Abstract
Habenular (Hb) processes negative emotions that may drive compulsive food-intake. Its functional changes were reported following laparoscopic-sleeve-gastrectomy (LSG). However, structural connectivity (SC) of Hb-homeostatic/hedonic circuits after LSG remains unclear. We selected regions implicated in homeostatic/hedonic regulation that have anatomical connections with Hb as regions-of-interest (ROIs), and used diffusion-tensor-imaging with probabilistic tractography to calculate SC between Hb and these ROIs in 30 obese participants before LSG (PreLSG) and at 12-month post-LSG (PostLSG12) and 30 normal-weight controls. Three-factor-eating-questionnaire (TFEQ) and Dutch-eating-behavior-questionnaire (DEBQ) were used to assess eating behaviors. LSG significantly decreased weight, negative emotion, and improved self-reported eating behavior. LSG increased SC between the Hb and homeostatic/hedonic regions including hypothalamus (Hy), bilateral superior frontal gyri (SFG), left amygdala (AMY), and orbitofrontal cortex (OFC). TFEQ-hunger negatively correlated with SC of Hb-Hy at PostLSG12; and increased SC of Hb-Hy correlated with reduced depression and DEBQ-external eating. TFEQ-disinhibition negatively correlated with SC of Hb-bilateral SFG at PreLSG. Increased SC of Hb-left AMY correlated with reduced DEBQ-emotional eating. Higher percentage of total weight-loss negatively correlated with SC of Hb-left OFC at PreLSG. Enhanced SC of Hb-homeostatic/hedonic regulatory regions post-LSG may contribute to its beneficial effects in improving eating behaviors including negative emotional eating, and long-term weight-loss.
Keywords: habenula, homeostasis/hedonic, laparoscopic sleeve gastrectomy, structural connectivity
Introduction
Laparoscopic sleeve gastrectomy (LSG), one of the most effective procedures for treating obesity, produces sustained weight loss and reduces craving for high-calorie food and negative emotions after surgery (Mack et al. 2016). Neuroimaging studies have demonstrated that LSG-induced improvements in eating behaviors and weight loss were associated with brain functional/structural alterations in regions/circuits implicated in hemostatic control (e.g. hypothalamus, Hy), reward processing (e.g. striatum), and cognitive control (e.g. prefrontal cortex) (Rao 2012; Li et al. 2018; Hu et al. 2021). However, little attention has been paid to identify LSG-induced alterations in brain regions/circuits involved with negative emotional processing.
The habenula (Hb), is involved with negative reinforcement and has been implicated in the enhanced sensitivity to negative reinforcement in addiction and mood disorders (Batalla et al. 2017; Koob 2021) and might also contribute to negative emotion-related overeating in obesity. A recent study reported lower resting-state functional connectivity (FC) between homeostatic/hedonic regions and the Hb in patients with cancer-associated weight loss compared with noncancer controls, suggesting the Hb acts as a link between homeostatic and hedonic pathways in regulating eating behaviors (Maldonado et al. 2018). Our recent study showed that LSG significantly increased brain resting-state activity in Hb, and enhanced FC of Hb-mediodorsal thalamic nucleus, which correlated with reduced body mass index (BMI) after LSG, suggesting LSG-induced weight loss in part may reflect rebalanced function in brain regions that process positive and negative rewards (Wang et al. 2021).
The structural connectivity (SC) between brain regions plays a prominent role in their functional coupling and are crucial to behavior (Bonnelle et al. 2012; Leong et al. 2016; Zhang et al. 2016). Thus, recovery of obesity-related brain structural abnormalities following LSG might underlie previously observed changes in function and associated behavioral outcomes. Another recent study showed LSG-induced sustained improvements in FC/SC between dorsolateral prefrontal cortex (DLPFC) and pregenual anterior cingulate cortex (pgACC), and reductions in BMI were correlated with increases in FC/SC of DLPFC-pgACC (Hu et al. 2021). Further, the relationship between lower ghrelin levels and greater cognitive control was mediated by SC of DLPFC-ACC, suggesting enhanced FC/SC in prefrontal regions contributes to long-term reductions in weight loss and food craving following LSG (Hu et al. 2021). However, to our knowledge, no systematic analysis has been conducted to investigate LSG-induced alterations in SC of the Hb-homeostatic/hedonic circuits and their association with negative emotional-related behavioral changes and weight loss after LSG.
Importantly, previous studies indicate that the Hb functionally and anatomically links cortical and subcortical structures that modulate diverse brain functions relevant to food/drug addiction and emotional dysfunctions (Volkow et al. 2013; Grodd et al. 2020; Koob 2021). Particularly, the Hb receives afferents primarily from limbic brain regions that are directly or indirectly innervated by the cerebral cortex, such as the hypothalamus (Hy), basal forebrain, anterior cingulate cortex (ACC), prefrontal cortex, and basal ganglia. It projects to several midbrain areas, most importantly the rostromedial tegmental nucleus, which contains mainly inhibitory GABAergic cells and regulates the activity of monoaminergic nuclei, including the dopaminergic ventral tegmental area (Hikosaka et al. 2008; Loonen and Ivanova 2016; Batalla et al. 2017; Grodd et al. 2020). In these regions, homeostatic feeding is mainly regulated by the Hy to control food intake and maintain energy balance for survival (Rossi and Stuber 2018). Brain regions involved with hedonic feeding are more complex and support distinct aspects of food reward and motivation. Key components of this hedonic pathway include basal ganglia and ventral tegmental area (core regions of the dopamine reward pathway); ACC, amygdala, and hippocampus (emotion regulation, memory); prefrontal cortex and orbital frontal gyrus (cognitive/executive control, salience attribution) (Volkow et al. 2013). Despite this distinction, there is significant functional and anatomical overlap between the homeostatic and the hedonic circuitry. Normally, these two regulatory systems are dynamically coordinated in order to meet metabolic demands and ensure survival (Rossi and Stuber 2018). Thus, revealing the interconnectivity between Hb and homeostatic/hedonic circuits before and after LSG is of value for identifying potential targets for neuromodulation or medications for weight loss therapy.
To address this neglect, we selected regions implicated in homeostatic/hedonic food regulation that have anatomical connections with Hb as regions of interest (ROIs). Thirty participants with obesity undergoing LSG performed behavioral measurements and magnetic resonance imaging (MRI) scans at pre-LSG (PreLSG) and 12 months post-LSG (PostLSG12), and we compared this group with 30 normal weight (NW) controls who had mirroring behavioral and MRI assessments at baseline. Then we used diffusion tensor imaging (DTI) with probabilistic tractography to calculate SC between the Hb and these ROIs, and analyzed their association with eating behaviors in all participants. The three-factor eating questionnaire (TFEQ) and the Dutch eating behavior questionnaire (DEBQ) were used to assess eating behaviors. We hypothesized that LSG would improve SC of Hb-homeostatic/hedonic circuits in participants with obesity and these improvements would be associated with decreased negative emotional-related eating behavior and weight loss.
Materials and methods
Participants
The experiments were conducted in accordance with the Helsinki Declaration and the protocol was approved by the Institutional Review Board of Xijing Hospital and registered in the Chinese Clinical Trial Registry Center as ChiCTR-OOB-15006346 (http://www.chictr.org.cn). Group 1 (LSG group): 43 participants with obesity (BMI ≥ 35 kg/m2, age between 18 and 60 years) were recruited for LSG at Xijing Hospital of Digestive Diseases affiliated with the Air Force Medical University in Xi’an, China, from 11 April 2015 to 31 December 2016. Participants were screened to ensure no history of psychiatric and/or neurological diseases, previous intestinal surgery and/or inflammatory intestinal disease, organ dysfunction, or taking any current medication that could affect the central nervous system. Other exclusion criteria: (1) participants who had a waist circumference greater than the interior diameter of the MRI scanner (Zhang et al. 2016; Zhang et al. 2019). (2) participants not willing to be part of the experiment group. (3) participants could not return for long-term follow-up after LSG. Given these criteria, 13 participants were disqualified. Among them were 6 participants whose waist circumference was greater than the interior diameter of the MRI scanner; 7 participants who were unable to return for the follow-up MRI assessments due to long-distance travel. Thus, 30 remaining participants with obesity (mean age: 28.00 ± 1.31 years) completed the baseline MRI scans (PreLSG) and underwent LSG surgeries all performed by the same surgeon. Eighteen participants were female (mean age: 28.88 ± 1.57 years) and 12 were male (mean age: 26.92 ± 2.31 years). Written informed consent was obtained from all participants. Identical MRI scans were performed at PostLSG12. Group 2 (NW group): 36 NW (BMI: 18.50–22.90 kg/m2, age between 18 and 60 years) were recruited from the local community in Xi’an, China, from 1 January 2016 to 31 December 2016. The inclusion criteria were in line with the LSG group, participants had no history of brain and intestinal diseases, organ dysfunction, and were not taking any current medication. One female and 2 male participants were excluded due to the contraindications to MRI, and 3 subjects’ imaging data were lost due to technical problems. Thus, 30 remaining NW participants qualified for inclusion criteria as a part of the control group (mean age: 28.00 ± 1.11 years), among whom 19 were female (mean age: 26.89 ± 1.31 years) and 11 were male (mean age: 29.91 ± 1.94 years). After written informed consent was obtained from all participants, participants were instructed to perform identical assessments and MRI scans mirroring the LSG group at baseline (NW).
Experimental design
All participants underwent 12 hours of overnight fasting, and MRI scans were performed between 9 and 10 A.M. to ensure consistency across assessments and to minimize circadian variability.
Behavioral measurements
All clinical measurements were identically conducted in participants with obesity at PreLSG and PostLSG12, and in NW at baseline (Table 1). Both groups completed the Hamilton Anxiety Rating Scale (HAMA) (Hamilton 1959), Hamilton Depression Rating Scale (HAMD) (Hamilton 1960), Yale Food Addiction Scale (YFAS) (Gearhardt et al. 2009), TFEQ, and DEBQ. Specifically, the TFEQ measures eating behaviors and quantifies three sub-factors for cognitive control, disinhibition, and hunger (Stunkard and Messick 1985), and the DEBQ assesses restrained-, emotional-, and external-eating behavior (Wallis and Hetherington 2009). The TFEQ and DEBQ data were only available for 23 participants, and so analyses with the TFEQ and DEBQ were limited to these 23 participants.
Table 1.
Demographic and clinical information of participants with obesity and NW.
| NW (n = 30) (Mean ± SE) |
LSG (n = 30) (Mean ± SE) |
PreLSG vs. NW | PreLSG vs. PostLSG12 | PostLSG12 vs. NW | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PreLSG | PostLSG12 | T | P | T | P | T | P | |||
| Age (years) | 28.00 ± 1.11 | 28.00 ± 1.31 | 29.10 ± 1.31 | 0.058 | 0.954 | N/A | N/A | 0.642 | 0.523 | |
| *Gender | 11 M/19F | 12 M/18F | 12 M/18F | N/A | N/A | N/A | N/A | N/A | N/A | |
| Weight (kg) | 58.67 ± 1.91 | 106.99 ± 3.38 | 74.56 ± 3.16 | 12.435 | < 0.001 | 20.828 | < 0.001 | 4.303 | < 0.001 | |
| %TWL | N/A | N/A | 30.66 ± 1.34 | N/A | N/A | N/A | N/A | N/A | N/A | |
| BMI (kg/m2) | 21.42 ± 0.46 | 38.06 ± 0.82 | 26.40 ± 0.81 | 17.612 | < 0.001 | 20.648 | < 0.001 | 5.340 | < 0.001 | |
| HAMA | 5.30 ± 0.82 | 10.17 ± 1.18 | 3.97 ± 0.53 | 3.375 | 0.001 | 5.746 | <0.001 | −1.362 | 0.178 | |
| YFAS | 1.43 ± 0.18 | 5.30 ± 0.42 | 1.87 ± 0.26 | 8.416 | <0.001 | 7.691 | <0.001 | 1.372 | 0.175 | |
| HAMD | 5.03 ± 0.81 | 9.53 ± 1.21 | 5.13 ± 0.80 | 3.092 | 0.003 | 3.751 | <0.001 | 0.088 | 0.930 | |
| * *TFEQ | Cognitive control | 8.65 ± 0.99 | 8.78 ± 0.87 | 13.48 ± 0.94 | 0.099 | 0.922 | −3.956 | <0.001 | 3.547 | <0.001 |
| Disinhibition | 5.87 ± 0.48 | 8.70 ± 0.51 | 5.48 ± 0.35 | 4.041 | <0.001 | 6.119 | <0.001 | −0.663 | 0.511 | |
| Hunger | 3.40 ± 0.52 | 7.30 ± 0.52 | 3.35 ± 0.34 | 5.334 | <0.001 | 7.899 | <0.001 | −0.070 | 0.944 | |
| * *DEBQ | Restrain eating | 2.39 ± 0.19 | 2.93 ± 0.14 | 3.54 ± 0.16 | 2.324 | 0.025 | −3.557 | 0.002 | 4.708 | <0.001 |
| Emotional eating | 2.11 ± 0.15 | 2.48 ± 0.15 | 2.02 ± 0.13 | 1.733 | 0.090 | 2.916 | 0.008 | −0.487 | 0.629 | |
| External eating | 2.90 ± 0.11 | 3.36 ± 0.10 | 2.79 ± 0.11 | 3.072 | 0.004 | 4.871 | <0.001 | −0.688 | 0.495 | |
Note: *Gender: chi-square test. **TFEQ, DEBQ: NW (n = 23), LSG (n = 23).
Abbreviation: %TWL, the percentage of total weight loss; BMI, body mass index; HAMA, Hamilton Anxiety Rating Scale; YFAS, Yale Food Addiction Scale; HAMD, Hamilton Depression Rating Scale; TFEQ, three Factor Eating Questionnaire; DEBQ, Dutch Eating Behavior Questionnaire; NW, normal weight; LSG, laparoscopic sleeve gastrectomy; PreLSG, participants with obesity who received MRI scan before surgery; PostLSG12, participants with obesity who received MRI scan at 12 months after surgery; SE, standard error.
MRI acquisition
The experiment was performed on a 3.0 T Signa Excite HD (GE) MRI scanner. The 3D-T1 weighted images for each participant were acquired using a 3D magnetization-prepared rapid acquisition gradient-echo sequences pulse sequence with a voxel size of 1 mm3 and with an axial fast spoiled gradient-echo sequence (repetition time (TR) = 7.8 ms; echo time (TE) = 3.0 ms; data matrix = 256 × 256; slices = 166; field of view (FOV) = 256 × 256 mm2). The diffusion weighted imaging scan with a single-shot spin-echo echo-planar-imaging sequence, and diffusion sensitizing gradients were applied along 60 noncollinear directions (b = 1,000 s/mm2) with 10 acquisitions without diffusion weighting (b = 0 s/mm2). The imaging parameters were 75 continuous axial slices, TR = 9,400 ms, TE = 84 ms, matrix size = 128 × 128, and field-of-view = 256 × 256 mm2, resulting in 2-mm isotropic voxels. We used restraining pads to minimize head motion.
DTI-data preprocessing and definition of the regions of interest
Preprocessing of DTI data was performed with the FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl). Brain extraction, head motion, and eddy-current correction were applied to the DTI images by using the FSL Brain Extractor Tool (Smith 2002) and the FMRIB’s Diffusion Toolbox. The diffusion tensor was then calculated for each voxel. Diffusion maps, including the fractional anisotropy (FA), and mean diffusivity, axial diffusivity, and radial diffusivity, were derived in individual diffusion space for each participant. Here, we used the coordinates of the Hb provided by Lawson et al. (2013) who proposed an approach to locate and manually trace the Hb in humans. In detail, the normalized right Hb coordinates in MNI space: [x = 4.8 (range 4.3–6.0), y = −24.1 (range −23.4 to −25.3), and z = 2.2 (range 1.4–3.5)]; the normalized left Hb coordinates: [x = −2.8 (range −2.0 to −3.7), y = −24.4 (range −23.4 to −25.6), and z = 2.3 (range 1.3–3.7)]. The Hb template in 2 mm space for nonzero voxels were 29.3 and 29.4 mm3 for right and left respectively, and given the small size of Hb, we then combined them for analysis. Regions implicated in homeostatic/hedonic regulation and have anatomical connections with Hb were selected as ROIs (Grodd et al. 2020). Specifically, the bilateral ROIs, including hippocampus, caudate, amygdala (AMY), prefrontal cortex, orbito-frontal cortex (OFC), ACC, were extracted from the automated anatomical labeling map (van den Bos et al. 2015). Wherein prefrontal cortex consisting of Frontal_Sup (SFG), Frontal_Mid, Frontal_Inf_Oper, Frontal_Inf_Tri, and Frontal_Sup_Medial. The ROIs of ventral tegmental area, nucleus accumbens were generated from the Harvard-Oxford subcortical structural atlas in standard space (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases/). The ROIs of the Hy were provided by Boes et al. (2018) who defined ROIs on the standard MNI space. In details, the center of anterior hypothalamic region coordinates in MNI space (x = ±4.4, y = 1.33, z = −14.67), and the posterior hypothalamic coordinates (x = ±6.6, y = −7.33, z = −12.67), which covers the whole Hy region. With regards to the distinct functions of subregions of OFC, we then used the seed mask provided by Lei et al. (2014) to parcellate the OFC into medial OFC (mOFC) and lateral OFC (lOFC) and reperformed the identical analysis of fiber tracking, wherein the mOFC consisting of Frontal_Sup_Orb, Rectus and Frontal_Med_Orb, the lOFC consisting of Frontal_Mid_Orb and Frontal_Inf_Orb.
Probabilistic tractography
We used a two-mask seeding approach to ensure that the probabilistic tractography maps only included streamlines passing through both the Hb and the target ROIs. The distributions of fiber orientations at each voxel were calculated with BEDPOSTX (Bayesian Estimation of Diffusion Parameters Obtained using Sampling Techniques). The probabilistic tractography from each voxel within the seed ROI was initiated with the probtrackx2 command (parameters: streamlines = 5,000; step length = 0.5 mm; curvature threshold = 0.2). Fiber tracking could only be conducted in native diffusion space, so all ROIs in standard space were transformed to native diffusion space (Lei et al. 2014). Briefly, the individual raw DTI images were first coregistered to the T1-weighted images using FSL’s Linear Image Registration Tool (FLIRT) with mutual information used as a cost function, and then they were normalized into MNI space using Linear (FLIRT) and FMRIB’s Nonlinear Image Registration Tool (http://www.fmrib.ox.ac.uk/fsl). Finally, the inverse transformations were applied to the ROI in MNI space to obtain the ROIs in individual diffusion space. For each participant, only fibers that passed through both ROIs were included in the reconstructed fiber tract. The fiber tracts were binarized and then overlapped in individual diffusion space; the binarized maps were then transformed to standard space and combined. The final tract masks connecting the Hb and ROIs in each hemisphere were generated by keeping the voxels with values larger than 40% of the total number of the participants in each group (Yuan et al. 2018). Finally, the values of the mean FA of the remaining fiber tracts connecting each pair of ROIs were extracted for statistical analysis.
Statistical analysis
Paired t-tests were implemented in IBM SPSS (Statistical Package for Social Sciences, Release 22.0, Chicago: SPSS, IL) to assess time effects on clinical measures and brain SC. Two-sample t-tests were also performed to examine the differences between NW and each of the two timepoints for the LSG group (PreLSG, PostLSG12). In addition, partial correlation with age and gender as covariates was used to assess the association between the values of the mean FA of the Hb-homeostatic/hedonic tracts and behavioral data with significant time effects in LSG. Since there were 96 correlation analyses in each group (12 (behavioral variables) * 8 (imaging variables)), we selected a standard method (false discovery rate, FDR, P < 0.05) to correct for multiple comparisons instead of Bonferroni correction, which is often considered too strict (P < 0.0005).
Results
Demographic characteristics
At baseline, there were no significant differences in age and gender between LSG and NW groups. The PreLSG relative to NW group had significantly higher weight (t = 12.435, P < 0.001), BMI (t = 17.612, P < 0.001), HAMA (t = 3.375, P = 0.001), YFAS (t = 8.416, P < 0.001), HAMD (t = 3.092, P = 0.003), TFEQ-disinhibition (t = 4.041, P < 0.001), TFEQ-hunger (t = 5.334, P < 0.001), DEBQ-restrain eating (t = 2.324, P = 0.025), and DEBQ-external eating (t = 3.072, P = 0.004, Table 1), as expected.
LSG significant decreased weight (t = 20.828, P < 0.001), BMI (t = 20.648, P < 0.001), HAMA (t = 5.746, P < 0.001), YFAS (t = 7.691, P < 0.001), HAMD (t = 3.751, P < 0.001), TFEQ-disinhibition (t = 6.119, P < 0.001), TFEQ-hunger (t = 7.899, P < 0.001), DEBQ-emotional eating (t = 2.916, P = 0.008), DEBQ-external eating (t = 4.871, P < 0.001), and increased TFEQ-cognitive control (t = −3.956, P < 0.001) and DEBQ-restrain eating (t = −3.557, P = 0.002, Table 1). Excluding weight (t = 4.303, P < 0.001), BMI (t = 5.340, P < 0.001), TFEQ-cognitive control (t = 3.547, P < 0.001), and DEBQ-restrain eating (t = 4.708, P < 0.001, Table 1), other demographic characteristics were equivalent to that in NW at PostLSG12.
LSG-induced changes in SC
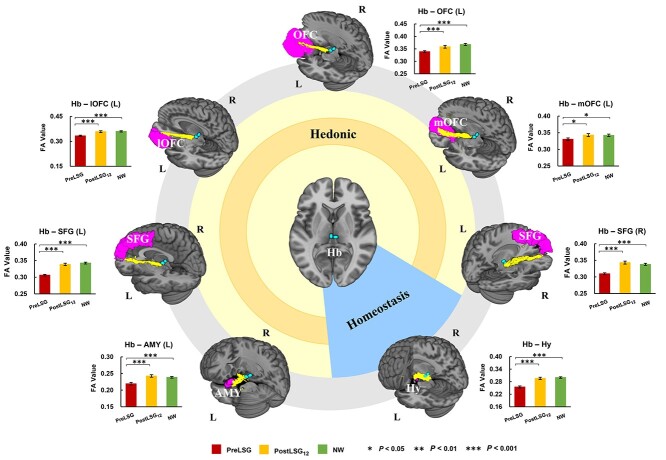
Probabilistic tractography identified increased SC between the Hb and several ROIs including Hy, bilateral SFG, left AMY, and left OFC, which on average became roughly equivalent to those of NW at PostLSG12 (Fig. 1).
Fig. 1.
LSG-induced increases in SC between the Hb and ROIs including Hy, bilateral SFG, left AMY, and left OFC, which on average became roughly equivalent to those of NW at PostLSG12. The error bars indicate standard error of the mean. Abbreviation: Hb, habenula; Hy, hypothalamus; SFG, superior frontal gyri; AMY, amygdala; OFC, orbito-frontal cortex; mOFC, medial regions of the orbito-frontal cortex; lOFC, lateral regions of the orbito-frontal cortex; FA, fractional anisotropy; SC, structural connectivity; L, left; R, right; LSG, laparoscopic sleeve gastrectomy; PreLSG, participants with obesity who received MRI scan before surgery; PostLSG12, participants with obesity who received MRI scan at 12 months after surgery; NW, normal weight.
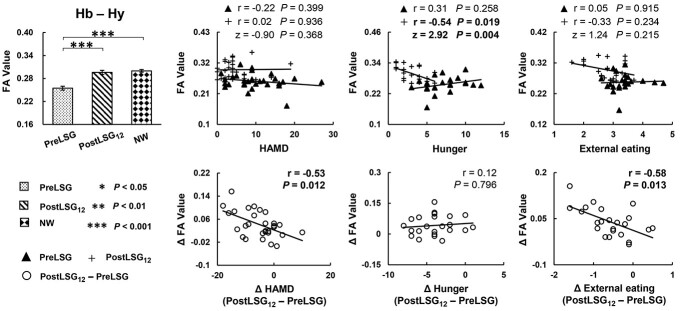
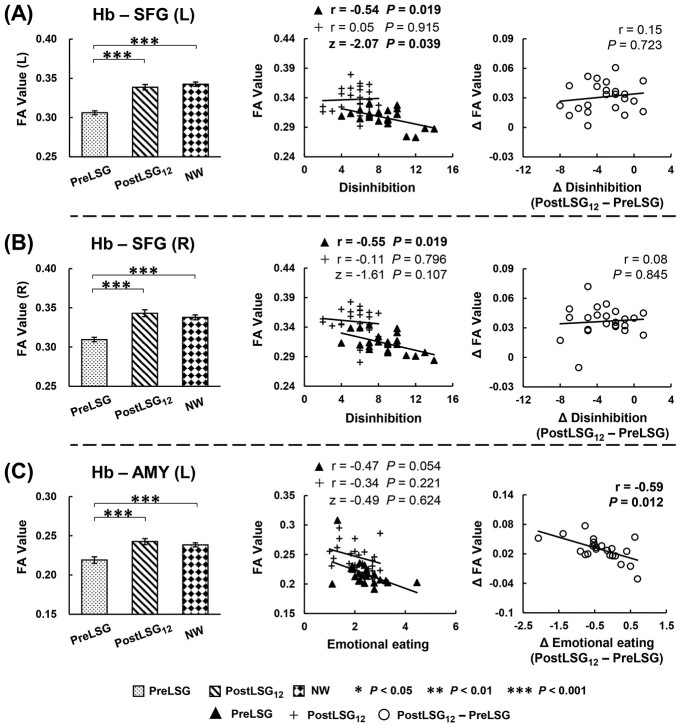
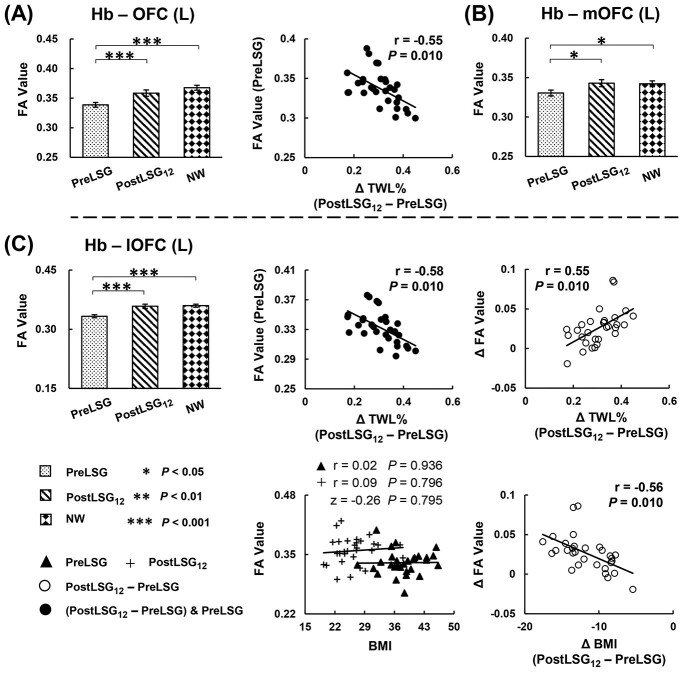
Significant correlations between these SC and behavioral measures were listed below: Firstly, LSG-induced increases in SC of Hb-Hy correlated with reductions in HAMD (r = −0.53, P = 0.012) and DEBQ-external eating (r = −0.58, P = 0.013, Fig. 2). Increases in SC of Hb-left AMY correlated with reductions in DEBQ-emotional eating (r = −0.59, P = 0.012, Fig. 3C). Increases in SC of Hb-left lOFC correlated with reductions in BMI (r = −0.56, P = 0.010, Fig. 4C), and increases in %TWL (r = 0.55, P = 0.010, Fig. 4C). Secondly, at PreLSG, SC of Hb-bilateral SFG was negatively correlated with TFEQ-disinhibition (r = −0.54, P = 0.019, Fig. 3A; r = −0.55, P = 0.019, Fig. 3B). At PostLSG12, SC of Hb-Hy was negatively correlated with TFEQ-hunger (r = −0.54, P = 0.019, Fig. 2). Finally, at PreLSG, SC of Hb-left OFC (r = −0.55, P = 0.010, Fig. 4A) and Hb-left lOFC (r = −0.58, P = 0.010, Fig. 4C) were negatively correlated with higher %TWL.
Fig. 2.
LSG-induced increases in SC of the Hb-Hy. Increases in SC of Hb-Hy were correlated with reductions in HAMD and DEBQ-external eating at PostLSG12. TFEQ-hunger was negatively correlated with SC of Hb-Hy at PostLSG12. Only the correlation slope between SC of Hb-Hy and TFEQ-hunger was significantly different. The error bars indicate standard error of the mean. Abbreviation: Hb, habenula; Hy, hypothalamus; FA, fractional anisotropy; SC, structural connectivity; HAMD, Hamilton depression; TFEQ, three factor eating questionnaire; DEBQ, Dutch Eating Behavior Questionnaire; LSG, laparoscopic sleeve gastrectomy; PreLSG, participants with obesity who received MRI scan before surgery; PostLSG12, participants with obesity who received MRI scan at 12 months after surgery; NW, normal weight.
Fig. 3.
LSG-induced increases in SC of the Hb-bilateral SFG, and Hb-AMY. (A, B) TFEQ-disinhibition was negatively correlated with SC of Hb-bilateral SFG at PreLSG. (C) Increases in SC of Hb-left AMY were correlated with reductions in DEBQ-emotional eating. Only the correlation slope between SC of Hb-left SFG and TFEQ-disinhibition was significantly different. The error bars indicate standard error of the mean. Abbreviation: Hb, habenula; SFG, superior frontal gyri; AMY, amygdala; FA, fractional anisotropy; SC, structural connectivity; TFEQ, three factor eating questionnaire; DEBQ, Dutch Eating Behavior Questionnaire; L, left; LSG, laparoscopic sleeve gastrectomy; PreLSG, participants with obesity who received MRI scan before surgery; PostLSG12, participants with obesity who received MRI scan at 12 months after surgery; NW, normal weight.
Fig. 4.
LSG-induced increases in SC of the Hb-OFC, and Hb SC with lOFC show a significant association with weight loss. (A) Higher %TWL was negatively correlated with SC of Hb-left OFC at PreLSG. (B) LSG-induced increases in SC of the Hb-mOFC. (C) Higher %TWL was negatively correlated with SC of Hb-left OFC at PreLSG. LSG-related increases in SC in the Hb-left lOFC were correlated with increases in %TWL and reductions in BMI at PostLSG12. The correlation slope between SC of Hb-left OFC and BMI was not significantly different. The error bars indicate standard error of the mean. Abbreviation: Hb, habenula; OFC, orbito-frontal cortex; mOFC, medial regions of the orbito-frontal cortex; lOFC, lateral regions of the orbito-frontal cortex; FA, fractional anisotropy; SC, structural connectivity; %TWL, the percentage of total weight loss; BMI, body mass index; L, left; LSG, laparoscopic sleeve gastrectomy; PreLSG, participants with obesity who received MRI scan before surgery; PostLSG12, participants with obesity who received MRI scan at 12 months after surgery; NW, normal weight.
In addition, when compared the correlation slopes of PreLSG and PostLSG12, only SC of Hb-left SFG and TFEQ-disinhibition (z = −2.07, P = 0.039, Fig. 3A), and SC of Hb-Hy and TFEQ-hunger (z = 2.92, P = 0.004, Fig. 2) were significantly different.
Discussion
Our study shows increases in SC of the Hb with brain regions implicated in homeostatic/hedonic regulation 12-month post-LSG that were associated with weight loss and with decreases in negative emotional-related eating behaviors. At PostLSG12, participants had significant decreases in weight and BMI, anxiety, depression, and improvement in eating behaviors. These changes in weight, mood, and eating behaviors were associated with increases in SC of Hb-Hy, Hb-SFG, Hb-AMY, and Hb-OFC one year after surgery. Our findings highlight the critical role of the Hb in mediating negative emotional-related eating behavior, and improvements in SC of Hb-homeostatic/hedonic circuits could serve as a biomarker for the long-term efficacy of LSG in appetite regulation and weight loss.
LSG-induced increases in SC of the Hb-Hy
The Hy is a small but highly versatile structure implicated in energy homeostasis, endocrine activity, and food-intake control, and its abnormalities can result in eating disorders (Sousa-Ferreira et al. 2014). Anatomical connection analysis revealed that projections from the lateral hypothalamic area to the lateral habenula encoded for maladaptive emotional states in rats and humans, including depression and anxiety (Kowski et al. 2008; Batalla et al. 2017). Furthermore, negative emotion regulation strategies often motivate individuals to engage in emotional eating to escape from such unpleasant feelings (Konttinen 2020). This negative reinforcement process driving individuals to seek and consume high-calorie foods is one of the hallmarks of food addiction, which increases risk for developing obesity (Volkow et al. 2013). Further support for the connection of Hb-Hy comes from a pilot study, when participants with cancer-associated weight loss and healthy controls were compared. Cancer-induced decreases in resting-state FC of Hb-Hy were observed, suggesting a disruption of satiety and hunger signaling (Maldonado et al. 2018). The current study showed that LSG increased SC of Hb-Hy at PostLSG12, at which time point it was equivalent to that in NW, and correlation analysis showed that it negatively correlated with hunger ratings at PostLSG12. Recovery of structural abnormalities of Hb-Hy in obese participants following LSG also accompanied decreased hunger levels, reflecting the association between brain SC changes and improvement in eating behaviors. These data further strengthen the evidence that brain structure plays a prominent role in mediating functional coupling between brain regions and behavior. At PostLSG12, enhanced SC of Hb-Hy was also negatively correlated with reduced depression ratings and external eating scores, which is consistent with recent evidence that the Hb and the hypothalamo–pituitary–adrenal axis are involved in mood and eating disorders (Batalla et al. 2017; Bao and Swaab 2019). Recently, a study showed that Hb-Hy circuit encoded negative valence and computed a prediction signal for negative events, providing further evidence for the role of the Hb-Hy circuit in mood and aversion (Lazaridis et al. 2019). Together our findings suggesting that recovery of brain circuitry abnormalities in Hb-Hy circuit might underlie the improved negative emotional-related eating behavior in obese participants following LSG.
LSG-induced increases in SC of the Hb-SFG
As a subregion of the dorsolateral prefrontal cortex (Lei et al. 2014; Liu et al. 2019), the SFG appears to be specifically involved in emotional/behavioral regulation that may affect appetite (Koush et al. 2019). Studies from our group using structural MRI and surface-based morphometry analysis showed LSG increased cortical thickness in the SFG is association with decreased BMI (Liu et al. 2019). In addition, neuroimaging studies reported that interactions between Hb and prefrontal cortex–midbrain circuits signal emotional demand and mediate value-based decision-making (Orsini et al. 2015). Our results showed a negative correlation between the SC of Hb-bilateral SFG and disinhibition ratings at PreLSG, suggesting that the weaker the SC of Hb-bilateral SFG the higher the disinhibition ratings. Critically, LSG enhanced the SC of this circuit, and the association between disinhibition and SC disappeared following surgery.
LSG-induced increases in SC of the Hb-AMY
Based on the potential involvement of AMY in emotional-related behavior and food preference, anatomic connections to and from this nucleus have been mapped in rodents and humans, including sensory, prefrontal, and reward systems (Tiedemann et al. 2020). Notably, the Hb with its direct and indirect connections to AMY, appears to play a similar role with regards to antireward responses, and may be involved in negative emotion-related overeating (Loonen and Ivanova 2016; Grodd et al. 2020). However, it is debated whether the Hb is functionally coupled with AMY. Previous high-resolution resting-state fMRI studies did not report significant Hb-AMY FC, which is consistent with rodent findings that there is a lack of afferent and efferent connections between both regions (Quina et al. 2015; Torrisi et al. 2017). In contrast to these reports, one study showed that participants with low subclinical depression ratings exhibited greater Hb FC with right AMY (Ely et al. 2016); another inconsistency with task fMRI study reported increased connectivity between the right Hb and bilateral AMY as a function of cues predicting increasingly aversive outcomes (Lawson et al. 2014). In fact, the strength of emotional response was modulated by the connection of AMY-hippocampal-Hb, which offers a potential channel implicated in emotional behavior (Loonen and Ivanova 2016). In the current study, due to the small size of the Hb, we combined right and left regions as a whole region to perform SC analysis between the Hb and bilateral AMY, and found LSG-induced significant increases in SC only between Hb and left AMY at PostLSG12, which was negatively correlated with reduced emotional eating scores. Thus, although the above findings cannot speak to whether this circuit is lateralized (Hb-left AMY), it provides a basis for future high-resolution (e.g. 7 T MRI) studies to investigate the presence of such a lateralized circuit in humans. These data also suggest LSG-induced improvements in SC of Hb-AMY may inhibit the intensity of negative emotions and thereby decrease negative emotion-related overeating behaviors.
LSG-induced increases in SC of the Hb-OFC
The OFC is an important region of reward and motivation circuits implicated in various aspects of salience attribution and decision-making, and impairments in OFC function contribute to compulsive palatable food-intake behaviors (Volkow et al. 2013). Anatomical evidence indicates that the OFC mainly connects with the Hb via the stria medullaris and fornix (Grodd et al. 2020). Neuroimaging evidence also suggests that participants with greater substance use compared to others showed higher resting-state FC between Hb and left OFC (Oh et al. 2020), which might contribute to the negative motional state that drives compulsive behaviors in addiction. Similarly, disruption of neuronal circuits underlying negative emotional states that include amygdala and Hb may confer vulnerability to compulsive overeating in part through engagement of the OFC, thus increasing the risk for eating disorders and obesity (Moore et al. 2017). These prior studies provide evidence in line with the current finding that higher %TWL was negatively correlated with SC of Hb-left OFC at PreLSG, suggesting that disrupted Hb-OFC connectivity contributes to the negative emotional states that drive emotional-related eating behaviors that increase obesity risk.
The OFC is a relatively large region, and previous studies have demonstrated regional specialization between lOFC and mOFC, wherein the lOFC is more engaged with reward-guided learning, whereas the mOFC plays a greater role in reward-guided decision making (Noonan et al. 2017; Noonan et al. 2010). Thus, we also investigated contributions from OFC subregions, showing higher %TWL were negatively correlated with SC of Hb-left lOFC at PreLSG. In addition, we only found that LSG-induced increased SC of Hb-left lOFC showed a negative correlation with reduced BMI, and positive correlations with increased %TWL at PostLSG12, suggesting LSG-induced improvement in circuits implicated in negative emotion or motivation was associated with weight loss. One fMRI study reported a similar feedback-related functional coupling between lOFC and Hb when consistent stimulus-outcome mappings were present (Noonan et al. 2011), these regions play an important role in learning stimulus-reinforcement associations, and using the learned outcomes to guide eating behaviors. Since negative emotional states uncovered an association between weight and brain activation in the OFC in response to milkshake consumption (Rudenga et al. 2013), improvements in SC of Hb-lOFC may also contribute to weight loss and appetite regulation in obese patients after LSG.
In addition, Hb connectivity with various regions mediates different aspects of reward processing (Haber and Knutson 2010; Hikosaka 2010). However, we did not observe significant connectivity with some regions implicated in reward, such as the nucleus accumbens, consistent with findings in humans of an absence of afferent and efferent connections of Hb-nucleus accumbens (Grodd et al. 2020). We also tried the reverse tractography analysis (i.e. using striatal subregions as seed regions to calculate SC) and we were not able to observe significant tracts between Hb-putamen and Hb-pallidum. In addition, there were no significant changes in SC of Hb-caudate at PostLSG12.
It is worth noting that obesity is associated with inflammation, both in animal models (Guillemot-Legris and Muccioli 2017) and in human studies (Moreno-Navarrete et al. 2017), and that the inflammation can negatively affect brain structure. Specifically, a longitudinal study found that an inflammatory marker (lipopolysaccharide-binding protein, LBP) was associated with DTI-metrics in several white matter regions in obese subjects both at baseline and 2 years later, including the bilateral corticospinal tract, superior longitudinal fascicle, and cingulum (Moreno-Navarrete et al. 2017). Marques et al. (2014) assessed the effect of bariatric surgery in severely obese women, and found that obesity-related metabolic and inflammatory processes were associated with changes in cerebral metabolism amenable to reversal with weight loss, and accompanied with improved executive function. These findings raise the possibility that reduced inflammation following bariatric surgery might contribute to improvements in brain structure, cognitive function, and weight loss.
Limitations
There are several limitations: (1) Due to strict exclusion criteria and difficulty in retaining participants for long-term follow-up after LSG, we did not have a larger cohort for the LSG group and NW control groups, which limits the generalizability of our observations, and does not allow us to assess gender-related differences in responses to LSG. (2) Lack of data on the associations between brain SC and gut hormones, which might help to understand the potential neurophysiological mechanisms for improved emotional-eating and weight loss. (3) DTI tractography cannot provide information about neurotransmission. Future studies should use multimodal imaging to address whether these findings are related to, e.g. dopaminergic signaling differences in participants with obesity before and after LSG. (4) In this study, we did not include measures of neuroinflammation, which are likely to contribute to some of the adverse effects of obesity on brain tissue. Thus, future studies should include inflammation markers to evaluate their role in LSG associated improvement in brain structure, eating behaviors, and weight loss.
Conclusions
This study examined the LSG-induced alterations in SC of Hb-homeostatic/hedonic circuits and their association with negative emotional-related behavioral changes and weight loss in participants with obesity before and one year after LSG. Results showed that LSG significantly increased SC of the Hb-Hy, Hb-SFG, Hb-AMY, and Hb-OFC, regions that are implicated in energy homeostasis, emotional/behavioral regulation, salience attribution, and decision-making. The findings highlight that LSG improved SC of Hb-homeostatic/hedonic circuits contributing to improved eating behaviors and long-term weight loss.
Authors’ contribution
The authors’ responsibilities were as follows: Conceptualization, GJW, Yi Zhang, NDV, YN, and GJ; Data acquisition, JW, GL, Yang Hu, WZ, WJ, ZT, HL, FJ, Yaqi Zhang, FW, KMD, JY, Yu Han, and GC; Data analysis, JW, Yang Hu; Writing-Original Draft, JW, Yi Zhang, GJW; and Writing-Review & Editing, NDV, DT, and PM. All authors critically reviewed the content and approved the final version for publication.
Contributor Information
Jia Wang, Center for Brain Imaging, School of Life Science and Technology, Xidian University & Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment & Xi'an Key Laboratory of Intelligent Sensing and Regulation of trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Gang Ji, State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, Air Force Medical University, Xi'an, Shaanxi 710032, China.
Guanya Li, Center for Brain Imaging, School of Life Science and Technology, Xidian University & Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment & Xi'an Key Laboratory of Intelligent Sensing and Regulation of trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Yang Hu, Center for Brain Imaging, School of Life Science and Technology, Xidian University & Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment & Xi'an Key Laboratory of Intelligent Sensing and Regulation of trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Wenchao Zhang, Center for Brain Imaging, School of Life Science and Technology, Xidian University & Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment & Xi'an Key Laboratory of Intelligent Sensing and Regulation of trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Weibin Ji, Center for Brain Imaging, School of Life Science and Technology, Xidian University & Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment & Xi'an Key Laboratory of Intelligent Sensing and Regulation of trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Zongxin Tan, Center for Brain Imaging, School of Life Science and Technology, Xidian University & Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment & Xi'an Key Laboratory of Intelligent Sensing and Regulation of trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Hao Li, Center for Brain Imaging, School of Life Science and Technology, Xidian University & Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment & Xi'an Key Laboratory of Intelligent Sensing and Regulation of trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Fukun Jiang, Center for Brain Imaging, School of Life Science and Technology, Xidian University & Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment & Xi'an Key Laboratory of Intelligent Sensing and Regulation of trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Yaqi Zhang, Center for Brain Imaging, School of Life Science and Technology, Xidian University & Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment & Xi'an Key Laboratory of Intelligent Sensing and Regulation of trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Feifei Wu, Center for Brain Imaging, School of Life Science and Technology, Xidian University & Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment & Xi'an Key Laboratory of Intelligent Sensing and Regulation of trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Karen M von Deneen, Center for Brain Imaging, School of Life Science and Technology, Xidian University & Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment & Xi'an Key Laboratory of Intelligent Sensing and Regulation of trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Juan Yu, State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, Air Force Medical University, Xi'an, Shaanxi 710032, China.
Yu Han, Department of Radiology, Tangdu Hospital, Air Force Medical University, Xi'an, Shaanxi 710038, China.
Guangbin Cui, Department of Radiology, Tangdu Hospital, Air Force Medical University, Xi'an, Shaanxi 710038, China.
Peter Manza, Laboratory of Neuroimaging, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD 20892, USA.
Dardo Tomasi, Laboratory of Neuroimaging, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD 20892, USA.
Nora D Volkow, Laboratory of Neuroimaging, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD 20892, USA.
Yongzhan Nie, State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, Air Force Medical University, Xi'an, Shaanxi 710032, China.
Yi Zhang, Center for Brain Imaging, School of Life Science and Technology, Xidian University & Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment & Xi'an Key Laboratory of Intelligent Sensing and Regulation of trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Gene-Jack Wang, Laboratory of Neuroimaging, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD 20892, USA.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 82172023); Natural Science Basic Research Program of Shaanxi (grant numbers 2022JC-44, 2022JQ-622); National Clinical Research Center for Digestive Diseases (grant number 2015BAI13B07); the Fundamental Research Funds for the Central Universities (grant number XJS211201); and support in part from the Intramural Research Program of the National Institute on Alcoholism and Alcohol Abuse (grant number Y1AA3009 to P.M., D.T., N.D.V., G.J.W.).
Conflict of interest statement. The authors declared no conflict of interest.
References
- Bao AM, Swaab DF. The human hypothalamus in mood disorders: the HPA axis in the center. IBRO Rep. 2019:6:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalla A, Homberg JR, Lipina TV, Sescousse G, Luijten M, Ivanova SA, Schellekens A, Loonen A. The role of the habenula in the transition from reward to misery in substance use and mood disorders. Neurosci Biobehav Rev. 2017:80:276–285. [DOI] [PubMed] [Google Scholar]
- Boes AD, Fischer D, Geerling JC, Bruss J, Saper CB, Fox MD. Connectivity of sleep- and wake-promoting regions of the human hypothalamus observed during resting wakefulness. Sleep. 2018:41:zsy108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012:109:4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely BA, Xu J, Goodman WK, Lapidus KA, Gabbay V, Stern ER. Resting-state functional connectivity of the human habenula in healthy individuals: associations with subclinical depression. Hum Brain Mapp. 2016:37:2369–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009:52:430–436. [DOI] [PubMed] [Google Scholar]
- Grodd W, Kumar VJ, Schuz A, Lindig T, Scheffler K. The anterior and medial thalamic nuclei and the human limbic system: tracing the structural connectivity using diffusion-weighted imaging. Sci Rep. 2020:10:10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot-Legris O, Muccioli GG. Obesity-induced neuroinflammation: beyond the hypothalamus. Trends Neurosci. 2017:40:237–253. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010:35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959:32:50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960:23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010:11:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008:28:11825–11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Ji G, Li G, Manza P, Zhang W, Wang J, Lv G, He Y, Zhang Z, Yuan K, et al. Brain connectivity, and hormonal and behavioral correlates of sustained weight loss in obese patients after laparoscopic sleeve gastrectomy. Cereb Cortex. 2021:31:1284–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen H. Emotional eating and obesity in adults: the role of depression, sleep and genes. Proc Nutr Soc. 2020:79:283–289. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drug addiction: hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol Rev. 2021:73:163–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koush Y, Pichon S, Eickhoff SB, Van De Ville D, Vuilleumier P, Scharnowski F. Brain networks for engaging oneself in positive-social emotion regulation. NeuroImage. 2019:189:106–115. [DOI] [PubMed] [Google Scholar]
- Kowski AB, Geisler S, Krauss M, Veh RW. Differential projections from subfields in the lateral preoptic area to the lateral habenular complex of the rat. J Comp Neurol. 2008:507:1465–1478. [DOI] [PubMed] [Google Scholar]
- Lawson RP, Drevets WC, Roiser JP. Defining the habenula in human neuroimaging studies. NeuroImage. 2013:64:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson RP, Seymour B, Loh E, Lutti A, Dolan RJ, Dayan P, Weiskopf N, Roiser JP. The habenula encodes negative motivational value associated with primary punishment in humans. Proc Natl Acad Sci U S A. 2014:111:11858–11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaridis I, Tzortzi O, Weglage M, Martin A, Xuan Y, Parent M, Johansson Y, Fuzik J, Furth D, Fenno LE, et al. A hypothalamus-habenula circuit controls aversion. Mol Psychiatry. 2019:24:1351–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Chen C, Xue F, He Q, Chen C, Liu Q, Moyzis RK, Xue G, Cao Z, Li J, et al. Fiber connectivity between the striatum and cortical and subcortical regions is associated with temperaments in Chinese males. NeuroImage. 2014:89:226–234. [DOI] [PubMed] [Google Scholar]
- Leong JK, Pestilli F, Wu CC, Samanez-Larkin GR, Knutson B. White-matter tract connecting anterior insula to nucleus accumbens correlates with reduced preference for positively skewed gambles. Neuron. 2016:89:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Ji G, Hu Y, Xu M, Jin Q, Liu L, von Deneen KM, Zhao J, Chen A, Cui G, et al. Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self-referential processing. Hum Brain Mapp. 2018:39:4755–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Ji G, Li G, Hu Y, Jin Q, Hu C, Zhao J, Meng Q, von Deneen KM, Chen A, et al. Structural changes in brain regions involved in executive-control and self-referential processing after sleeve gastrectomy in obese patients. Brain Imag Behav. 2019:13:830–840. [DOI] [PubMed] [Google Scholar]
- Loonen AJ, Ivanova SA. Circuits regulating pleasure and happiness: the evolution of the amygdalar-hippocampal-habenular connectivity in vertebrates. Front Neurosci. 2016:10:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack I, Olschlager S, Sauer H, von Feilitzsch M, Weimer K, Junne F, Peeraully R, Enck P, Zipfel S, Teufel M. Does laparoscopic sleeve gastrectomy improve depression, stress and eating behaviour? A 4-year follow-up study. Obes Surg. 2016:26:2967–2973. [DOI] [PubMed] [Google Scholar]
- Maldonado M, Molfese DL, Viswanath H, Curtis K, Jones A, Hayes TG, Marcelli M, Mediwala S, Baldwin P, Garcia JM, et al. The habenula as a novel link between the homeostatic and hedonic pathways in cancer-associated weight loss: a pilot study. J Cachexia Sarcopenia Muscle. 2018:9:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques EL, Halpern A, Correa MM, de Melo ME, Horie NC, Buchpiguel CA, Martins NCA, Ono CR, Prando S, Santo MA, et al. Changes in neuropsychological tests and brain metabolism after bariatric surgery. J Clin Endocrinol Metab. 2014:99:E2347–E2352. [DOI] [PubMed] [Google Scholar]
- Moore CF, Sabino V, Koob GF, Cottone P. Pathological overeating: emerging evidence for a compulsivity construct. Neuropsychopharmacology. 2017:42:1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Blasco G, Puig J, Biarnes C, Rivero M, Gich J, Fernandez-Aranda F, Garre-Olmo J, Ramio-Torrenta L, Alberich-Bayarri A, et al. Neuroinflammation in obesity: circulating lipopolysaccharide-binding protein associates with brain structure and cognitive performance. Int J Obes. 2017:41:1627–1635. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Walton ME, Behrens TE, Sallet J, Buckley MJ, Rushworth MF. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci U S A. 2010:107:20547–20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Mars RB, Rushworth MF. Distinct roles of three frontal cortical areas in reward-guided behavior. J Neurosci. 2011:31:14399–14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Chau B, Rushworth M, Fellows LK. Contrasting effects of medial and lateral orbitofrontal cortex lesions on credit assignment and decision-making in humans. J Neurosci. 2017:37:7023–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Lee J, Gosnell SN, Patriquin M, Kosten T, Salas R. Orbitofrontal, dorsal striatum, and habenula functional connectivity in psychiatric patients with substance use problems. Addict Behav. 2020:108:106457. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB. Neural mechanisms regulating different forms of risk-related decision-making: insights from animal models. Neurosci Biobehav Rev. 2015:58:147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quina LA, Tempest L, Ng L, Harris JA, Ferguson S, Jhou TC, Turner EE. Efferent pathways of the mouse lateral habenula. J Comp Neurol. 2015:523:32–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RS. Bariatric surgery and the central nervous system. Obes Surg. 2012:22:967–978. [DOI] [PubMed] [Google Scholar]
- Rossi MA, Stuber GD. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab. 2018:27:42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenga KJ, Sinha R, Small DM. Acute stress potentiates brain response to milkshake as a function of body weight and chronic stress. Int J Obes. 2013:37:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002:17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Ferreira L, de Almeida LP, Cavadas C. Role of hypothalamic neurogenesis in feeding regulation. Trends Endocrinol Metab. 2014:25:80–88. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985:29:71–83. [DOI] [PubMed] [Google Scholar]
- Tiedemann LJ, Alink A, Beck J, Buchel C, Brassen S. Valence encoding signals in the human amygdala and the willingness to eat. J Neurosci. 2020:40:5264–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S, Nord CL, Balderston NL, Roiser JP, Grillon C, Ernst M. Resting state connectivity of the human habenula at ultra-high field. NeuroImage. 2017:147:872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM. Adolescent impatience decreases with increased frontostriatal connectivity. Proc Natl Acad Sci U S A. 2015:112:E3765–E3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013:14:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis DJ, Hetherington MM. Emotions and eating. Self-reported and experimentally induced changes in food intake under stress. Appetite. 2009:52:355–362. [DOI] [PubMed] [Google Scholar]
- Wang J, Li G, Hu Y, Zhang W, Zhang L, Tan Z, Li H, Jia Z, von Deneen KM, Li X, et al. Habenular and mediodorsal thalamic connectivity predict persistent weight loss after laparoscopic sleeve gastrectomy. Obesity (Silver Spring). 2021:30:172–182. [DOI] [PubMed] [Google Scholar]
- Yuan K, Yu D, Zhao M, Li M, Wang R, Li Y, Manza P, Shokri-Kojori E, Wiers CE, Wang GJ, et al. Abnormal frontostriatal tracts in young male tobacco smokers. NeuroImage. 2018:183:346–355. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ji G, Xu M, Cai W, Zhu Q, Qian L, Zhang YE, Yuan K, Liu J, Li Q, et al. Recovery of brain structural abnormalities in morbidly obese patients after bariatric surgery. Int J Obes. 2016:40:1558–1565. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ji G, Li G, Hu Y, Liu L, Jin Q, Meng Q, Zhao J, Yuan K, Liu J, et al. Ghrelin reductions following bariatric surgery were associated with decreased resting state activity in the hippocampus. Int J Obes. 2019:43:842–851. [DOI] [PubMed] [Google Scholar]