Abstract
Interventional electrophysiology offers a great variety of treatment options to patients suffering from symptomatic cardiac arrhythmia. Catheter ablation of supraventricular and ventricular tachycardia has globally evolved a cornerstone in modern arrhythmia management. Complex interventional electrophysiological procedures engaging multiple ablation tools have been developed over the past decades. Fluoroscopy enabled interventional electrophysiologist throughout the years to gain profound knowledge on intracardiac anatomy and catheter movement inside the cardiac cavities and hence develop specific ablation approaches. However, the application of X-ray technologies imposes serious health risks to patients and operators. To reduce the use of fluoroscopy during interventional electrophysiological procedures to the possibly lowest degree and to establish an optimal protection of patients and operators in cases of fluoroscopy is the main goal of modern radiation management. The present manuscript gives an overview of possible strategies of fluoroscopy reduction and specific radiation protection strategies.
Keywords: Radiation protection, Ultra low-dose protocols, Radiation awareness, Radiation dosage reduction
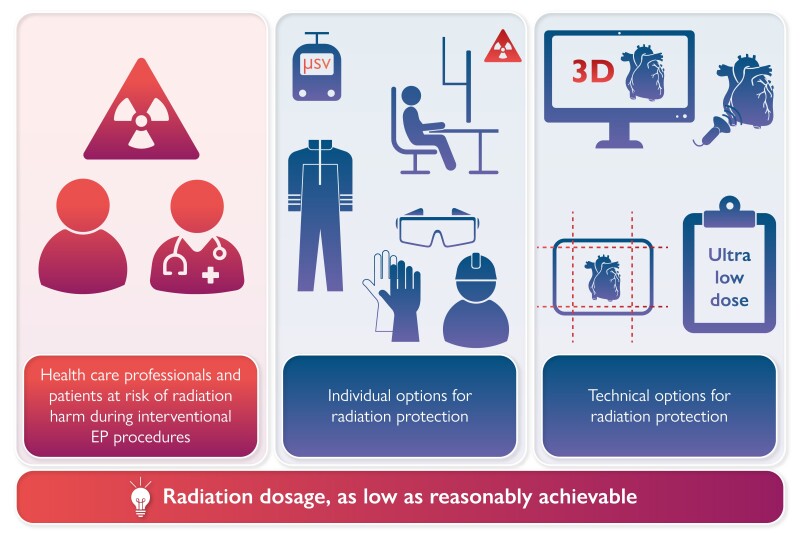
Graphical Abstract
Graphical Abstract.
3D, 3-dimensional; EP, electrophysiology; μSv, μSievert.
What’s new?
The as low as reasonably achievable principle is still valid—also in the light of new ultra low-dose protocols.
X-ray exposure in electrophysiology procedures can be minimized using easy-to-apply rules.
Technological tools make zero-fluoroscopy procedures possible.
Introduction
Typical conversation if two electrophysiologists meet: ‘How much radiation do you have in a PVI?’ Answer: ‘Around 7 min.’ And the dose? Answer: ‘About 400.’
Are we communicating on the right level with regards to this important topic? At the end, it is the radiation exposure that is part of the bodily harm we are causing to our patients (besides vascular puncture etc.). And importantly, radiation exposure is not only relevant to our patients but also to ourselves and the staff in the electrophysiology (EP) lab. X-ray technology has evolved dramatically in the recent decades—resulting in a significant reduction of radiation dose emitted by the X-ray systems. In parallel, an increasing awareness of the potentially harming effects can be observed especially among the younger generation of EP again resulting in reduced radiation usage.
The purpose of this manuscript is to create awareness of the potentially harming effects of using X-ray for our procedures, to quantify the risk of causing malignancies, to demonstrate ways of self-protection by reducing exposure or even better by reducing the radiation dose emitted by the X-ray system, and to show alternative imaging modalities.
Finally, we will try to answer the question if radiation exposure still is a cause for concern or if current technologies overcame this issue.
Medical radiation hazards
X-rays have marked a turning point in medical advancement since introduction to clinical practice. The increasing use and complexity of imaging and interventional techniques has introduced undesirable hazards. Complex catheter-based procedures performed by interventional cardiologists commonly expose to relevant radiation doses.1
Operators in interventional catheter labs have traditionally focused on potential harms to patients. Radiation-induced deterministic effects related to predictable changes in the tissues (e.g. skin injuries and cataract) and occurring above specific dose thresholds are well known.2–4 To limit stochastic effects related to an unpredictable, dose-independent potential future harm to tissues (e.g. radiation-induced carcinogenesis and germ cell mutagenesis), unjustified and non-optimized radiation use is carefully avoided.5–7
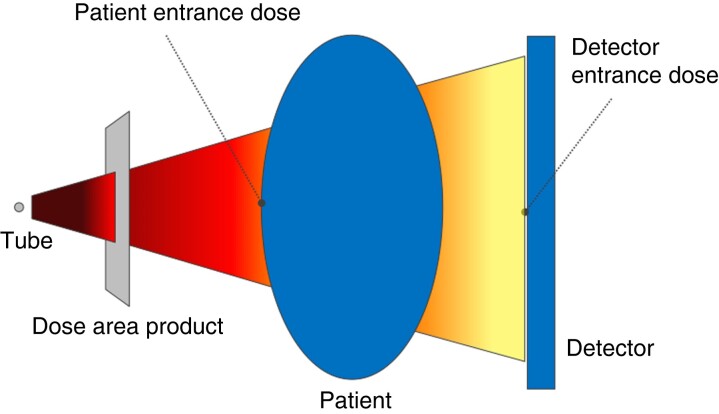
On the other side, operators and paramedical staff have posed minor attention on protecting themselves from radiation exposure harms. Fortunately, professional societies have started to promote awareness and provide specific recommendations to limit radiation exposure’s detrimental effects.8,9Figure 1 shows a schematic set up of X-ray systems. However, thorough consciousness and knowledge by prescribers and practitioners is lacking. As an example, most physicians, including interventional cardiologists, grossly underestimate the lifetime cancer risk related to medical radiation exposure.10,11
Figure 1.
Schematic setup of X-ray systems. The patient entrance dose is relevant for deterministic radiation injuries and is measured in Gy (air kerma). The dose area product is measured between the tube and the patient’s body, and the unit is Gy*cm2.
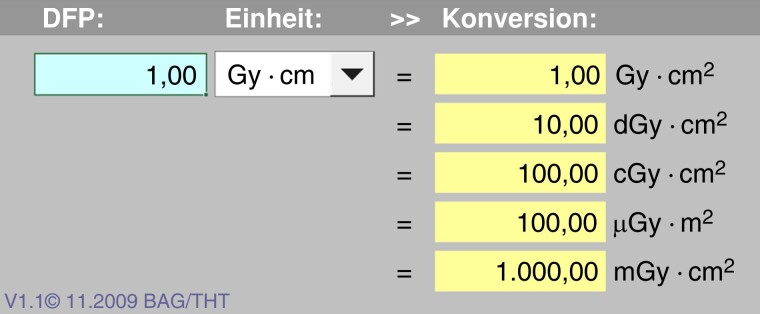
Radiation exposure confers distinct professional health risks to operators and paramedical staff in the catheter lab.12 Typical lifetime work-related exposure is in the range of 50–200 mSv, corresponding to a whole-body dose equivalent of 2500–10 000 chest X-rays (CXR) and an attributable excess cancer risk of 1 in 100.13 Notably, dose area product (DAP) units of the different manufacturers vary between one another (Table 1 and Figure 2). Despite carcinogenesis affecting exposed patients, repetitive low-dose occupational radiation exposure has also been related to several non-carcinogenic effects (Table 2).15 Long-term cancer risk is a feared consequence of X-ray professional exposure.12 More specifically, tumours on the left side of the brain, which is commonly more exposed and least protected by traditional shielding,24 have been reported.16,25 Extremely large cohort studies would be necessary to establish a clear epidemiological association. Biomarkers may overcome this limitation: telomere shortening and circulating microRNAs have recently shown great potential. To date, they are considered suitable to serve as early markers of most common cancers (e.g. breast, colon, lung, prostate, pancreas, gastric, ovarian, oesophagus, and liver). Interventional cardiologists, compared to non-exposed colleagues or controls, have reported significantly increased cell-free DNA or mitochondrial DNA plasma levels and chromosomal damage in circulating lymphocytes.26,27
Table 1.
Dose area product units of the different manufacturers
| Company | Siemens (µGy*m2) | Philips (cGy*cm2) | GE (Gy*cm2) | Toshiba/Canon (Gy*cm2) |
|---|---|---|---|---|
| Siemens (µGy*m2) | NA | NA | ×100 | ×100 |
| Philips (cGy*cm2) | NA | NA | ×100 | ×100 |
| GE (Gy*cm2) | :100 | :100 | NA | NA |
| Toshiba (Gy*cm2) | :100 | :100 | NA | NA |
DAP unit is modifiable in modern X-ray systems. The standard unit is Gy*cm2. Start with the line and look for the factor in the column. Example: the DAP from Siemens needs to be multiplied by 100 to give an equivalent to GE. NA, not available.
Figure 2.
Calculator for different units used for the dose area product. Typically, µGy*m2 or Gy*cm2 are used. The multiple manufacturers use different units. © www.bfs.de (Bundesamt für Strahlenschutz).
Table 2.
Medical radiation hazards within catheter lab operators and paramedical staff
| Radiation-induced effects | Description | Author, year |
|---|---|---|
| Cancerogenesis | Fifteen cardiac cath lab personnel, median individual effective dose 46 mSv (IR 24–64)→risk of (fatal and nonfatal) cancer 1 in 192 (IR 1 in 137–370) | Venneri et al.13 |
| Two brain cancers in Ontario cardiologists→an unusual cluster | Finkelstein14 | |
| Six interventional cardiologists and 3 interventional radiologists with brain malignancies in the left hemisphere | Roguin et al.16 | |
| Non-cancerogenic | ||
| Reproductive | Eighty-three exposed and 51 non-exposed subjects→different semen motility, viability, morphological abnormalities, and DNA fragmentation (P < 0.05–0.0001); higher hypermethylated spermatozoa (P < 0.05) | Kumar et al.17 |
| Teratogenic | Ninety-three cath lab personnel (exposed 3 months prior to conception) and 164 age-matched unexposed subjects→low birth weight in offspring 13% vs. 5.3%, P = 0.02; increased azoospermia factor region c microdeletion and microduplications (1.53 ± 0.8 vs. 1.02 ± 0.4, P = 0.0005) | Andreassi et al.18 |
| Neurodegenerative | One hundred and thirty-two interventional cardiologists and 83 non-exposed subjects→brain-specific miR-134 (P = 0.002) and miR-2392 (P = 0.003) significantly down-regulated | Borghini et al.19 |
| Cardiovascular | Two hundred and twenty-three cath lab personnel and 222 unexposed subjects→left and right carotid intima-media thickness significantly increased (P < 0.04); leucocyte telomere length significantly reduced (P = 0.008) | Andreassi et al.20 |
| Cataractogenesis | A total of 67 246 eligible US radiologic technologists→5-year lagged exposure related with self-reported cataract, an excess hazard ratio/mGy of 0.69 × 10−3 (P < 0.001) | Little et al.21 |
| Survey on 1478 board-certified cardiologists→above 10 000 h radiation exposure independently related to cataracts (adjusted OR 1.72; 95% CI 1.24–2.37, P = 0.001) | Thirumal et al.22 | |
| Pooled analysis on 2559 subjects (exposed = 1224)→posterior lens opacity higher in exposed subjects (RR = 3.21; 95% CI 2.14–4.83, P < 0.00001) | Elmaraezy et al.23 | |
IR, interquartile ranges; OR, odds ratio; RR, relative risk; CI, confidence interval.
In terms of non-cancerogenic consequences, potentially affecting fertility and future child’s health, a direct effect on the gonads and the hypothalamic-pituitary-gonadal axis in women and men, is a matter of concern.28,29 Exposed male health workers have shown worse semen quality than unexposed controls, with higher incidence of DNA fragmentation and hypermethylated spermatozoa.17 Pilot data show higher prevalence of genomic instability in specific ‘hot-spots’ of the Y chromosome, a genetic marker of impaired spermatogenesis that can also be transmitted to offspring and predispose to congenital abnormalities when spermatozoa are formed.18,30–32 Chronic occupational radiation exposure may also be associated with an increased incidence of cataract, early atherosclerosis, accelerated vascular aging and vascular, as well as neurocognitive decline.19–23
Eventually, indirect occupational radiation exposure effects have been described. Orthopaedic injuries and musculoskeletal pain have been related to wearing heavy leaded aprons, while high arousal inherent to radiation managing for continued periods has been related to psychological states as stress and anxiety.33–35
Technical options for radiation protection
Introduction
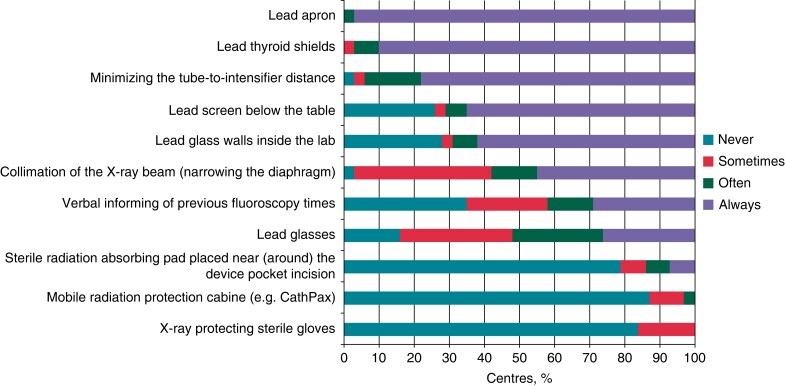
Although the best option would be a zero-fluoroscopy procedure, the use of radiation is crucial for most EP procedures. Therefore, effort should be taken to optimize radiation protection. The radiation exposure is even more relevant to the operator than the patient. First, the patient undergoes only one or a few EP procedures in life, whereas the operator experiences a daily exposure to radiation during the complete physician’s life. Second, the fluoroscopy exposure to the patient is part of the procedure and the potential cancer risk is part of the interventional risk, whereas for the operator, it is an occupational health risk. Generally, in every EP lab, the top priority should be to minimize radiation dose and to use all technical options aiming at protection of the patient and the operator. A variety of technical tools have been developed and are currently used in different ways to reduce fluoroscopy exposure. Radiation exposure can be reduced either by technical settings such as frame rates and collimation or technical tools such as led shields and led apron. A recent European Heart Rhythm Association (EHRA) survey demonstrated a very different adoption of technical options for radiation protection (Figure 3).36 The use is limited by costs and applicability. The Zero-Gravity system (Biotronik SE & Co KG, Berlin, Germany) for instance is a highly effective method to minimize fluoroscopy exposure to the operator. However, the system is costly and requires adoption of the individual workflow. Lead-containing gloves are several times more expensive than conventional gloves. In addition, the tactile feedback is impaired due to the thickness of the gloves. Fluoroscopy system customization is often a simple and cheap method to reduce radiation exposure at a price of a lower image quality. Table 3 summarizes recent available options for fluoroscopy protection.
Figure 3.
Safety measures and techniques used to decrease X-ray radiation during electrophysiology procedures.36
Table 3.
Recent available options for fluoroscopy protection
| Technical options for radiation protection | Patient protection/benefit | Operator protection/benefit | Type of radiation protection | Costs |
|---|---|---|---|---|
| Frame rates | + | + | System customization | No cost |
| Collimation (maximize) | + | + | Workflow | No cost |
| Magnification (minimize) | + | + | Workflow | No cost |
| Beam angulation | + | + | Workflow | No cost |
| Cine vs. storage of the last fluoroscopic sequence | + | + | Workflow | No cost |
| Lead apron | − | + | Technical option | Low |
| Thyroid collar | − | + | Technical option | Low |
| Protective glasses, visors, and headwear | − | + | Technical option | Low |
| Lead gloves | − | + | Technical option | Moderate |
| Table-suspended lead | − | + | Technical option | Low |
| Lead glass (separate stand or ceiling suspended) | − | + | Technical option | Low |
| Separate stand lead for allied professionals | − | + | Technical option | Low |
| Radioprotective cabin | − | + | Technical option | High |
| Ceiling-mounted suspended radiation protection system | − | + | Technical option | High |
| Led mattress | + | + | Technical option | Low |
| 3D-mapping systems | + | + | Technical option | Moderate |
| MRI-guided ablation | + | + | Technical option | High |
| Robotic magnetic navigation | − | + | Technical option | High |
| Robotic navigation | − | + | Technical option | High |
System customization and workflow adoption
The amount of radiation for both the patient and the operator as well as the image quality depends on multiple parameters including the tube current, the pulse duration, tube voltage, and the copper filtration. A higher image quality often results in a higher amount of increasing radiation. Different settings with different image qualities can often be preselected aiming at an optimal balance between image quality and radiation amount for each individual procedure. For instance, a high-resolution image is required for visualization of the coronary arteries in epicardial ablation procedures, whereas a low-resolution image is required sufficient in patients undergoing common type flutter ablation.
Frame rate settings
Many fluoroscopy units have a default setting of 12.5–25 frames per second (fps). For instance, a reduction from 25 to 3.75 fps is associated with a six- to eight-fold reduction of the radiation dose.
Collimation
Collimating the area of interest has two advantages. First, it results in dose reduction and second X-ray scatter reduction, thereby improving image quality. It is one of the most significant and efficient techniques to reduce the radiation dose to the patient and the operator. The collimation field should be continuously adjusted throughout the procedure.
Magnification
Magnification increases the dose. Therefore, magnification should be minimized.
Beam angulation
Scattered radiation is the main source of exposure for the operator and other persons in the lab. Most scatter originates from the beam entrance site. Therefore, the operator is exposed to more scattered radiation in left anterior oblique (LAO) projection when the operator is positioned on the right side of the table (e.g. procedures performed from the groin, right-sided device implantations) and in right anterior oblique (RAO) projection when the operator is positioned on the left side of the table (e.g. left-sided device implantation).
A recent study demonstrated that LAO caudal angulation is associated with the greatest increase in radiation dose compared to the posterior–anterior angulation, while LAO cranial angulation and RAO cranial and caudal angulations increase radiation dose to a lesser extent.37
Cine vs. storage of the last fluoroscopic sequence
The radiation dose is about 10-fold higher in cine mode as compared to the fluoroscopic mode. Therefore, whenever possible, storing the last fluoroscopic sequence should always be preferred to cine.
Technical tools
Personal Shielding
The minimum protection for everyone inside the lab should be a lead apron and thyroid collar. Importantly, the lead apron requires regular inspections (e.g. twice annually) as it may break over time. Mostly, lead is used as a shielding material for protection against X-ray. Recently, the high-density metal wolfram carbide has been introduced as a light, environmentally friendly and non-toxic alternative to lead. Other polymeric composite materials for radiation shielding are under evaluation.38 Optional protective shielding includes protective goggles, visors, headwear, and gloves (Figure 4). Glasses provide excellent eye protection with a typical lead thickness of 0.5–0.75 mm. Visors protect the entire face and brain, but lead thickness is only 0.1 mm. Caps fully protect the skull but additional weight and limited heat dissipation impair the operator’s comfort. Lead-containing gloves are significantly thicker thus impairing the tactile feedback. In addition, they reduce the radiation exposure only by 10–30%.
Figure 4.
Left operator protected with protective visors. Right operator protected with protective gloves (dark grey), protective goggles, and a brain cap.
Lab shielding including suspended radiation protection system and radioprotection cabin
Two shields should be used during each procedure. A lead glass shield above the patient (Figure 5) and a lead shield below the table including an overlapping shield flap. The lower shield is typically fixed at the table and moving with the table. The lead glass shield above the patient is either fixed at the ceiling or at a separate stand. Scattered radiation can further be reduced by up to 70% by using a simple lead mattress above or below the patient’s pelvis. The main disadvantage is that a fluoroscopic visualization of this region is not possible. More advanced tools include the ceiling-mounted suspended radiation protection system ‘Zero-Gravity’ or the radioprotection cabin. It optimizes shielding and reduces radiation exposure to background levels, including complete protection of eyes, brain, and the axillary region (Figure 6). Another major advantage of these systems is the orthopaedic relief avoiding orthopaedic injuries resulting from routinely wearing heavy protective apparel. Additional lead shields for allied professionals or anaesthesiologists should be provided. Particularly in younger patients, gonad protection should be used (Figure 7).
Figure 5.
Lead shield below the table including an overlapping shield flap (grey). Scattered radiation can further be reduced using a simple lead mattress.
Figure 6.
Suspended radiation protection system ‘Zero-gravity’.
Figure 7.
Gonad protection (female right, male left).
3D-mapping systems
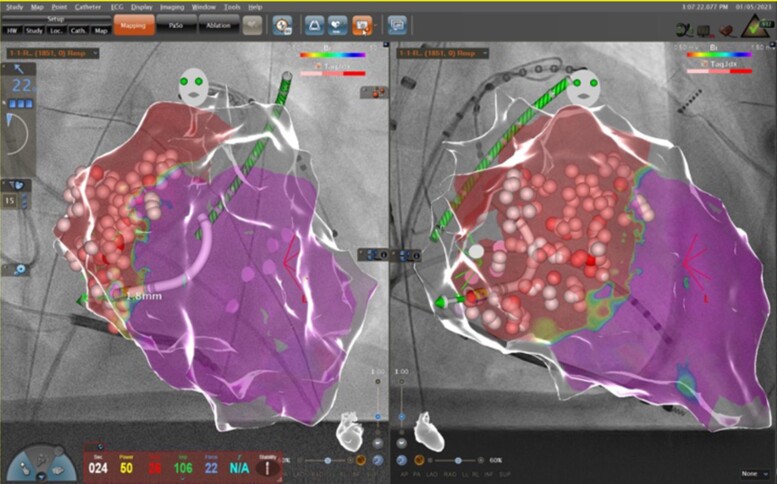
Introduction of 3D-mapping systems has resulted in a dramatic reduction of radiation dose. These systems include the CARTO system (Biosense Webster), the Ensite X system (Abbott Medical), and the Rhythmia system (Boston Scientific). 3D-mapping systems do not only allow for non-fluoroscopic catheter visualization (NFCV) but also for 3D-mapping of complex arrhythmias. In addition, other image modalities such as computed tomography, magnetic resonance imaging, or intracardiac echocardiography can be integrated and allow precise visualization of the heart and adjacent structures. Hence, 3D-mapping systems are recommended for most ablation procedures.39 Novel tools such as the CARTOUNIVUTM module combine fluoroscopic imaging and 3D-mapping (Figure 8).
Figure 8.
Combination of a 3D map (CARTO) with a fluoroscopic image. 3D map of the left ventricle in left anterior oblique (left image) and right anterior oblique projection (right image).
Robotic magnetic navigation systems/robotic ablation
Robotic magnetic navigation systems and robotic navigation systems have been developed aiming at more accurate and precise catheter navigation without radiation exposure to the operator. Robotic magnetic navigation systems (Stereotaxis) use powerful magnets to control catheter movement. Mechanical robotic navigation systems (Hansen) were used to robotically steer sheaths. For both systems, the operator steers the catheter in the control room. Remote navigation systems are complex and expensive. A clinical benefit has not been proven.40 Therefore, these systems are only used in a minority of EP centres.
Cardiac magnetic resonance imaging
Interventional cardiac magnetic resonance imaging (MRI) has been established as a radiation-free alternative compared to standard fluoroscopy-guided catheter ablation. Until today, this technique has only been used for cavotricuspid isthmus-dependent atrial flutter ablation. Although this technique requires extensive material effort, it allows detailed anatomic visualization and observation of lesion formation during ablation.41
How low can we go (with unchanged workflow) by optimizing the X-ray system?
There are several well-established radiation protection strategies to minimize radiation exposure, such as use of collimation, short detector-to-patient distance, avoiding LAO angulation, X-ray ray shielding/protection, or reduced fluoroscopy duration. These conventional strategies have the common aim to reduce the proportion of produced radiation hitting the operator or the patient. However, a more recent technological achievement in EP is the development of ultra low-dose (ULD) fluoroscopy protocols, which are technically optimized for their use in EP laboratories. In addition to conventional radiation protection strategies, ULD fluoroscopy significantly reduces the dose of generated radiation in the X-ray tube.42–44
Ultra low-dose fluoroscopy—technically optimized for electrophysiology
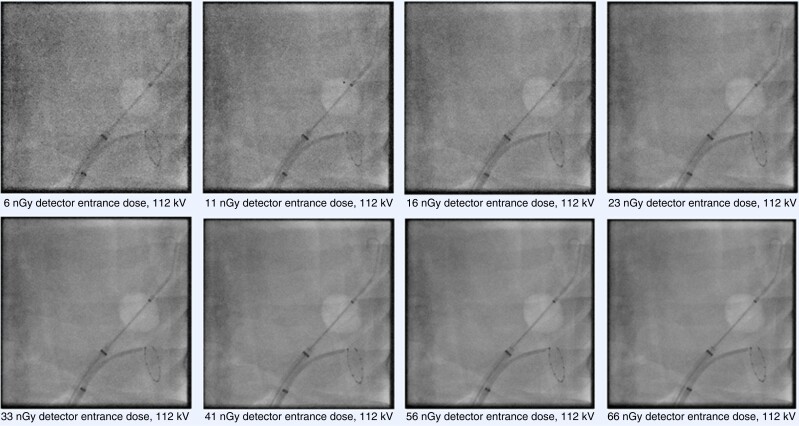
Though fluoroscopy systems were technically optimized for high-resolution coronary angiography, there is no need for high-resolution fluoroscopic imaging in EP, where visualization of electrodes, catheters, sheaths, and the cardiac silhouette is the main purpose of using fluoroscopy. Therefore, ULD fluoroscopy protocols are technically designed and optimized for their use in EP procedures. They acquire images at radiation doses just sufficient to perform EP procedures. In ULD fluoroscopy, the detector entrance dose per fluoroscopic pulse, necessary to create a fluoroscopic image, is dramatically reduced compared to conventional fluoroscopy. Notably, in ULD protocols, the X-ray tube voltage is identically chosen (for example 81 kV for cine-loops and 90 kV for pulsed fluoroscopy) compared to conventional fluoroscopy in order to prevent that softer X-ray radiation (lower kV) might create better image quality but be more harmful to tissue. Figure 9 depicts fluoroscopic acquisitions of EP catheters at systematically varied detector entrance doses in a phantom model experiment.42
Figure 9.
Fluoroscopic acquisitions of electrophysiology catheters (cryoballoon and circular mapping catheter) placed inside a thoracic phantom model. The detector entrance doses of the fluoroscopy system (Artis zee, Siemens AG, Forchheim, Germany) were systematically increased from 6 nGy per pulse to 66 nGy per pulse at a constant X-ray tube voltage of 112 kV. Increasing the dose results in improvement of overall image quality, however relevant details (markers and electrodes) can still be identified at the lowest dose level (6 nGy).
Clinical application
From an operator standpoint, ULD fluoroscopy creates a different, reduced image quality (Figure 10). Although unfamiliar, the reduced image quality did not significantly affect procedure times, clinical outcome, or radiofrequency (RF) times and did not result in higher complications. Implementing ULD fluoroscopy in the clinical workflow results in a highly-significant reduction of X-ray doses. Recent data show a 30-fold reduction of DAP using ULD fluoroscopy in complex ablation procedures compared to standard fluoroscopy.36,42,45
Figure 10.
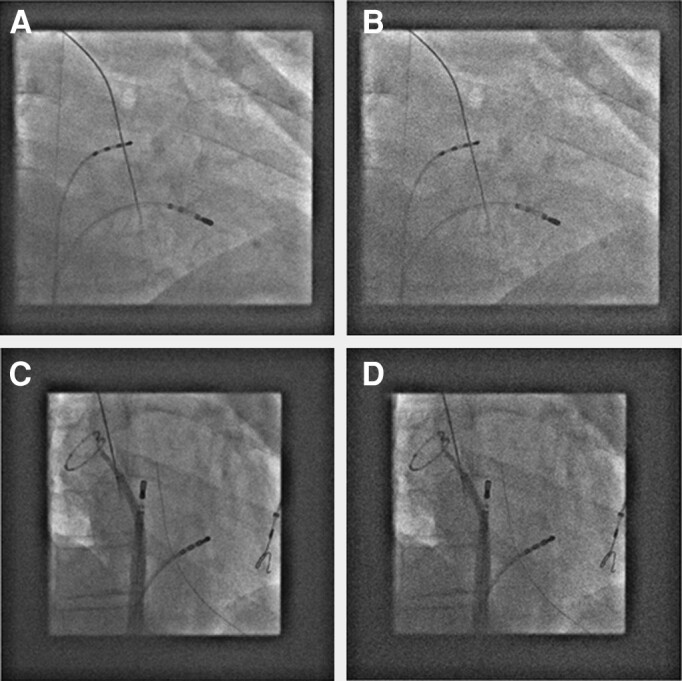
Corresponding clinical fluoroscopic images displayed in standard (A, C) and ULD (B, D) settings.
Radiation doses in atrial fibrillation ablation procedures in 2005–2022: looking at facts
Reviewing data on radiation during atrial fibrillation (AF) ablation over the past years, there are a few explanatory statements and definitions to consider. First, the following studies were selected as the majority addressed radiation reduction by specific interventions. Therefore, they may deviate from real-life data but on the other hand may demonstrate achievable reduction in radiation dose. Second, radiation dose data are provided as published, namely fluoroscopy time and kerma area product (KAP). The latter, formerly known as DAP, was converted into a uniform unit (cGycm2) for comparability. In addition, the estimated effective dose (eED), which is an indicator of the risk of tumour development, was calculated from the published data [eED = KAP (in cGycm2) × 0.002; the correction factor for women (1.38) was neglected for reasons of simplification]. This calculated eED was put into perspective with the eED of a CXR (0.02mSv) and thus converted to the unit ‘equivalent number of CXR’ in order to illustrate the meaning and magnitude of these values.46 Hence, for the presented studies in the subsequent paragraphs, one may find the data on the respective low-dose cohort in parenthesis and in the following order: analysed study period, fluoroscopy time, KAP, factor of KAP reduction, eED, and CXR equivalents (Table 4).
Table 4.
Summary of study indication changes in radiation dosages during atrial fibrillation ablations by radiofrequency ablation (A) and single-shot devices (B)
| Author | System/technique | Year of inclusion | Fluoroscopy time in min | KAP change | KAP in cGycm2 | eED in mSv | CXR equivalents |
|---|---|---|---|---|---|---|---|
| (A) 3D-mapping system (radiofrequency ablation, point-by-point) | |||||||
| Estner et al.47 (doi:10.1093/europace/eul079) | 3D-mapping vs. fluoroscopy only | 2005 | 38.9 | −39% | 5660 | 11.3 | 566 |
| Lee et al.48 (doi: 10.1093/europace/euv186) | Contact force vs. non-contact force | 2009–2014 | 9.5 | −70% | 104 | 0.2 | 10 |
| Christoph et al.43 (doi:10.1093/europace/euu334) | NFCV vs. conventional 3D-mapping | 2013 | 6.4 | −49% | 3726 | 7.5 | 373 |
| Huo et al.49 (doi:10.1016/j.hrthm.2015.05.018) | NFCV vs. conventional 3D-mapping | 2014 | 1.8 | −73% | 652 | 1.3 | 65 |
| Sommer et al.50 (doi:10.1093/europace/eux378) | NFCV: last 250 vs. first 250 patients | 2012–2017 | 0.5 | −94% | 152 | 0.3 | 15 |
| Khalaph et al.51 (doi: 10.1111/pace.14555) | Visualizable steerable sheath vs. non-visualizable sheath | 2019–2021 | 7.0 | −35% | 507 | 1.0 | 51 |
| Knecht et al.52 (doi:10.1093/europace/euv006) | Zero-Fluoro after TSP vs. ‘normal-fluoro’ after TSP | 2014 | 4.2 | −25% | 1320 | 2.6 | 132 |
| Lehrmann et al.53 (doi:10.1093/europace/euw334) | Radiation dose over time | 2005 | 53.0 | 4635 | 9.3 | 464 | |
| 2015 | 5.0 | −96% | 185 | 0.4 | 19 | ||
| Voskoboinik et al.54 (doi:/10.1016/j.hrthm.2017.02.014) | Radiation dose over time | 2010 | 30.6 | 9.0 | 450 | ||
| 2015 (CF PVI only) | 11.4 | −66% | 3.1 | 155 | |||
| Bourier et al.42 (doi:10.1093/europace/euv364) | Optimized X-ray programme vs. previous settings | 2014–2015 | 8.6 | −77% | 200 | 0.4 | 20 |
| Attanasio et al.45 (doi:10.1111/pace.14205) | Optimized X-ray programme | 2015–2018 | 6.2 | 91 | 0.2 | 9 | |
| Schreiber et al.55 (doi:/10.1007/s00399-021-00762-7) | Optimized X-ray programme | 2020 | 9.4 | 128 | 0.3 | 13 | |
| (B) Single-shot devices for AF ablation | |||||||
| Hoffmann et al.56 (doi:10.1093/europace/euz155) | Cryoballoon vs. RF | 2011–2016 | 23.4 | +39% | 2487 | 5.0 | 249 |
| Rubesch-Kütemeyer et al.57 (doi:/10.1007/s10840-019-00564-5) | Cryoballoon over time | 2013 | 11.7 | 1428 | 2.9 | 143 | |
| 2017 | 5.1 | −57% | 617 | 1.2 | 62 | ||
| Reissmann et al.58 (doi:10.1093/europace/eux066) | Cryoballoon optimized vs. standard X-ray programme | 2016 | 10.0 | −82% | 389 | 0.8 | 39 |
| Kühne et al.59 (doi: 10.3389/fcvm.2021.664538) | Cryoballoon without PV occlusion testing vs. standard | 2017–2019 | 11.0 | −81% | 368 | 0.7 | 37 |
| Rottner et al.60 (doi:/10.3389/fcvm.2022.967341) | Cryoballoon Kodex-EPD version 1.4.6 vs. 1.4.8 | 2019–2021 | 10.9 | −58% | 294 | 0.6 | 29 |
| Huang et al.61 (doi:10.1111/jce.14546) | Laserballoon low dose (ICE, 3D-mapping system) vs. standard | 2018–2019 (standard) | 16.9 | 1980 | 4.0 | 198 | |
| 2018–2019 (low dose) | 1.7 | −91% | 181 | 0.4 | 18 | ||
| Magni et al.62 (doi:/10.3389/fcvm.2022.959186) | Pulsed-field ablation | 2021–2022 | 13.5 | 658 | 1.3 | 66 | |
| Lemoine et al.63 (doi:/10.1007/s00392-022-02091-2) | Pulsed-field ablation | 2021–2022 | 16.0 | 505 | 1.0 | 51 | |
| Bohnen et al.64 (doi/10.1093/europace/euac111) | Pulsed-field ablation | 2021 | 16.0 | 125 | 0.3 | 13 | |
The table only includes studies that reported kerma area product (KAP). The bold numbers show the reduction of Kerma area product (KAP) by the use of 3 D mapping systems.
CXR, chest X-ray; eED, estimated effective dose; ICE, intracardiac echocardiography; NFCV, non-fluoroscopic catheter visualization; PV, pulmonary vein; RF, radiofrequency; TSP, transseptal puncture.
Point-by-point ablation using 3D-mapping systems
3D-mapping systems have revolutionized radiation dose reduction in point-by-point AF ablation. One of the earliest studies by Estner et al. showed 49% reduction in fluoroscopy time with the NavX system.47 An 86% decrease in fluoroscopy time was also demonstrated for the Carto system several years later.65 Since then, several technical innovations, namely (i) contact force, (ii) NFCV technology, (iii) visualizable steerable sheaths, and (iv) improvements in transseptal puncture (TSP) technique, enabled further reductions of radiation dose.
Contact force
In an ‘all comers’ retrospective study cohort of over 1500 patients, using contact force ablation catheters resulted in ∼19% reduction of procedure time, ∼77% decrease of fluoroscopy time, and ∼71% reduction of radiation dose. Despite a relatively long fluoroscopy time, excellent X-ray settings led to one of the lowest radiation doses published to date, apart from zero-fluoroscopy AF ablation.48
Non-fluoroscopic catheter visualization technology
The NFCV technology enables integration of fluoroscopy images into 3D-mapping systems. This technology enables real-time visualization of catheters superimposed on previously acquired X-ray images, allowing catheter movement in X-ray environment without repeat fluoroscopy. Several smaller studies that evaluated the CartoUnivu™ system showed varying amounts of fluoroscopy time and dose reduction.43,49 The largest study to date in 1000 patients using the Mediguide™ system could nearly omit fluoroscopy. After a learning curve, comparing the first 250 with the last 250 patients, the need for fluoroscopy markedly decreased with experience.50
Visualizable steerable sheaths
Steerable sheaths support precise positioning and stabilization of the ablation catheter during pulmonary vein isolation (PVI). In general, placement is guided fluoroscopically. Recently, steerable sheaths have been introduced capable of being visualized in 3D-mapping systems. Compared to a matched control cohort, their use could significantly reduce fluoroscopy time by 30% as well as radiation dose.51,66
Transseptal access
Several publications have identified catheter placement and TSP as the main source of radiation during AF ablation.54 After TSP, fluoroscopy could be completely avoided in 29 of 30 patients in a study by Knecht et al., using a 3D-mapping system together with a radiation awareness protocol.52 In addition, using a patent foramen ovale (PFO) for left access, a zero-fluoroscopy approach of the entire PVI was possible in 87% of patients in a study by Kühne et al.67 Therefore, a further reduction of radiation during PVI may be achieved by zero-fluoroscopy TSP. Apart from guidance by intracardiac (ICE) or transoesophageal echocardiography, recent studies investigated the feasibility of a fluoroscopy-free TSP by direct visualization of the needle on the 3D-mapping system.68,69 The advantage of these approaches is the widespread availability of these systems while avoiding additional costs, patient discomfort or the need for general anaesthesia.
Ablation in magnetic resonance interventional suites (iCMR)
Another way of coming down to zero fluoroscopy is to treat patients in a non-fluoroscopic environment like MRI. Since 2010, constant progress has been made in this field and recently, a MR-conditional ablation system has received CE mark and is available for ablation of typical atrial flutter. In the first series of patients being published, the procedure seemed safe and feasible70—but still further progress has to be made until the potential advantages of tissue visualization will become a relevant factor in ablation procedures.
Variability in published radiation doses for radiofrequency atrial fibrillation ablation procedures
Studies investigating radiation in AF ablation consistently show a dose reduction over time. Lehrmann et al. showed a decrease in radiation dose in 2005–2015 from an eED of 9.3 to 0.4 mSV and in 2010–2015 from 9.0 to 3.1 mSv, respectively.53,54 Even with optimal use of 3D-mapping systems including all technical advances, the resulting radiation dose still varies greatly between centres despite reporting the same fluoroscopy time. This may be explained by two reasons: lack of radiation awareness and different X-ray settings. Regarding lack of radiation awareness, Estner et al. published the results of an EHRA survey in 2015. More than 25% of operators used an unnecessarily high frame rate of ≥12 fps, and more than 40% of centres did not use collimation or only occasionally.36 On the other hand, some centres have developed special EP low-dose programmes with improved image post processing [typical optimized EP programme: reduced frame rate 2–3 fps (fluoroscopy) and 7.5 fps (cine-angiography); 8 and 36 nGy detector entrance dose for fluoroscopy and cine-angiography, respectively]. Three exemplary studies show very low radiation doses using this optimized approach for AF ablation, despite a considerable amount of fluoroscopy time.45,55,71Table 4A summarizes data on changes in fluoroscopy times in RF-PVI.
Single-shot devices for atrial fibrillation ablation
Most single-shot devices are not integrated into 3D-mapping systems and rely on fluoroscopy.72 The effect of radiation dose reduction by optimized X-ray settings is therefore aggravated. Avoiding repeated cine-angiography for assessment of pulmonary venous anatomy/occlusion and/or replacing it by fluoroscopic film imagery can further reduce radiation dose.58 Various studies reported shorter fluoroscopy times during RF-PVI compared to cryoballoon (CB) ablation, e.g. the Fire and Ice trial or the Circa-Dose trial.73,74 Radiation doses, unfortunately, were not published. The FREEZE cohort study, a multicentre trial comparing RF to CB ablation, reported X-ray data in >4100 patients. Atrial fibrillation ablation showed significant reduction in fluoroscopy time and radiation dose compared to a CB procedure.56 A smaller single-centre study presented data on CB-PVI between 2013 and 2017 in >1000 patients.57 Procedural changes enabled radiation dose reduction in two recent studies.57,59 Recently, a new 3D-mapping system (Kodex-EPD™) was used in conjunction with the CB and the circular mapping catheter (Achieve™) for PVI, leading to further reduction of radiation dose.60 Comparison of CB-PVI and laserballoon (LB) PVI at highly experienced centres revealed similar fluoroscopy times between both techniques in a cohort of 200 patients.75 Huang et al. reported fluoroscopy time and radiation dose during conventional LB-PVI and during low-dose LB-PVI utilizing ICE, a 3D-mapping system and a multipolar mapping catheter.61 In a multi-national pulsed-field ablation survey, mean fluoroscopy time was 13.7 min.76 Three studies reported similar fluoroscopy times but varying radiation doses.62–64 The RF balloon on the other hand is integrable into a 3D-mapping system. However, currently published fluoroscopy times in a multicentre trial vary between centres and appear to differ by type of sedation.77Table 4B summarizes data on changes in fluoroscopy times in single-shot ablation.
Safety and efficacy of radiation reduction efforts
The aforementioned studies demonstrated similar efficacy and safety in the conventional or low-dose radiation arm. This is also confirmed in a meta-analysis of more than 2200 patients comparing low-dose or zero fluoroscopy to conventional AF ablation procedures. The 1-year risk of AF recurrence, repeat procedures, and procedural complications were similar between the groups. The weighted mean difference in fluoroscopy time and KAP were significantly reduced.78
Zero-fluoroscopic procedures using intracardiac echocardiography—worth the effort?
The ICE represents a major advancement in cardiac imaging. Due to its potential to guide TSP, visualize ablation catheter, and to reveal complications (e.g. cardiac tamponade, thrombus formation, microbubbles during overheating with imminent danger ‘pops’, and pulmonary vein stenosis), it makes part of percutaneous interventional and EP procedures.79 Moreover, ICE can be easily performed using conscious sedation unlike transoesophageal echocardiography, which requires intubation and general anaesthesia in most cases with its inherent risks including oesophageal injury by the probe.80
Available intracardiac echocardiography technologies
Two types of ICE are available: (i) rotational ICE using a piezoelectric crystal mounted on the tip of the catheter providing cross-sectional images in a 360° radial plane and (ii) phased-array ICE using a 64-element transducer mounted on the distal end of an 8- or 10-French steerable catheter. The catheter can be deflected aft and forward in two planes and produces a wedge-shaped image that is displayed on an ultrasound workstation. Due to easier probe handling, intuitive imaging and possible acquisition of Doppler and colour flow imaging, phased-array ICE represents a prevailing ICE modality (Table 5). The fact that ICE is the only interventional tool offering real-time imaging predestines this technology as suitable to reduce or even eliminate fluoroscopic exposure during catheter ablation.
Table 5.
Presently available intracardiac imaging devices and their capabilities
| Device | Company | Description |
|---|---|---|
| ACUSON AcuNav Volume ICE Catheter | Siemens Healthineers | Side-looking, 64-element phased-array, 4 planes steerability, 8F and 10F, greyscale, colour Doppler, tissue Doppler, compatible with ACUSON SC2000 PRIME Ultrasound System |
| ViewFlex Xtra ICE Catheter | Abbott Vascular | Side-looking, 64-element phased-array, 4 planes steerability, 8F, compatible with the ViewMate Z and ViewMate II ultrasound consoles |
| CartoSound | Biosense Webster | Side-looking, 64-element phased-array, 4 planes steerability 10F device with integrated ultrasound array with the CARTO magnetic sensors in the tip, which permits integration of ICE and 3D electroanatomical maps |
| Foresight ICE system | Conavi Medical | Forward-looking ICE, provides colour Doppler, pulsed wave Doppler, 2-D and 3-D measurements and electrocardiogram-gated 3D image acquisition |
Barriers to zero-fluoroscopic approach
Two barriers prevent total elimination of fluoroscopy during catheter ablation despite using 3D-mapping systems with advanced possibilities of catheter visualization: (i) introduction of catheters into the heart and (ii) performance of TSP for left access. Once all catheters are deployed, the use of 3D-mapping may omit fluoroscopy regardless of ICE, especially when contact force-sensing catheters are used.81–83 However, ICE may help with safe navigation of the catheters. While fluoroscopy during catheter introduction will probably not be completely eliminated, performance of contemporary impedance-based 3D-mapping systems, allowing continuous tracking of catheters from the groins to the heart, can eliminate use of fluoroscopy in the majority (i.e. 94–97%) of cases.81,84 Using a PFO to access the left atrium can be an alternative to TSP, but it is only feasible in a limited number of cases.85 Moreover, such an access to the left atrium is usually too superior and anterior to allow for a comfortable manipulation with the ablation catheter towards the left pulmonary veins. The TSP can be fully accomplished without fluoroscopy under guidance of ICE as shown in many studies.81,84,86 Not only that ICE allows for more direct visual guidance, but it also allows for the assessment of difficult septal anatomies.87 A recently published trial including almost 750 ICE-only guided transseptal procedures at five institutions showed that all punctures could be accomplished with 100% success without fluoroscopy with no periprocedural complications related to puncture itself in average of 19 ± 10 min (skin-to-LA access).88 Even in patients with intracardiac devices, no device-related complications were observed. Representative ICE images during the most important steps of zero-fluoroscopic TSP are shown in Figure 11.
Figure 11.
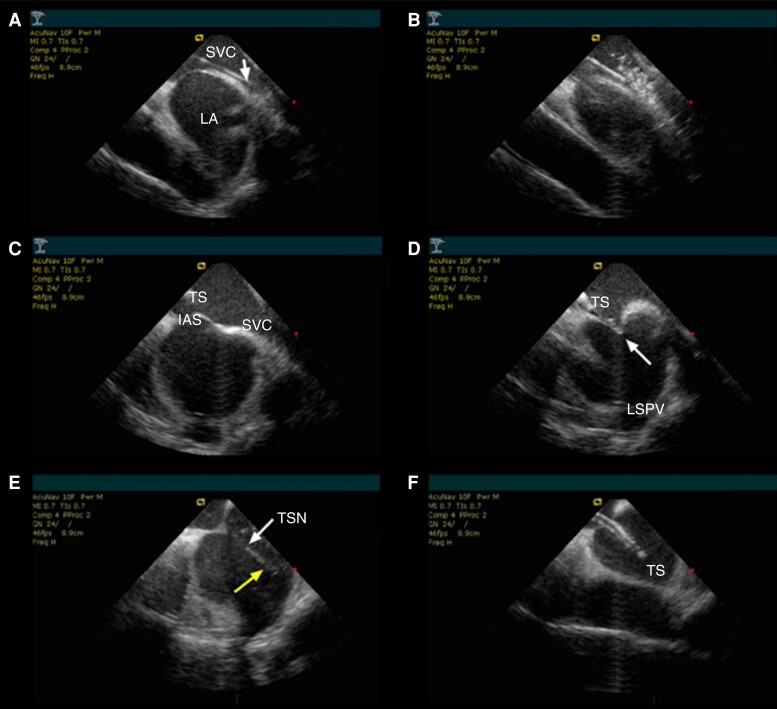
Crucial phases of ICE-guided transseptal puncture. (A) The guide wire is directed to the superior vena cava. (B) The transseptal sheath is advanced by the visual control over the wire, and its position is ascertained by the saline injection which forms ‘bubbles’ on the ICE image. (C) Then the needle is loaded and the whole instrument is pulled back while maintaining rotation towards the septum. (D) Gentle rotations clockwise and counterclockwise allow for proper positioning of the transseptal sheath towards the septum, showing typical tenting sign (arrow). (E) The needle is advanced (upper arrow), and penetration to the left atrium may be clearly visualized by the saline injection forming ‘bubbles’ on ICE image (lower arrow). (F) Once the dilator is placed together with the needle in the left atrium, the sheath is advanced while keeping the dilator in a stable position with the needle retracted. IAS, interatrial septum; LA, left atrium; LSPV, left superior pulmonary vein; SVC, superior vena cava; TSN, transseptal needle; TS, tenting on the septum; TSS, transseptal sheath.
Limitations of intracardiac echocardiography usage
The limiting factors for complete zero-fluoroscopic procedures are the following: (i) absence of large-scale randomized trials (RCT) showing clear-cut benefits of complete zero-fluoroscopic approach for patients and EP personnel, (ii) additional costs of ICE, and (iii) unwillingness to change the routine, which has been exercised for few decades using fluoroscopy as the only navigation tool for catheter deployment. In our opinion, no RCTs are realistically needed to prove enhanced safety of using ICE during complex ablation procedures. Seeing how the catheter moves inside the heart, the catheter–tissue contact, and all intracardiac structures is impressive and persuasive that even a limited experience with ICE is sufficient for any electrophysiologist to allow him or her from using this technology. Published data show that ICE not only cuts down the radiation exposure but also shortens the ablation procedure with the possibility of treating more patients during working hours. The ICE in combination with 3D-mapping can eliminate fluoroscopy completely. The attitudinal aspects toward X-ray could be overcome by a generation change in the EP with more progressive thinking (‘the best radiation bill is zero bill’) and expertise. Finally, additional costs of ICE could be overcome if worldwide consumption of probes puts pressure on producers to lower prices. The answer to the ultimate question whether ICE is worth the effort is definitely positive. It is worth the price: 34% relative reduction of complications reported by Goya et al. speaks for itself; however, not every health care system is willing to pay for it.89 There are at least three categories of patients who might have clear benefit from zero- or limited-fluoroscopy procedures: (i) obese patients (due to inherently higher doses of radiation), (ii) pregnant women (we need to avoid radiation completely), and (iii) children (we might feel compelled to eliminate any stochastic adverse events of radiation, i.e. eliminate the lifetime risk of cancer).
Summary
Two factors have contributed to a significant decrease in radiation exposure during EP interventions in the past decade:
Technological improvements with ULD protocols allowing to perform complex procedures with <10% of former radiation doses.
General awareness about this topic especially by the younger generation in the EP labs worldwide.
Do we still have to do better?
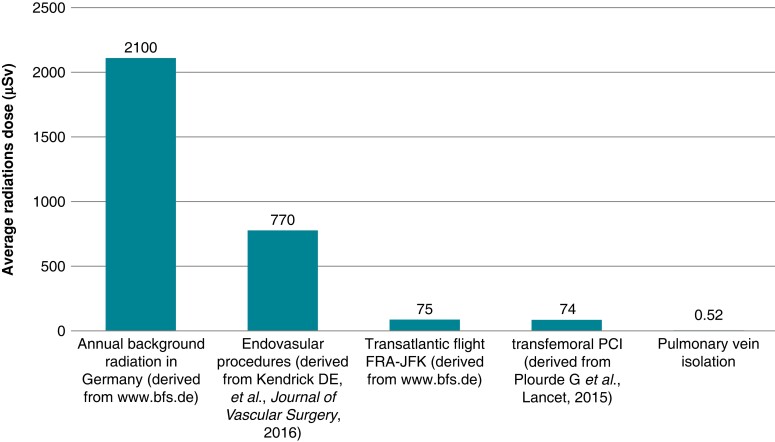
Using technological innovations and considering fluoroscopy time and dosage as a quality marker finally let us achieve the goal of exposing ALARA—as low as reasonably achievable. And today, we can almost eliminate every potentially harming side-effect of using radiation (see Figure 12). Still, keeping the stochastic effects in mind, the only way to come to zero risk is exposing zero ionizing radiation by adding an additional real-time imaging modality: for example, by using 3D-mapping systems and ICE.
Figure 12.
Comparison between annual background radiation and different interventions. PCI, percutaneous coronary intervention; FRA-JFK, Frankfurt to John F. Kennedy (adapted from Schreiber et al.55).
Acknowledgements
The authors thank the EHRA Scientific Document Committee: Prof Katja Zeppenfeld, Prof Jens Cosedis Nielsen, Dr Alireza Sepehri Shamloo, Prof Kristina Hermann Haugaa, Prof Isabel Deisenhofer, Prof Silvia Priori, Prof Markus Stühlinger, Prof Christian Meyer, Prof Petr Peichel, Dr Daniel Keene, Prof Jacob Tfelt Hansen, Dr Luigi di Biase, and Prof Arthur Wilde.
Contributor Information
Philipp Sommer, Clinic for Electrophysiology, Herz- und Diabeteszentrum NRW, Georgstr. 11, Bad Oeynhausen 32545, Germany.
Vanessa Sciacca, Clinic for Electrophysiology, Herz- und Diabeteszentrum NRW, Georgstr. 11, Bad Oeynhausen 32545, Germany.
Matteo Anselmino, Division of Cardiology, Department of Medical Sciences, ‘Citta della Salute e della Scienza di Torino’ Hospital, University of Turin, Torino, Italy.
Roland Tilz, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Luebeck, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Lübeck, Germany.
Felix Bourier, Department of Electrophysiology, German Heart Center, Technical University, Munich, Germany.
Heiko Lehrmann, Department of Cardiology and Angiology (Campus Bad Krozingen), University Hospital Freiburg, Bad Krozingen, Germany.
Alan Bulava, Department of Cardiology, Ceske Budejovice Hospital and Faculty of Health and Social Sciences, University of South Bohemia, Ceske Budejovice, Czech Republic.
Funding
This document is supported by SIEMENS HEALTHCARE GmbH in the form of an educational grant. The scientific content has not been influenced in any way by its sponsor.
Data availability
All relevant data are within the manuscript and its supporting information files.
References
- 1. Walters TE, Kistler PM, Morton JB, Sparks PB, Halloran K, Kalman JM. Impact of collimation on radiation exposure during interventional electrophysiology. Europace 2012;14:1670–3. [DOI] [PubMed] [Google Scholar]
- 2. Suzuki S, Furui S, Yamakawa T, Isshiki T, Watanabe A, Iino Ret al. Radiation exposure to patients’ skin during cardiac resynchronization therapy. Europace 2009;11:1683–8.. [DOI] [PubMed] [Google Scholar]
- 3. Rehani MM, Vano E, Ciraj-Bielac O, Kleiman NJ. Radiation and cataract. Radiat Prot Dosimetry 2011;147:300–4. [DOI] [PubMed] [Google Scholar]
- 4. Stewart FA, Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, Macvittie TJet al. ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs—threshold doses for tissue reactions in a radiation protection context. Ann ICRP 2012;41:1–322. [DOI] [PubMed] [Google Scholar]
- 5. Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GBet al. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 2013;346:f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- 7. Picano E, Vañó E, Rehani MM, Mont L, Bodi V, Bar Oet al. The appropriate and justified use of medical radiation in cardiovascular imaging. A position document of the ESC associations of cardiovascular imaging, percutaneous cardiovascular interventions and electrophysiology. Eur Heart J 2014;35:665–72. [DOI] [PubMed] [Google Scholar]
- 8. Klein LW, Miller DL, Balter S, Laskey W, Haines D, Norbash Aet al. Joint inter-society task force on occupational hazards in the interventional laboratory occupational health hazards in the interventional laboratory: time for a safer environment. J Vasc Interv Radiol 2009;20:S278–83. [DOI] [PubMed] [Google Scholar]
- 9. Hirshfeld JW J, Ferrari VA, Bengel FM, Bergersen L, Chambers CE, Einstein AJet al. 2018 ACC/HRS/NASCI/SCAI/SCCT expert consensus document on optimal use of ionizing radiation in cardiovascular imaging-best practices for safety and effectiveness, part 2: radiological equipment operation, dose-sparing methodologies, patient and medical personnel protection: a report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol 2018;71:2829–55. [DOI] [PubMed] [Google Scholar]
- 10. Giaccardi M, Anselmino M, Del Greco M, Mascia G, Paoletti Perini A, Mascia Pet al. Radiation awareness in an Italian multispecialist sample assessed with a web-based survey. Acta Cardiol 2021;76:307–11. [DOI] [PubMed] [Google Scholar]
- 11. Einstein AJ, Tilkemeier P, Fazel R, Rakotoarivelo H, Shaw LJ; American Society of Nuclear Cardiology . Radiation safety in nuclear cardiology—current knowledge and practice: results from the 2011 American Society of Nuclear Cardiology member survey. JAMA Intern Med 2013;173:1021–3. [DOI] [PubMed] [Google Scholar]
- 12. Andreassi MG, Piccaluga E, Guagliumi G, Del Greco M, Gaita F, Picano E; on behalf of the Healthy Cath lab Study Group. Occupational health risks in cardiac catheterization laboratory workers. Circ Interv 2016;9: e003273. [DOI] [PubMed] [Google Scholar]
- 13. Venneri L, Rossi F, Botto N, Andreassi MG, Salcone N, Emad Aet al. Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: insights from the National Research Council’s Biological Effects of Ionizing Radiation VII Report. Am Heart J 2009;157:118–24. [DOI] [PubMed] [Google Scholar]
- 14. Finkelstein MM. Cancer incidence among Ontario police officers. Am J Ind Med 1998;34:157–62. [DOI] [PubMed] [Google Scholar]
- 15. Zanzonico P, Dauer L, Strauss HW. Radiobiology in cardiovascular imaging. JACC Cardiovasc Imaging 2016;9:1446–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roguin A, Goldstein J, Bar O. Brain tumors among interventional cardiologists: a cause for alarm? Report of four new cases from two cities and a review of the literature. EuroIntervention 2012;7:1081–6. [DOI] [PubMed] [Google Scholar]
- 17. Kumar D, Skalthur G, Kalthur G, Uppangala S, Kumari S, Challapalli Set al. Semen abnormalities, sperm DNA damage and global hypermethylation in health workers occupationally exposed to ionizing radiation. PLoS One 2013;7:e69927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andreassi MG, Borghini A, Vecoli C, Piccaluga E, Guagliumi G, el Greco Met al. Reproductive health risks and Y chromosome genomic instability in male staff working in cardiac catheterization laboratory with chronic low-dose X-ray exposure. Eur Heart J 2018; 39(suppl_1):ehy563.P4570 [Google Scholar]
- 19. Borghini A, Vecoli C, Mercuri A, Carpeggiani C, Piccaluga E, Guagliumi Get al. Low-dose exposure to ionizing radiation deregulates the brain-specific miR-134 in interventional cardiologists. Circulation 2017;136:2516–8. [DOI] [PubMed] [Google Scholar]
- 20. Andreassi MG, Piccaluga E, Gargani L, Sabatino L, Borghini A, Faita Fet al. Subclinical carotid atherosclerosis and early vascular aging from chronic low-dose ionizing radiation exposure: a genetic, telomere and vascular ultrasound study in cardiac catheterization laboratory staff. JACC Cardiovasc Interv 2015;8:616–27. [DOI] [PubMed] [Google Scholar]
- 21. Little MP, Kitahara CM, Cahoon EK, Bernier M-O, Velazquez-Kronen R, Doody MMet al. Occupational radiation exposure and risk of cataract incidence in a cohort of US radiologic technologists. Eur J Epidemiol 2018;33:1179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thirumal R, Vanchiere C, Bhandari R, Jiwani S, Horswell R, Chu Set al. The inverse correlation between the duration of lifetime occupational radiation exposure and the prevalence of atrial arrhythmia. Front Cardiovasc Med 2022;9:863939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elmaraezy A, Ebraheem Morra M, Tarek Mohammed A, Al-Habaa A, Elgebaly A, Ghazy AAet al. Risk of cataract among interventional cardiologists and catheterization lab staff: a systematic review and meta-analysis. Catheter Cardiovasc Interv 2017;90:1–9. [DOI] [PubMed] [Google Scholar]
- 24. Anselmino M, Marcantoni L, Agresta A, Chieffo E, Floris R, Racheli Met al. Radiation awareness area of the Italian Association of Arrhythmology and Cardiac Pacing (AIAC). Interventional cardiology and X-ray exposure of the head: overview of clinical evidence and practical implications. J Cardiovasc Med 2022;23:353–8. [DOI] [PubMed] [Google Scholar]
- 25. Filkelstein MM. Is brain cancer an occupational disease of cardiologists? Can J Cardiol 1998;14:1385–8. [PubMed] [Google Scholar]
- 26. Borghini A, Mercuri A, Turchi S, Chiesa MR, Piccaluga E, Andreassi MG. Increased circulating cell-free DNA levels and mtDNA fragments in interventional cardiologists occupationally exposed to low levels of ionizing radiation. Environ Mol Mutagen 2015;56:293–300. [DOI] [PubMed] [Google Scholar]
- 27. Andreassi MG, Cioppa A, Botto N, Joksic G, Manfredi S, Federici Cet al. Somatic DNA damage in interventional cardiologists: a case-control study. FASEB J 2005;19:998–9. [DOI] [PubMed] [Google Scholar]
- 28. Sarkozy A, De Potter T, Heidbuchel H, Ernst S, Kosiuk J, Vano Eet al. Occupational radiation exposure in the electrophysiology laboratory with a focus on personnel with reproductive potential and during pregnancy: A European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS). Europace 2017;19:1909–22. [DOI] [PubMed] [Google Scholar]
- 29. Lassi ZS, Imam AM, Bhutta ZH. Preconception care: caffeine, smoking, alcohol, drugs and other environmental chemical/radiation exposure. Reprod Health 2014;11:S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lu C, Jiang J, Zhang R, Wang Y, Xu M, Qin Yet al. Gene copy number alterations in the azoospermia-associated AZFc region and their effect on spermatogenic impairment. Mol Hum Reprod 2014;20:836–43. [DOI] [PubMed] [Google Scholar]
- 31. Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohleet Get al. European Association of Urology guidelines on male infertility: the 2012 update. Eur Urol 2012;62:324–32. [DOI] [PubMed] [Google Scholar]
- 32. Diemer T, Desjardins C. Developmental and genetic disorders in spermatogenesis. Hum Reprod 1999;5:120–40. [DOI] [PubMed] [Google Scholar]
- 33. Orme NM, Rihal CS, Gulati R, Holmes DR Jr, Lennon RJ, et al. Occupational health hazards of working in the interventional laboratory: a multisite case control study of physicians and allied staff. J Am Coll Cardiol 2015;65:820–6. [DOI] [PubMed] [Google Scholar]
- 34. Klein LW, Tra Y, Garratt KN, Powell W, Lopez-Cruz G, Chambers Cet al. Society for Cardiovascular Angiography and Interventions. Occupational health hazards of interventional cardiologists in the current decade: results of the 2014 SCAI membership survey. Catheter Cardiovasc Interv 2015;86:913–24. [DOI] [PubMed] [Google Scholar]
- 35. Detling N, Smith A, Nishimura R, Keller S, Martinez M, Young Wet al. Psychophysiologic responses of invasive cardiologists in an academic catheterization laboratory. Am Heart J 2006;15:522–8. [DOI] [PubMed] [Google Scholar]
- 36. Estner HL, Grazia Bongiorni M, Chen J, Dagres N, Hernandez-Madrid A, Blomström-Lundqvist Cet al. Use of fluoroscopy in clinical electrophysiology in Europe: results of the European Heart Rhythm Association Survey. Europace 2015;17:1149–52. [DOI] [PubMed] [Google Scholar]
- 37. Kuon E, Dahm J, Empen K, Robinson D, Reuter G, Wucherer Met al. Identification of less-irradiating tube angulations in invasive cardiology. J Am Coll Cardiol 2004;44:1420–8. [DOI] [PubMed] [Google Scholar]
- 38. More CV, Alsayed Z, Badawi MS, Thabet AA, Pawar PP. Polymeric composite materials for radiation shielding: a review. Environ Chem Lett 2021;19:2057–90.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim YH, Chen SA, Ernst S, Guzman CE, Han S, Kalarus Zet al. 2019 APHRS expert consensus statement on three-dimensional mapping systems for tachycardia developed in collaboration with HRS, EHRA, and LAHRS. J Arrhythm 2020;36:215–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rillig A, Schmidt B, Di Biase L, Lin T, Scholz L, Heeger CHet al. Manual versus robotic catheter ablation for the treatment of atrial fibrillation: the man and machine trial. JACC Clin Electrophysiol 2017;3:875–83. [DOI] [PubMed] [Google Scholar]
- 41. Lichter J, Kholmovski EG, Coulombe N, Ghafoori E, Kamali R, MacLeod Ret al. Real-time magnetic resonance imaging-guided cryoablation of the pulmonary veins with acute freeze-zone and chronic lesion assessment. Europace 2019;21:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bourier F, Reents T, Ammar-Busch S, Buiatti A, Kottmaier M, Semmler Vet al. Evaluation of a new very low dose imaging protocol: feasibility and impact on X-ray dose levels in electrophysiology procedures. Europace 2016;18:1406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Christoph M, Wunderlich C, Moebius S, Forkmann M, Sitzy J, Salmas Jet al. Fluoroscopy integrated 3D mapping significantly reduces radiation exposure during ablation for a wide spectrum of cardiac arrhythmias. Europace 2015;17:928–37. [DOI] [PubMed] [Google Scholar]
- 44. Sommer P, Rolf S, Piorkowski C, Gaspar T, Huo Y, Piedra Cet al. Nonfluoroscopic catheter visualization in atrial fibrillation ablation: experience from 375 consecutive procedures. Circ Arrhythm Electrophysiol 2014;7:869–74. [DOI] [PubMed] [Google Scholar]
- 45. Attanasio P, Huemer M, Kaehler N, Keller T, Schreiber T, Niehues Ret al. Safe procedures despite ultra low radiation doses during catheter ablations of atrial and ventricular arrhythmias—a multicenter experience. Pacing Clin Electrophysiol 2021;44:807–13. [DOI] [PubMed] [Google Scholar]
- 46. Heidbuchel H, Wittkampf FHM, Vano E, Ernst S, Schilling R, Picano Eet al. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace 2014;16:946–64. [DOI] [PubMed] [Google Scholar]
- 47. Estner HL, Deisenhofer I, Luik A, Ndrepepa G, von Bary C, Zrenner Bet al. Electrical isolation of pulmonary veins in patients with atrial fibrillation: reduction of fluoroscopy exposure and procedure duration by the use of a non-fluoroscopic navigation system (NavX®). Europace 2006;8:583–7. [DOI] [PubMed] [Google Scholar]
- 48. Lee G, Hunter RJ, Lovell MJ, Finlay M, Ullah W, Baker Vet al. Use of a contact force-sensing ablation catheter with advanced catheter location significantly reduces fluoroscopy time and radiation dose in catheter ablation of atrial fibrillation. Europace 2016;18:211–8. [DOI] [PubMed] [Google Scholar]
- 49. Huo Y, Christoph M, Forkmann M, Pohl M, Mayer J, Salmas Jet al. Reduction of radiation exposure during atrial fibrillation ablation using a novel fluoroscopy image integrated 3-dimensional electroanatomic mapping system: a prospective, randomized, single-blind, and controlled study. Heart Rhythm 2015;12:1945–55. [DOI] [PubMed] [Google Scholar]
- 50. Sommer P, Bertagnolli L, Kircher S, Arya A, Bollmann A, Richter Set al. Safety profile of near-zero fluoroscopy atrial fibrillation ablation with non-fluoroscopic catheter visualization: experience from 1000 consecutive procedures. Europace 2018;20:1952–8. [DOI] [PubMed] [Google Scholar]
- 51. Khalaph M, Sommer P, Lucas P, Guckel D, Fink T, Sciacca Vet al. First clinical experience using a visualized sheath for atrial fibrillation ablation. Pacing Clin Electrophysiol 2022;45:922–9. [DOI] [PubMed] [Google Scholar]
- 52. Knecht S, Sticherling C, Reichlin T, Pavlovic N, Mühl A, Schaer Bet al. Effective reduction of fluoroscopy duration by using an advanced electroanatomic-mapping system and a standardized procedural protocol for ablation of atrial fibrillation: ‘the unleaded study’. Europace 2015;17:1694–9. [DOI] [PubMed] [Google Scholar]
- 53. Lehrmann H, Jadidi AS, Minners J, Keyl C, Hochholzer W, Carrapatoso Fet al. Important reduction of the radiation dose for pulmonary vein isolation using a multimodal approach. Europace 2018;20:279–87. [DOI] [PubMed] [Google Scholar]
- 54. Voskoboinik A, Kalman ES, Savicky Y, Sparks PB, Morton JB, Lee Get al. Reduction in radiation dose for atrial fibrillation ablation over time: a 12-year single-center experience of 2344 patients. Heart Rhythm 2017;14:810–6. [DOI] [PubMed] [Google Scholar]
- 55. Schreiber T, Kähler N, Biewener S, Tscholl V, Nagel P, Attanasio Pet al. Results from a real-time dosimetry study during left atrial ablations performed with ultra-low dose radiation settings. Herzschrittmachertherapie und Elektrophysiologie 2021;32:244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hoffmann E, Straube F, Wegscheider K, Kuniss M, Andresen D, Wu LQet al. Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace 2019;21:1313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rubesch-Kütemeyer V, Fischbach T, Guckel D, Körber B, Horstkotte D, Gutleben K-Jet al. Long-term development of radiation exposure, fluoroscopy time and contrast media use in daily routine in cryoballoon ablations after implementation of intracardiac echocardiography and other radioprotective measures: experiences from a large single-centre cohort. J Interv Card Electrophysiol 2020;58:169–75. [DOI] [PubMed] [Google Scholar]
- 58. Reissmann B, Maurer T, Wohlmuth P, Krüger M, Heeger C, Lemes Cet al. Significant reduction of radiation exposure in cryoballoon-based pulmonary vein isolation. Europace 2018;20:608–13. [DOI] [PubMed] [Google Scholar]
- 59. Kühne M, Knecht S, Spies F, Aeschbacher S, Haaf P, Zellweger Met al. Cryoballoon ablation of atrial fibrillation without demonstration of pulmonary vein occlusion—the simplify cryo study. Front Cardiovasc Med 2021;8:664538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rottner L, Obergassel J, My I, Kirchhof P, Ouyang F, Reissmann Bet al. Cryoballoon ablation guided by a novel wide-band dielectric imaging system. Front Cardiovasc Med 2022;9:967341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huang HD, Rodriguez JM, Serafini NJ, Macias C, Winterfield J, Sharma PSet al. Comparison between minimal fluoroscopy and conventional approaches for visually guided laser balloon pulmonary vein isolation ablation. J Cardiovasc Electrophysiol 2020;31:1608–15. [DOI] [PubMed] [Google Scholar]
- 62. Magni FT, Mulder BA, Groenveld HF, Wiesfeld ACP, Tieleman RG, Cox MGet al. Initial experience with pulsed field ablation for atrial fibrillation. Front Cardiovasc Med 2022;9:959186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lemoine MD, Fink T, Mencke C, Schleberger R, My I, Obergassel Jet al. Pulsed-field ablation-based pulmonary vein isolation: acute safety, efficacy and short-term follow-up in a multi-center real world scenario. Clin Res Cardiol 2023;112:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bohnen M, Weber R, Minners J, Jadidi A, Eichenlaub M, Neumann FJet al. Characterization of circumferential antral pulmonary vein isolation areas resulting from pulsed-field catheter ablation. Europace 2022;25:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Scaglione M, Biasco L, Caponi D, Anselmino M, Negro A, di Donna Pet al. Visualization of multiple catheters with electroanatomical mapping reduces X-ray exposure during atrial fibrillation ablation. Europace 2011;13:955–62. [DOI] [PubMed] [Google Scholar]
- 66. Fitzpatrick N, Mittal A, Galvin J, Jauvert G, Keaney J, Keelan Eet al. The impact of steerable sheath visualization during catheter ablation for atrial fibrillation. Europace 2023:25:1345–51.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kühne M, Knecht S, Mühl A, Reichlin T, Pavlović N, Kessel-Schaefer Aet al. Fluoroscopy-free pulmonary vein isolation in patients with atrial fibrillation and a patent foramen ovale using solely an electroanatomic mapping system. PLoS One 2016;11:e0148059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yu R, Liu N, Lu J, Zhao X, Hu Y, Zhang Jet al. 3-Dimensional transseptal puncture based on electrographic characteristics of Fossa Ovalis: a fluoroscopy-free and echocardiography-free method. JACC Cardiovasc Interv 2020;13:1223–32. [DOI] [PubMed] [Google Scholar]
- 69. Bohnen M, Minners J, Eichenlaub M, Weber R, Allgeier H-J, Jadidi Aet al. Feasibility and safety of a three-dimensional anatomic map-guided transseptal puncture for left-sided catheter ablation procedures. Europace 2023. ;25:1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Paetsch I, Jahnke C, Hilbert S, Krueger S, Weiss S, Smink Jet al. Cardiovascular magnetic resonance-guided electrophysiological interventions: radiofrequency ablation of typical atrial flutter. Circ Cardiovasc Imaging 2017;10:e005780.. [DOI] [PubMed] [Google Scholar]
- 71. Zhang Z, Phang CC, Tan RY, Pang SC, Chandramohan S, Zhuang KDet al. Does reducing radiation levels for procedures affect image quality and radiation to proceduralists? A double-blinded randomised study of two protocols. Clin Radiol 2021;76:157.e1–157.e10.. [DOI] [PubMed] [Google Scholar]
- 72. Maurer T, Schlüter M, Kuck KH. Keeping it simple: balloon devices for atrial fibrillation ablation therapy. JACC Clin Electrophysiol 2020;6:1577–96. [DOI] [PubMed] [Google Scholar]
- 73. Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun Jet al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. [DOI] [PubMed] [Google Scholar]
- 74. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle Let al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation 2019; 140:1779–88. [DOI] [PubMed] [Google Scholar]
- 75. Chun JKR, Bordignon S, Last J, Mayer L, Tohoku S, Zanchi Set al. Cryoballoon versus laserballoon: insights from the first prospective randomized balloon trial in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2021;14:E009294. [DOI] [PubMed] [Google Scholar]
- 76. Ekanem E, Reddy VY, Schmidt B, Reichlin T, Neven K, Metzner Aet al. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022;24:1256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schilling R, Dhillon GS, Tondo C, Riva S, Grimaldi M, Quadrini Fet al. Safety, effectiveness, and quality of life following pulmonary vein isolation with a multi-electrode radiofrequency balloon catheter in paroxysmal atrial fibrillation: 1-year outcomes from SHINE. Europace 2021;23:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Huang HD, ul ainAbid Q, Ravi V, Sharma P, Larsen T, Krishnan Ket al. Meta-analysis of pulmonary vein isolation ablation for atrial fibrillation conventional vs low- and zero-fluoroscopy approaches. J Cardiovasc Electrophysiol 2020;31:1403–12. [DOI] [PubMed] [Google Scholar]
- 79. Kautzner J, Haskova J, Lehar F. Intracardiac echocardiography to guide non-fluoroscopic electrophysiology procedures. Card Electrophysiol Clin 2021;13:399–408. [DOI] [PubMed] [Google Scholar]
- 80. Falasconi G, Penela D, Soto-Iglesias D, Jauregui B, Chauca A, Antonio RSet al. A standardized stepwise zero-fluoroscopy approach with transesophageal echocardiography guidance for atrial fibrillation ablation. J Interv Card Electrophysiol 2022;64:629–39. [DOI] [PubMed] [Google Scholar]
- 81. Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol 2015;38:797–806. [DOI] [PubMed] [Google Scholar]
- 82. Lyan E, Tsyganov A, Abdrahmanov A, Morozov A, Bakytzhanuly A, Tursunbekov Aet al. Nonfluoroscopic catheter ablation of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2018;41:611–9. [DOI] [PubMed] [Google Scholar]
- 83. Zei PC, Hunter TD, Gache LM, O'Riordan G, Baykaner T, Brodt CR. Low-fluoroscopy atrial fibrillation ablation with contact force and ultrasound technologies: a learning curve. Pragmat Obs Res 2019;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tahin T, Riba A, Nemeth B, Arvai F, Lupkovics G, Szeplaki Get al. Implementation of a zero fluoroscopic workflow using a simplified intracardiac echocardiography guided method for catheter ablation of atrial fibrillation, including repeat procedures. BMC Cardiovasc Disord 2021;21:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kuhne M, Knecht S, Muhl A, Reichlin T, Pavlovic N, Kessel-Schaefer Aet al. Fluoroscopy-free pulmonary vein isolation in patients with atrial fibrillation and a patent foramen ovale using solely an electroanatomic mapping system. PLoS One 2016;11:e0148059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jan M, Zizek D, Kuhelj D, Lakic N, Prolic Kalinsek T, Stublar Jet al. Combined use of electro-anatomic mapping system and intracardiac echocardiography to achieve zero-fluoroscopy catheter ablation for treatment of paroxysmal atrial fibrillation: a single centre experience. Int J Cardiovasc Imaging 2020;36:415–22. [DOI] [PubMed] [Google Scholar]
- 87. Lakkireddy D, Rangisetty U, Prasad S, Verma A, Biria M, Berenbom Let al. Intracardiac echo-guided radiofrequency catheter ablation of atrial fibrillation in patients with atrial septal defect or patent foramen ovale repair: a feasibility, safety, and efficacy study. J Cardiovasc Electrophysiol 2008;19:1137–42. [DOI] [PubMed] [Google Scholar]
- 88. Baykaner T, Quadros KK, Thosani A, Yasmeh B, Mitra R, Liu Eet al. Safety and efficacy of zero fluoroscopy transseptal puncture with different approaches. Pacing Clin Electrophysiol 2020;43:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Goya M, Frame D, Gache L, Ichishima Y, Tayar DO, Goldstein Let al. The use of intracardiac echocardiography catheters in endocardial ablation of cardiac arrhythmia: meta-analysis of efficiency, effectiveness, and safety outcomes. J Cardiovasc Electrophysiol 2020;31:664–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and its supporting information files.