Abstract
Background
Remote measurement technology (RMT) has the potential to address current research and clinical challenges of attention-deficit/hyperactivity disorder (ADHD) symptoms and its co-occurring mental health problems. Despite research using RMT already being successfully applied to other populations, adherence and attrition are potential obstacles when applying RMT to a disorder such as ADHD. Hypothetical views and attitudes toward using RMT in a population with ADHD have previously been explored; however, to our knowledge, there is no previous research that has used qualitative methods to understand the barriers to and facilitators of using RMT in individuals with ADHD following participation in a remote monitoring period.
Objective
We aimed to evaluate the barriers to and facilitators of using RMT in individuals with ADHD compared with a group of people who did not have a diagnosis of ADHD. We also aimed to explore participants’ views on using RMT for 1 or 2 years in future studies.
Methods
In total, 20 individuals with ADHD and 20 individuals without ADHD were followed up for 10 weeks using RMT that involved active (questionnaires and cognitive tasks) and passive (smartphone sensors and wearable devices) monitoring; 10 adolescents and adults with ADHD and 12 individuals in a comparison group completed semistructured qualitative interviews at the end of the study period. The interviews focused on potential barriers to and facilitators of using RMT in adults with ADHD. A framework methodology was used to explore the data qualitatively.
Results
Barriers to and facilitators of using RMT were categorized as health-related, user-related, and technology-related factors across both participant groups. When comparing themes that emerged across the participant groups, both individuals with and without ADHD experienced similar barriers and facilitators in using RMT. The participants agreed that RMT can provide useful objective data. However, slight differences between the participant groups were identified as barriers to RMT across all major themes. Individuals with ADHD described the impact that their ADHD symptoms had on participating (health-related theme), commented on the perceived cost of completing the cognitive tasks (user-related theme), and described more technical challenges (technology-related theme) than individuals without ADHD. Hypothetical views on future studies using RMT in individuals with ADHD for 1 or 2 years were positive.
Conclusions
Individuals with ADHD agreed that RMT, which uses repeated measurements with ongoing active and passive monitoring, can provide useful objective data. Although themes overlapped with previous research on barriers to and facilitators of engagement with RMT (eg, depression and epilepsy) and with a comparison group, there are unique considerations for people with ADHD, for example, understanding the impact that ADHD symptoms may have on engaging with RMT. Researchers need to continue working with people with ADHD to develop future RMT studies for longer periods.
Keywords: attention-deficit/hyperactivity disorder, ADHD, remote measurement technology, engagement, barriers and facilitators, qualitative analysis, mobile phone
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common psychiatric disorder, with a prevalence of 2.5% among adults [1]. ADHD is diagnosed based on impairing levels of inattentive, hyperactive, and impulsive behaviors [2], but most adolescents and adults with ADHD present with additional co-occurring mental health problems manifesting in anxiety, depression, and poor sleep [1,3,4]. Although individual long-term outcomes are highly variable, adult ADHD has been associated with an increased risk of detrimental outcomes, including educational and occupational difficulties and antisocial behavior [3].
Individuals with ADHD are often assessed in a clinic or research laboratory setting, providing a snapshot of the symptoms and impairments they experience. Recent developments in remote measurement technology (RMT; including smartphone apps and wearable devices) offer new opportunities to address current research and clinical challenges [5,6]. For research, a remote ADHD assessment and monitoring battery will enable detailed, frequent, long-term, and real-world data collection on the clinical symptoms of ADHD and co-occurring disorders, functional and cognitive impairments, and health behaviors (such as exercise and sleep) in large sample sizes [5,7]. Longer term, an evidence-based remote ADHD assessment and monitoring battery also has the potential to transform clinical practice by offering the clinician easy and time-saving access to frequent detailed data on symptoms and impairments [5,8]. We have recently developed a new RMT system, the ADHD Remote Technology (ART) system, for adolescents and adults (aged ≥16 years) with ADHD that incorporates active (questionnaires and cognitive tasks) and passive (smartphone apps and a wearable device) monitoring.
Research using RMT has been successfully applied to many populations [9-11]. Despite participants and clinicians often reporting positively on the use of RMT [6,12-16], adherence and attrition are potential obstacles when applying RMT to clinical populations [11]. Individuals with ADHD often display difficulties with organization, following through on instructions, remembering daily tasks, and maintaining attention on difficult or boring tasks [2]. Although RMT allows for continuous, real-time data collection, it also requires frequent interaction and engagement from the participant, such as regular charging of devices and troubleshooting of technical malfunctions [17]. A substantial proportion of missing data or high dropout rates could result in the loss of statistical power and concerns about possible bias.
Hypothetical views and attitudes toward RMT in a population with ADHD have been explored previously [18]. Focus groups involving individuals with ADHD were positive about using RMT in clinical practice [18]. Despite positive reports, the study relied on theoretical scenarios, so we were unable to generalize these findings to the actual implementation of RMT. To our knowledge, no previous study has used qualitative methods to understand the end-point acceptability of RMT in individuals with ADHD. A strength of this study is that the individuals with ADHD all participated in the ART pilot study, which involved a 10-week remote monitoring period before a discussion on the barriers to and facilitators of RMT; thus, all participants had direct experience of using remote monitoring measures, including smartphone apps and wearable devices. The addition of a comparison group is also a novel approach to qualitative feedback on RMT. By including a comparison group, we were able to draw comparisons between individuals with ADHD and those without ADHD. The purpose of this study was to evaluate the barriers to and facilitators of using RMT in individuals with ADHD, compared with a group of people who did not have a diagnosis of ADHD. We also aimed to explore participants’ views on the use of RMT in future studies with a longer duration.
Methods
Design
The ART pilot study was an observational study involving a 10-week RMT study period, using active (questionnaires and cognitive tasks) and passive (wearable devices and smartphones) monitoring. The ART pilot study involved 2 participant groups: adolescents and adults with ADHD and a comparison group (individuals without ADHD). Semistructured interviews were completed after participating in the study, following a topic guide on the barriers to and facilitators of using RMT in individuals with ADHD and in those without ADHD.
Participants
Participants were eligible to participate in the ART pilot study if they were aged between 16 and 60 years, able to provide informed consent, fluent in English, and willing to wear a wearable device and use an Android phone during the 10-week remote monitoring period. Participants with ADHD were eligible if they met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria for ADHD, and participants in the comparison group were eligible if they did not reach the threshold for ADHD on the Barkley symptom and impairment scale [19]. Participants in the ART pilot study were recruited from previous studies (where they had indicated that they were willing to be contacted regarding future research studies), via the Attention Deficit Disorder Information and Support Service, social media, and King’s Volunteer circular and on the “Call for Participants” website.
Procedure
Participants attended 2 remote baseline sessions with a research worker using Microsoft Teams. The first remote baseline session with the participants with ADHD included the administration of the Diagnostic Interview for ADHD in adults [20] to confirm ADHD diagnosis and cognitive and self-report measures, which are beyond the scope of this study (Figure 1). Participants without ADHD in the comparison group were assessed in the same way, except that instead of the full ADHD diagnostic interview, they completed the ADHD symptom and impairment questionnaire [19].
Figure 1.
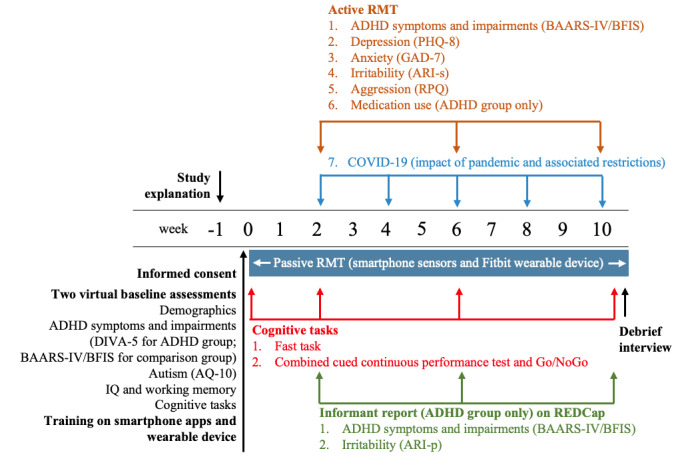
ADHD Remote Technology pilot study design. ADHD: attention-deficit/hyperactivity disorder; AQ-10: autism spectrum quotient; ARI-p: Affective Reactivity Index-parent-report; ARI-s: Affective Reactivity Index- self report; BAARS-IV/BFIS: Barkley Adult ADHD Rating Scale—IV/Barkley Functional Impairment Scale; DIVA-5: Diagnostic Interview for Adult ADHD; GAD-7: Generalized Anxiety Disorder-7; PHQ-8: Patient Health Questionnaire-8; REDCap: Research Electronic Data Capture; RPQ: Reactive-Proactive Aggression Questionnaire; RMT: remote measurement technology.
The second session was administered once participants had received their wearable device (Fitbit Charge 3) and Android smartphone via post (if they were not using their own compatible Android device) approximately a week after the first session. It included training on the use of wearable devices and on the smartphone Passive and Active Apps. The participants also received a leaflet summarizing key information (Participant Technology User Guide) and researcher contact details for future reference.
All participants then participated in a 10-week remote monitoring period using a wearable device and smartphone. RMT incorporated active (questionnaires and cognitive tasks) and passive (wearable device and smartphone sensors) monitoring. Active monitoring involved the participants completing clinical symptom questionnaires on the smartphone Active App and cognitive tasks on their home PC or laptop during weeks 2, 6, and 10 (Figure 1). Participants completed 2 computer-based cognitive tasks that were sensitive to differences between individuals with ADHD and those without ADHD [21]. Passive App monitoring data (data that do not require any conscious engagement from the participant) were collected continuously on a 24/7 basis through a smartphone and a Fitbit device (Fitbit Charge 3). The smartphone Passive App collected data on a range of measures, such as ambient noise, ambient light, phone use information (eg, which apps were used and how long and when the phone was unlocked), and relative location. In this study, we used participants’ personal Android devices where available and provided a participant with a Motorola G7 Play or G7 Power if they had an iPhone or did not have a smartphone. Participants were asked to wear the Fitbit on their nondominant hand.
At the end of the 10-week remote monitoring period, participants were invited to participate in an optional “debrief” session, which included a qualitative interview to investigate their experiences of participating. The data collected during the interviews formed the basis of this study. The interviews, which lasted 25 to 50 minutes, were conducted by 2 research workers (QD and HD) and focused on potential barriers to and facilitators of RMT using a semistructured framework that covers participant experience, enrolling into the study and support, working the study into daily life, experience of using the devices, data privacy and sharing, and future uses.
Ethics Approval, Informed Consent, and Participation
The interview and study procedures were approved by the North East—Tyne and Wear South Research Ethics Committee (20/NE/0034; IRAS ID: 278126). Informed consent was obtained from all participants before the assessments started. To maintain participant anonymity, their data were pseudonymized. The participants’ names were replaced with a code. Data collected from apps, wearable devices, and interviews were only associated with this code and stored separately from any personally identifiable information. Participants were compensated £30 (US $37) after the completion of the baseline sessions, £20 (US $25) after the first remote active monitoring follow-up (end of week 5), and a further £50 (US $62) at the study end point (end of week 10). The participants did not receive additional compensation for completing the debrief interviews.
Data Analysis
Descriptive statistics were calculated for gender, age, and ethnicity. All interviews were audio recorded and transcribed verbatim. A framework methodology was used to qualitatively explore the data [12]: (1) transcription, (2) familiarization with the interviews, (3) coding by 2 researchers working independently (HD and AA) using the qualitative software program NVivo Software (version 2020), (4) developing a working analytical framework (HD and AA), (5) applying the analytical framework (HD), (6) charting data into a framework matrix (HD), and (7) interpreting the data (HD and SS). The analytical framework was built on barriers to and facilitators of engagement with RMT identified in a recent systematic review [9]. We then identified themes that deviated across participant groups using the charted framework matrix.
The framework methodology is suitable for the analysis of interview data, where it is desired to make comparisons within and between cases [22]. Percentages are used to compare the themes that deviated between individuals with ADHD and individuals without ADHD in a comparison group.
Results
Participants
In total, 20 individuals with ADHD and 20 individuals without ADHD in a comparison group participated in the ART pilot study (Table 1). Of the participants who participated in the ART pilot study, 22 participants (10 individuals with ADHD and 12 individuals in the comparison group) opted to complete audio recorded debrief interviews at the end of the study period. Of these participants, 91% (20/22) completed the debrief interview within 2 months of completing their remote monitoring period, and all participants completed the debrief interview within 6 months. Interviews were completed until data saturation, which refers to a point in the data collection process where the interviewer keeps hearing repeated information or when no new information is identified [23,24].
Table 1.
Participant characteristics.
|
|
ADHDa Remote Technology pilot study | Debrief interviews | ||||
|
|
ADHD group (n=20) | Comparison group (n=20) | ADHD group (n=10) | Comparison group (n=12) | ||
| Gender (female), n (%) | 15 (75) | 15 (75) | 8 (80) | 11 (91) | ||
| Age (years), mean (SD) | 27.49 (6.04) | 27.79 (6.17) | 28.03 (6.36) | 27.75 (6.74) | ||
| Ethnicity, n (%) | ||||||
|
|
Asian | 1 (5) | 3 (15) | 0 (0) | 3 (25) | |
|
|
Black | 1 (5) | 0 (0) | 1 (10) | 0 (0) | |
|
|
White | 16 (80) | 17 (85) | 9 (90) | 9 (75) | |
|
|
Mixed or multiple ethnic groups | 2 (10) | 0 (0) | 0 (0) | 0 (0) | |
aADHD: attention-deficit/hyperactivity disorder.
Barriers to and Facilitators of RMT
Multimedia Appendix 1 presents the 3 themes of barriers to and facilitators of RMT that emerged across participant groups. These themes have been subdivided into health-related, user-related, and technology-related themes to guide the understanding of which aspects of the system we need to target to improve engagement.
Table 2 presents the fourth theme that is based on hypothetical views of what participants would want from future research.
Table 2.
Hypothetical views on what participants would want from the study in the future.
| Future studies | ADHDa group (n=10), n (%) | Comparison group (n=12), n (%) | Total (n=22), n (%) | ||||||
| Willingness | |||||||||
|
|
Positive about using the system for 1 or 2 years |
|
8 (80) | 10 (83) | 18 (82) | ||||
| Sharing data with general practitioners | |||||||||
|
|
Acceptable | 5 (50) | 11 (92) | 16 (73) | |||||
|
|
|
Benefit their care | 6 (60) | 7 (58) | 13 (59) | ||||
|
|
|
Access to objective data |
|
2 (20) | 4 (33) | 6 (27) | |||
|
|
|
Real-time access |
|
2 (20) | 1 (8) | 3 (14) | |||
|
|
|
Taking action |
|
5 (50) | 5 (43) | 10 (45) | |||
|
|
Questioned whether all RMTb data are relevant |
|
5 (50) | 1 (8) | 6 (27) | ||||
|
|
Choice |
|
3 (30) | 3 (25) | 6 (27) | ||||
| Improvements needed | |||||||||
|
|
Feedback | ||||||||
|
|
|
Wanted more feedback |
|
4 (40) | 2 (17) | 6 (27) | |||
|
|
|
Visual feedback |
|
4 (40) | 5 (42) | 9 (41) | |||
|
|
|
Verbal feedback |
|
2 (20) | 1 (8) | 3 (14) | |||
|
|
|
Feedback on active data |
|
5 (50) | 3 (25) | 8 (36) | |||
|
|
|
Feedback on passive data |
|
3 (30) | 7 (58) | 10 (45) | |||
|
|
|
During study period |
|
2 (20) | 2 (17) | 4 (18) | |||
|
|
|
End of study |
|
4 (40) | 5 (42) | 9 (41) | |||
|
|
More information |
|
4 (40) | 1 (8) | 5 (23) | ||||
|
|
Passive monitoring |
|
|||||||
|
|
|
Passive app syncing issues |
|
3 (30) | 5 (42) | 8 (36) | |||
|
|
|
Using own phone |
|
3 (30) | 2 (17) | 5 (23) | |||
|
|
Cognitive tasks |
|
|||||||
|
|
|
Shortening the length |
|
5 (50) | 2 (17) | 7 (32) | |||
|
|
|
Less frequent |
|
3 (30) | 2 (17) | 5 (23) | |||
aADHD: attention-deficit/hyperactivity disorder.
bRMT: remote measurement technology.
Health-Related Theme
Insight
For participants with ADHD, RMT provided better insight into their health-related behaviors and lifestyle. For health-related behaviors, RMT (specifically the wearable device) provided better insights into their sleep and physical activity levels, and participants with ADHD described improvements in these health-related behaviors. In terms of lifestyle, RMT provided participants with ADHD better insight into their routines and phone use, and some participants also described an improvement in these areas. For example, 1 participant with ADHD explained as follows:
I’ve definitely found it helpful in sort of motivating me to keep a good routine and try and get my steps in every day. So, like exercise and health wise, it’s been really good.
ADHD03
Participants in the comparison group also agreed that RMT gave them better insight into their sleep and physical activity and noted improvements in these health-related behaviors:
Well, having the Fitbit data that’s very helpful...I’ve been using the Fitbit data actively to monitor my exercises or meditation, sleep quality, so that was very useful.
COMP21
Impact of ADHD Symptoms on RMT
Participants with ADHD commented on the impact of ADHD symptoms on taking part in the study. Participants with ADHD described difficulties with attention, forgetfulness, motivation to complete demanding tasks, and organization, which impacted their experiences in the study. Difficulties with forgetfulness included remembering to complete questionnaires and cognitive tasks and to charge the devices. One participant explained as follows:
I’m not really organised with things. Things happen at the spare of the moment...I’m not someone that can pre-think that my Fitbit’s gonna run out of charge, it would be like “oh, now it has now run out of charge.”
ADHD01
User-Related Theme
Perceived Costs
Perceived costs relate to any aspects of RMT that made participation challenging or difficult. The most prominent perceived cost for participants with ADHD was the cognitive tasks. There were also some perceived costs relating to passive monitoring measures, such as charging the devices, adapting to the new study phone, and phone connectivity issues (eg, synchronizing data from the wearable device to the phone). The comparison group reported fewer personal costs.
Compatibility
There was a consensus among participants in both groups that the ART pilot study fit into their daily life. One participant explained as follows:
The overall experience was like very easy...I was just carrying on with my normal life.
ADHD04
Another participant described that they “could honestly just go about my daily life and participate, yeah, passively” (ADHD09). Despite the study blending into participants’ daily life, some participants with ADHD mentioned difficulty finding the time to complete the cognitive tasks in a quiet and distraction-free environment. This was also mentioned in the comparison group:
I did sometimes struggle to find like an hour that I could just block off and have no distractions and things, that was a bit more tricky.
COMP13
Intrinsic Value
There was agreement among participants with ADHD that the benefits of participating outweighed the costs of participating in the study. For example, 1 participant with ADHD explained as follows:
Oh no, definitely benefits outweigh, like there wasn’t really any cons, it was very simple and straightforward. But then like benefits, yup definitely more aware of my sleep and my exercise. So really, really outweighed the cons, definitely.
ADHD04
Participants in the comparison group also agreed that the benefits outweighed the costs, with 1 participant stating, “100%, like, it’s definitely worth taking part” (COMP16).
Technology Acceptance
All participants with and without ADHD commented on the acceptability and ease of collecting the passive data, with 1 participant explaining as follows:
Yeah, I mean that’s easier to be honest, um, as long as I know what data is being collected and it’s not particularly, um, kind of sensitive data, it’s not looking at kind of what’s going in and out of text message conversations, for example. Um, it’s easier to have that collected passively for me personally, rather than to actively remember to upload something.
COMP19
Participants explained that they accepted RMT measures as technology nowadays collects these data. Participants with and without ADHD mentioned that the study had been well explained and were reassured that the study had gone through the correct reviews, so they could trust that the study was ethical, that it would be safe for them to take part, and that their data would be kept anonymous.
Overall Experience
Overall, the participants’ experience using the smartphone and wearable device in the ART system was positive. Participants both with and without ADHD described the study as easy, enjoyable, and interesting. One participant with ADHD explained as follows:
You know ’cause it gave me a very new exciting toy to play with...and I mean it was very exciting. You know, the study wasn’t too long that it got boring, you know?
ADHD02
Participants with and without ADHD also noted that the study period went quickly and that the study was well organized.
Few participants with ADHD described the study as demanding or that it required a short-term adjustment period. However, more participants in the comparison group described the study as long, demanding, or requiring an adjustment period. For example, 1 participant in the comparison group explained as follows:
I guess it was, it was more of an intensive or like a like a longer study than ones I’ve done before.
COMP13
Despite these comments, these participants also reported that the overall experience of the study was positive and that the benefits outweighed any costs.
Technology-Related Theme
Value in Gathering RMT Data
There was a consensus among participants that there was value in gathering RMT data. One participant with ADHD explained as follows:
I think it’s really great. Um, I think it really, sort of improves the ability to get accurate and up-to-date data. I think it’s great.
ADHD10
In general, participants described passive data as collecting objective measures of their health and that such data are more accurate than “manually record[ing] it yourself” (ADHD03).
Participants with and without ADHD commented that passive monitoring was better than manually providing the data, as it improved the accuracy, the amount of data collected, and the ability to observe behavioral patterns and to compare variables. A participant with ADHD commented as follows:
I mean, it certainly made it more accurate ’cause I’m very aware now that if I’d been filling out forms, they would not have been accurate at all because I had absolutely no awareness of how much sleep I was getting.
ADHD10
Convenience
Participants with ADHD expressed the view that the passive monitoring aspect made it easier for the participant to participate, for example:
With the passive monitoring so like when you’re monitoring sleep, the fact that you’re not kind of having to manually record it yourself, and it is done all automatically, I think that sort of, umm, I think if you were having to like manually record it and go to that effort, you would, I think it would have an influence on your, sort of, approach to kind of going to sleep and things like that. Whereas when it’s done passively, like you’re not having that kind of, um, that you’re not putting that input in yourself.
ADHD03
Participants in the comparison group also commented that passive monitoring made it easier for them to participate.
Intrusiveness
Cognitive tasks were described as the most intrusive aspect of technology-related barriers to RMT. All participants with ADHD described the cognitive tasks as tedious, for example:
Yeah, I’m not sure if there’s anything that can be done about it...but the tasks were really difficult...I’m not sure how like a neurotypical person would find it, but sort of sitting and looking at a screen with no stimulation other than it for close to an hour was really hard!
ADHD10
In general, participants with ADHD viewed cognitive tasks as being too long or commented that the frequency of completing the tasks was too often. The cognitive tasks were also described by the participants in the comparison group as tedious, the tasks were viewed as too long, and participants commented on the frequency of the tasks being too often.
Some participants expressed concerns over monitoring; for example, they had initial concerns that the research team would be monitoring their location. In total, 3 participants questioned the relevance of the aggression questionnaire they were asked to complete.
Usability
Participants described in detail the usability of the Active App, Fitbit wearable device, and study Android phone. For the Fitbit, participants with ADHD noted that it was comfortable; however, they reported some adherence difficulties and technical issues during the study period. Adherence difficulties included remembering to charge and remembering to wear the Fitbit again after charging or showering. Technical issues described by participants with ADHD were related to charging issues and syncing the Fitbit with the smartphone. Participants in the comparison group also commented on their comfort, adherence difficulties, and technical issues.
Technical challenges with the study Android phone were reported by individuals with ADHD; these included problems with the Passive App including synchronizing and transfer issues. Despite reporting some technical issues with the Passive App, participants with ADHD mentioned that the phone was easy to use and that they were able to use the phone normally. The questionnaires on the Active App were described as being easy to complete.
Future Study Theme
Willingness
Participants were asked for their views on using the ART system in the longer term. Overall, participants with ADHD were positive about using the system for 1 or 2 years. One participant explained as follows:
Yeah, I think because it was so behind the scenes, I didn’t have to do anything. You were just sort of looking at what I’m doing on my normal day-to-day. Yeah, it wouldn’t bother me [to take part for] a year.
ADHD04
Participants in the comparison group also agreed with this; for example, 1 participant explained as follows:
Yes,...I wouldn’t find it like a very big strain to participate in the study if it was exactly as it was for a longer period of time.
COMP20
Sharing Data With General Practitioners
Participants were asked to consider how they would feel about RMT data being shared with the general practitioner (GP). There was some discrepancy among participants with ADHD regarding whether they would find it acceptable to share these data with GPs, whereas the comparison group agreed that this would be acceptable. In general, those who would be willing to share RMT data felt it could benefit the care they received, as it would provide GPs real-time access to objective data that they could then take action on. One participant with ADHD explained as follows:
I think that could be sort of quite beneficial ’cause then it would then help, say the GP or whoever, to understand a bit better exactly what’s going on...They can kind of see from the data...what you’re saying is, is how it is.
ADHD03
Those who would be less willing to share RMT data with GPs questioned whether all RMT data would be relevant to share with their GPs. Despite this, participants with ADHD emphasized they would find it more acceptable if they had a choice in what aspects of RMT would be shared, for example:
I would prefer to choose as some of it obviously, I would feel isn’t relevant to me and they don’t need to [know].
ADHD09
Only 1 participant in the comparison group expressed concerns about whether the data collected were relevant to their health care.
Improvements Needed
Participants with and without ADHD described improvements that could be made to the ART system for future long-term studies. This included additional feedback during the study period. Participants suggested that feedback should be given on both active and passive monitoring data in either a visual or verbal format, which would be shared both during and at the end of the study.
Some participants with ADHD suggested that providing more information would be beneficial for future studies, such as information on adapting to the Android phone, data use, video explanations, and leaflets for technology instructions. However, 1 participant with ADHD commented that the information sheets contain too much information and should be shortened.
The participants suggested changes specific to the active and passive monitoring aspects of ART system, including cognitive tasks (active monitoring) and the smartphone (passive monitoring). The participants with ADHD suggested two ways to improve the administration of the cognitive tasks: (1) shortening the length, for example, 1 participant explained, “anything that could be done, just like you know shorter but more frequent—that would’ve been great!” (ADHD10); and (2) less-frequent administration, for example, “Yeah, I think if you’re doing it for a much longer period, I would definitely say needs to be a longer gap between them” (ADHD03). Participants in the comparison group also agreed with these suggestions; for example, 1 participant in the comparison group explained as follows:
You know, maybe if you did a yearlong study, you maybe wouldn’t need to do [the cognitive tasks] quite so often.
COMP13
Improvements in passive monitoring aspects of the ART system were related to the study smartphone. Participants with ADHD suggested improving syncing issues of the Passive App and that it would be better to be able to use their own phone during the study period, instead of a study Android phone. Participants in the comparison group also made these suggestions.
Differences Between Individuals With and Without ADHD
When comparing themes that emerged across the participant groups (Multimedia Appendix 1 and Table 2), individuals with and without ADHD experienced similar barriers to and facilitators of using RMT. However, slight differences between participant groups were identified across all 4 themes. In the health-related theme, all participants with ADHD identified the impact of ADHD symptoms as a potential barrier to RMT. Examples of the impact of ADHD symptoms included forgetfulness, disorganization, and difficulties with attention. In comparison, only 1 participant in the comparison group reported that forgetfulness was a potential barrier to RMT. Some participants with ADHD reported improvements in lifestyle; however, participants in the comparison group did not report this.
In the user-related theme, more individuals with ADHD noted that the cognitive tasks (active monitoring) were a perceived cost compared with the comparison group. This would be expected because of the demand of the cognitive tasks in individuals who have difficulties with attention to demanding tasks, that is, the cognitive tasks have been specifically designed to be challenging for individuals with ADHD in order for them to be informative about the differences between people with and without ADHD. Another perceived cost for individuals with ADHD was the charging of the devices, whereas participants in the comparison group did not mention this. In the overall experience minor theme, the negative subtheme highlighted some differences between individuals with and without ADHD. Compared with participants with ADHD, more participants in the comparison group described the study as long, demanding, or requiring an adjustment period.
In the technology-related theme, individuals with ADHD described more technical challenges related to the study Android phone, compared with those in the comparison group. In the future studies theme, there was disagreement among the individuals with ADHD about sharing RMT data with the GP, whereas participants in the comparison group found this acceptable.
Discussion
Principal Findings
This study builds on a growing number of RMT studies in clinical populations. Our findings provide novel insights into the barriers to and facilitators of using RMT in individuals with ADHD who have completed a remote monitoring study period that uses repeated measurements with ongoing active (eg, questionnaires and cognitive tasks) and passive (eg, smartphone sensors and wearable devices) monitoring. This study highlights the similarities and some, albeit few, differences between individuals with and without ADHD interpreted as barriers to and facilitators of RMT. The ART pilot study also shares similar methods and measures as the RMT in individuals with depression and epilepsy [25,26], and the topic guide and coding frame were adapted from a study that explored barriers to and facilitators of using RMT in individuals with depression [9], so comparisons can be drawn with other clinical populations.
Insight Into Health-Related Behaviors
Individuals with ADHD, as well as the comparison group, commented on the benefits of using the wearable device, including them obtaining better insight and being able to observe improvements in their sleep and physical activity. Although these insights may have helped maintain participant engagement with the study, we note that using the devices to change behavior was not an aim in the ART pilot study (ie, the research team had emphasized that the participants should continue their behaviors and daily lives as normal). The ART pilot study involved a remote monitoring period of 10 weeks, and over a longer remote monitoring period, these effects may be reduced, as participants have longer to acclimatize to wearing and using the devices, so its novelty of receiving insight into health-related behaviors may reduce. Future research should consider the type of feedback provided by the wearable devices.
Concerns Over Core ADHD Symptoms
Owing to individuals with ADHD often reporting difficulties with following through on instructions, remembering daily tasks, and maintaining attention on demanding tasks [2], we hypothesized that this could be a potential barrier when developing a remote measurement system for individuals with ADHD. All participants with ADHD spoke about the difficulties their ADHD symptoms had on participating, including difficulties with organization, motivation to complete demanding tasks, and forgetfulness. Symptoms such as forgetfulness have also been raised as a potential barrier to RMT in other psychiatric disorders (including depression) [9]. Only 1 individual from the comparison group commented that forgetfulness was a potential barrier to the ART system. It could be suggested that forgetfulness and other related ADHD symptoms may be a barrier to future RMT studies, and further support should be in place to help support individuals with ADHD in RMT studies. The ART system included reminders for questionnaire and cognitive task completions; however, future RMT studies with individuals with ADHD should go further, for example, by providing notifications to charge and replace the wearable device. We have already incorporated this consideration in our ongoing European Union–funded clinical study “ADHD Remote Technology Study of Cardiometabolic Risk Factors and Medication Adherence,” where reminders are set up to notify the participant after a prolonged period of not wearing the device and when their wearable device is fully charged [27].
Cognitive Tasks
Barriers to using RMT in individuals with ADHD are mainly related to the perceived cost and intrusiveness of cognitive tasks (the frequency of administration and time taken to complete them). The tasks were completed during weeks 2, 6, and 10; this is the first study in which multiple measurements using cognitive tasks were collected in a 10-week period. Future studies should allow more time between the tasks to ensure participants are not fatigued and their continued participation in the study.
Importance of Compatibility
Participants noted that the benefits of participating in an RMT study outweighed any costs. This finding is positive for future studies using RMT in individuals with ADHD. Despite initial concerns that using RMT with individuals with ADHD relies on the participant to engage with the technology and researchers, all participants with ADHD described the study as fitting into their daily lives. The compatibility of RMT was also described by all individuals in the comparison group. The importance of compatibility with one’s existing routine was also described in individuals with major depressive disorder [9].
Collecting and Sharing of RMT Data
As RMT collects vast amounts of data on a variety of different measures, it is expected that concerns about data privacy may arise. Privacy concerns have also been raised in other RMT studies, including individuals with depression and epilepsy [9,28]. Although we observed some concerns over monitoring and adherence difficulties, participants explained that they were reassured, as the study was explained well, and they trusted that the research had undergone the right ethical checks. This is positive as the interest in RMT studies has gained momentum. Future RMT studies explicitly need to consider the importance of choice and control around data sharing when designing an RMT study.
Individuals with epilepsy have reported interest in using RMT as a method to remember and communicate with health care professionals [28]. Individuals with ADHD also described the potential use of presenting RMT data to their GP or health care professionals as a way of remembering or providing evidence about their ADHD symptoms or co-occurring health behaviors. In contrast, there were some concerns about sharing RMT data with their health care professionals. However, it is important to note that despite asking participants, “How would you feel about the information collected during the study going to your GP or healthcare provider?” those who questioned whether all RMT data would be relevant to their care only referred to GPs and did not refer to other health care professionals. Our findings may not apply to views of sharing data with other health care professionals, such as clinicians involved in medication titration, who may be in more frequent contact with individuals with ADHD and where it may benefit the care they receive if their clinical care team had access to frequent questionnaire completion or sleep behaviors.
Future Research
Building on the barriers to and facilitators of RMT described by individuals with ADHD, future research using RMT should incorporate our suggestions for long-term monitoring of individuals with ADHD (Textbox 1). These recommendations have been considered at the individual and group levels. At an individual level, individuals with ADHD emphasized some privacy concerns and the importance for the individual to have a choice over the types of RMT data that are collected and then shared with health care professionals. Individuals with ADHD also seemed to report varying levels of technical issues and an adjustment period for the devices provided. The type of data (active or passive data), the format (visual or verbal), and the frequency (during or at the end of the study period) that feedback is provided should be tailored to the individual to help with continued engagement with RMT.
Researchers’ suggestions for future studies based on the barriers to and facilitators of using remote measurement technology (RMT).
Privacy concerns
Emphasis should be placed on the participants’ control over their data and their choice to share data with health care professionals.
Technical issues
Technical issues will happen, but the overall consensus is that the smartphone apps and wearable devices allow for objective and accurate data collection.
Adjustment period
Allow for an adjustment period while the participant becomes familiar with the new devices. In addition, provide self-help guides and videos to aid with the introduction of new devices
Feedback
Feedback to individuals with attention-deficit/hyperactivity disorder should be personalized based on the types of RMT data that individual would like to see and in the form they would find most useful (eg, visual or verbal feedback).
Adherence
Reminders throughout the study period, including to complete questionnaires, are vital for successful completion rates, but reminders should also be extended to reminding the participant to replace the wearable device after it is removed for charging or showering.
Cognitive tasks
Fewer cognitive tasks during a study period with longer completion times
At a group level, individuals with ADHD were positive about using the ART system for 1 or 2 years. There was agreement that despite some technical issues, RMT collected objective data and had the potential to help provide evidence about their ADHD symptoms or co-occurring health behaviors to their GP or health care professionals. On the basis of the reports that individuals with ADHD felt their symptoms impacted their study experience, future research should incorporate ADHD-specific support from researchers to help with the core symptoms of ADHD (eg, reminders) to promote with adherence. There was consensus that future studies using cognitive tasks should be conducted less frequently.
Strengths and Limitations
The use of qualitative methods allows for an in-depth exploration and discussion of the potential barriers to and facilitators of using RMT, such as smartphone apps and wearable devices, for a remote assessment and monitoring system that can be applied to both research and clinical long-term monitoring of symptoms, impairments, and health-related behaviors associated with ADHD.
Previous qualitative research has relied on hypothetical views of using RMT [18,28,29]. A strength of this study is that the individuals with ADHD participated in a 10-week remote monitoring period before this discussion, so they had direct experience of using remote monitoring measures, including smartphone apps and wearable devices. We still introduced a hypothetical scenario of longer future RMT studies, as this is beneficial to gain insight into what participants would want from future research. Future research should complete debrief interviews after participants have completed longer RMT studies to understand whether barriers to and facilitators of RMT changes when participating for a year or two. We have already incorporated this consideration into our “ADHD Remote Technology Study of Cardiometabolic Risk Factors and Medication Adherence” study, where individuals with ADHD will be measured for 12 months and will provide detailed qualitative feedback on their participation [27].
The gender imbalance in this study is also a limitation that should be acknowledged. In adulthood, the prevalence rate of ADHD is similar for male individuals and female individuals [30]; however, in this study, only 3 (two participants with ADHD and 1 participant in the comparison group) of 22 participants who completed the interviews were male. It could be that female individuals have views on RMT that may not be shared with male individuals. Owing to the imbalance of genders, we were unable to compare the views between female individuals and male individuals; this is an important topic for future research.
Conclusions
This study builds on emerging research on the barriers to and facilitators of using RMT in psychiatric disorders in health care and long-term monitoring. We have shown the barriers to and facilitators of using RMT in people with ADHD following a 10-week remote monitoring study period. The themes that emerged during this qualitative analysis suggest that people with ADHD agree that RMT can provide useful objective data that uses repeated measurements with ongoing active (eg, questionnaires) and passive (eg, smartphone sensors and wearable devices) monitoring. Although themes overlapped with previous research on barriers and facilitators of engagement with RMT (eg, depression and epilepsy) and with a comparison group, there are unique considerations for people with ADHD, for example, understanding the impact of ADHD symptoms has with using RMT and reducing the frequency of cognitive task administration. Researchers need to continue to work with people with ADHD to develop future RMT studies for longer periods.
Acknowledgments
The authors thank all those who made this research possible: all participants who contributed their time to the study; AB, Chief Executive of the Attention-Deficit/Hyperactivity Disorder (ADHD) Information Services; and the funders. The ADHD Remote Technology pilot study was funded by a King’s Together Strategic Grant. HD is supported by the UK Medical Research Council (MR/N013700/1) and King’s College London member of the MRC Doctoral Training Partnership in Biomedical Sciences. QD is supported by the China Scholarship Council (201908440362).
Abbreviations
- ADHD
attention-deficit/hyperactivity disorder
- ART
ADHD Remote Technology
- GP
general practitioner
- RMT
remote measurement technology
Final major, minor, and subthemes of barriers to and facilitators of remote measurement technology that emerged across participant groups.
Data Availability
For open access, the author has applied a Creative Commons Attribution (CC BY) license to any author-accepted manuscript version. The data supporting this study are not openly available but may be shared on request following a formal review process by the chief investigator and coinvestigators.
Footnotes
Conflicts of Interest: JK has given talks at educational events sponsored by Medice; all funds are received by King’s College London and used for studies of attention-deficit/hyperactivity disorder (ADHD). PA has received funding for research by Vifor Pharma, has given sponsored talks, and been an advisor for Shire, Janssen-Cilag, Eli-Lilly, Flynn Pharma, and Pfizer regarding the diagnosis and treatment of ADHD; all funds are received by King’s College London and used for studies of ADHD. The other authors report no conflicts of interest.
References
- 1.Faraone SV, Banaschewski T, Coghill D, Zheng Y, Biederman J, Bellgrove MA, Newcorn JH, Gignac M, Al Saud NM, Manor I, Rohde LA, Yang L, Cortese S, Almagor D, Stein MA, Albatti TH, Aljoudi HF, Alqahtani MM, Asherson P, Atwoli L, Bölte S, Buitelaar JK, Crunelle CL, Daley D, Dalsgaard S, Döpfner M, Espinet S, Fitzgerald M, Franke B, Gerlach M, Haavik J, Hartman CA, Hartung CM, Hinshaw SP, Hoekstra PJ, Hollis C, Kollins SH, Sandra Kooij JJ, Kuntsi J, Larsson H, Li T, Liu J, Merzon E, Mattingly G, Mattos P, McCarthy S, Mikami AY, Molina BS, Nigg JT, Purper-Ouakil D, Omigbodun OO, Polanczyk GV, Pollak Y, Poulton AS, Rajkumar RP, Reding A, Reif A, Rubia K, Rucklidge J, Romanos M, Ramos-Quiroga JA, Schellekens A, Scheres A, Schoeman R, Schweitzer JB, Shah H, Solanto MV, Sonuga-Barke E, Soutullo C, Steinhausen HC, Swanson JM, Thapar A, Tripp G, van de Glind G, van den Brink W, Van der Oord S, Venter A, Vitiello B, Walitza S, Wang Y. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021 Sep;128:789–818. doi: 10.1016/j.neubiorev.2021.01.022. http://hdl.handle.net/2318/1791803 .S0149-7634(21)00049-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th edition. Washington, DC, USA: American Psychiatric Association; 2013. [Google Scholar]
- 3.Asherson P, Buitelaar J, Faraone SV, Rohde LA. Adult attention-deficit hyperactivity disorder: key conceptual issues. Lancet Psychiatry. 2016 Jun;3(6):568–78. doi: 10.1016/S2215-0366(16)30032-3.S2215-0366(16)30032-3 [DOI] [PubMed] [Google Scholar]
- 4.Franke B, Michelini G, Asherson P, Banaschewski T, Bilbow A, Buitelaar JK, Cormand B, Faraone SV, Ginsberg Y, Haavik J, Kuntsi J, Larsson H, Lesch KP, Ramos-Quiroga JA, Réthelyi JM, Ribases M, Reif A. Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur Neuropsychopharmacol. 2018 Oct;28(10):1059–88. doi: 10.1016/j.euroneuro.2018.08.001. https://linkinghub.elsevier.com/retrieve/pii/S0924-977X(18)30303-1 .S0924-977X(18)30303-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao Y, Thompson C, Peterson S, Mandrola J, Beg MS. The future of wearable technologies and remote monitoring in health care. Am Soc Clin Oncol Educ Book. 2019 Jan;39:115–21. doi: 10.1200/EDBK_238919. https://ascopubs.org/doi/10.1200/EDBK_238919?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alpert JM, Manini T, Roberts M, Kota NS, Mendoza TV, Solberg LM, Rashidi P. Secondary care provider attitudes towards patient generated health data from smartwatches. NPJ Digit Med. 2020 Mar 3;3:27. doi: 10.1038/s41746-020-0236-4. doi: 10.1038/s41746-020-0236-4.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matcham F, Leightley D, Siddi S, Lamers F, White KM, Annas P, de Girolamo G, Difrancesco S, Haro JM, Horsfall M, Ivan A, Lavelle G, Li Q, Lombardini F, Mohr DC, Narayan VA, Oetzmann C, Penninx BW, Bruce S, Nica R, Simblett SK, Wykes T, Brasen JC, Myin-Germeys I, Rintala A, Conde P, Dobson RJ, Folarin AA, Stewart C, Ranjan Y, Rashid Z, Cummins N, Manyakov NV, Vairavan S, Hotopf M, RADAR-CNS consortium Remote Assessment of Disease and Relapse in Major Depressive Disorder (RADAR-MDD): recruitment, retention, and data availability in a longitudinal remote measurement study. BMC Psychiatry. 2022 Feb 21;22(1):136. doi: 10.1186/s12888-022-03753-1. https://bmcpsychiatry.biomedcentral.com/articles/10.1186/s12888-022-03753-1 .10.1186/s12888-022-03753-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Insel TR. Digital phenotyping: technology for a new science of behavior. JAMA. 2017 Oct 03;318(13):1215–6. doi: 10.1001/jama.2017.11295.2654782 [DOI] [PubMed] [Google Scholar]
- 9.Simblett S, Greer B, Matcham F, Curtis H, Polhemus A, Ferrão J, Gamble P, Wykes T. Barriers to and facilitators of engagement with remote measurement technology for managing health: systematic review and content analysis of findings. J Med Internet Res. 2018 Jul 12;20(7):e10480. doi: 10.2196/10480. https://www.jmir.org/2018/7/e10480/ v20i7e10480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White KM, Dawe-Lane E, Siddi S, Lamers F, Simblett S, Riquelme Alacid G, Ivan A, Myin-Germeys I, Haro JM, Oetzmann C, Popat P, Rintala A, Rubio-Abadal E, Wykes T, Henderson C, Hotopf M, Matcham F. Understanding the subjective experience of long-term remote measurement technology use for symptom tracking in people with depression: multisite longitudinal qualitative analysis. JMIR Hum Factors. 2023 Jan 26;10:e39479. doi: 10.2196/39479. https://humanfactors.jmir.org/2023//e39479/ v10i1e39479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sieber C, Haag C, Polhemus A, Sylvester R, Kool J, Gonzenbach R, von Wyl V. Feasibility and scalability of a fitness tracker study: results from a longitudinal analysis of persons with multiple sclerosis. Front Digit Health. 2023 Feb 28;5:1006932. doi: 10.3389/fdgth.2023.1006932. https://europepmc.org/abstract/MED/36926468 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Zeev D, Scherer EA, Gottlieb JD, Rotondi AJ, Brunette MF, Achtyes ED, Mueser KT, Gingerich S, Brenner CJ, Begale M, Mohr DC, Schooler N, Marcy P, Robinson DG, Kane JM. mHealth for schizophrenia: patient engagement with a mobile phone intervention following hospital discharge. JMIR Ment Health. 2016 Jul 27;3(3):e34. doi: 10.2196/mental.6348. https://mental.jmir.org/2016/3/e34/ v3i3e34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis MM, Freeman M, Kaye J, Vuckovic N, Buckley DI. A systematic review of clinician and staff views on the acceptability of incorporating remote monitoring technology into primary care. Telemed J E Health. 2014 May;20(5):428–38. doi: 10.1089/tmj.2013.0166. https://europepmc.org/abstract/MED/24731239 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naslund JA, Aschbrenner KA, Barre LK, Bartels SJ. Feasibility of popular m-health technologies for activity tracking among individuals with serious mental illness. Telemed J E Health. 2015 Mar;21(3):213–6. doi: 10.1089/tmj.2014.0105. https://europepmc.org/abstract/MED/25536190 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holtz B, Vasold K, Cotten S, Mackert M, Zhang M. Health care provider perceptions of consumer-grade devices and apps for tracking health: a pilot study. JMIR Mhealth Uhealth. 2019 Jan 22;7(1):e9929. doi: 10.2196/mhealth.9929. https://mhealth.jmir.org/2019/1/e9929/ v7i1e9929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews JA, Craven MP, Jamnadas-Khoda J, Lang AR, Morriss R, Hollis C, RADAR-CNS Consortium Health care professionals' views on using remote measurement technology in managing central nervous system disorders: qualitative interview study. J Med Internet Res. 2020 Jul 24;22(7):e17414. doi: 10.2196/17414. https://www.jmir.org/2020/7/e17414/ v22i7e17414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Druce KL, Dixon WG, McBeth J. Maximizing engagement in mobile health studies: lessons learned and future directions. Rheum Dis Clin North Am. 2019 May;45(2):159–72. doi: 10.1016/j.rdc.2019.01.004. https://linkinghub.elsevier.com/retrieve/pii/S0889-857X(19)30004-3 .S0889-857X(19)30004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simons L, Valentine AZ, Falconer CJ, Groom M, Daley D, Craven MP, Young Z, Hall C, Hollis C. Developing mHealth remote monitoring technology for attention deficit hyperactivity disorder: a qualitative study eliciting user priorities and needs. JMIR Mhealth Uhealth. 2016 Mar 23;4(1):e31. doi: 10.2196/mhealth.5009. https://mhealth.jmir.org/2016/1/e31/ v4i1e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barkley RA, Murphy KR. Attention-Deficit Hyperactivity Disorder: A Clinical Workbook. 3rd edition. New York, NY, USA: Guilford Press; 2006. [Google Scholar]
- 20.Kooij JJ, Francken MH. DIVA 2.0 diagnostic interview for ADHD in adults. DIVA Foundation. 2010. [2019-10-01]. https://www.advancedassessments.co.uk/resources/ADHD-Screening-Test-Adult.pdf .
- 21.Sun S, Denyer H, Sankesara H, Deng Q, Ranjan Y, Conde P, Rashid Z, Bendayan R, Asherson P, Bilbow A, Groom M, Hollis C, Folarin AA, Dobson RJ, Kuntsi J. Remote administration of ADHD-sensitive cognitive tasks: a pilot study. J Atten Disord (Forthcoming) 2023 Jun 02;:10870547231172763. doi: 10.1177/10870547231172763. https://journals.sagepub.com/doi/abs/10.1177/10870547231172763?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013 Sep 18;13:117. doi: 10.1186/1471-2288-13-117. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-13-117 .1471-2288-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saunders B, Sim J, Kingstone T, Baker S, Waterfield J, Bartlam B, Burroughs H, Jinks C. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52(4):1893–907. doi: 10.1007/s11135-017-0574-8. https://europepmc.org/abstract/MED/29937585 .574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glaser BG, Strauss AL. Discovery of Grounded Theory: Strategies for Qualitative Research. New York, NY, USA: Routledge; 1999. [Google Scholar]
- 25.Matcham F, Barattieri di San Pietro C, Bulgari V, de Girolamo G, Dobson R, Eriksson H, Folarin AA, Haro JM, Kerz M, Lamers F, Li Q, Manyakov NV, Mohr DC, Myin-Germeys I, Narayan V, Bwjh P, Ranjan Y, Rashid Z, Rintala A, Siddi S, Simblett SK, Wykes T, Hotopf M, RADAR-CNS consortium Remote assessment of disease and relapse in major depressive disorder (RADAR-MDD): a multi-centre prospective cohort study protocol. BMC Psychiatry. 2019 Feb 18;19(1):72. doi: 10.1186/s12888-019-2049-z. https://bmcpsychiatry.biomedcentral.com/articles/10.1186/s12888-019-2049-z .10.1186/s12888-019-2049-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruno E, Biondi A, Böttcher S, Vértes G, Dobson R, Folarin A, Ranjan Y, Rashid Z, Manyakov N, Rintala A, Myin-Germeys I, Simblett S, Wykes T, Stoneman A, Little A, Thorpe S, Lees S, Schulze-Bonhage A, Richardson M. Remote assessment of disease and relapse in epilepsy: protocol for a multicenter prospective cohort study. JMIR Res Protoc. 2020 Dec 16;9(12):e21840. doi: 10.2196/21840. https://www.researchprotocols.org/2020/12/e21840/ v9i12e21840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denyer H, Ramos-Quiroga JA, Folarin A, Ramos C, Nemeth P, Bilbow A, Woodward E, Whitwell S, Müller-Sedgwick U, Larsson H, Dobson RJ, Kuntsi J. ADHD remote technology study of cardiometabolic risk factors and medication adherence (ART-CARMA): a multi-centre prospective cohort study protocol. BMC Psychiatry. 2022 Dec 20;22(1):813. doi: 10.1186/s12888-022-04429-6. https://bmcpsychiatry.biomedcentral.com/articles/10.1186/s12888-022-04429-6 .10.1186/s12888-022-04429-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simblett SK, Bruno E, Siddi S, Matcham F, Giuliano L, López JH, Biondi A, Curtis H, Ferrão J, Polhemus A, Zappia M, Callen A, Gamble P, Wykes T, RADAR-CNS Consortium Patient perspectives on the acceptability of mHealth technology for remote measurement and management of epilepsy: a qualitative analysis. Epilepsy Behav. 2019 Aug;97:123–9. doi: 10.1016/j.yebeh.2019.05.035.S1525-5050(19)30123-4 [DOI] [PubMed] [Google Scholar]
- 29.Simblett SK, Evans J, Greer B, Curtis H, Matcham F, Radaelli M, Mulero P, Arévalo MJ, Polhemus A, Ferrao J, Gamble P, Comi G, Wykes T, RADAR-CNS consortium Engaging across dimensions of diversity: a cross-national perspective on mHealth tools for managing relapsing remitting and progressive multiple sclerosis. Mult Scler Relat Disord. 2019 Jul;32:123–32. doi: 10.1016/j.msard.2019.04.020.S2211-0348(19)30180-4 [DOI] [PubMed] [Google Scholar]
- 30.Matte B, Anselmi L, Salum GA, Kieling C, Gonçalves H, Menezes A, Grevet EH, Rohde LA. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015 Jan;45(2):361–73. doi: 10.1017/S0033291714001470. https://europepmc.org/abstract/MED/25066615 .S0033291714001470 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Final major, minor, and subthemes of barriers to and facilitators of remote measurement technology that emerged across participant groups.
Data Availability Statement
For open access, the author has applied a Creative Commons Attribution (CC BY) license to any author-accepted manuscript version. The data supporting this study are not openly available but may be shared on request following a formal review process by the chief investigator and coinvestigators.


