Abstract
PURPOSE
Personal continuity between patient and physician is a core value of primary care. Although previous studies suggest that personal continuity is associated with fewer potentially inappropriate prescriptions, evidence on continuity and prescribing in primary care is scarce. We aimed to determine the association between personal continuity and potentially inappropriate prescriptions, which encompasses potentially inappropriate medications (PIMs) and potential prescribing omissions (PPOs), by family physicians among older patients.
METHODS
We conducted an observational cohort study using routine care data from patients enlisted in 48 Dutch family practices from 2013 to 2018. All 25,854 patients aged 65 years and older having at least 5 contacts with their practice in 6 years were included. We calculated personal continuity using 3 established measures: the usual provider of care measure, the Bice-Boxerman Index, and the Herfindahl Index. We used the Screening Tool of Older Person’s Prescriptions (STOPP) and the Screening Tool to Alert doctors to Right Treatment (START) specific to the Netherlands version 2 criteria to calculate the prevalence of potentially inappropriate prescriptions. To assess associations, we conducted multilevel negative binomial regression analyses, with and without adjustment for number of chronic conditions, age, and sex.
RESULTS
The patients’ mean (SD) values for the usual provider of care measure, the Bice-Boxerman Continuity of Care Index, and the Herfindahl Index were 0.70 (0.19), 0.55 (0.24), and 0.59 (0.22), respectively. In our population, 72.2% and 74.3% of patients had at least 1 PIM and PPO, respectively; 30.9% and 34.2% had at least 3 PIMs and PPOs, respectively. All 3 measures of personal continuity were positively and significantly associated with fewer potentially inappropriate prescriptions.
CONCLUSIONS
A higher level of personal continuity is associated with more appropriate prescribing. Increasing personal continuity may improve the quality of prescriptions and reduce harmful consequences.
Key words: personal continuity; drug prescriptions; inappropriate prescribing; deprescribing; potentially inappropriate medication list; practice patterns, physicians’; family practice; primary care; geriatrics; health services for the aged; continuity of care; adverse events; polypharmacy; chronic disease
INTRODUCTION
Personal continuity is considered one of the core values of primary care.1-5 This form of continuity, also known as relational continuity, implies familiarity and mutual confidence between patient and physician that can and usually do arise from repeated contacts over time.6 Reported benefits include reduced mortality rates,7,8 fewer hospital admissions,9 reduced health care costs,10 a better patient-physician relationship,11,12 improved preventive care,13 fewer emergency department visits,14 greater patient and physician satisfaction,12,15,16 and better medication use and compliance.13,17-22 Adverse outcomes of personal continuity could include frustrating or difficult patient-physician relationships and delayed diagnosis or referral.23 In recent years, personal continuity has declined in primary care.24 This decline has been due to a variety of changes in society and health care, including family physicians increasingly working part time and in larger practices.1,2,5,25
Managing prescriptions is an important aspect of providing primary care for older adults, because inappropriate prescribing potentially leads to avoidable adverse drug events such as hospitalizations, falls, and acute kidney injury.26,27 The primary care population is aging,28 and older patients have more chronic conditions and use more medication compared with younger patients. Additionally, older adults have a higher risk of harm due to drug-drug and drug-disease interactions,29 and age-related pharmacokinetic and pharmacodynamic changes.30 Dutch family physicians are therefore expected to regularly evaluate medication with their older patients.31 We hypothesized that when personal continuity is higher, prescription management is better.
Previous studies have shown that personal continuity in primary care is associated with improved statin use,32 heart failure medication adherence,33 and diabetes monitoring.34 Among patients with a dementia diagnosis, a recent study shows that personal continuity is associated with improved prescribing in primary care.35 In addition, higher pharmacy continuity is associated with less inappropriate medication use and improved adherence.36 On the other hand, to avoid frustrating or difficult patient-physician interactions, family physicians may prescribe inappropriate medication to meet a patient’s demands.
The actual association between personal continuity and potentially inappropriate prescriptions (PIPs) by family physicians is largely unknown. We therefore aimed to study the association between personal continuity and prescriptions with potentially harmful consequences, as defined by the Screening Tool of Older Person’s Prescriptions (STOPP) criteria and the Screening Tool to Alert doctors to Right Treatment (START) criteria, in primary care.37
METHODS
Setting, Study Population, and Data Collection
We conducted an observational cohort study using anonymized routine care data, extracted from the database of the Academic Network of Primary Care (ANHA) at Amsterdam University Medical Centers during a 6-year period (2013-2018). These 48 family practices were located in the urban area in and around Amsterdam and Haarlem, The Netherlands. Patients were included if they were enlisted at least 1 year with the same family practice; had at least 5 contacts (ie, telephone calls, home visits, e-mails, and/or face-to-face consultations) with their practice over 6 years, of which at least 2 were with a family physician; and were aged 65 years or older in 2013 (Figure 1).
Figure 1.
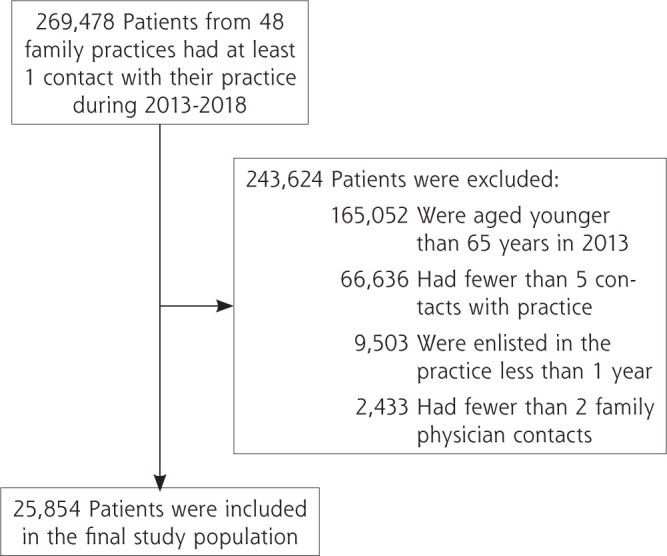
Selection of the study population.
Potential covariates included sex, age, number of chronic conditions, and measures of personal continuity (described below). Medications were included as 5th-level Anatomic Therapeutic Chemical codes based on the STOPP and START criteria.38 Conditions were ascertained from International Classification of Primary Care codes.39 We defined chronic conditions according to definitions of the Netherlands Institute for Health Services Research (Supplemental Table 1 and Supplemental Table 2).
Personal Continuity Measures
No consensus exists on the preferred way to measure our main determinant, personal continuity.40 To optimize the validity and robustness of our results,41 we therefore decided to use 3 established measures: the usual provider of care (UPC) measure and the Herfindahl Index (HI), both of which capture the density of contacts with family physicians, and the Bice-Boxerman Index (BBI), also known as the Continuity of Care Index, which captures the dispersion of contacts among family physicians (Table 1).7,40,42-47 For each patient, we used all contacts that were registered in the electronic health record by any family physician during our study period. Values range from 0 to 1 for all 3 personal continuity measures, with higher values denoting greater personal continuity.
Table 1.
Continuity Measures, Examples, and Calculation
| Measure and Examplesa | Calculation |
|---|---|
| Usual provider of care (this measure is based on the fraction of contacts with a particular family physician) Example A: 6/10 = 0.60 Example B: 6/10 = 0.60 |
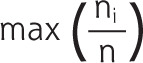
|
| Herfindahl Index (this measure is based on the fraction of contacts with all family physicians) Example A: (62/102) + (42/102) = 0.52 Example B: (62/102) + (32/102) + (12/102) = 0.46 |
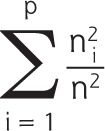
|
| Bice-Boxerman Index (also known as Continuity of Care Index; this measure is based on the fraction of contacts with all family physicians who had at least several contacts) Example A: [(6 × 5)/(10 × 9)] + [(4 × 3)/(10 × 9)] = 0.47 Example B: [(6 × 5)/(10 × 9)] + [(3 × 2)/(10 × 9)] + [(1 × 0)/(10 × 9)] = 0.40 |

|
p = total number of different family physicians; n = total number of contacts with any family physician; ni = number of contacts with family physician i.
Example A: the patient had 10 contacts, of which 6 were with family physician A and 4 were with family physician B. Example B: the patient had 10 contacts, of which 6 were with family physician A, 3 were with family physician B, and 1 was with family physician C.
Definitions of Inappropriate Prescribing
Potentially inappropriate prescriptions can be categorized as potentially inappropriate medications (PIMs) and potential prescribing omissions (PPOs).48 To determine our main outcomes of PIMs and PPOs, we used the STOPP and START specific to the Netherlands version 2 criteria, respectively.49 These validated instruments for identifying inappropriate prescriptions contain 108 criteria and have been applied in various international health care settings.37 The Netherlands-specific versions of the criteria, created in 201250 and revised in 2015 and 2020,49 are currently included in multidisciplinary guidelines on polypharmacy among older adults.31 Of the 108 items defined by Huibers et al38 and Damoiseaux-Volman et al,51 we were able to program applicable scripts for 100 items (68 PIMs and 32 PPOs) for our study (Supplemental Table 3 and Supplemental Table 4).
We did not find any literature on weighting of a previous prescription that was repeated and therefore based our decision on consensus expert opinion. Using the expertise of several family physicians, a clinical statistician, a clinical geriatrician, and a pharmacist, we agreed that without additional knowledge of the family physicians’ motivation, repeated prescriptions should not be weighted equally as the prescribing of multiple different PIMs or PPOs. We therefore determined the number of PIMs and PPOs by adding the unique number of each per patient during our study period.
Statistical Analysis
We used R version 4.0.3 in R-studio (R Foundation for Statistical Computing) with the packages readr, dplyr, lubridate, stringr, tidyr, and haven to program the STOPP and START criteria scripts and count the number of PIPs per patient. We used SPSS version 28 (IBM Corp) to create descriptive plots and to conduct subsequent analyses. Descriptive statistics were used to summarize percentages or ranges for categorical variables and means with SDs for noncategorical variables. As the linearity assumption was not met for the continuity measures and for age, we categorized UPC, BBI, and HI values into tertiles (low, intermediate, and high personal continuity), and age into decades.
Multilevel negative binomial regression analyses were conducted to analyze the main associations between personal continuity and PIPs. We accounted for correlation among patients of the same practice by including practice as level. Because the associations were modified by number of chronic conditions for PIMs, we stratified the patient population into tertiles (0-2, 3-4, or 5-18 chronic conditions). Additional covariates consisted of age, sex, and number of chronic conditions. We present the crude and adjusted stratified results as rate ratios, which represent the relative differences in incidence of PIMs or PPOs between 2 groups. We compared the high- and intermediate-continuity groups with the low-continuity group.
Ethical Approval
The ANHA database is run according to Dutch privacy legislation and contains pseudonymized primary care data from all patients of the participating family practices, except for those who decline participation. The medical ethics committee of VU University Medical Center (now Amsterdam University Medical Center–location VUmc) confirmed that the Medical Research Involving Human Subjects Act does not apply to observational studies based on anonymized data from the ANHA database, and these studies are therefore exempt from the patient informed consent (protocol no. VUmc2015-260).
RESULTS
A total of 269,478 patients enlisted in 48 practices had at least 1 contact with their practice over our 6-year study period (Figure 1). Our final analytic sample contained 25,854 older adult patients having a total of 1,198,861 contacts, of which 674,627 were with family physicians. The mean number of family physician contacts was 26.1 per patient (range = 2-1,115) (Table 2). The patients had a mean age of 74.6 years in 2013 (range = 65-94).
Table 2.
Baseline Characteristics of the Study Population
| Characteristic | By Number of Chronic Conditions | Total Population (N = 25,854) |
||
|---|---|---|---|---|
| 0 to 2 (n = 7,992) |
3 or 4 (n = 8,349) |
5 to 18 (n = 9,513) |
||
| Male, % | 45.5 | 42.8 | 38.3 | 42.0 |
| Age group,a % | ||||
| 65-69 years | 45.5 | 33.7 | 22.5 | 33.2 |
| 70-74 years | 24.2 | 24.7 | 21.9 | 23.5 |
| 75-79 years | 13.6 | 17.0 | 20.9 | 17.4 |
| 80-84 years | 8.1 | 12.3 | 17.3 | 12.8 |
| ≥85 years | 8.6 | 12.2 | 17.4 | 13.0 |
| Prescriptions over 6 years,b % | ||||
| 0-4 medications | 39.8 | 13.6 | 4.1 | 18.2 |
| 5-9 medications | 37.5 | 35.0 | 17.8 | 29.4 |
| 10-14 medications | 15.8 | 29.1 | 28.3 | 24.7 |
| ≥15 medications | 6.9 | 22.3 | 49.8 | 27.7 |
| Chronic conditions, % | ||||
| Oncologic disease | 16.4 | 33.1 | 47.4 | 33.2 |
| Coronary heart disease | 7.1 | 17.6 | 36.2 | 21.2 |
| Psychiatric disease | 11.4 | 14.4 | 18.1 | 14.8 |
| Diabetes mellitus | 7.6 | 22.1 | 36.6 | 23.0 |
| Usual provider of care, No. (%)c | ||||
| Low tertile | 2,699 (33.8) | 2,871 (34.4) | 3,253 (34.2) | 8,823 (34.1) |
| Intermediate tertile | 2,598 (32.5) | 2,732 (32.7) | 3,289 (34.6) | 8,619 (33.3) |
| High tertile | 2,695 (33.7) | 2,746 (32.9) | 2,971 (31.2) | 8,412 (32.5) |
| Bice-Boxerman Index, No. (%)d | ||||
| Low tertile | 2,766 (34.6) | 2,783 (33.3) | 3,069 (32.3) | 8,618 (33.3) |
| Intermediate tertile | 2,479 (31.0) | 2,763 (33.1) | 3,376 (35.5) | 8,618 (33.3) |
| High tertile | 2,747 (34.4) | 2,803 (33.6) | 3,068 (32.3) | 8,618 (33.3) |
| Herfindahl Index, No. (%)e | ||||
| Low tertile | 2,458 (30.8) | 2,804 (33.6) | 3,356 (35.3) | 8,618 (33.3) |
| Intermediate tertile | 2,694 (33.7) | 2,733 (32.7) | 3,188 (33.5) | 8,615 (33.3) |
| High tertile | 2,840 (35.5) | 2,812 (33.7) | 2,969 (31.2) | 8,621 (33.3) |
| Enlistment,f mean (SD), y | 16.8 (8.7) | 17.0 (8.8) | 16.8 (8.9) | 16.9 (8.8) |
| Contacts in 6 years, mean (SD) | ||||
| Total | 28.6 (23.0) | 43.5 (31.6) | 63.8 (49.7) | 46.4 (40.1) |
| With family physician | 16.8 (14.6) | 24.5 (19.7) | 35.4 (29.7) | 26.1 (24.0) |
| PIMs, mean (SD) | 1.0 (1.3) | 1.8 (1.7) | 2.9 (2.3) | 2.0 (2.0) |
| PPOs, mean (SD) | 1.1 (1.3) | 2.0 (1.8) | 3.2 (2.4) | 2.2 (2.1) |
PIM = potentially inappropriate medication; PPO = potential prescribing omission; START = Screening Tool to Alert doctors to Right Treatment; STOPP = Screening Tool of Older Person’s Prescriptions.
On January 1, 2013.
Based on unique Anatomic Therapeutic Chemical codes from STOPP and START criteria.
Low, 0.12-0.59; intermediate, 0.60-0.79; high, 0.80-1.00.
Low, 0.00-0.41; intermediate, 0.42-0.64; high, 0.65-1.00.
Low, 0.09-0.46; intermediate, 0.47-0.66; high, 0.67-1.00.
On December 31, 2018. Follow-up usually spanned 6 years (2013-2018) unless the patient was partially enlisted elsewhere during this period.
Baseline Characteristics
On average, the older the patients, the more chronic conditions they had (Table 2). Patients were enlisted with their practice for a mean (SD) of 16.9 (8.8) years. During our 6-year study period, patients having 5 to 18 chronic conditions had more contacts with their family physician, 35.4 (29.7), than the patients having 0 to 2 chronic conditions or 3 or 4 chronic conditions, 16.8 (14.6) and 24.5 (19.7), respectively.
The patients’ mean (SD) values for the UPC measure, the BBI, and the HI were 0.70 (0.19), 0.55 (0.24), and 0.59 (0.22), respectively. In the total population, the cutoffs for the low, intermediate, and high tertiles were 0.12, 0.60, and 0.80 for the UPC; 0.00, 0.42, and 0.65 for the BBI; and 0.09, 0.47, and 0.67 for the HI. The mean numbers of PIMs and PPOs were lower in the groups with fewer chronic conditions.
Incidences of PIMs and PPOs
Over 6 years, the total number of unique PIMs and PPOs was 56,605 and 55,578, respectively; the 10 most frequently observed of each type are shown in Table 3 and Table 4, respectively. In our study population, 72.2% and 74.3% of patients had at least 1 PIM and PPO, respectively, and 30.9% and 34.2% had at least 3 PIMs and PPOs, respectively. The most frequently observed PIM was “stop benzodiazepines” (15.7% of all PIMs) and the most frequently observed PPO was “start laxatives in patients receiving opioids regularly” (14.7% of all PPOs).
Table 3.
The 10 Most Frequently Observed PIMs
| PIM Description | Percentage of Total PIMs (N = 56,605)a | |
|---|---|---|
| 1. | Stop benzodiazepines (sedative, may cause reduced sensorium, impair balance) | 15.7 |
| 2. | Stop benzodiazepines taken for ≥4 weeks (no indication for longer treatment; risk of prolonged sedation, confusion, impaired balance, falls, road traffic accidents; all benzodiazepines should be withdrawn gradually if taken for >4 weeks as there is a risk of causing a benzodiazepine withdrawal syndrome if stopped abruptly) | 11.9 |
| 3. | Stop drugs likely to cause constipation (eg, antimuscarinic/anticholinergic drugs, oral iron, opioids, verapamil, aluminum antacids) in patients with chronic constipation where nonconstipating alternatives are available (risk of exacerbation of constipation) | 5.1 |
| 4. | Aspirin, clopidogrel, dipyridamole, vitamin K antagonists, direct thrombin inhibitors, or factor Xa inhibitors with concurrent substantial bleeding risk, that is, uncontrolled severe hypertension, bleeding diathesis, recent nontrivial spontaneous bleeding (high risk of bleeding) | 4.6 |
| 5. | Stop PPI for uncomplicated peptic ulcer disease or erosive peptic esophagitis at full therapeutic dosage for >8 weeks (dose reduction or earlier discontinuation indicated) | 4.5 |
| 6. | Stop colchicine if eGFR <10 mL/min/1.73m2 (risk of colchicine toxicity) | 4.0 |
| 7. | Stop NSAIDs if eGFR <50 mL/min/1.73m2 (risk of deterioration in renal function) | 3.6 |
| 8. | Stop use of oral or transdermal strong opioids (morphine, oxycodone, fentanyl, buprenorphine, diamorphine, methadone, tramadol, pethidine, pentazocine) as first-line therapy for mild pain (WHO analgesic ladder not observed) | 3.5 |
| 9. | Stop neuroleptic drugs (may cause gait dyspraxia, parkinsonism) | 3.2 |
| 10. | Stop hypnotic Z-drugs such as zopiclone, zolpidem, zaleplon (may cause protracted daytime sedation, ataxia) | 3.2 |
eGFR = estimated glomerular filtration rate; NSAID = nonsteroidal anti-inflammatory drug; PIM = potentially inappropriate medication; PPI = proton pump inhibitor; WHO = World Health Organization.
Note: PIMs provide information on correct deprescribing according to the Screening Tool of Older Person’s Prescriptions (STOPP) criteria.
Unique PIMs per patient over 6 years.
Table 4.
The 10 Most Frequently Observed PPOs
| PPO Description | Percentage of Total PPOs (N = 55,578)a | |
|---|---|---|
| 1. | Start laxatives in patients receiving opioids regularly | 14.7 |
| 2. | Start ACE inhibitor with systolic heart failure and/or documented coronary artery disease | 7.9 |
| 3. | Start statin therapy with a documented history of coronary, cerebral, or peripheral vascular disease, unless patient has end-of-life status or is >85 years old | 7.4 |
| 4. | Start ACE inhibitor or ARB (if intolerant of ACE inhibitor) in diabetes with evidence of renal disease, that is, dipstick proteinuria or microalbuminuria (>30 mg/24 hours) with or without serum biochemical renal impairment | 7.1 |
| 5. | Start metformin twice a day with diabetes mellitus type 2 if eGFR is 30-50 mL/min/1.73m2, not if < 30 mL/min/1.73m2 | 6.5 |
| 6. | Start antiplatelet therapy (aspirin, clopidogrel, prasugrel, or ticagrelor) with a documented history of coronary, cerebral, or peripheral vascular disease | 5.9 |
| 7. | Start aspirin (75-160 mg once daily) in the presence of chronic atrial fibrillation, where vitamin K antagonists or direct thrombin inhibitors or factor Xa inhibitors are contraindicated | 5.6 |
| 8. | Start β-blocker with ischemic heart disease | 5.1 |
| 9. | Start vitamin D supplement in older adults who are housebound, are experiencing falls, or have osteopenia (bone mineral density T-score is greater than −2.5 but less than −1.0 in multiple sites) | 4.9 |
| 10. | Start regular inhaled β2 agonist or antimuscarinic bronchodilator (eg, ipratropium, tiotropium) for mild to moderate asthma or COPD | 4.8 |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; COPD = chronic obstructive pulmonary disease; eGFR = estimated glomerular filtration rate; PPO = potential prescribing omission.
Note: PPOs provide information on correct prescribing according to the Screening Tool to Alert doctors to Right Treatment (START) criteria.
Unique PPOs per patient over 6 years.
Associations of Continuity With PIMs and PPOs
Higher UPC, BBI, and HI values, reflecting greater personal continuity, were associated with a lower rate of PPOs after adjusting for age, sex, and number of chronic conditions (Table 5; crude analysis is shown in Supplemental Table 5). For PPOs, the rate ratio of high vs low UPC was 0.91; in other words, as this ratio is less than 1, the incidence of PPO is lower in the high UPC group than in the low UPC group. The respective rate ratios were 0.93 for the BBI and 0.88 for the HI. Rate ratios comparing the intermediate vs low category were 0.96 for the UPC, 1.00 for the BBI, and 0.95 for the HI. The PPO incidence was highest in the low continuity groups, followed by the intermediate groups, and then by the high continuity groups.
Table 5.
Adjusted Associations Between Personal Continuity Measures and Incident PPO
| Continuity Measure | RR (95% CI) for PPO | P Value |
|---|---|---|
| Usual provider of care | <.001 | |
| Low tertile | ref | |
| Intermediate tertile | 0.96 (0.94-0.99) | |
| High tertile | 0.91 (0.89-0.94) | |
| Bice-Boxerman Index | <.001 | |
| Low tertile | ref | |
| Intermediate tertile | 1.00 (0.97-1.02) | |
| High tertile | 0.93 (0.90-0.96) | |
| Herfindahl Index | <.001 | |
| Low tertile | ref | |
| Intermediate tertile | 0.95 (0.92-0.98) | |
| High tertile | 0.88 (0.86-0.91) |
PPO = potential prescribing omission; ref = reference category; RR = rate ratio.
Notes: Results of multilevel negative binomial regression analysis with adjustment for sex, age, and number of chronic conditions. The RRs are exponential regression coefficients. Total population was 25,854.
For the outcome of PIMs, we found a similar association, although mainly observed in the group of patients with 5 to 18 chronic conditions (Table 6; crude analysis is shown in Supplemental Table 6). In this group, for PIM, the rate ratio of high vs low UPC was 0.90; in other words, the incidence of PIM was lower in the high UPC group compared with the low UPC group. The respective rate ratios were 0.93 for the BBI and 0.87 for the HI. Corresponding values comparing the intermediate vs low category were 0.94 for the UPC, 0.99 for the BBI, and 0.92 for the HI. In the group with 3 or 4 chronic conditions, we found a similar trend, although it was not significant. In the group with 0 to 2 chronic conditions, the rate ratio of high vs low BBI was 1.13; the ratio was not significant for the UPC and HI measures.
Table 6.
Adjusted Associations Between Personal Continuity Measures and Incident PIM
| Continuity Measure | 0-2 Chronic Conditions | 3-4 Chronic Conditions | 5-18 Chronic Conditions | Total Population | ||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) for PIM |
P Value | RR (95% CI) for PIM |
P Value | RR (95% CI) for PIM |
P Value | RR (95% CI) for PIM |
P Value | |
| Usual provider of care | .002 | .44 | <.001 | <.001 | ||||
| Low tertile | ref | ref | ref | ref | ||||
| Intermediate tertile | 1.12 (1.05-1.18) | 0.97 (0.92-1.03) | 0.94 (0.91-0.98) | 0.97 (0.94-1.00) | ||||
| High tertile | 1.04 (0.98-1.12) | 0.96 (0.92-1.02) | 0.90 (0.87-0.94) | 0.92 (0.89-0.95) | ||||
| Bice-Boxerman Index | <.001 | .21 | .002 | <.001 | ||||
| Low tertile | ref | ref | ref | ref | ||||
| Intermediate tertile | 1.23 (1.16-1.31) | 1.05 (0.99-1.10) | 0.99 (0.95-1.03) | 1.07 (1.04-1.10) | ||||
| High tertile | 1.13 (1.06-1.20) | 1.01 (0.96-1.07) | 0.93 (0.90-0.97) | 0.99 (0.95-1.02) | ||||
| Herfindahl Index | .04 | .01 | <.001 | <.001 | ||||
| Low tertile | ref | ref | ref | ref | ||||
| Intermediate tertile | 1.01 (0.95-1.08) | 0.96 (0.91-1.01) | 0.92 (0.89-0.96) | 0.92 (0.89-0.94) | ||||
| High tertile | 0.94 (0.88-1.00) | 0.92 (0.87-0.97) | 0.87 (0.83-0.90) | 0.85 (0.82-0.88) | ||||
PIM = potentially inappropriate medication; ref = reference category; RR = rate ratio.
Notes: Results of multilevel negative binomial regression analysis adjusted for sex and age, and stratified by number of chronic conditions. The RRs are exponential regression coefficients. Total population was 25,854; there were 7,992 patients with 0-2 chronic conditions, 8,349 patients with 3-4 chronic conditions, and 9,513 patients with 5-18 chronic conditions.
DISCUSSION
Summary
We found that higher personal continuity (defined by UPC, BBI, or HI) was associated with a lower rate of PPOs among older adults in primary care (P <.001). For PIMs, this association was observed only among the group having 5 to 18 chronic conditions. In our primary care population, the prevalence of PIMs and PPOs was high: three-fourths of older patients had at least 1 PIM or PPO, and one-third had at least 3 PIMs or PPOs.
The differing results for PPOs and PIMs might be explained by the differences in definitions. PPOs provide information on correct prescribing (START criteria), whereas PIMs provide information on correct deprescribing (STOPP criteria). Previous literature shows that family physicians experience organizational, interpersonal, and individual socioecologic barriers in deprescribing.52 Perhaps these barriers overwhelm the potential benefits of personal continuity.
Comparison With Existing Literature
To our knowledge, ours is the first study published on the association of personal continuity between patient and family physician and the occurrence of PIPs in primary care. Recently, Delgado et al35 found that higher personal continuity is associated with fewer potentially inappropriate prescriptions and fewer adverse events among patients with dementia. Our results are similar, although our study included older primary care patients generally, regardless of diagnoses.
In our study, personal continuity levels were intermediate, on average, and similar to those in other international studies.47,53 Initially, we also included the Modified Continuity Index to assess personal continuity.40 This measure does not take into account the number of encounters with a single clinician, however, which is essential to calculating personal continuity. We therefore decided not to include this measure in the results.
The prevalence of PIMs and PPOs was 72.2% and 74.3%, respectively, among all patients. The PIM prevalence was higher and the PPO prevalence was lower than those in another study in Dutch family practices (PIM 34.7% and PPO 84.8%).54 The STOPP and START versions used in that study did not include our first and second most frequent PIMs, pertaining to benzodiazepine use, which could explain the differing prevalences. Other studies have also differed from ours regarding the most frequently observed PIMs and PPOs. Those studies by Nauta et al55 and O’Riordan et al,56 however, used different versions of the STOPP and START criteria and included fewer criteria (39 PIMs and 18 PPOs in the former study, and 48 PIMs and 22 PPOs in the latter study, as compared with 68 PIMs and 32 PPOs in our study).
A Dutch longitudinal hospital study51 used criteria definitions similar to ours. That study found that the PIM and PPO prevalences were lower, possibly because their follow-up was shorter than ours. Nonetheless, the most frequently observed PIMs and PPOs were similar to those in our study.51
Strengths and Limitations
This study was based on longitudinal real-life health care data from 48 family practices covering all family physician contacts over 6 years, which is a major strength. The included practices varied in list sizes, patient populations, and practice organization, and had similar continuity levels compared with practices in other studies.47,53 We therefore consider our results generalizable for Dutch family practices, in particular in urban areas. Another strength is that we used 3 established personal continuity measures.
We were able to program scripts for 100 of 108 STOPP and START criteria. These criteria are used as established, well-known tools to identify inappropriate prescriptions among older patients.37 The criteria are limited, however, by indicating potentially inappropriate prescribing and lack information on possible careful considerations by the prescriber.50 Despite inclusion of these criteria in Dutch guidelines in 2012,50 the prevalence of PIMs and PPOs in our study population was still high; however, this could be the result of our exclusion of patients having fewer than 5 contacts with their practice or fewer than 2 family physician contacts (Figure 1), which was required to accurately calculate personal continuity. Similarly, if our follow-up period had been shorter, we would not have been able to accurately calculate personal continuity for most patients with fewer contacts. In addition, we determined the number of unique PIPs per patient during 6 years to avoid overestimating the effect. From a clinical perspective, however, any PIP should be prevented to provide optimal care.
Finally, we focused on personal continuity between family physician and patient, whereas other health care professionals in primary care (eg, family physician trainees, practice nurses) may also provide personal continuity and prescribe medication. This is especially true among patients with certain chronic conditions, including diabetes and other cardiovascular risk factors, because care for these conditions is partially provided by practice nurses; contacts with clinicians other than family physicians were not included in this study.57
Implications for Research and Practice
This study adds to the currently known benefits of personal continuity in primary care. We recommend that family physicians improve personal continuity because it is associated with PIP. Team-based care with multiple clinicians may be detrimental. In particular, older patients with many chronic conditions could benefit from personal continuity to reduce inappropriate prescribing and possibly prevent adverse events. This proposal is in line with findings of other studies in primary care. Family physicians should therefore encourage older patients to schedule appointments with the same family physician and discuss prescribing and deprescribing to reduce potential barriers. The possible causal association could be determined by a targeted randomized intervention, that is, by specifically allocating 1 prescriber per patient.
In addition, the prevalence of PIPs in primary care is high. Perhaps, the practical use of the STOPP and START criteria to identify PIPs could be improved by developing and implementing a user-friendly, time-efficient (digital) tool to support family physicians and their patients in prescription management, including deprescribing.
Finally, by initially using 4 established measures to calculate continuity, we found inconsistent results depending on the measure. We are therefore working on a clinimetric study to provide recommendations for applying the right continuity measure in the right situation. In addition, future studies should use qualitative research (eg, semistructured interviews) to include patients’ perspectives on personal continuity.
Supplementary Material
Acknowledgments
The authors are grateful to all participating family physicians and the data managers of the Academic Network of Primary Care (ANHA) for their time and effort in contributing the routine care data.
Footnotes
Conflicts of interest: authors report none.
Funding support: Marije T. te Winkel and Otto R. Maarsingh were supported by the Stichting Beroepsopleiding Huisartsen in the Netherlands.
Disclaimer: The Stichting Beroepsopleiding Huisartsen was not involved in the design of the study; collection, analysis, and interpretation of the data; or writing of the manuscript.
Previous presentation: Portions of the findings reported have been presented at the 50th NAPCRG Annual Meeting; November 18-22, 2022; Phoenix, Arizona.
References
- 1.Guthrie B, Saultz JW, Freeman GK, Haggerty JL.. Continuity of care matters. BMJ. 2008; 337: a867. 10.1136/bmj.a867 [DOI] [PubMed] [Google Scholar]
- 2.Stokes T, Tarrant C, Mainous AG III, Schers H, Freeman G, Baker R.. Continuity of care: is the personal doctor still important? A survey of general practitioners and family physicians in England and Wales, the United States, and The Netherlands. Ann Fam Med. 2005; 3(4): 353-359. 10.1370/afm.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman GK, Olesen F, Hjortdahl P.. Continuity of care: an essential element of modern general practice? Fam Pract. 2003; 20(6): 623-627. 10.1093/fampra/cmg601 [DOI] [PubMed] [Google Scholar]
- 4.Van der Horst HE, Dijkstra R.. Woudschoten 2019: Huisarts-geneeskundige kernwaarden en kerntaken herijkt [General practitioner-medical core values and core tasks reassessed]. Huisarts Wet. 2019; 62. 10.1007/s12445-019-0260-2 [DOI] [Google Scholar]
- 5.Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R.. Continuity of care: a multidisciplinary review. BMJ. 2003; 327(7425): 1219-1221. 10.1136/bmj.327.7425.1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starfield B, Horder J.. Interpersonal continuity: old and new perspectives. Br J Gen Pract. 2007; 57(540): 527-529. [PMC free article] [PubMed] [Google Scholar]
- 7.Leleu H, Minvielle E.. Relationship between longitudinal continuity of primary care and likelihood of death: analysis of national insurance data. PLoS One. 2013; 8(8): e71669. 10.1371/journal.pone.0071669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maarsingh OR, Henry Y, van de Ven PM, Deeg DJ.. Continuity of care in primary care and association with survival in older people: a 17-year prospective cohort study. Br J Gen Pract. 2016; 66(649): e531-e539. 10.3399/bjgp16X686101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker I, Steventon A, Deeny SR.. Association between continuity of care in general practice and hospital admissions for ambulatory care sensitive conditions: cross sectional study of routinely collected, person level data. BMJ. 2017; 356: j84. 10.1136/bmj.j84 [DOI] [PubMed] [Google Scholar]
- 10.Shin DW, Cho J, Yang HK, et al. Impact of continuity of care on mortality and health care costs: a nationwide cohort study in Korea. Ann Fam Med. 2014; 12(6): 534-541. 10.1370/afm.1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frederiksen HB, Kragstrup J, Dehlholm-Lambertsen B.. Attachment in the doctor-patient relationship in general practice: a qualitative study. Scand J Prim Health Care. 2010; 28(3): 185-190. 10.3109/02813432.2010.505447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridd M, Shaw A, Salisbury C.. ‘Two sides of the coin’—the value of personal continuity to GPs: a qualitative interview study. Fam Pract. 2006; 23(4): 461-468. 10.1093/fampra/cml010 [DOI] [PubMed] [Google Scholar]
- 13.Saultz JW, Lochner J.. Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med. 2005; 3(2): 159-166. 10.1370/afm.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohnke H, Zielinski A.. Association between continuity of care in Swedish primary care and emergency services utilisation: a population-based cross-sectional study. Scand J Prim Health Care. 2017; 35(2): 113-119. 10.1080/02813432.2017.1333303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridd MJ, Ferreira DL, Montgomery AA, Salisbury C, Hamilton W.. Patient-doctor continuity and diagnosis of cancer: electronic medical records study in general practice. Br J Gen Pract. 2015; 65(634): e305-e311. 10.3399/bjgp15X684829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raivio R, Jääskeläinen J, Holmberg-Marttila D, Mattila KJ.. Decreasing trends in patient satisfaction, accessibility and continuity of care in Finnish primary health care - a 14-year follow-up questionnaire study. BMC Fam Pract. 2014; 15: 98. 10.1186/1471-2296-15-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maciejewski ML, Hammill BG, Bayliss EA, et al. Prescriber continuity and disease control of older adults. Med Care. 2017; 55(4): 405-410. 10.1097/MLR.0000000000000658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maciejewski ML, Hammill BG, Voils CI, et al. Prescriber continuity and medication availability in older adults with cardiometabolic conditions. SAGE Open Med. 2018; 6: 2050312118757388. 10.1177/2050312118757388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matulis JC III, Schilling JJ, North F.. Primary care provider continuity is associated with improved preventive service ordering during brief visits for acute symptoms. Health Serv Res Manag Epidemiol. 2019; 6: 2333392819826262. 10.1177/2333392819826262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallvik SE, Geissert P, Wakeland W, et al. Opioid-prescribing continuity and risky opioid prescriptions. Ann Fam Med. 2018; 16(5): 440-442. 10.1370/afm.2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerse N, Buetow S, Mainous AG III, Young G, Coster G, Arroll B.. Physician-patient relationship and medication compliance: a primary care investigation. Ann Fam Med. 2004; 2(5): 455-461. 10.1370/afm.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan KS, Wan EY, Chin WY, et al. Effects of continuity of care on health outcomes among patients with diabetes mellitus and/or hypertension: a systematic review. BMC Fam Pract. 2021; 22(1): 145. 10.1186/s12875-021-01493-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira Gray D, Sidaway-Lee K, White E, Thorne A, Evans P.. Improving continuity: THE clinical challenge. InnovAiT. 2016; 9(10): 635-645. 10.1177/1755738016654504 [DOI] [Google Scholar]
- 24.Tammes P, Morris RW, Murphy M, Salisbury C.. Is continuity of primary care declining in England? Practice-level longitudinal study from 2012 to 2017. Br J Gen Pract. 2021; 71(707): e432-e440. 10.3399/BJGP.2020.0935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Versteeg S, Vis E, van der Velden L, Batenburg R.. De werkweek van de Nederlandse huisarts in 2018: en een vergelijking met 2013 [The working week of the Dutch GP in 2018: and a comparison with 2013]. Nivel. Published Nov 2018. Accessed 2022. https://www.nivel.nl/sites/default/files/bestanden/Werkweek_van_de_nederlandse_huisarts_2018.pdf
- 26.Hamilton H, Gallagher P, Ryan C, Byrne S, O’Mahony D.. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011; 171(11): 1013-1019. 10.1001/archinternmed.2011.215 [DOI] [PubMed] [Google Scholar]
- 27.Moriarty F, Bennett K, Cahir C, Kenny RA, Fahey T.. Potentially inappropriate prescribing according to STOPP and START and adverse outcomes in community-dwelling older people: a prospective cohort study. Br J Clin Pharmacol. 2016; 82(3): 849-857. 10.1111/bcp.12995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Wit NJ, Schuurmans MJ.. Future care for older people in general practice: paradigm shifts are needed. Br J Gen Pract. 2017; 67(664): 500-501. 10.3399/bjgp17X693221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Marum RJ. Medicatie stoppen bij ouderen: een checklist kan handig zijn [Discontinuing medication in elderly: a checklist may be useful]. Ned Tijdschr Geneeskd. 2011; 155(36): A3802. [PubMed] [Google Scholar]
- 30.Mangoni AA, Jackson SH.. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004; 57(1): 6-14. 10.1046/j.1365-2125.2003.02007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NHG richtlijn [NHG Guidelines] . Polyfarmacie bij ouderen [Polypharmacy in the elderly]. Updated Dec 2020. Accessed 2022. https://richtlijnen.nhg.org/multidisciplinaire-richtlijnen/polyfarmacie-bij-ouderen
- 32.Youens D, Doust J, Robinson S, Moorin R.. Regularity and continuity of GP contacts and use of statins amongst people at risk of cardiovascular events. J Gen Intern Med. 2021; 36(6): 1656-1665. 10.1007/s11606-021-06638-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uijen AA, Bosch M, van den Bosch WJ, Bor H, Wensing M, Schers HJ.. Heart failure patients’ experiences with continuity of care and its relation to medication adherence: a cross-sectional study. BMC Fam Pract. 2012; 13: 86. 10.1186/1471-2296-13-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youens D, Robinson S, Doust J, Harris MN, Moorin R.. Associations between regular GP contact, diabetes monitoring and glucose control: an observational study using general practice data. BMJ Open. 2021; 11(11): e051796. 10.1136/bmjopen-2021-051796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delgado J, Evans PH, Gray DP, et al. Continuity of GP care for patients with dementia: impact on prescribing and the health of patients. Br J Gen Pract. 2022; 72(715): e91-e98. 10.3399/BJGP.2021.0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi E, Lee IH.. Relational continuity of care in community pharmacy: a systematic review. Health Soc Care Community. 2022; 30(1): e39-e50. 10.1111/hsc.13428 [DOI] [PubMed] [Google Scholar]
- 37.Cooper JA, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open. 2015; 5(12): e009235. 10.1136/bmjopen-2015-009235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huibers CJA, Sallevelt BTGM, de Groot DA, et al. Conversion of STOPP/START version 2 into coded algorithms for software implementation: A multidisciplinary consensus procedure. Int J Med Inform. 2019; 125: 110-117. 10.1016/j.ijmedinf.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 39.Lamberts H, Wood M.. International Classification of Primary Care. Oxford University Press; 1987. [Google Scholar]
- 40.Jee SH, Cabana MD.. Indices for continuity of care: a systematic review of the literature. Med Care Res Rev. 2006; 63(2): 158-188. 10.1177/1077558705285294 [DOI] [PubMed] [Google Scholar]
- 41.Schloss PD. Identifying and overcoming threats to reproducibility, replicability, robustness, and generalizability in microbiome research. mBio. 2018; 9(3): e00525-18. 10.1128/mBio.00525-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hussain T, Chang HY, Luu NP, Pollack CE.. The value of continuity between primary care and surgical care in colon cancer. PLoS One. 2016; 11(5): e0155789. 10.1371/journal.pone.0155789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hussey PS, Schneider EC, Rudin RS, Fox DS, Lai J, Pollack CE.. Continuity and the costs of care for chronic disease. JAMA Intern Med. 2014; 174(5): 742-748. 10.1001/jamainternmed.2014.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryvicker M, Russell D.. Individual and environmental determinants of provider continuity among urban older adults with heart failure: a retrospective cohort study. Gerontol Geriatr Med. 2018; 4: 2333721418801027. 10.1177/2333721418801027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tammes P, Purdy S, Salisbury C, MacKichan F, Lasserson D, Morris RW.. Continuity of primary care and emergency hospital admissions among older patients in England. Ann Fam Med. 2017; 15(6): 515-522. 10.1370/afm.2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker J, Payne B, Clemans-Taylor BL, Snyder ED.. Continuity of care in resident outpatient clinics: a scoping review of the literature. J Grad Med Educ. 2018; 10(1): 16-25. 10.4300/JGME-D-17-00256.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bazemore A, Petterson S, Peterson LE, Bruno R, Chung Y, Phillips RL Jr.. Higher primary care physician continuity is associated with lower costs and hospitalizations. Ann Fam Med. 2018; 16(6): 492-497. 10.1370/afm.2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P.. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015; 44(2): 213-218. 10.1093/ageing/afu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knol W, Verduijn MM, Lelie-van der Zande AC, et al. [Detecting inappropriate medication in older people: the revised STOPP/START criteria]. Ned Tijdschr Geneeskd. 2015; 159: A8904. [PubMed] [Google Scholar]
- 50.Vermeulen Windsant-van den Tweel AM, Verduijn MM, Derijks HJ, van Marum RJ.. [Detection of inappropriate medication use in the elderly; will the STOPP and START criteria become the new Dutch standards?]. Ned Tijdschr Geneeskd. 2012; 156(40): A5076. [PubMed] [Google Scholar]
- 51.Damoiseaux-Volman BA, Medlock S, Raven K, et al. Potentially inappropriate prescribing in older hospitalized Dutch patients according to the STOPP/START criteria v2: a longitudinal study. Eur J Clin Pharmacol. 2021; 77(5): 777-785. 10.1007/s00228-020-03052-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doherty AJ, Boland P, Reed J, et al. Barriers and facilitators to deprescribing in primary care: a systematic review. BJGP Open. 2020; 4(3): bjgpopen20X101096. 10.3399/bjgpopen20X101096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coma E, Mora N, Peremiquel-Trillas P, et al. Influence of organization and demographic characteristics of primary care practices on continuity of care: analysis of a retrospective cohort from 287 primary care practices covering about 6 million people in Catalonia. BMC Fam Pract. 2021; 22(1): 56. 10.1186/s12875-021-01414-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruin-Huisman L, Abu-Hanna A, van Weert HCPM, Beers E.. Potentially inappropriate prescribing to older patients in primary care in the Netherlands: a retrospective longitudinal study. Age Ageing. 2017; 46(4): 614-619. 10.1093/ageing/afw243 [DOI] [PubMed] [Google Scholar]
- 55.Nauta KJ, Groenhof F, Schuling J, et al. Application of the STOPP/START criteria to a medical record database. Pharmacoepidemiol Drug Saf. 2017; 26(10): 1242-1247. 10.1002/pds.4283 [DOI] [PubMed] [Google Scholar]
- 56.O Riordan D, Aubert CE, Walsh KA, et al. Prevalence of potentially inappropriate prescribing in a subpopulation of older European clinical trial participants: a cross-sectional study. BMJ Open. 2018; 8(3): e019003. 10.1136/bmjopen-2017-019003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faber MJ, Burgers JS, Westert GP.. A sustainable primary care system: lessons from the Netherlands. J Ambul Care Manage. 2012; 35(3): 174-181. 10.1097/JAC.0b013e31823e83a4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


