Abstract
Background
There are thousands of species of known medicinal plants in the world. Ruta angustifolia L. has been widely used in traditional medication for jaundice and liver disease. Previous studies have shown that R. angustifolia leaves can inhibit the hepatitis C virus in Huhit culture cells, reduce the value of NS3 protein, and possess a synergistic effect in combination with antiviral drugs. Therefore, this plant is potential to be developed as a drug candidate. Characteristics of plants including microscopic, physicochemical properties, and phytochemical profiles are necessary information to ensure the quality of raw material in drug development.
Objective
This study was carried out to examine the microscopic and physicochemical including the standardized parameter of R. angustifolia leaves to fulfil the quality raw materials as traditional medicine.
Methods
Simplicia of R. angustifolia leaves obtained from Jombang, East Java, were observed under a microscope and determined its physicochemical properties referred to the Materia Medica Indonesia V. The TLC and HPLC profiles of extract were determined as well.
Results
Microscopic analysis were conducted by transfection sections and the presence of epidermis cells, palisade, mesophyll with stomata, and Ca-oxalate crystal were found. The standard parameter obtained value of loss of drying, extractive value in water and ethanol, and ash value. The TLC and HPLC profiles of leaves extract demonstrated to contain with flavonoid, terpenoids, and alkaloids.
Conclusion
Ruta angustifolia obtained from Jombang, east Java, has a specific character in microscopic analysis. The physicochemical properties analysis of R. angustifolia leaves as a raw material met the requirements according to Materia Medica Indonesia V.
Key words: Ruta angustifolia, Medicinal plants, Raw material, Infectious disease
Introduction
Thousands of plant species were known as medicinal plants in the world. Approximately 30,000 species of medicinal plants were allegedly located in Indonesia. From the abundant medicinal plants present, only 1,200 types of plants have been used as raw materials for herbal medicines.1,2 Medicinal plants can be used as a source of drug candidates for infection with the hepatitis C virus (HCV).3 Apart from having active chemical components, medicinal plants are also exhibited as inhibitors in the replication of various DNA and RNA viruses.4 Various treatments have traditionally been used as a therapy for HCV infection treatment, and some of these treatments have gone through various tests including clinical trials and some have theoretical efficacy.5 It was also reported several medicinal plants possess anti-HCV activities with various mechanisms. 3,6,7
Ruta angustifolia L. is a plant of Rutaceae and is locally known as inggu. Ruta has been reported to be used as various traditional medicine including antiseptic, anti-inflammatory, pain relief, respiratory tract treatment, nervous system, and musculoskeletal.8 Meanwhile, it was used as a traditional remedy for jaundice and liver disease in Indonesia.9
Ruta angustifolia L. plant contains alkaloids, coumarin, and flavonoids. Our previous studies have isolated chalepin and pseudane IX which exhibited strong antiHCV activity.10 Ruta angustifolia extract was able to decrease the protein value of NS3 HCV and work at the post-entry step. A synergistic effect was shown when R. angustifolia extract was combined with the antiviral HCV drugs.11 Moreover, it was also reported that chalepin and rutamarin can significantly inhibit the growth of cancer cells.12 Those suggested that R. angustifolia L. is the potential to be developed into a product of traditional medicine, including standardized herbal medicine. In its development, raw materials products had certain requirements which are important for their pharmacological and safe effects. Thus, to ensure the constancy and clarity of the ingredients contained in preparations from natural raw materials so that it is expected to have a fixed pharmacological effect from time to time, evaluation of drug raw materials following standard parameters is needed. The raw material must meet the requirements listed in the monograph published by the Ministry of Health of the Republic of Indonesia, namely Materia Medica Indonesia and Indonesian Herbal Pharmacopoeia. These requirements include raw material identity, microscopic test, organoleptic, water-soluble extract, ethanol-soluble extract, loss on drying, total ash content, acid-insoluble ash content, water-soluble ash content, and determination of certain chemicals.
Therefore, this research aims to conduct the standard parameters of inggu leaves (R. angustifolia L.) as the raw material in the development of traditional medicines through microscopic and physicochemical properties evaluation to guarantee the quality of the medicinal product.
Materials and Methods
Materials
This study was inggu leaves that were cultivated in Jombang, East Java, Indonesia. The plant was determined by a botanist of Materia Medica Indonesia with specimen number 074/319A/ 102.7/2019. The plants were collected at the age of 10-12 months. It was sorted, cleaned, and dried at room temperature and protected from direct sunlight for 3 weeks. The dried simplicia is made into a powder by grinding and followed by further analysis.
Microscopic analyses
The test is carried out under a light microscope. The magnification is adjusted to the need of the study. The anatomy of the inggu leaves and the fragments present in the leaves powder were examined and compared to the reference. Water was used as media and chloral hydrate was used as a reagent to dissolve the cytoplasm.
Physicochemical properties
Physicochemical properties were evaluated including the percentage of substance dissolve in water and ethanol, total ash content, acid-insoluble ash, and loss of drying.13
Water-soluble extract determination
Five g powder of inggu was macerated with 100 mL of saturated water, shaken repeatedly for the first 6 hours, and further macerate during 18 hours. A number of 20 mL of filtrate was evaporated, dried in a shallow dish that has been heated at 105°C several times to obtaine the constant balance. The percentage of the watersoluble extract was calculated compared to the reference.
Ethanol-soluble extract determination
Five g of inggu dried powder was macerated with 100 mL of 95% ethanol in flask, and shaken repeatedly for the first 6 hours and 18 hours. The 20 mL of the filtrate was evaporated to obtain the dried material in a shallow dish at 105°C and tapped, heated in the same temperature until the constant weight. The value in % of ethanol soluble extract was determined.
Determination of dry loss
As much as one to two g of simplicia is placed in a shallow, weighed bottle that has been preheated at fixing temperature and set as tare. Flatten the ingredients in the weighing bottle, shaking until got a layer about 5 to 10 mm thick. It was heated at 105°C in the oven for one hour and following by cooling in desiccators. It was dried chamber, opened the lid, and dried at a fixed temperature to a fixed weight. While drying, the bottle was closed and started cooling in the desiccator to room temperature. Loss in weight was called as moisture content that was used to calculate its percentation by comparing to to initial weight of simplicia.
Total ash determination
The total ash was conducted by weighing two g of inggu powder that has been mashed and put into crushed porcelain crucibles. It was incinerated slowly at 600 ºC until the charcoal runs out, cool, and weighed. Additional hot water was necessary If the charcoal cannot be removed. It was stired and filtered through ash-free filter paper. It was spread in the crucible with the filter, steamed, and heated it until the weight remains. The percentage of total ash was calculated compared to the weight of the powder.
Acid-insoluble ash determination
The obtained ash from the total ash calculatio was washed with 25 mL of dilute hydrochloric acid (2 N) and boiled for five minutes. Gathering the unsoluble part in acid which were filtered through ash-free filter paper. It was washed with hot water and sprinkled in crushers until the weight remains. Ash content which is insoluble in acid is calculated against the weight of the crude material.
Determination of water-soluble ash
In a crucible containing total ash, add 25 mL of water and boil for five minutes. Insoluble substances are filtered using ash-free filter paper. Washed with hot water and incubated in a crucible for 15 minutes at a temperature of no more than 450°C, open the lid of the porcelain crucible. Spread to constant weight. Water-soluble ash content is calculated compared to the weight of the crude material.
Table 1.
Physicochemical characteristic of R. angustifolia leaves.
| Parameters | Value* (% w/w) | Requirement (materia medica ndonesia V) (%) |
|---|---|---|
| Water-soluble extract | 27.14±0.47 | Not less than 28 |
| Ethanol-soluble extract | 20.17±0.43 | Not less than 8 |
| Total ash | 10.75±0.01 | Not less than 10 |
| Water-soluble ash | 2.67±0.47 | No specific parameter |
| Acid-insoluble ash | 0.52±0.05 | Not more than 3 |
| Loss on drying | 10.02±0.21 | Not more than 10 |
*Mean±SD in triplicate.
Table 2.
Determination of heavy metal.
| Test | Result | Requirements |
|---|---|---|
| Pb | 0.12 ppm | ≤10 ppm |
| Cd | 0.01 ppm | ≤0.3 ppm |
| Hg | 0.00013 ppm | ≤0.5 ppm |
| As | 0.00002 ppm | ≤5 ppm |
Determination of heavy metal
The presence of heavy metal (lead (Pb), cadmium (Cd), mercury (Hg), and arsenic (As)) were determined by Atomic Absorbance Spectrophotometer (AAS). Five g samples were digested with suitable reagents such as 9 mL HNO3 and 3 mL H2O2 and heated at 180°C for 25 min. After this destruction process, the sample was diluted and analyzed. A calibration curve is made by absorbing the standard series which has been corrected to the blank, and the metal content. The metal content in the sample is calculated.
Phytochemical profile with thin layer chromatography and high-performance liquid chromatography
The phytochemical screening was conducted by TLC using silica gel GF254 as a stationary phase, hexane-acetone (3/2 v/v) as a mobile phase, and spray reagent FeCl3, borate citrate, and dragendorff for identification of polyphenol, flavonoid and alkaloid compounds, respectively. Meanwhile, terpenoid identification using silica gel GF254 as a stationary phase, hexane-ethyl acetate (7/3 v/v) as a mobile phase, and spray reagent anisaldehyde sulphate.
Ruta angustifolia extract was subjected to HPLC to determine the peaks’ chromatogram profile and UV spectra. HPLC Shimadzu was used for this experiment, completed with RP-18 column (Merck, 4.6x250 mm, 5 μm), using acetonitrile-water (80%-20% v/v) as mobile phase, flowrate 0.5 mL/min. The extract (1,000 ppm) was injected with a volume of 40 μL and a chromatogram was observed at UV 254 nm for 30 minutes.
Results
Microscopic analysis
The plant R. angustifolia showed as an herb with small leaves (Figure 1). This plant was found less than 1 meter high, the leaves are green, oblong to an oval and smooth surface. A microscopic profile of the transection section of R. angustifolia leaves was shown to have an epidermis, upper and lower palisade tissue, spongy tissue, lysigen gland, stomata, and calcium oxalate rosette-shaped crystal (Figure 2). Further evaluation of its leaves powder by observing under the microscope found tissue of palisade, mesophyll, epidermis, stomata, crystal, oil gland, and tissue vessel (Figure 3).
Figure 1.

Ruta angustifolia plants. A) parts of the plant as a whole; B) part of the leaves.
Figure 2.
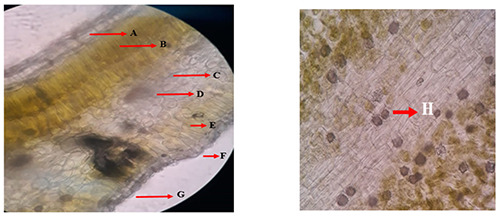
Microscopic analysis on the simplicia powder of R. angustifolia leaves. A: Upper epidermis. B: Upper palisade tissue. C: Spongy tissue. D: Lysigen gland. E: Lower palisade tissue. F: Stomata. G: Lower epidermis. H: Calcium oxalate rosette-shaped crystal.
Physicochemical properties
Physicochemical properties determination of R. angustifolia leaves was obtained from the parameter value of water-soluble extract, ethanol-soluble extract, total ash, water-soluble ash, and acid-insoluble ash. The water-soluble extract value obtained was 27.14±0.47%, which indicated the amount of polar substance of the plant which dissolve in the water. While the ethanol-soluble was 20.17±0.43%, indicating the extracted components of the plant in ethanol. Total ash is important to evaluate the amount of external and internal minerals in simplicia. The total value of ash obtained was 10.75±0.01%. The ash was dissolved in water and water-soluble ash was 2.67±0.47%. It was also dissolved in acid to determine the acid-insoluble ash, the value was 0.52±0.05%. The acid-insoluble parameter indicated the impurities such as silicate in the material. Another tested parameter was the loss of drying that measured the value of the compound due to the process of drying and it was revealed of 10.02±0.21%. Those results were relatively within the limit of Materia Medica Indonesia V (Table 1).
Heavy metal evaluation
Heavy metals were external contamination that need to be measured to guarantee the safety of the material. Heavy metal contamination testing aims to determine the value of metal content including Pb, Cd, Hg, and As in the simplicia of R. angustifolia. Those metals contributed dangerously to human health. The value of Pb, Cd, Hg, and As were 0.12 ppm, 0.01 ppm, 0.00013 ppm, and 0.00002 ppm, respectively (Table 2).
TLC and HPLC profile
The phytochemical evaluations of leaves extract were conducted by TLC and HPLC. Based on the TLC profile, it was found that leaves of R. angustifolia contain an alkaloid, flavonoid, terpenoid, and polyphenol compounds (Figure 4). While the HPLC profile of the extract detected several peaks at Retention Time (Rt) of 3.9 min, 5.2 min, 6.0 min, 7.4 min, 8.7 min, and 10.5 min. Those peaks were further observed in the UV spectrum to predict the groups of compounds contained in the extract (Figure 5).
Figure 3.
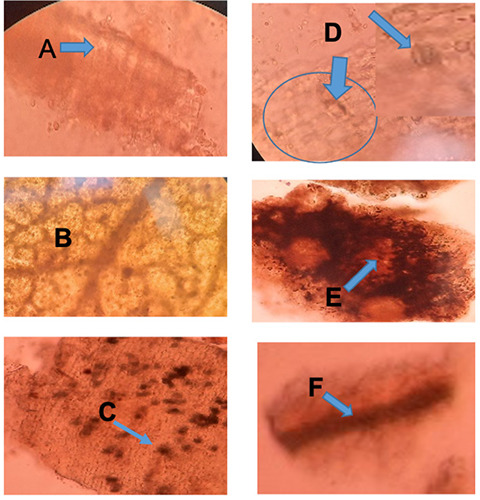
Microscopic profile of Ruta angustifolia. A: Palisade; B: Mesophyll; C: Epidermis with crystal; D: Stomata; E: Volatile oil cavity; F: Vessel fragment.
Discussion
Microscopic analysis
Microscopic tests are characteristic elements of anatomy or tissue which identify the types of raw material based on the specific identification fragments.14 It was found that the upper and lower epidermis consisted of one rectangular cell layer, the upper palisade tissue consisting of two long cylindrical cell layers, the lower palisade tissue consisting of one cylindrical cell layer, and slightly bent long, essential oil glands, spongy tissue consisting of several layers of cells that are irregular in shape and there are many air cavities, idioblasts with calcium oxalate rosette-shaped crystals, vascular bundles, and anomocytic stomata. The fragments found from the observations match the identification fragments listed in the Materia Medica Indonesia V, namely the lower epidermis, upper epidermis, calcium oxalate rosette-shaped crystal, and vessel bundles.15,16
Physicochemical properties
Water-soluble extract value is used for evaluating crude drugs which indicated an approximate measure of their chemical constituents. Water-soluble extract value aims to determine the number of polar compounds that can be extracted by water solvents.13 In determining the concentration of water-soluble extract, the result was 27.14% ±0.47. The polar compounds found in Ruta angustifolia L. include rutin, tannins, and saponins which may extracted in the water solvent.17 The soluble extractive of ethanol aims to determine the amount of semi-polar or non-polar compounds that can be extracted using ethanol as a solvent.18 For the determination of the content of extracts that dissolve in ethanol, the result was 20.17% ±0.42, this result met the requirements listed in Materia Medica Indonesia V, which is not less than 8%. The semipolar or non-polar compounds found in the R. angustifolia L. plant include essential oils, sterols, triterpenoids, chalepin, γ-fagarine, arborinine, and kokusaginine.10 Loss on drying provides an overview of the maximum limits on the number of compounds lost during the drying process, which can be lost after heating at 105°C including water and volatile compounds such as essential oils.6,13 In the determination of loss on drying, the yield was 10.02±0.21%. The value obtained is due to the loss on drying for not only water but volatile compounds such as essential oils as well. This can happen because the raw material is still not completely dry.
Figure 4.
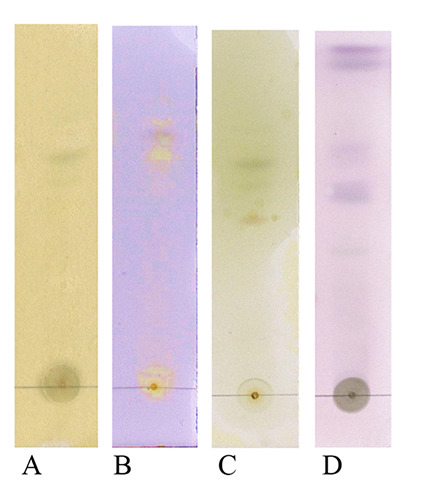
The TLC profile of R. angustifolia extract using silica gel GF254 as a stationary phase, hexane-acetone (3/2 v/v) (A-C), and hexane-ethyl acetate (7/3 v/v) (D) as a mobile phase; spray reagent FeCl3 (A), borate citrate (B), dragendorff (C), and anisaldehyde sulphate (D) for identification of polyphenol, flavonoid, alkaloid, and terpenoid, respectively
Figure 5.
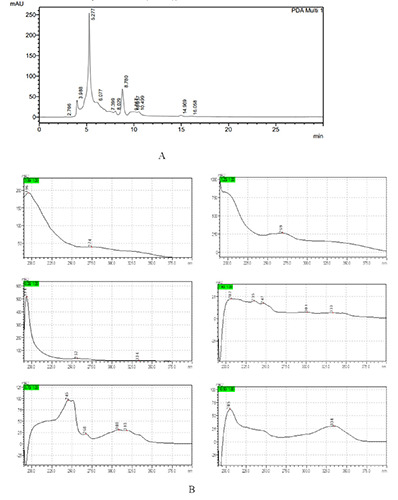
The HPLC profile of R. angustifolia extract observed under UV 254 nm using RP-18 column (Merck, 4.6x250 mm, 5 μm) and acetonitrile-water (80%-20% v/v) as mobile phase with a flow rate of 0.5 mL/min (a), The UV spectra of several peaks at Rt 3.9 min, 5.2 min, 6.0 min, 7.4 min, 8.7 min and 10.5 min (b)
The total ash content shows the internal and external mineral content in the raw material and extract, if the total ash content value is high it indicates the mineral content in the raw material. In determining the total ash content, the result obtained was 10.75±0.01%. This result has met the requirements set out in Materia Medica Indonesia V, which is not less than 10%. The higher the total ash content, the higher the mineral content in the raw material.6,13,19
Acid-insoluble ash content indicates the amount of mineral content that is insoluble in acid. If the value of acid-insoluble ash content is high, it shows that the raw material and extracts studied contain silicate content from soil or sand, silver metal, lead, and mercury.6,17,20 For the determination of the acid-insoluble ash content, the result was 0.52±0.05%. This result has met the requirements set out in Materia Medica Indonesia V, which is not more than 3%.
Heavy metal evaluation
The heavy metal contamination test aims to assure that raw material does not contain certain heavy metals such as Hg, Pb, Cd, and As which exceed the predetermined value because they are harmful (toxic) to health.20 Hg (mercury) can damage the nervous system, digestive system, immune system, kidneys, and lungs. Meanwhile, Pb (lead) and Cd can cause abdominal pain, constipation, headaches, frequent forgetting, and pain in the hands or feet. The high amount can cause anemia, and kidney and brain damage. Moreover, arsenic exposure may cause cancer.21,22
In the determination of heavy metal contamination for Pb, Cd, Hg, and As, the results showed the content was 0.12 ppm, 0.01 ppm, 0.00013 ppm, and 0.00002 ppm, respectively. The results of this determination have met the requirements for raw material, namely for Pb metal ≤10 ppm, Cd metal ≤0.3 ppm, Hg metal ≤0.5 ppm, and metal As ≤5 ppm.19,20
TLC and HPLC profile
The phytochemistry screening of R. angustifolia extract by TLC in this study showed the presents of polyphenols, alkaloids, flavonoids, and terpenoids groups of compounds. The compounds were indicated by yellow spots for polyphenols and flavonoids, orange to brown spots for alkaloids, and violet spots for terpenoids after being sprayed with FeCl3 and borate citrate, dragendorff, and anisaldehyde sulphate, respectively (Figure 4). Meanwhile, the HPLC chromatogram profile of R. angustifolia extract showed several peaks under 30 min observation (Figure 5A). Each peak was observed with its UV spectra to predict the group of compounds contained in the extract (Figure 5B). The TLC results were in accordance with the UV spectra of HPLC chromatogram peaks detected in the extract. The UV spectra have λmaks at 269 and 274 nm which represent peaks detected at Rt 5.2 min and 3.9 min. These UV spectra possibly represent alkaloids due to the dihydroindole chromophore of alkaloids absorption maximal near about 250 and 300 nm.23 The main absorption features of this phenolic subfamily are given by an intense absorption band at 280 nm.24 The UV spectra have λmaks at 235 nm which represent peaks detected at Rt 7.4 min. The terpenoid types were reported to show a simple absorption at 235.60 nm. Furthermore, other UV spectra have λmaks at 194, 257, and 334 nm for a peak at Rt 6.0 min; λmaks at 247, 301, and 333 nm for a peak at Rt 7.4 min; λmaks at 245, 268, 308, and 318 nm for a peak at Rt 8.7 min; and λmaks at 205 and 334 nm for a peak at Rt 10.5 min. These four UV spectra possibly represent flavonoid compounds. The typical UV spectra of flavonoids include two absorbance bands. In flavanones and dihydroflavonols, band A lies at 300-330 nm and band B at 277-295 nm range.25 The results suggested that alkaloids, terpenoids, and flavonoids were present in the extract.
Conclusions
The results suggest that R. angustifolia leaves simplicia obtained from Jombang, East Java, revealed the specific characteristic and possesses standard parameters according to Materia Medica Indonesia V to ensure the quality of the raw material of the drug product.
Acknowledgments
The author would like to thank the Ministry of Research and Technology for funding through the Penelitian Dasar scheme with grant number 4/AMD/E1/KP.PTNBH/2020.
Funding Statement
Funding: None.
References
- 1.Sen T, Samanta SK. Medicinal plants, human health, and biodiversity: a broad review. Adv Biochem Eng Biotechnol 2015;147:59-110. [DOI] [PubMed] [Google Scholar]
- 2.Yacoub K, Cibis K, Risch C. Biodiversity of Medicinal Plants. Biodiversity, Natural Products and Cancer Treatment 1-32. [Google Scholar]
- 3.Wahyuni TS, Utsubo CA, Hotta H. Promising Anti-Hepatitis C Virus Compounds from Natural Resources. Natural product communications 2016;11:1193-200. [PubMed] [Google Scholar]
- 4.Javed T, Ashfaq UA, Riaz S, Rehman S, Riazuddin S. In-vitro antiviral activity of Solanum nigrum against Hepatitis C Virus. Virol J 2011;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milliman WB, Lamson DW, Brignall MS. Hepatitis C; a retrospective study, literature review, and naturopathic protocol. Altern Med Rev 2000;5:355-71. [PubMed] [Google Scholar]
- 6.Sumbul S, Ahmad MA, Asif M, et al. Physicochemical and phytochemical standardization of berries of Myrtus communis Linn. J Pharm Bioallied Sci 2012;4:322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahyuni T, Azmi D, Permanasari AA, Adianti M, Tumewu L, Widiandani T, et al. Anti-viral activity of Phyllanthus niruri against hepatitis C virus. Malaysian Applied Biology 2019;48:105-11. [Google Scholar]
- 8.Pollio A, De Natale A, Appetiti E, et al. Continuity and change in the Mediterranean medical tradition: Ruta spp. (Rutaceae) in Hippocratic medicine and present practices. J Ethnopharm 2008;116:469-82. [DOI] [PubMed] [Google Scholar]
- 9.Richardson JS, Sethi G, Lee GS, Malek SN. Chalepin: isolated from Ruta angustifolia L. Pers induces mitochondrial mediated apoptosis in lung carcinoma cells. BMC Complement Altern Med 2016;16:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahyuni TS, Widyawaruyanti A, Lusida MI, et al. Inhibition of hepatitis C virus replication by chalepin and pseudane IX isolated from Ruta angustifolia leaves. Fitoterapia 2014;99:276-83. [DOI] [PubMed] [Google Scholar]
- 11.Wahyuni TS, Mahfud H, Permatasari AA, et al. Synergistic anti-hepatitis C virus activity of Ruta angustifolia extract with NS3 protein inhibitor. J basic clin physiol and pharmacol 2019. [DOI] [PubMed] [Google Scholar]
- 12.Fakai MI, Karsani S.A, Malek SNA. Chalepin And Rutamarin Isolated from Ruta angustifolia Inhibit Cell Growth in Selected Cancer Cell Lines (MCF7, MDA-MB-231, HT29, and HCT116). JISTM 2017;2:10. [Google Scholar]
- 13.Mukherjee PK. Chapter 3 - Quality Evaluation of Herbal Medicines: Challenges and Opportunities. Quality Control Evaluation of Herbal Drugs. Elsevier 2019;53-77. [Google Scholar]
- 14.Nazish I, Ali M, Mir SR, et al. Application of Microscopy in Authentication of Unani Traditional Plant Ruta graveolens 2010. [Google Scholar]
- 15.Mukherjee PK. Chapter 5 - Morphological and Microscopical Evaluations. In: Mukherjee PK, editor. Quality Control and Evaluation of Herbal Drugs: Elsevier; 2019;151-93. [Google Scholar]
- 16.Mattummal R, Divya K, Kn SK. Comparison of the Leaf Drug Ruta graveolens and Its Substitute Ruta chalepensis. Intern J Res Ayurveda Pharma 2018;9:100-10. [Google Scholar]
- 17.Alotaibi S, Saleem M, Al-humaidi J. Phytochemical contents and Biological Evaluation of Ruta chalepennsis L growing in Saudi Arabia. Saudi Pharma J. 2018;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tutik Sri W, Adita Ayu P, Lidya T, et al. Qualitative and Quantitative Analysis of 70% Ethanol Extract from Ruta angustifolia for Developing Anti-Hepatitis C Agents. Pharmacognosy J. 2021;13. [Google Scholar]
- 19.Organization WHO. Quality control methods for medicinal plant materials. 1998. [Google Scholar]
- 20.Mukherjee PK. Chapter 4 - Qualitative Analysis for Evaluation of Herbal Drugs. In: Mukherjee PK, editor. Quality Control and Evaluation of Herbal Drugs: Elsevier 2019;79-149. [Google Scholar]
- 21.Bernard A. Cadmium & its adverse effects on human health. Indian J Med Res 2008;128:557-64. [PubMed] [Google Scholar]
- 22.Hong YS, Song KH, Chung JY. Health effects of chronic arsenic exposure. J Prev Med Public Health 2014;47:245-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govindachari TR, Rajappa S. Ultraviolet absorption spectra of dihydroindole alkaloids. Proceedings of the Indian Academy of Sciences - Section A 1960;51:319-21. [Google Scholar]
- 24.Wessel JR. An adaptive orienting theory of error processing. Psychophysiol 2018;55. [DOI] [PubMed] [Google Scholar]
- 25.Tsimogiannis D, Samiotaki M, Panayotou G, Oreopoulou V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules 2007;12:593-606. [DOI] [PMC free article] [PubMed] [Google Scholar]


