off threshold, rendering t-values on or above this cut-off significant at a family-wise error corrected level of p < .05 (Kimberg et al., 2007).
We also plotted SIVT performance against volume of the highest max T regions for the entire sample (N = 171) to control for co-atrophy effects by illustrating how each diagnostic group contributes to the main effect, and we used whole brain VBM to identify the pattern of gray matter volume reduction in the bvFTD and svPPA disease groups specifically, by dummy coding the diagnostic groups against healthy controls.
3. Results
3.1. Demographics and clinical characteristic
Compared to normal controls, svPPA patients performed significantly worse on the SIVT (p = .013) and bvFTD patients showed a trend towards significance (p = .074). The svPPA patients also performed significantly worse on the BNT compared to normal controls (p < .001), as well as PSP patients (p = .007, table 1). All patient groups were significantly older than the normal control group. For the patient groups, the Global CDR® plus NACC FTLD score ranged between an average of 0.9 to 1.4, indicating that the patients in our sample had mild dementia. Pairwise comparison between diagnostic groups showed that on the Global CDR® plus NACC FTLD, bvFTD patients scored significantly higher than CBS (p = .0076) and nfvPPA (p = .039). The CDR® plus NACC FTLD Sum of Boxes score showed a similar pattern, where bvFTD patients scored significantly higher than CBS (p = .0052) and nfvPPA (p = .0081). On the MMSE, only lvPPA patients scored significantly lower than nfvPPA patients (p = .039).
3.2. Neuroimaging results
3.2.1. Group voxel-wise variance map
The whole-brain gray matter variance map revealed higher variance in regions that are typically affected in our patient cohort, such as the bilateral anterior insula, thalamus, caudate, bilateral temporal pole, bilateral hippocampus and bilateral entorhinal area, see supplementary figure 1. These findings suggest that the relative higher level of variance is driven by the difference in atrophy patterns between the neurodegenerative syndromes included in this study, which is difficult to avoid in groups with widespread brain atrophy. Furthermore, the results do not indicate evident signs of floor effects; besides higher variance regions, the level of variance is adequately spread across the brain.
3.2.2. Gray matter volume predicting SIVT score
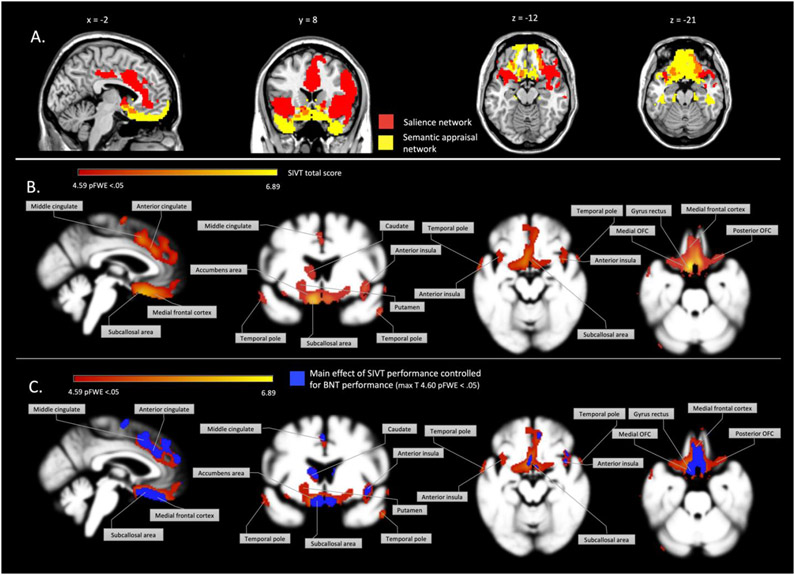
Using whole-brain VBM analysis to model brain regions with a significant linear relationship to SIVT Total score, we found that lower scores predicted lower gray matter volume predominantly in the subgenual anterior cingulate cortex (sgACC), medial frontal cortex and gyrus rectus, posterior and medial orbitofrontal cortex (OFC), middle and anterior cingulate cortex, anterior insula, polar, superior, middle and inferior (anterior) temporal cortex, nucleus accumbens, putamen and left head of the caudate (pFWE < .05), see figure 1B and table 2. Weaker but significant involvement was also found for the posterior insula, thalamus, lateral OFC, fusiform gyrus and supplementary motor cortex. Volume reduction in the right hemisphere predicted SIVT scores more for frontal regions and bilateral for temporal regions. Volume reduction showed a statistically stronger relationship in the right hemisphere for the majority of the frontal structures, and bilateral involvement for temporal structures. Furthermore, these results do not mimic the voxel-wise variance map, which indicates that we have evenly distributed variance (supplementary figure 1). Thus, it is unlikely that important regions were left out of this result due to inadequate variance of atrophy in our patient sample.
Figure 1.
structural atrophy related to SIVT total score. A. Visualization of the intrinsically connected semantic appraisal network identified by Yeo and colleagues (2011) and the salience network identified by Toller and colleagues (2018) that highly overlap with the VBM results. The limbic (here referred to as the semantic appraisal) is represented in light yellow, and salience networks in magenta. B. Gray matter volumes that significantly correlate with performance on the SIVT (yellow-red spectrum). C. Main effect of gray matter volume correlated with SIVT performance (yellow-red spectrum), overlayed with the main analysis controlled for BNT performance (in blue).
Table 2.
Atrophy in structures related to social semantic processing
| Left hemisphere | Right hemisphere | |||||||
|---|---|---|---|---|---|---|---|---|
| Max T | x | y | z | Max T | x | y | z | |
| Frontal | ||||||||
| Subgenual cingulate gyrus | 6.89 | −6 | 12 | −18 | 6.34 | 4 | 18 | −22 |
| Medial frontal cortex | 6.05 | −3 | 21 | −18 | 6.20 | 3 | 21 | −21 |
| Gyrus rectus | 5.86 | −6 | 19 | −22 | 6.21 | 3 | 21 | −22 |
| Medial orbital gyrus | 5.83 | −13 | 21 | −25 | 5.40 | 9 | 19 | −21 |
| Anterior cingulate gyrus | 5.49 | −2 | 28 | 24 | ||||
| Posterior orbital gyrus | 5.42 | −24 | 18 | −22 | 5.09 | 21 | 19 | −22 |
| Superior frontal gyrus medial segment | 5.16 | −1 | 37 | 31 | 5.26 | 3 | 31 | −13 |
| Orbital part of the inferior frontal gyrus | 4.82 | 39 | 22 | −10 | ||||
| Central operculum | 4.81 | 43 | −6 | 7 | ||||
| Frontal operculum | 4.80 | 40 | 13 | 1 | ||||
| Precentral gyrus | 4.73 | 54 | 1 | 40 | ||||
| Lateral orbital gyrus | 4.61 | −36 | 24 | −9 | 4.76 | 37 | 24 | −10 |
| Temporal | ||||||||
| Anterior insula | 4.96 | −33 | 12 | −13 | 5.81 | 39 | 12 | −6 |
| Middle temporal gyrus | 5.57 | −58 | −6 | −28 | 5.06 | 55 | 4 | −28 |
| Temporal pole | 5.04 | −54 | 12 | −16 | 5.38 | 52 | 9 | −30 |
| Superior temporal gyrus | 5.24 | −58 | 1 | −12 | 4.81 | 58 | 7 | −9 |
| Inferior temporal gyrus | 5.26 | −49 | −7 | −39 | 4.65 | 54 | −7 | −36 |
| Posterior insula | 4.62 | −42 | −10 | −6 | 4.82 | 43 | −6 | 6 |
| Fusiform gyrus | 4.68 | −40 | −40 | −27 | 4.64 | 39 | −39 | −28 |
| Subcortical | ||||||||
| Accumbens Area | 5.71 | −10 | 7 | −13 | 5.12 | 9 | 7 | −13 |
| Putamen | 5.25 | −15 | 6 | −13 | 5.05 | 24 | 7 | −9 |
| Caudate | 5.09 | −12 | 3 | 12 | ||||
| Thalamus | 4.73 | −10 | −3 | 13 | ||||
| Parietal | ||||||||
| Middle cingulate gyrus | 5.64 | −1 | 13 | 37 | 5.05 | 1 | 15 | 39 |
| Supplementary motor cortex | 5.53 | −3 | −6 | 58 | 5.05 | 1 | 18 | 42 |
Visual inspection of brain/behavior relationships with the BNT revealed that predominantly the left temporal lobe correlates strongly with BNT performance. The main effect, i.e., correlation of gray matter volume with the SIVT remained very similar when adding the BNT as an additional covariate in VBM analysis, though the max-T was reduced after family wise error-correction. After this correction, primarily involvement of the temporal lobes fell below the cut-off (max T = 4.60, see Supplementary figure 2C). To error-check whether disease severity had an influence on the main effect, we ran a VBM that included the CDR® plus NACC FTLD score as an additional covariate, and found similar results when adding the BNT as a covariate. The main effect remained present, though the overall effect was reduced in strength. These results indicate that neither general semantic knowledge or disease severity have significant influence on the main effect, and illustrates that inclusion of an additional control variable reduces the variance between brain/behavior relationships and therefore the strength of the effects.
Additionally, by plotting the relationship of each group (N = 171) between SIVT performance and volume of the most significant Neuromorphometric region, we found that there is no clear co-atrophy effect present, i.e., no single diagnostic group membership seems to drive the main effect (supplementary figure 3). Then, for informational purposes, we illustrated how these specific atrophy patterns of bvFTD and svPPA patients relates to the relationship between gray matter atrophy and SIVT total score, we overlayed patient patterns with SIVT results (see supplementary figure 4).
4. Discussion
In this paper, we show that additional to anterior temporal structures known to mediate semantic knowledge, gray matter volume in paralimbic structures such as the sgACC, OFC, anterior cingulate, and insula provide key input to allow comprehension of vocabulary in the socioemotional domain. We also show that volume in both left and right ATL structures correlate with correct identification of these social concepts. The contribution of these regions to the recognition of social concepts remained after controlling for performance on a non-social semantic task, which suggests that these structures make a unique contribution to social concept processing. These results provide support for the hub-and-spoke theory of semantic processing (Lambon Ralph et al., 2010b; Patterson et al., 2007; Rogers et al., 2004), suggesting that hubs in the left and right temporal cortex and spokes in frontal limbic areas aid social concept understanding.
4.1. Importance of personalized hedonic evaluations for accurate social concept identification
The sgACC, striatum, and OFC all play different roles in the evaluation system of the brain. The sgACC and dorsal striatum (the head of the caudate and putamen) are involved in reward expectation, where the putamen helps associate actions with reward, while the caudate nucleus and the sgACC identify the difference between predicted reward and received reward, i.e., prediction error (O’Doherty et al., 2004). The ventral striatum, specifically the nucleus accumbens (NA) is suggested to play an important role in motivation by predicting the amount of reward that will become available when conducting a certain action (Liljeholm and O’Doherty, 2012; Salgado and Kaplitt, 2015). During anticipation of small rewards the level of activity in the NA is lower than when anticipating larger rewards, indicating that NA activity is proportional to the intensity of anticipated reward (Schmidt et al., 2012). These reward prediction processes support the ventromedial PFC and OFC in evaluating the hedonic value of a goal, and together this system drives the selection of the action that an individual judges to be most beneficial to them (O’Doherty, 2011; Rudebeck and Rich, 2018; Wilson et al., 2014). These neurologic structures also are capable of rapidly updating these reward computations, allowing his selection process to be adjusted moment-by-moment depending on situational factors (O’Doherty et al., 2021). During evaluation of these outcomes, the medial portion of the OFC seems to be more sensitive to positive or rewarding outcomes, whereas the lateral portion seems more sensitive for punishment (O’Doherty et al., 2001; O’Doherty, 2007; Ursu and Carter, 2005). A similar spatial specialization seems to exist on the posterior-anterior axis, where primary reinforcers are generally processed more posteriorly and secondary reinforcers more anteriorly (Sescousse et al., 2010), though these dichotomies likely underestimate the complexity of reinforcer representations in the OFC.
These frontal limbic structures are structurally tightly connected with regions in the temporal cortex through the UF white matter tract. The bidirectional connectedness between the ATLs and frontal orbital regions likely allows for dynamic adaptation of (social) concepts in the temporal lobes based on value provided by the frontal orbital regions (von der Heide et al., 2013). By investigating task-free functional brain connectivity, Yeo and colleagues (2011) identified these structures as part of an intrinsically connected network they referred to as the “limbic network”, which we and others have termed the semantic appraisal network (SAN), (Seeley et al., 2012b; Toller et al., 2019; Yang et al., 2021). This network is selectively targeted in svPPA and some bvFTD patients, as is described by Seeley and colleagues (2009). Generally, the SAN is defined as having major hubs in the polar region of the bilateral ATLs and frontal limbic structures, along with subcortical structures including the head of the caudate, putamen, nucleus accumbens, and amygdala bilaterally (Rankin, 2020; Yeo et al., 2011). As is displayed in figure 1 a and b, these SAN structures highly overlap with structures that we identified in our study to be important for correctly recognizing social interaction words. Because of the role of these main hubs in making value-based judgements (e.g., OFC and striatum) as well as in the integration of information in bilateral ATLs to create coherent semantic concepts, the SAN functions to provide a personalized hedonic value to semantic concepts. In other words, it attaches ‘emotional’ weight to semantic representations, which may be particularly important for socioemotional concepts (Yang et al., 2021). Our study’s brain-behavior results showing that these structures are involved in assigning meaning to social concepts is consistent with this theoretical understanding of the role of the SAN, with both right and left temporal poles seeming to contribute equally.
4.2. Relevance of sensitivity to social cues for accurate identification of social concepts
Our results also underline the role of the anterior insula and dorsal ACC in social concept identification. These structures are reciprocally connected, and both contain neuroanatomically distinct Von Economo and fork neurons (Seeley et al., 2012a), which are proposed to transmit neuronal signals more rapidly due to their longer axon size (Allman et al., 2010). The insula is a major afferent hub that is known to receive signals from the autonomic nervous system through the posterior part of the insula, and brings this autonomic state of the body to awareness through medial and anterior parts (Uddin et al., 2017). The dorsal portion of the ACC plays an important role in cognitive control by generating continuous predictions about the cognitive load that actions will require, which supports optimization of behavioral responses (Sheth et al., 2012). Combined, these nodes are the main hubs of the salience network, another intrinsically connected network of which the primary function is to apply selective attention to stimuli (external or internal) that are acutely relevant for an individual’s safety, security, or survival (Seeley et al., 2007; Uddin, 2015). Similar to the SAN, the SN can be identified in healthy brain functioning (Yeo et al., 2011) and is selectively targeted in disease, particularly in bvFTD syndrome (Seeley et al., 2009). Behaviorally, bvFTD patients present with prominent disturbances in social behavior such as social inappropriateness, disinhibition, apathy, and lack of empathic sensitivity. These research results, which directly connect SN with behavior, indicate that the break-down of selective attention processes that enable discrimination of subtle social cues has substantial influence on social functioning. As illustrated in figure 1 a and b, the ACC and insula of the SN were identified in this study as being involved in understanding social interaction concepts. This suggests that selective attention to subtle social cues is important when linking linguistic and visual representations of a social concept.
4.3. Impaired identification of social concepts in neurodegenerative disease
In our study, the ability to correctly identify social interaction concepts was significantly impaired in neurodegenerative disease patients with svPPA and (trend towards) bvFTD. To elucidate how the overall disease-related atrophy patterns typical of each patient group corresponds with the atrophy patterns in our results, we overlayed atrophy maps of the svPPA (green) and bvFTD (blue) patient groups with our VBM maps of SIVT performance (see supplementary figure 4). This revealed that both disease groups have substantial overlap with the structures important for social concepts. Specifically, there is overlap in the sgACC, medial, lateral, and posterior OFC, insula, caudate, and inferior and polar regions of the temporal cortex, with more overlap in the left hemisphere for svPPA patients, and more bilateral overlap for bvFTD patients. Although aberrations in social behavior are foundational for bvFTD disease diagnosis (Rascovsky et al., 2011), disturbances in social behavior are also increasingly recognized in svPPA. For example, a meta-analysis of social cognition in svPPA showed that, independent from language comprehension levels, emotion recognition, theory of mind, and empathy processes are impaired (Fittipaldi et al., 2019). A similar pattern exists for a subgroup of bvFTD patients with focal degeneration only in SAN nodes, sometimes called “right temporal” FTD patients, who show impairments in emotion naming and perspective taking (Ranasinghe et al., 2016). Our results linking lesions in the SAN with an impaired ability to correctly identify social concepts suggest that the hedonic evaluation processes mediated by the SAN play a significant and somewhat under-reported role in the social function deficits observed in these neurodegenerative syndromes.
4.4. Relationship with previous work on social concepts generation
Research investigating social concept generation requires a clear understanding of how social concepts are different from other, non-social concepts. To establish a better working definition of social versus non-social words, Diveica and colleagues (2022) quantified the ‘socialness’ of words through study participant ratings. From a range of lexical, semantic and affective properties, socialness ratings correlated most with the valence, arousal and abstractness of words. While on a continuum, the majority of social concepts seem more abstract and higher in valence. In addition, increased socialness predicted a decrease in time required to recognize a word. These results validate that social concepts can be quantitively distinguished from non-social concepts. This difference can also be observed in our study, where the anatomical correlates of a semantic task with no social content, the BNT, are different from the anatomical correlates of a social semantic task, the SIVT. Mainly the left temporal lobe correlated with performance on the BNT, and unlike brain correlation with the SIVT, no orbitofrontal structures were involved.
Neural systems that represent social semantic concepts, with varying levels of specificity, have been investigated in previous work using different forms of task design and imaging techniques, which resulted in different brain-behavior outcomes. For example, fMRI studies contrasting positive-social, negative-social, and non-social (animal) concepts reported activation in the bilateral superior and polar ATL, as well as OFC, medial PFC and the temporoparietal junction (TPJ) (Ross and Olson, 2010; Zahn et al., 2007). In a different fMRI task participants were asked to decide how semantically related social versus non-social words were, which activated regions in the dorsomedial and ventromedial PFC, posterior cingulate cortex, middle temporal cortex, and TPJ (Contreras et al., 2012; Wang et al., 2019). A study of social action-verb matching used fMRI to show the involvement of the bilateral ATLs, TPJ, inferior frontal gyrus/OFC, and ventromedial PFC/sgACC (Lin et al., 2015). In an attempt to reconcile these different studies, Arioli and colleagues (2020) performed a meta-analysis. While they discriminated similar brain regions in i.e., the superior and medial frontal gyrus, middle temporal gyrus, PCC, ACC and precuneus, they noted that large differences in task design between studies has complicated the ability to identify a ‘core’ social semantic representation system. Besides task design, many studies use a functional model of brain-behavior relationships, while in this study we used a structural design. Conventional fMRI designs may have the disadvantage of signal loss in the ATLs due to its close location to bone and air in the brain (Olman et al., 2009; Visser et al., 2010), which may explain discrepancies within functional imaging approaches as well as between structural and functional imaging results. This limitation can however be overcome by using analysis sequences optimized to increase signal in the ATL (Binney et al., 2016; Persichetti et al., 2021; Visconti di Oleggio Castello et al., 2021).
4.5. Limitations and Conclusions
Our study was limited by the usual issues involved with VBM analyses of brain-behavior correlations, such as the fact that structural damage leading to volume loss does not necessarily have a direct correspondence with functional changes. Furthermore, we did maximize variability of affected brain regions in our sample by including neurodegenerative disease patients with diverse syndromes and performed additional error check analyses to confirm that we had adequate variance across the brain, particularly in temporal and frontal regions, but there is always the possibility that our use of the VBM method failed to reveal additional regions also involved in social concept processing. Another limitation inherent in this study is that the SIVT, as is the case with many language tasks, is subject to ceiling effects in healthy individuals and patients with no semantic loss for social concepts. However, we performed error checks and found that despite this ceiling effect, performance was variable enough across patients and across patient groups that linear modeling of brain-behavior relationships was still appropriate.
In future studies of this topic, more realistic test paradigms, such as task based functional MRI using videos, may better reflect the mechanisms by which large quantities of information are integrated by the semantic representation system in real life. Using more naturalistic social stimuli for experiments may help us understand on a more granular level which parts of the brain provide domain-specific (i.e., spoke-level) information for a concept, and perhaps reveal the neural mechanisms behind how domain-specific elements combine to create concepts that are weakly or strongly semantically connected. Such naturalistic approaches would also better enable insight into the dynamic temporal influence that hubs of different intrinsically connected networks have on each other. As our study further elucidated, the SAN and major hubs of the SN play important roles in social concept generation, potentially stemming from the causal influence that the SN has on downstream networks such as the default mode and frontoparietal networks (Chiong et al., 2013; Rijpma et al., 2021; Uddin, 2015). Analysis of directional influence (i.e., effective connectivity) between main hubs of the SN and SAN networks during social concept processing can clarify how neural information flow contributes to accurate semantic cognition. Furthermore, investigating the directionality of intra-network information flow, specifically through the UF tract, would give the opportunity to understand how dynamics between bottom-up and top-down processes contribute to the formation and optimization of (social) semantic concepts. Additionally, the CSC framework’s control system (Lambon Ralph et al., 2017), which is hypothesized to support generation of semantic representations through executive control and working memory processes, could also be evaluated using more naturalistic social cognition tasks to examinate the directional connections between the adaptive (frontoparietal) and stable (task control) networks.
Taken together, this study shows that the role of the SAN in attaching hedonic value to semantic representations seems to be particularly important for the accuracy of social concept generation, and damage to regions comprised in this network is a key mechanism for social deficits observed in some neurodegenerative syndromes like bvFTD and svPPA. Our results further support the hub-and-spoke framework as the neural organization underlying semantic cognition, and social semantics in particular, since connections between the frontal limbic and subcortical regions with the ATL were required for social comprehension on our task, likely because these spoke regions convey unique category-specific information for the generation of social concepts. Furthermore, our finding that both the left and right ATL were involved in social concept identification is consistent with the theory that this hub-and-spoke semantic system is bilateral and graded (Lambon Ralph et al., 2017).
Supplementary Material
Highlights.
Brain structures that assign value to information predict comprehension of social concepts
Both left and right anterior temporal lobes seem involved in processing semantic information
Semantic and behavioral variant frontotemporal dementia patients show deficits in identifying social interaction words
The identified atrophy patterns support the hub-and-spoke model
The identified atrophy patterns overlap with key regions of the semantic appraisal network
Acknowledgements
Voxel-based morphometry analyses were performed using the UCSF Brainsight system, developed at the UCSF Memory and Aging Center by Katherine P. Rankin, Cosmo Mielke, and Paul Sukhanov, and powered by the VLSM script written by Stephen M. Wilson, with funding from the Rainwater Charitable Foundation and the UCSF Chancellor’s Fund for Precision Medicine. We would furthermore like to thank all participants, their family members and the volunteers that participated in this research, and the project’s research coordinators who helped us with data collection.
Funding
This work was supported by the National Institutes of Health under Grant [numbers 2RF1AG029577-11 (PI: Rankin), P01AG019724 (PI: Miller), P30AG062422-01 (PI: Miller)]; Larry L. Hillblom foundation under Grant [number 2002/2j (PI: Rankin), 2014-A-004-NET (PI: Kramer)].
Footnotes
Declarations of interest: none.
Pre-registration
No part of the study procedures or analyses was pre-registered prior to the research being conducted.
Data availability
All code used for the analyses is available at https://github.com/MyrtheGwenRijpma/SIVT_VBM_project.git. The participant data cannot be placed in an open archive because it is not permitted under the study’s IRB approval due to the sensitive nature of patients’ data. However, all data is available to any interested research via a request submitted through a public-facing resource request portal at: http://memory.ucsf.edu/resources/data. Following a UCSF-regulated procedure, access will be granted to designated individuals in line with ethical guideline on the reuse of sensitive data. This would require submission of the Material Transfer Agreement, available at https://icd.ucsf.edu/material-transfer-and-data-agreements.
References
- Acosta-Cabronero J, Williams GB, Pereira JMS, Pengas G, Nestor PJ, 2007. The impact of skull-stripping and radio-frequency bias correction on grey-matter segmentation for voxel-based morphometry. Neuroimage 39, 1654–1665. 10.1016/j.neuroimage.2007.10.051 [DOI] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR, 2010. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct 214, 495–517. 10.1007/s00429-010-0254-0 [DOI] [PubMed] [Google Scholar]
- Arioli M, Gianelli C, Canessa N, 2020. Neural representation of social concepts: a coordinate-based meta-analysis of fMRI studies. Brain Imaging and Behavior 2020 1–10. 10.1007/s11682-020-00384-6 [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich Stephen G., Riley DE, Tolosa E, Tröster AI, Vidailhet M, Weiner WJ, 2013. Criteria for the diagnosis of corticobasal degeneration. Neurology 80, 496–503. 10.11477/mf.1416200168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Barnes G, Chen C, Deaunizeau J, Flandin G, Friston K, Kiebel S, Kilner J, Litvak V, Moran R, Penny W, 2016. SPM12 manual [WWW Document]. URL http://www.fil.ion.ucl.ac.uk/spm/doc/spm12manual [Google Scholar]
- Binney RJ, Hoffman P, Lambon Ralph MA, 2016. Mapping the Multiple Graded Contributions of the Anterior Temporal Lobe Representational Hub to Abstract and Social Concepts: Evidence from Distortion-corrected fMRI. Cerebral Cortex 26, 4227–4241. 10.1093/cercor/bhw260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Ramsey R, 2020. Social Semantics: The role of conceptual knowledge and cognitive control in a neurobiological model of the social brain. Neurosci Biobehav Rev 112, 28–38. 10.1016/j.neubiorev.2020.01.030 [DOI] [PubMed] [Google Scholar]
- Chiong W, Wilson SM, D’Esposito M, Kayser AS, Grossman SN, Poorzand P, Seeley WW, Miller BL, Rankin KP, 2013. The salience network causally influences default mode network activity during moral reasoning. Brain 136, 1929–1941. 10.1093/brain/awt066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JA, Montal V, Hochberg D, Quimby M, Mandelli ML, Makris N, Seeley WW, Gorno-Tempini ML, Dickerson BC, 2017. Focal temporal pole atrophy and network degeneration in semantic variant primary progressive aphasia. Brain 140, 457–471. 10.1093/brain/aww313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JM, Banaji MR, Mitchell JP, 2012. Dissociable neural correlates of stereotypes and other forms of semantic knowledge. Soc Cogn Affect Neurosci 7, 764–770. 10.1093/scan/nsr053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Brosch T, 2012. Motivational salience: Amygdala tuning from traits, needs, values, and goals. Curr Dir Psychol Sci 21, 54–59. 10.1177/0963721411430832 [DOI] [Google Scholar]
- Diveica V, Pexman PM, Binney RJ, 2022. Quantifying social semantics: An inclusive definition of socialness and ratings for 8388 English words. Behav Res Methods, 10.3758/s13428-022-01810-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldaief MC, Perez DL, Quimby M, Hochberg D, Touroutoglou A, Feldman Barrett L, Dickerson BC, 2021. Atrophy in Distinct Corticolimbic Networks Subserving Socioaffective Behavior in Semantic Variant Primary Progressive Aphasia. Dement Geriatr Cogn Disord 49, 589–597. 10.1159/000511341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittipaldi S, Ibanez A, Baez S, Manes F, Sedeno L, Garcia AM, 2019. More than words: Social cognition across variants of primary progressive aphasia. Neurosci Biobehav Rev 100, 263–284. 10.1016/j.neubiorev.2019.02.020 [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M, 2011. Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014. 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser J, Koenigs M, 2018. The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol Psychiatry 83, 638–647. 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Müller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I, Wenning GK, Höglinger GU, Morris HR, Litvan I, Kassubek J, Corvol JC, Whitwell JL, Levin J, van Swieten J, Bhatia KP, Josephs KA, Seppi K, Golbe LI, Grossman M, Dodel R, Lorenzl S, van Eimeren T, Arzberger T, Müller U, Poewe W, Oertel WH, Compta Y, Bordelon Y, 2017. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Movement Disorders 32, 853–864. 10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RL, Hoffman P, Pobric G, Lambon Ralph MA, 2016. The semantic network at work and rest: Differential connectivity of anterior temporal lobe subregions. Journal of Neuroscience 36, 1490–1501. 10.1523/JNEUROSCI.2999-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, 2010. Functional specificity in the human brain: A window into the functional architecture of the mind. Proc Natl Acad Sci U S A 107, 11163–11170. 10.1073/pnas.1005062107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF, 2007. Power in Voxel-based Lesion – Symptom Mapping. J Cogn Neurosci 19, 1067–1080. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, Miller BL, Mercaldo N, 2008. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 131, 2957–2968. 10.1093/brain/awn234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Weintraub S, Pankratz VS, 2011. Language and behavior domains enhance the value of the clinical dementia rating scale. Alzheimer’s and Dementia 7, 293–299. 10.1016/j.jalz.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Cipolotti L, Manes F, Patterson K, 2010a. Taking both sides: Do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain 133, 3243–3255. 10.1093/brain/awq264 [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Jefferies E, Patterson K, Rogers TT, 2017. The neural and computational bases of semantic cognition. Nat Rev Neurosci 18, 42–55. 10.1038/nrn.2016.150 [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Sage K, Jones RW, Mayberry EJ, 2010b. Coherent concepts are computed in the anterior temporal lobes. Proc Natl Acad Sci U S A 107, 2717–2722. 10.1073/pnas.0907307107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeholm M, O’Doherty JP, 2012. contributions of the striatum to learning, motivation, and performance: An associative account. Trends Cogn Sci 16, 467–475. 10.1016/j.tics.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N, Bi Y, Zhao Y, Luo C, Li X, 2015. The theory-of-mind network in support of action verb comprehension: Evidence from an fMRI study. Brain Lang 141, 1–10. 10.1016/j.bandl.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, Henderson VW, 1992. Boston Naming Test: Shortened versions for use in Alzheimer’s disease. Journals of Gerontology 47, 154–158. 10.1093/geronj/47.3.P154 [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, 2011. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia 7, 263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa T, Brushaber D, Syrjanen J, Kremers W, Fields J, Forsberg LK, Heuer HW, Knopman D, Kornak J, Boxer A, Rosen HJ, Boeve BF, Appleby B, Bordelon Y, Bove J, Brannelly P, Caso C, Coppola G, Dever R, Dheel C, Dickerson B, Dickinson S, Dominguez S, Domoto-Reilly K, Faber K, Ferrell J, Fishman A, Fong J, Foroud T, Gavrilova R, Gearhart D, Ghazanfari B, Ghoshal N, Goldman JS, Graff-Radford J, Graff-Radford N, Grant I, Grossman M, Haley D, Hsiung R, Huey E, Irwin D, Jones D, Jones L, Kantarci K, Karydas A, Kaufer D, Kerwin D, Kraft R, Kramer J, Kukull W, Litvan I, Lucente D, Lungu C, Mackenzie I, Maldonado M, Manoochehri M, McGinnis S, McKinley E, Mendez MF, Miller B, Multani N, Onyike C, Padmanabhan J, Pantelyat A, Pearlman R, Petrucelli L, Potter M, Rademakers R, Ramos EM, Rankin K, Rascovsky K, Roberson ED, Rogalski E, Sengdy P, Shaw L, Tartaglia MC, Tatton N, Taylor J, Toga A, Trojanowski JQ, Wang P, Weintraub S, Wong B, Wszolek Z, 2020. Utility of the global CDR® plus NACC FTLD rating and development of scoring rules: Data from the ARTFL/LEFFTDS Consortium. Alzheimer’s and Dementia 16, 106–117. 10.1002/alz.12033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ, 2004. Dissociable Role of Ventral and Dorsal Striatum in Instrumental Conditioning. Science (1979) 304, 452–454. 10.1126/science.1094285 [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C, 2001. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci 4, 95–102. 10.1038/82959 [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, 2011. Contributions of the ventromedial prefrontal cortex to goal-directed action selection. Ann N Y Acad Sci 1239, 118–129. 10.1111/j.1749-6632.2011.06290.x [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, 2007. Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Ann N Y Acad Sci 1121, 254–272. 10.1196/annals.1401.036 [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Rutishauser U, Iigaya K, 2021. The hierarchical construction of value. Curr Opin Behav Sci 41, 71–77. 10.1016/j.cobeha.2021.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olman CA, Davachi L, Inati S, 2009. Distortion and signal loss in medial temporal lobe. PLoS One 4. 10.1371/journal.pone.0008160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, McCoy D, Klobusicky E, Ross LA, 2013. Social cognition and the anterior temporal lobes: A review and theoretical framework. Soc Cogn Affect Neurosci 8, 123–133. 10.1093/scan/nss119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papinutto N, Galantucci S, Mandelli ML, Gesierich B, Jovicich J, Caverzasi E, Henry RG, Seeley WW, Miller BL, Shapiro KA, Gorno-Tempini ML, 2016. Structural connectivity of the human anterior temporal lobe: A diffusion magnetic resonance imaging study. Hum Brain Mapp 37, 2210–2222. 10.1002/hbm.23167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual B, Masdeu JC, Hollenbeck M, Makris N, Insausti R, Ding SL, Dickerson BC, 2015. Large-scale brain networks of the human left temporal pole: A functional connectivity MRI study. Cerebral Cortex 25, 680–702. 10.1093/cercor/bht260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT, 2007. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 8, 976–987. 10.1038/nrn2277 [DOI] [PubMed] [Google Scholar]
- Persichetti AS, Denning JM, Gotts SJ, Martin A, 2021. A data-driven functional mapping of the anterior temporal lobes. Journal of Neuroscience 41, 6038–6049. 10.1523/JNEUROSCI.0456-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut DC, 2002. Graded modality-specific specialisation in semantics: A computational account of optic aphasia, Cognitive Neuropsychology. 10.1080/02643290244000112 [DOI] [PubMed] [Google Scholar]
- Ranasinghe KG, Rankin KP, Pressman PS, Perry DC, Lobach I. v., Seeley WW, Coppola G, Karydas AM, Grinberg LT, Shany-Ur T, Lee SE, Rabinovici GD, Rosen HJ, Gorno-Tempini ML, Boxer AL, Miller ZA, Chiong W, DeMay M, Kramer JH, Possin KL, Sturm VE, Bettcher BM, Neylan M, Zackey DD, Nguyen LA, Ketelle R, Block N, Wu TQ, Dallich A, Russek N, Caplan A, Geschwind DH, Vossel KA, Miller BL, 2016. Distinct subtypes of behavioral variant frontotemporal dementia based on patterns of network degeneration. JAMA Neurol 73, 1078–1088. 10.1001/jamaneurol.2016.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, 2020. Brain Networks Supporting Social Cognition in Dementia. Curr Behav Neurosci Rep 203–211. 10.1007/s40473-020-00224-3 [DOI] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EGP, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL, 2011. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GE, Hoffman P, Lambon Ralph MA, 2015a. Graded specialization within and between the anterior temporal lobes. Ann N Y Acad Sci 1359, 84–97. 10.1111/nyas.12951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GE, Lambon Ralph MA, Hoffman P, 2015b. The roles of left versus right anterior temporal lobes in conceptual knowledge: An ALE meta-analysis of 97 functional neuroimaging studies. Cerebral Cortex 25, 4374–4391. 10.1093/cercor/bhv024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpma MG, Shdo SM, Shany-Ur T, Toller G, Kramer JH, Miller BL, Rankin KP, 2021. Salience driven attention is pivotal to understanding others’ intentions. Cogn Neuropsychol 38, 88–106. 10.1080/02643294.2020.1868984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K, 2004. Structure and Deterioration of Semantic Memory: A Neuropsychological and Computational Investigation. Psychol Rev 111, 205–235. 10.1037/0033-295X.111.1.205 [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL, 2002. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 58, 198–208. 10.1212/WNL.58.2.198 [DOI] [PubMed] [Google Scholar]
- Ross LA, Olson IR, 2010. Social cognition and the anterior temporal lobes. Neuroimage 49, 3452–3462. 10.1016/j.neuroimage.2009.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Rich EL, 2018. Orbitofrontal cortex. Current Biology 28, R1083–R1088. 10.1016/j.cub.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado S, Kaplitt MG, 2015. The nucleus accumbens: A comprehensive review. Stereotact Funct Neurosurg 93, 75–93. 10.1159/000368279 [DOI] [PubMed] [Google Scholar]
- Schapiro AC, McClelland JL, Welbourne SR, Rogers Timothy, T., Lambon Ralph MA, 2013. Why Bilateral Damage Is Worse than Unilateral Damage to the Brain. Washington, DC: APA, Guideline Development Panel for the Treatment of Posttraumatic Stress Disorder in Adults. 25, 2107–2123. 10.1162/jocn [DOI] [PubMed] [Google Scholar]
- Schmidt L, Lebreton M, Cléry-Melin ML, Daunizeau J, Pessiglione M, 2012. Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biol 10. 10.1371/journal.pbio.1001266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini ML, 2008. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol 65, 249–255. 10.1001/archneurol.2007.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD, 2009. Neurodegenerative Diseases Target Large-Scale Human Brain Networks. Neuron 62, 42–52. 10.1016/j.neuron.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27, 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Merkle FT, Gaus SE, Craig AD, Allman JM, Hof PR, 2012a. Distinctive neurons of the anterior cingulate and frontoinsular cortex: A historical perspective. Cerebral Cortex 22, 245–247. 10.1093/cercor/bhr005 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Zhou J, Kim E-J, 2012b. Frontotemporal Dementia: What Can the Behavioral Variant Teach Us about Human Brain Organization? The Neuroscientist 18, 373–385. 10.1177/1073858411410354 [DOI] [PubMed] [Google Scholar]
- Sescousse G, Redouté J, Dreher JC, 2010. The architecture of reward value coding in the human orbitofrontal cortex. Journal of Neuroscience 30, 13095–13104. 10.1523/JNEUROSCI.3501-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN, 2012. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 488, 218–221. 10.1038/nature11239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toller G, Yang WFZ, Brown JA, Ranasinghe KG, Shdo SM, Kramer JH, Seeley WW, Miller BL, Rankin KP, 2019. Divergent patterns of loss of interpersonal warmth in frontotemporal dementia syndromes are predicted by altered intrinsic network connectivity. Neuroimage Clin 22, 101729. 10.1016/j.nicl.2019.101729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ, 1992. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 40, 922–935. 10.1111/j.1532-5415.1992.tb01992.x [DOI] [PubMed] [Google Scholar]
- Uddin LQ, 2015. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 16, 55–61. 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O, 2017. Structure and Function of the Human Insula. Physiol Behav 34, 300–306. 10.1097/WNP.0000000000000377.Structure [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu S, Carter CS, 2005. Outcome representations, counterfactual comparisons and the human orbitofrontal cortex: Implications for neuroimaging studies of decision-making. Cognitive Brain Research 23, 51–60. 10.1016/j.cogbrainres.2005.01.004 [DOI] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K, 2011. Journal of Statistical Software mice: Multivariate Imputation by Chained Equations in R. [Google Scholar]
- Visconti di Oleggio Castello M, Borghesani V, Rankin KP, Gallant JL, Vu AT, 2021. Functional imaging of the anterior temporal lobe: effects of acceleration, multi-echo acquisition, and reconstruction methods. International Society for Magnetic Resonance in Medicine. https://archive.ismrm.org/2021/2681.html. [Google Scholar]
- Visser M, Jefferies E, Lambon Ralph MA, 2010. Semantic processing in the anterior temporal lobes: A meta-analysis of the functional neuroimaging literature. J Cogn Neurosci 22, 1083–1094. 10.1162/jocn.2009.21309 [DOI] [PubMed] [Google Scholar]
- von der Heide RJ, Skipper LM, Klobusicky E, Olson IR, 2013. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain, 10.1093/brain/awt094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang B, Bi Y, 2019. Close yet independent: Dissociation of social from valence and abstract semantic dimensions in the left anterior temporal lobe. Hum Brain Mapp 40, 4759–4776. 10.1002/hbm.24735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Takahashi YK, Schoenbaum G, Niv Y, 2014. Orbitofrontal cortex as a cognitive map of task space. Neuron 81, 267–279. 10.1016/j.neuron.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WFZ, Toller G, Shdo S, Kotz SA, Brown J, Seeley WW, Kramer JH, Miller BL, Rankin KP, 2021. Resting functional connectivity in the semantic appraisal network predicts accuracy of emotion identification. Neuroimage Clin 31, 102755. 10.1016/j.nicl.2021.102755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106, 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J, 2007. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci U S A 104, 6430–6435. 10.1073/pnas.0607061104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All code used for the analyses is available at https://github.com/MyrtheGwenRijpma/SIVT_VBM_project.git. The participant data cannot be placed in an open archive because it is not permitted under the study’s IRB approval due to the sensitive nature of patients’ data. However, all data is available to any interested research via a request submitted through a public-facing resource request portal at: http://memory.ucsf.edu/resources/data. Following a UCSF-regulated procedure, access will be granted to designated individuals in line with ethical guideline on the reuse of sensitive data. This would require submission of the Material Transfer Agreement, available at https://icd.ucsf.edu/material-transfer-and-data-agreements.