Abstract
Background
Aged skin is characterized by wrinkles, hyperpigmentation, and roughness. Collagen is the most abundant protein in our body and it’s responsible for skin health and it’s mostly influenced by factors that accelerated aging such as UV.
Objective
This study aimed to identify the potential use of collagen as skin supplementation and the challenges and strategies for its delivery.
Methods
The articles were first searched through the existing database with the keyword of “collagen antiaging”. The 585 articles were then screened by year of publication (2012-2022) resulted in 475 articles. The articles were then selected based on the delivery of collagen either orally or topically, resulted in 12 articles for further analysis.
Results
Collagen has important roles in skin physiology by involving some mechanisms through inhibition of Mitogen- Activated Protein Kinase, induction of Tissue Growth Factor β (TGF-β), and inhibition of Nuclear Factor kappa beta (NF-κβ). The oral administration of collagen has an effective biological activity but requires large doses (up to 5 g daily). Meanwhile, the topical administration of collagen is limited by poor permeability due to high molecular weight (±300 kDa). Several strategies need to be carried out mainly by physical modification such as hydrolyzed collagen or entrapment of collagen using a suitable delivery system.
Conclusions
Collagen could improve the skin properties, but further research should be conducted to increase its penetration either by physical modification or entrapment into suitable carrier
Key words: Collagen, Oral, Topical, Scoping review, Tissue regeneration
Introduction
Skin is the largest organ in our body. Skin is composed of epidermis (upper layer), dermis, and subcutaneous. The epidermis is composed of proliferating cells (basal), differentiated cells (keratinocytes), or squamous. In the dermis layer, a cell-producing extracellular matrix (fibroblast) including collagen is found.1 Skin aging can be caused by external and internal factors or a combination of them. The external factor is often related to pollutants, UV radiation and inappropriate lifestyles. Of these factors, UV radiation (UVA at 314-400 nm wavelength, UVB at 290-320 nm wavelength, and UVC at 100-280 nm wavelength) is the primary cause to skin aging. UV radiation have shown significant impacts on skin aging through increased oxidative stress, damaged DNA, released of inflammatory factors, decreased function of fibroblasts and keratinocytes, and breakdown of hyaluronic acid, collagen, heparan sulfate and elastin. The generation of Reactive Oxygen Species (ROS) by UV radiation will mediated the activation of signaling pathways such as Mitogen-Activated Protein Kinase (MAPK). This process further mediates the fibroblast apoptosis and degradation of ECM in dermis layer. The aged skin will result in phenotypes changes, such as pigmentation, skin dryness, wrinkles and decreased skin thickness (dermal and epidermal).2-7
Skin ages as the progressing time, the process called chronological aging, which is marked by tinner, laxer, finely lined, and evenly pigmented skin. The causes of chronologically aged skin are not well understood yet, but it’s correlated to free radicals. Mitochondrial oxidative energy generation (a process to produce body energy or ATP) will generate ROS. The excess ROS concentration resulted in the accumulation of cellular damage. The ageassociated cellular damage includes oxidation of lipids membrane results in altered signaling and transport efficiency, oxidation of protein results in a decrease in function and oxidation of DNA causes mutation. As a result of aging, the cell’s antioxidant capacity will be reduced. Moreover, the chronologically aged skin has lower fibroblast cells and less capacity to synthesize type I procollagen.8-12 Collagen is one of the most abundant proteins in our body. It is found in the connective tissue. Collagen, proteoglycans, hyaluronate (HA) and elastin form Extracellular Matrix (ECM). The ECM provides cells the mechanical strength and molecule cues for cell stabilization and functionalization.13,14 Collagen consists of several types, in which types I and III forms 95% skin collagen. The aged skin is mostly related to a reduction in collagen and elastin synthesis. This protein is produced by fibroblast cells but with the passage of time, aging elicits the reduction of 1% of collagen yearly.15
Collagen Peptide (CP) is a hydrolysis product of native collagen. CP is known to have antioxidant activities that provide cell regeneration. CP could be used in food supplement formulation and cosmeceutical skincare with better solubility and bioavailability along with lower allergenic properties compared to native collagen. Orally administrating collagen peptides increase collagen synthesis. The rate of activity is depended on molecular size and amino acid composition.16,17 CP stimulated the synthesis of ECM such as endogenous collagen by up-regulating several gene expressions related to collagen expression, a collagen-modifying enzyme in posttranslational modification, collagen cross-linking and collagen degradation.18
Since the aging process could reduce collagen synthesis and break down existing collagen, sufficient nutritional support for collagen is important factor in slowing down the aging process. Recently, the efforts made to maintain skin health and delay aging have become a research hotspot. This scoping review aims to study the potential use of collagen as skin supplementation and the challenges and strategies for its delivery for tissue regeneration.
Figure 1.
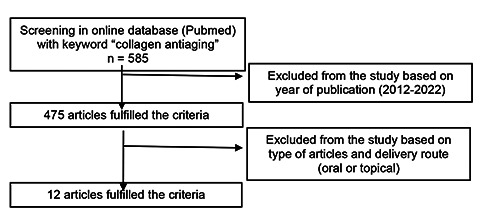
Consort diagram of article screening and selection.
Figure 2.
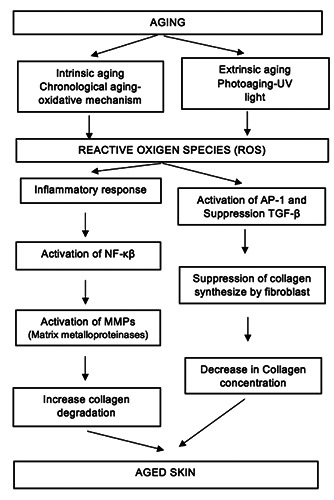
Skin aging and oxidative stress.37
Materials and Methods
This scoping review studied articles regarding the challenges and strategy of delivering collagen for tissue regeneration. The literature research was done through an existing database with the “collagen antiaging” as a key word. The database accessed for this study was PubMed. The search resulted in 585 articles. The articles further selected for year of publication (10 recent years, 2012-2022) resulted in 475 articles. The inclusion criteria of this study are research article, which administering collagen as an active ingredient topically or orally. The journal also checks for the predatory possibility by checking the DOAJ index and Beall’s list. The resulting 12 articles were reviewed in this study and the articles selection is showed in Figure 1.
Results
This scoping review contributes to the literature on collagen deliveries and activities as tissue regeneration. This study also discusses the present result in collagen mechanism for tissue regeneration. The paper reviewed in this study is presented in Table 1.
Discussion
The outermost layer of the human body consists of skin. It protects our body from any harm (mechanical, physical, thermal, and hazardous substances), prevents loss of moisture, and secretes metabolic products. As the other organ, skin ages through time. This process results from external, internal, and a combination of both factors. An internal factor, chronological aging is believed as a result of the generation of free radicals from mitochondrial oxidative energy generation and the decrease of body’s ability to produce collagen. While the external factors often related to UV irradiation, pollutants, and inappropriate lifestyles (Figure 2).
The mechanism in which supplementation of collagen facilitates collagen synthesis is mainly divided into two main mechanisms (Figure 3). First, the amino acid acts as a building block for collagen production. Second, the collagen peptide adheres to the fibroblast as a ligand. It then stimulates the collagen, elastin, and hyaluronic acid to synthesize.19
The antiaging activity of collagen has been extensively studied in vivo and in vitro methods. The in vitro experiment in fibroblast cells demonstrates the antiaging activity of hydrolyzed collagen by inhibition of ROS, synthesize of ECM including hyaluronic acid, elastin, and collagen, increasing fibroblast cell viability, improvement of protein folding and DNA repair mechanism (base excision repair, BER).20
The in vitro effect of Chicken Collagen Peptide (CCP) was studied in mice. The administration of CCP could alleviate UVinduced skin aging. First, CCP reduces skin oxidation, followed by inhibition of AP-1 (c-Jun and c-Fos) expression, and then activates the TGF-/Smad signaling pathway. This pathway results in the promotion of collagen synthesis, inhibition of MMP-1 and 3 activations, and reducing collagen breakdown. Interestingly, the effect of collagen supplementation could slightly differ with different sources of collagen.7
Figure 3.
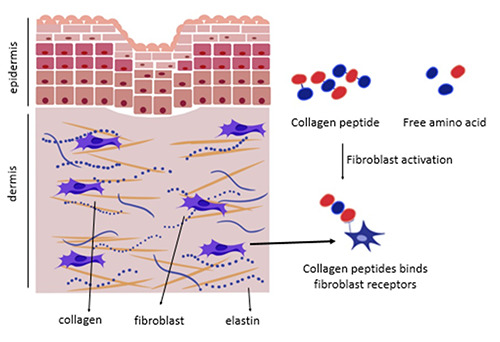
Mechanism of collagen supplementation in tissue generation.38,39
Table 1.
List of articles studied in this scoping review.
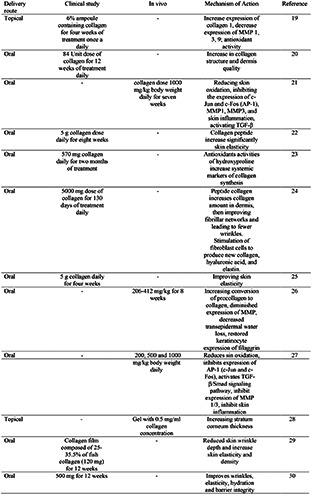
The Collagen Tripeptide (CT) was hydrolyzed product of native fish collagen isolated from Pangasius hypophthalmus which contains 4% Gly-Pro-Hyp. The in vitro study of CT was conducted using HDF cell culture and showed the increased expression of collagen 1 and decreased expression of MMP-1, 3, and 9 in a dosedependent manner. The doses used in that study were 250, 500, and 1000 μg/mL. The clinical trial for CT effectiveness was conducted with 22 subjects (asian women, with noticeable glabellar and periorbital wrinkles, and aged 30-54). The subjects received twice-aday topical treatment of CT ampoule. The administration of CT improved skin characteristics and reduced periorbital skin roughness and glabellar wrinkle. As much as 95.5% of subjects responded to being very satisfied and satisfied. The challenge of delivering CT through topical routes is mainly from poor permeability. The topical application of native collagen (1000 μg/ml) in reconstructed human-micro tissue was 0.425±0.100 % compared to the CT was 12.221±0.684%.21
The analysis of individual data from an open single-blind clinical study showed the oral administration of marine collagen peptide (570 mg) could improve collagen dermal structure and deposition as shown by the increase of hydroxyproline (a systemic marker of collagen synthesis).22 The other study reported the administration of collagen peptide (2.5 and 5.0 g/day) increased skin elasticity after 4 weeks of administration, but no statistically significant result from those doses.23 The other study showed a significant amount of hydrolyzed collagen appeared in the human blood that consumed hydrolyzed collagen after 12 hours of ingestion. The highest hydrolyzed collagen concentration was observed after 2 hours of ingestion, and half of the highest concentration of hydrolyzed collagen was observed after 4 hours. The concentration of a small peptide of Pro-Hyp (Proline-hydroxyproline) after ingesting 9.4–23 g of hydrolyzed collagen in human plasma was 25–60 nmol/mL.24
Collagen-containing products have become very popular recently. The important factor that influenced the activity of collagen from oral administration is absorption. The collagen should reach the bloodstream through an intestinal barrier in adequate quantity. Collagen supplementation could be given through oral and topical routes, given in the form of hydrolyzed collagen (Collagen Peptide, CP). The distribution of CP after oral ingestion was studied using 14C-labeled proline or CP administrated to rats. Observation in different tissue found that radioactivity was detected after 0-6 hours and remained detected after 14 days.25
The main concern related to the use of regular oral intake of collagen and its derivate has been raised from the fact that the collagen synthesis induction is marked by the increase of hydroxyproline which is associated with oxidative stress.26-28 This activity was related to the molecular size of peptide collagen. The peptide at size of 5 kDa showed a higher ability to donate hydrogen or electron. 29 The antioxidant activity of collagen peptides could arise from proline as a scavenger of hydroxyl radicals.30 Furthermore, the utilization of CP origin has been shown to activate the neutrophils and macrophage (innate immune response) through Tolllike receptor 4, which in turn activates NADPH-oxidase (NOX4) and overproduction of ROS.31,32
The native collagen could not absorb topically, the topical administration of CP should overcome the barrier of stratum corneum to reach the dermis layer (fibroblast cell). CP will reach the dermis through the transcellular route which is determined by its molecular size. CP will reach bloodstream through PEPT1 mediated transport of peptides, passive intracellular route, and transcytotic route.33,34 Two important key factors for CP penetration in topical administration are molecular volume (MV) and molecular weight (MW).35 The better penetration of CP was observed at 3500 and 4500 Da (compare to 1500 and 4500 Da) which may attributed to synergistic effect of structure features and lipopolicity.36 The main consideration in the topical use of collagen is the limited amount of penetrated collagen.
There were some limitations of the papers we studied, including a relatively short period of CP consumption and follow-up. Therefore, the long-term effects and durability of an improvement in elasticity could not be determined. The optimal dosage of CP is not yet identified. The absorption capacity of topical collagen application is limited by its molecular size. The manufacturing process of collagen with small molecular weight (1-10 kDa), higher permeability, higher biocompatibility, and lower antigenicity will be crucial for the optimal use of collagen for tissue regeneration.
Conclusions
The utilization of peptide collagen through oral and topical administration improves skin properties. However, only a small concentration of collagen can reach its active site due to its high molecular size. Increasing the dose of collagen will increase the risk of reactive oxygen species generation. Future development of collagen to increase absorption can be done by hydrolysis or entrapment of collagen using a suitable carrier system. With the increase in collagen concentration, tissue regeneration will also improve.
Funding Statement
Funding: None.
References
- 1.Champion RH, Rook A, Burton JL, et al. Textbook of Dermatology. Oxford, UK: Blackwell Scientific; 1992. [Google Scholar]
- 2.Ansary TM, Hossain MR, Kamiya K, et al. Inflammatory molecules associated with ultraviolet radiation mediated skin aging. Int. J. Mol. Sci. 202:3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsatsou F, Trakatelli M, Patsatsi A, et al. Extrinsic aging: UVmediated skin carcinogenesis, Dermatoendocrinology 2012:285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amaro-Ortiz A, Yan B, D'Orazio JA. Ultraviolet radiation, aging and the skin: prevention of damage by topical cAMP manipulation. Molecules 2014;19:6202–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann L. How to use oral and topical cosmeceuticals to prevent and treat skin aging. Facial Plast Surg Clin N Am 2018:407-13. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Su G, Zhou F, et al. Protective effect of bovine elastin peptides against photoaging in mice and identification of novel antiphotoaging peptides. J Agric Food Chem 2018:10760–8. [DOI] [PubMed] [Google Scholar]
- 7.Cao C, Xiao Z, Tong H, et al. Oral Intake of chicken bone collagen peptides anti-skin aging in mice by regulating collagen degradation and synthesis, inhibiting inflammation and activating lysosomes, Nutrients 2022;14:1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science 1996;273:59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hensley K, Floyd R. Reactive oxygen species and protein oxidation in aging: a look back, a look ahead. Arch Biochem Biophys 2002;397:377-83. [DOI] [PubMed] [Google Scholar]
- 10.Chamougrand N, Rigoulet M. Aging and oxidative stress: studies of some genes involved both in aging and in response to oxidative stress. Respir Physiol 2001;128:393-401. [DOI] [PubMed] [Google Scholar]
- 11.Ma W, Wlaschek M, Tantcheva-Poor I, et al., Chronological aging and photoaging of the fibroblasts and the dermal connective tissue. Clin Exp Dermatol 2001;26 :592-99. [DOI] [PubMed] [Google Scholar]
- 12.Herman D. Aging: overview. Ann N Y Acad Sci 2001;928:1-21. [DOI] [PubMed] [Google Scholar]
- 13.Sibilla S, Godfrey M, Brewer S, et al. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: scientific background and clinical studies. Open Nutraceuticals J 2015;8:29-42. [Google Scholar]
- 14.Poltavets V, Kochetkova M, Pitson SM, Samuel MS, The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front Oncol 2018;8:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganceviciene R, Liakou AI, Theodoridis A, et al. Skin antiaging strategies. Dermatoendocrinol 2012;4:308-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asserin J, Lati E, Shioya T, Prawitt J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo controlled clinical trials, J Cosmet Dermatol 2015;14:291-301. [DOI] [PubMed] [Google Scholar]
- 17.Sato K. The presence of food-derived collagen peptides in human body-structure and biological activity, Food Funct 2017;8:4325-30. [DOI] [PubMed] [Google Scholar]
- 18.Yamada H, Nagaoka M, Terajima N, et al. Effects of fish collagen peptides on collagen post-translational modifications and mineralization in an osteoblastic cell culture system, Dent Mater J 2013;32:88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito N, Seki S, Ueda F. Effects of composite supplement containing collagen peptide and ornithine on skin conditions and plasma IGF-1 levels—a randomized, double-blind, placebo- controlled trial, Marine Drugs 2018;16:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laing S, Bielfeldt S, Ehrenberg C, Wilhelm KP. A dermonutrient containing special collagen peptides improves skin structure and function: a randomized, placebo-controlled, tripleblind trial using confocal laser scanning microscopy on the cosmetic effects and tolerance of a drinkable collagen supplement, J Med Food 2020;23:147-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YI, Lee SG, Jung I, et al. Effect of a topical collagen tripeptide on antiaging and inhibition of glycation of the skin: A pilot study. Int J Mol Sci 2022;23:1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Luca C, Mikhal’chik EV, Suprun MV, et al. Skin antiageing and systemyc redox effect of supplementation with marine collagen peptides and plant-derived antioxidants: a single-blind case-control clinical study, Oxidative Medicine and Cellular Longevity 2016;14:2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proksch E, Segger D, Degwert J, et al., Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol 2014;27:47-55. [DOI] [PubMed] [Google Scholar]
- 24.Iwai K, Hasegawa T, Taguchi Y, et al., Identification of foodderived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem 2005;53:6531-36. [DOI] [PubMed] [Google Scholar]
- 25.Sai Y, Kajita M, Tamai I, et al., Adsorptive mediated endocytosis of a basic peptide in enterocyte-like Caco-2 cells. Am J .Physiol 1998;275:23. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Z, Yang G, Wang Y, et al. Suppression of thioredoxin system contributes to silica-induced oxidative stress and pulmonary fibrogenesis in rats, Toxicology Letters 2013;222:289-94. [DOI] [PubMed] [Google Scholar]
- 27.Zhou CF, Yu JF, Zhang JX. N-acetylcysteine attenuates subcutaneous administration of bleomycin-induced skin fibrosis and oxidative stress in a mouse model of scleroderma, Clin Exp Dermatol 2013;38:403–9. [DOI] [PubMed] [Google Scholar]
- 28.Cooper SK, Pandhare J, Donald SP, Phang JM. A novel function for hydroxyproline oxidase in apoptosis through generation of reactive oxygen species, J Biol Chem 2008;283:10485-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leon-Lopez A, Fuentes-Jimenez L., Hernandez-Fuentes AD, et al., Hydrolysed collagen from sheepskins as a source of functional peptides with antioxidant activity. Int. J. Mol. Sci. 2019,20:3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chalamaiah M, Dinesh KB, Hemalatha R, Jyothirmayi T. Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications—a review, Food Chemistry 2012;135:3020-38. [DOI] [PubMed] [Google Scholar]
- 31.Altenhöfer S, Radermacher KA, Kleikers PW, et al. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement, Antioxid Redox Signal 2015;23:406-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ott C, Jacobs K, Haucke E, et al. Role of advanced glycation end products in cellular signaling, Redox Biol 2014;2:411-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adson A, Raub TJ, Burton PS, et al. Quantitative approaches to delineate paracellular diffusion in cultured epithelial cell monolayers. J Pharm Sci 1994;83:1529–36. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe-Kamiyama M, Shimizu M, Kamiyama S, et al. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J Agric Food Chem 2010;58:835-41. [DOI] [PubMed] [Google Scholar]
- 35.Baert B, Deconinck E, Gele MV, et al. Transdermal penetration behavior of drugs: CART clustering, QSPR, and selection of model compounds. Bioorg Med Chem 2007;15:6943-55. [DOI] [PubMed] [Google Scholar]
- 36.Chai HJ, Li JH, Huang HN, et al. Effects of size and conformations of fish-scale collagen peptides in facial skin qualities and transdermal penetration efficiency, J Biomed Biotechnol 2010;2010:757301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lephart ED. Skin aging and oxidative stress: Equol’s antiaging effects via biochemical and molecular mechanisms, Ageing Res Rev 2016;31:36-54. [DOI] [PubMed] [Google Scholar]
- 38.Reilliy DM, Lozano J. Skin collagen through the lifestages: importance for skin health and beauty. Plastic Aesthetic Res 2020;2021. [Google Scholar]
- 39.Freitas-Rodríguez S, Folgueras AR, López-Otín C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond, Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2017;1864:2015-25. [DOI] [PubMed] [Google Scholar]


