Abstract
Metastatic colorectal cancer (mCRC) remains a lethal disease with ~14% of 5-year survival rate. While early-stage CRC can be cured by surgery with or without adjuvant chemotherapy, mCRC cannot be eradicated due to a large burden of disseminated cancer cells consisting of therapy-resistant metastasis-competent cells. To address this gap, recent studies have focused on further elucidating the molecular mechanisms underlying colorectal metastasis and recognizing the limitations of available therapeutic interventions. In this review, we discuss newfound factors that regulate CRC cell dissemination and colonization of distant organs, such as genetic mutations, identification of metastasis-initiating cells, epithelial-mesenchymal transition, and the tumor microenvironment. We review current treatments for mCRC, therapeutic regimens undergoing clinical trials, and trending pre-clinical studies being investigated to target treatment-resistant mCRC.
Keywords: Colorectal cancer, Metastasis, Cancer therapeutics, Targeted therapy, Immunotherapy
Significance of targeted and immunotherapies for metastatic colorectal cancer treatment
Colorectal cancer (CRC) is the third most common cause of cancer and the second most common cause of cancer-related death worldwide. Despite increasing survival rates, metastatic CRC (mCRC) remains a lethal disease with a 5-year survival rate of approximately 14% [1]. As the current therapeutic strategies are limited to a proportion of patients with certain types of CRC or can lead to severe side effects, new therapies targeting mCRC are a welcome addition to the current treatment regimen. In recent years, technologies such as whole-genome and single-cell sequencing, clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated gene 9 (Cas9) system, and generation of transgenic mouse models advanced our understanding of the mechanisms underlying CRC metastasis. This led to the development of targeted therapies (see Glossary) and immunotherapies which can circumvent undesirable cytotoxicity from and development of resistance to systemic chemotherapy. Multiple reviews provide an overview of existing targeted agents and immunotherapies for CRC [2,3]. However, guidelines are continuously updating the recommended therapies on the basis of the increasing number of clinical trials. To keep up to date on current studies and identify gaps and opportunities for advancements in the field, an evaluation of these recent developments is necessary. In this review, we discuss the latest discoveries on the mechanisms through which CRC metastasize and highlight treatment modalities that arose from those discoveries, such as therapies that are Food and Drug Administration (FDA)-approved or undergoing clinical trials. Moreover, we shed light on emerging technologies and innovations actively being researched to target mCRC.
Mechanisms of colorectal cancer metastasis
To understand how novel treatment modalities target mCRC, it is important to discern the complex mechanisms through which CRC cells metastasize. Recognizing the factors that regulate metastasis, including the cell-intrinsic factors (genetic abnormalities, heterogeneity of tumor cells, epithelial-mesenchymal transition (EMT) ) as well as the tumor microenvironment (TME), will provide the foundation onto which novel therapeutics can be tested in clinical trials and ultimately improve patient outcomes (Box 1).
Box 1: The invasion-metastasis cascade in colorectal cancer.
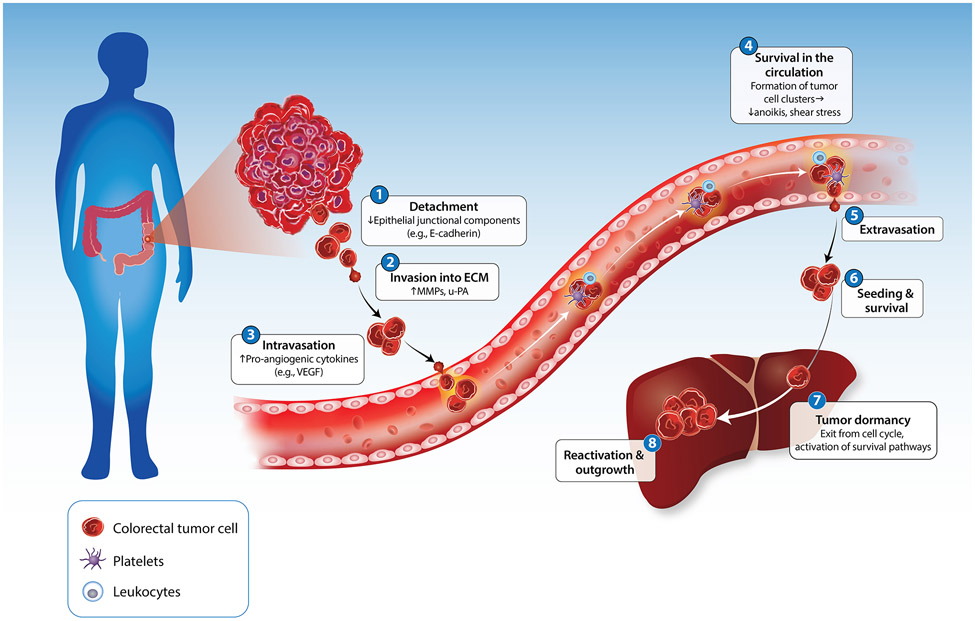
The invasion-metastasis cascade describes the changes in cancer cells and stroma that drive tumor progression to metastasis (Figure I). In the first step of the invasion-metastasis cascade, CRC cells detach from one another and adjacent normal or cancer cells by downregulating E-cadherin and other epithelial junctional components [82]. They then degrade the basement membrane by secreting or locally activating matrix metalloproteinases (MMPs) and urokinase plasminogen activator (u-PA) and invade through the underlying interstitial ECM [83]. Degradation of the pericellular and ECM releases pro-angiogenic factors such as VEGF and other pro-angiogenic cytokines, resulting in the formation of new blood and lymphatic vessels and facilitating the intravasation of tumor cells into the circulation [84]. Therefore, tumor cells can access the blood vasculature directly or by colonizing locoregional lymph nodes, although the proportion of CRC cells following one route or the other and the overall efficiency of either route is not known. Once in the circulation, tumor cells associate with leukocytes and platelets through various adhesion mechanisms. Evidence suggests that the resulting clusters resist shear stress and anoikis (apoptosis consequent to loss of proper adhesion to the ECM) more easily as compared to isolated CRC cells. Furthermore, tumor cell clusters are better suited to arrest in the microcirculation of the target organ, generating intravascular growths that infiltrate the stroma of the target organ [85]. Irrespective of whether tumor cells extravasate in the target organ as single cells or small clusters, disseminated tumor cells (DTCs) face enormous attrition due to maladaptation. Only a minority of cancer cells survive the stress of a foreign microenvironment and immune attack to colonize their secondary site and establish metastatic outgrowths [86]. Upon extravasation into the secondary site, in many cancer types, tumor cells that have disseminated to a target organ site (disseminated tumor cells; DTCs) enter a dormant state, characterized by exit from the cell cycle and activation of survival pathways. Interposed between initial extravasation and colonization and intimately linked to adaptation, solitary tumor cell dormancy has remained poorly understood [87]. Recent studies in mice suggest that DTCs can exit the cell cycle and survive for prolonged periods of time within specialized perivascular niches, which may be established by systemic signals prior to seeding by metastatic cells [88]. The signals that govern the survival of cancer cells during dormancy and, subsequently, reactivation and outgrowth mirror those that govern these processes in adult stem cells [89]. These studies suggest that cancer cells mold a supportive TME, including immune and non-immune elements, which co-evolve during tumor progression and metastasis [90]. The niches that support dormant and reactivated cells are likely to have distinct composition and to be regulated by distinct paracrine signals as compared to cancer stem cell niches and invasive niches at the primary site. Dissecting the metastatic ecosystems in mouse models and patient samples promises to enable the development of novel combination therapies capable of effectively targeting dormant and early reactivated metastatic cells.
Genetic abnormalities
Somatic mutations in the tumor protein p53 (TP53) tumor suppressor gene are the most frequent alterations in human cancers. TP53 mutations are found in 60% of mCRC patients and are associated with worse prognosis [4]. Interestingly, it was recently discovered that different TP53 missense mutations contribute differentially to CRC progression, suggesting that the type of mutation may impact the disease phenotype [5]. For example, TP53R273H loss-of-function (LOF) mutation promotes carcinogenesis via cell migration, invasion, and metastasis, consistent with the role of p53 as a tumor suppressor [5]. By contrast, p53 proteins with gain-of-function (GOF) mutations directly bind to the promoter sequences of known or putative CRC stem cell markers such as CD44, leucine rich repeat containing GPCR5 (LGR5), and aldehyde dehydrogenase (ALDH), enhancing their expression and facilitating tumor progression [6]. Nevertheless, both LOF and GOF mutations of p53 impact the prognosis of patients and have implications on the clinical treatment of mCRC. p53 mutations confer resistance to classical chemotherapy such as 5-fluorouracil (5-FU), oxaliplatin, and irinotecan [7]. With respect to targeted therapies, published clinical data suggest that p53 is not predictive of treatment response to anti-vascular endothelial growth factor (VEGF) drugs [7]. However, due to the potential role of p53 in epidermal growth factor receptor (EGFR) inactivation, EGFR-targeted therapies such as cetuximab and panitumumab show promise in p53-mutated, RAS-wildtype CRCs as demonstrated by the FIRE-3 trial and pre-clinical studies [7]. The effect of p53 mutation on immunotherapy is inconclusive, but evidence suggests that it may be a negative predictor for treatment response due to the association between mutant p53 and impaired antigen presentation and decreased immune cell infiltration [7]. The significance of mutant p53 in mCRC is spearheading the studies using various gene therapy technologies to target mutant p53. For example, Gendicine delivers wildtype p53 gene to the cancer cells to treat head and neck squamous cell carcinoma [8]. The success of Gendicine led to a clinical study conducted on the safety and efficacy of Gendicine combined with standard chemotherapy to treat mCRC with promising results [8]. However, standard treatment regimens utilizing p53 gene therapy still need to be established for advanced use in clinical practice.
In contrast to primary colorectal tumors, the genomic landscape of colorectal metastases has not been fully elucidated. Zehir et al. established a large-scale, prospective clinical sequencing initiative using 10,945 metastatic tumors, including 975 mCRC, to identify mutational signatures of metastases [9]. CRCs had one of the highest mutational burdens of all cancer types measured. Moreover, these cancers displayed a dominant DNA mismatch repair (MMR) signature that was associated with underlying LOF somatic mutations. Notably, APC, TP53, KRAS, and PIK3CA were identified as the top four recurrently mutated genes in mCRC [9]. Subsequent publications by other groups confirmed the top metastatic tumor-specific genes via targeted sequencing and whole genome sequencing [10-12]. The genetic alterations revealed by sequencing of mCRC led to clinical advancements in targeted and immunotherapies. For example, detection of mutated RAS and RAF predict lack of response to EGFR inhibitors, whereas RAS-wildtype tumors may show therapeutic promise with EGFR inhibitors, especially when the tumors harbor p53 mutations [8]. Mutations that lead to human epidermal growth factor receptor 2 (HER2) amplification predicts positive response to HER2-targeted therapies [13,14]. Additionally, sequencing can lead to detection of microsatellite instability (MSI) in which immunotherapy (e.g., immune checkpoint blockade) is the treatment of choice. It is important to note that the interpretation of data collected from most whole genome sequencing analyses of mCRC has significant limitations because the comparisons were made with either matched normal peripheral blood or normal colonic tissues rather than matched primary tumors. Further investigations should use matched primary colorectal tumors as a control group to identify mutational signatures associated with the cells’ ability to acquire metastatic capacity.
Colorectal metastasis-initiating cells
Identification of a subset of tumor cells harboring the capacity to initiate the metastatic process is crucial for the prevention of cancer metastasis and disease recurrence (Box 2). During development, Lgr5-expressing intestinal stem cells maintain the crypts by continuously replenishing the short-lived, specialized colonic epithelial cells [15]. This hierarchical organization is also maintained in primary tumors and metastases – lineage tracing experiments in mice demonstrated that only a small subset of tumor cells (a.k.a. cancer stem cells) can grow and maintain the tumors [16]. Surprisingly, a recent study conducted to investigate the cell of origin of colorectal metastases suggest that the vast majority of disseminating CRC cells do not express Lgr5, an established marker for cancer stem cells [16]. Once the cells formed metastases in the liver, however, some Lgr5- cells underwent plasticity and expressed Lgr5 to maintain the survival and growth of the metastases [16]. Similarly, observations in clinical samples identified L1 cell adhesion molecule (L1CAM)+ cells as colorectal tumor cells with metastasis-initiating capacity [17]. Recently, Canellas-Socias et al. identified a unique epithelial tumor cell population that expresses genes associated with poor prognosis in CRC patients [18]. They called the cells high-relapse cells (HRCs), marked by the expression of epithelial membrane protein 1 (EMP1). EMP1+ HRCs have the capacity to colonize the liver after surgical resection and are responsible for metastatic recurrence after surgical removal of the liver metastases. The authors showed that targeting EMP1+ cells effectively eliminated residual metastatic cells and prevented relapse after surgery in mice [18], further validating the importance of identifying and characterizing metastasis-initiating cells within the tumor to prevent metastasis.
Box 2: Metastasis-initiating cells in colorectal cancer.
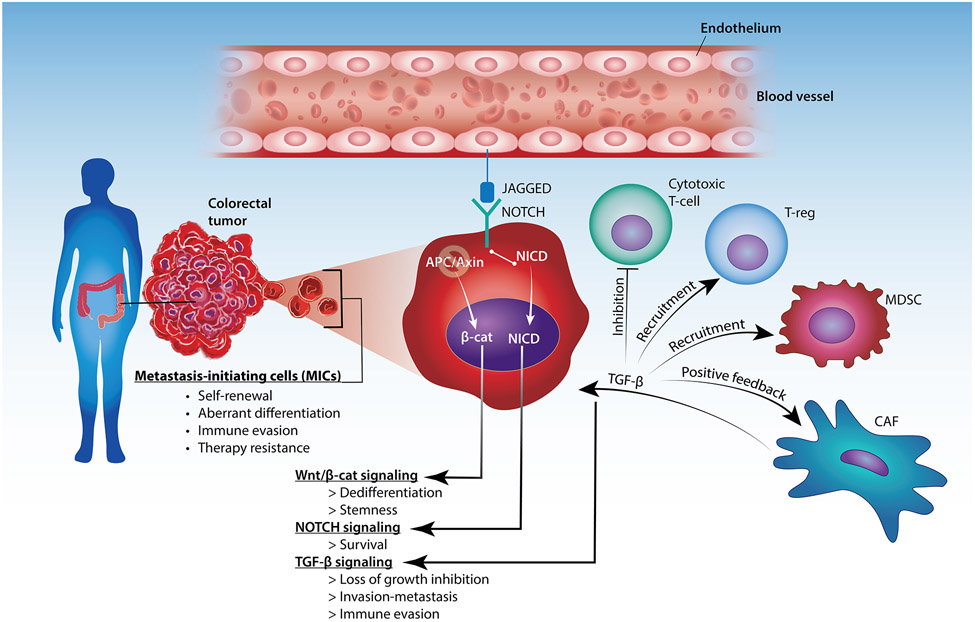
Current models suggest that metastases arise from a subpopulation of cancer stem or progenitor cells that have acquired invasive ability and are thus competent for dissemination and seeding of the target organ (Figure II). In addition to undergoing self-renewal and producing aberrantly differentiated progeny, the metastasis-initiating cells (MICs) coopt a supportive TME at tumor buds, located within the invasive front of primary CRCs as well as metastases at secondary sites [91]. Both invasive and metastatic niches often develop around tumor vessels, possibly because endothelial cell-derived JAGGED binds to NOTCH on CRC MICs and enhances their survival. The acquisition of metastatic competency in CRC may involve oncogene-induced dedifferentiation and acquisition of stemness traits through loss of the APC or AXIN tumor suppressors and, hence, activation of the WNT/β-catenin signaling pathway [92]. Inactivation of the TGF-β-responsive SMAD4 tumor suppressive transcription factor renders MICs resistant to the cytostatic effect of TGF-β, enabling CAFs to secrete and activate excessive amounts of TGF-β. Acting as a major driver of CRC metastasis, TGF-β consolidates the activation of CAFs in a positive feedback loop and contributes to the acquisition of mesenchymal and stemness traits by MICs [91]. Notably, TGF-β also creates an immune suppressive microenvironment by potently inhibiting cytotoxic T cells and enhancing the recruitment of myeloid-derived suppressor cells (MDSCs) and T-regs [93]. Although cancer stem cells and MICs share similar stemness traits, including regenerative capacity, resistance to therapy, and molding of a supportive microenvironment, the origin of MICs remains unclear. It is possible that MICs are the direct descendants of cancer stem cells or progenitors within primary tumors. However, it is also possible that aberrantly differentiated cancer cells acquire invasive capacity and revert to the progenitor state once they infiltrate a target organ. Finally, the molecular mechanisms that enable MICs to acquire the capacity to colonize the liver, the lung, or other organs are not known. Emerging evidence suggests that chromosomal instability and epigenetic reprogramming are both required for the acquisition of metastatic capacity [94]. Intriguingly, both processes may be driven by loss of TP53 in CRC [95,96].
Epithelial mesenchymal transition (EMT)
EMT is a process during which cells lose their epithelial characteristics, and they gain mesenchymal properties to increase their motility and develop an invasive phenotype [19]. Recently, the possibility that EMT may encompass a range of intermediate states has been proposed. This phenotype, referred to as “partial EMT” or “hybrid EMT”, describes cancer cells that exhibit both mesenchymal and epithelial characteristics due to internalization of the epithelial markers rather than transcriptional repression of the proteins [19,20]. More than 90% of human CRC cell lines exhibit a partial EMT, a status that favors formation of cell clusters during CRC dissemination [20]. Thus, EMT is a promising target to prevent primary tumors from acquiring invasive properties or to prevent recurrence after resection of the tumors and metastases. Development of drugs that target the EMT directly is challenging due to the plasticity and heterogeneity of the various pathways involved. However, potential therapeutic strategies may be to 1) combine EMT inhibitors with conventional chemotherapy agents to overcome pharmacological resistance in mCRC, and 2) use EMT inhibitors in the adjuvant setting to reduce recurrence after resection of the tumors [21].
The tumor microenvironment (TME)
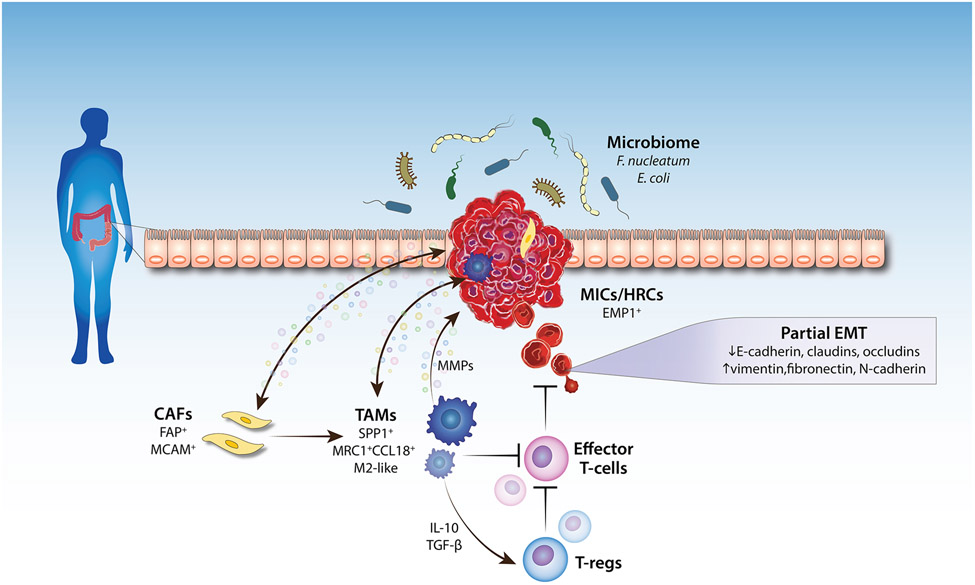
The TME is a heterogeneous collection of cancer cells, cancer-associated fibroblasts (CAFs), immune/inflammatory cells (e.g., tumor-associated macrophages (TAMs), T-cells, natural killer (NK) cells, myeloid-derived suppressor cells (MDSCs)), adipocytes, neurons, endothelial cells, secreted factors, and the extracellular matrix (ECM) that they secrete and mold into the extracellular space (Figure 1). All cells in the TME actively regulate cancer progression, making it an ideal target for a therapeutic approach.
Figure 1. Factors that affect colorectal cancer metastasis.
The tumor microenvironment (TME) consists of tumor cells, resident host cells (the colonic epithelium), immune cells, endothelial cells, neurons, adipocytes, secreted factors, and the extracellular matrix (ECM). The interplay between the cells within the TME and the tumor cells, as well as the interplay between the gut microbiome and the tumor cells, regulate tumor invasion and metastasis (namely, intravasation into blood vessels). Changes in the expression of epithelial-mesenchymal transition (EMT) genes occur within the subset of tumor cells that acquire metastatic properties. Abbreviations: MIC, metastasis-initiating cell; HRC, high-relapse cell; EMP1, epithelial membrane protein 1; MMP, matrix metalloproteinase; CAF, cancer-associated fibroblast; TAM, tumor-associated macrophage; T-reg, regulatory T-cell; TGF-β, transforming growth factor-β. Arrows pointing to the tumor indicate tumor-promoting effect.
CAFs are key components of the TME with diverse functions, including matrix deposition and remodeling, reciprocal signaling interactions with cancer cells, and crosstalk with infiltrating immune cells [22]. CAFs play a pro-tumorigenic role in CRC by secreting factors to sustain cell proliferation, evade cell death, and recruit immune cells, making CAFs a potential target for therapeutic strategies against CRC [23]. Recently, Kobayashi et al. identified pericryptal Lepr-lineage melanoma cell adhesion molecule (MCAM)-expressing fibroblasts as colorectal CAFs associated with poor patient survival [24]. MCAM+ CAFs recruit TAMs to promote colorectal tumor growth and metastasis by generating an inflammatory microenvironment in the tumors’ secondary site and by facilitating migration and invasion of the primary tumors by inducing EMT [24,25]. Furthermore, analyses of clinical CRC tumor samples show a positive correlation between CAFs and poor clinical outcome and recurrence of disease [25,26]. Indeed, a landmark study by Kraman et al. demonstrated that depleting fibroblast activation protein (FAP)+ CAFs improved anti-cancer vaccination efficacy [27]. Moreover, targeting the downstream functions of tumor promoting CAFs by blocking the interaction between the chemokine CXCL12 and its receptor CXCR4 have also led to promising results [27]. Endeavors such as these provide great cancer treatment opportunities and may provide a better clinical benefit to CRC patients.
TAMs regulate almost all steps of tumor metastasis. They maintain an immunosuppressive environment by their expression of inhibitory receptors such as programmed death-ligand 1 (PD-L1) and PD-L2. Additionally, they secrete interleukin (IL)-10 and transforming growth factor (TGF)-β to activate regulatory T-cells (T-regs), resulting in inhibition of antitumor immunity [28]. TAMs also release ECM remodeling factors and proteolytic enzymes to degrade ECM proteins, promoting primary colorectal tumor cell migration [29]. Recently, the use of single-cell analyses combined with spatiotemporal transcriptomics vastly expanded our ability to identify and characterize the subset of TAMs responsible for CRC metastasis. Comparison between primary colorectal tumors and matched liver metastases from patients revealed a subset of TAMs, marked by the expression of secreted phosphoprotein 1 (SPP1), as being associated with malignancy and unfavorable prognosis [30]. In the same year, it was discovered that SPP1+ TAMs interact with FAP+ fibroblasts, and this interaction may prevent lymphocyte infiltration and favor poor patient survival, displaying the complex interplay between stromal and immune cells within the TME [31]. Consistent with these findings, another group identified MRC1+CCL18+ M2-like TAMs with high SPP1 levels in colorectal liver metastases, and these macrophages harbored enhanced metabolic activity and immunosuppressive phenotypes [32]. These findings provide a potential therapeutic strategy by disrupting FAP+ fibroblasts and SPP1+ macrophages interaction to improve CRC therapy.
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells that are characterized by the ability to suppress immune responses; thus, they are implicated in immune regulation in many pathological conditions, including cancer. MDSCs are suggested to promote cancer metastasis by inducing EMT of primary tumors. It has been reported that the level of MDSCs is increased in the late stage of CRC, correlating with disease progression [33]. However, a recent study demonstrated elevated MDSC during colon polyposis, suggesting that the cells expand even in premalignant states. Although the role of MDSCs in regulating VEGF-induced angiogenesis have been implicated, further studies are required to decipher the role of MDSCs in the invasion-metastasis cascade [33]. Nevertheless, clinical relevance of MDSCs in mCRC cannot be ignored. Limagne et al. showed that accumulation of MDSC in mCRC was associated with poor outcome, and inhibition of PD-1/PD-L1 axis reversed the immunosuppressive activity of MDSCs [34]. Inhibiting PD-1/PD-L1 signaling may be a promising approach to target MDSCs in mCRC.
CD8+ T-cells, also known as cytotoxic T-cells, elicit their antitumor immunity by recognizing the tumor cells’ neoantigen and killing the tumor cells by releasing effector molecules (i.e., perforin, granzyme) or cytokines (i.e., tumor necrosis factor alpha (TNF-a), interferon gamma (IFN-γ)). Research on CD8+ T-cell–dependent antitumor immunity has classically focused on its role in the primary tumor. There is increasing evidence, however, that CD8+ T-cells prevent metastasis in many steps of the metastatic cascade. CD8+ T-cells primed by the primary tumor prevents invasion and extravasation of tumor cells in the circulation by directly eliminating mesenchymal cells [35]. In vitro studies using various cancer cell lines showed induction of EMT upon incubation with CD8+ T-cell-released cytokines such as TNF-α and IFN-γ, but whether this phenomenon occurs and leads to metastasis in vivo is unknown [35]. Once the tumor cells reach their target organ, they must survive the attack from CD8+ T-cells to successfully form metastases. Several studies have revealed that high densities of infiltrating CD8+ T-cells are associated with improved disease-free and overall survival in CRC [36]. Availability of tools such as tetramer assays are used to monitor CD8+ responses, further aiding in the clinical success of CD8+ T-cell-based therapies [37]. Further studies using metastatic CRC models are required to fill our knowledge gap on the role of CD8+ T-cells in each step of the CRC metastatic cascade.
Regulatory T-cells (T-regs) maintain immune homeostasis and prevent autoimmune responses by suppressing excessive immune activation. In addition to enabling tumor growth, T-regs may also initiate tumor metastasis by facilitating tumor dissemination and immune cell evasion [38]. Increased T-reg frequency is associated with clinical stage, poor prognosis, and a greater risk of CRC metastasis, and suppression of T-regs inhibits CRC metastasis [39]. Interestingly, however, recent studies have revealed a potential controversial role of T-regs in CRC. Intratumoral T-regs were associated with suppression of CRC metastasis, better response to chemotherapy, and improved overall patient survival [40,41]. The controversial functions of T-regs in cancer progression may be due to their highly specialized tissue-specific properties. Nevertheless, inhibiting T-reg function is currently the basis for immunotherapy (discussed in later section).
Therapeutic interventions targeting colorectal metastasis
For decades, systemic chemotherapy has been the primary treatment method used to prolong patient survival. For mCRC, the most widely used chemotherapy regimens are derived from the following drugs: 5-fluorouracil (5-FU), capecitabine (a pro-drug that is metabolized to 5-FU upon absorption), irinotecan, and oxaliplatin, along with folinic acid which synergizes with 5-FU. While highly effective, these traditional chemotherapeutic drugs are DNA damaging agents and thus affect all rapidly dividing cells, leading to significant toxicity and limiting its duration of use. Furthermore, CRC cells inevitably develop resistance to chemotherapy agents, at which point additional lines of therapies are needed.
Unlike systemic therapy, targeted therapy recognizes and targets tumor cells by their cell membrane receptors or signaling pathways that are specific to tumor cells; thus, targeted drugs only impact a small subset of healthy tissue, minimizing the side effects. Over the past years, researchers explored adding targeted therapies to traditional first line chemotherapies and demonstrated improved outcomes (Table 1) (Figure 2). Drugs targeting processes or pathways that are elevated in tumors cell such as angiogenesis and EGFR-mediated mitogen-activated protein kinase (MAPK) pathway are currently used in clinic with great success. Additionally, immune checkpoint inhibitors targeting PD-1, PD-L1, and CTLA-4 have opened opportunities for use of immunotherapy in mCRC treatment.
Table 1.
Therapies used for metastatic colorectal cancer
| Systemic chemotherapy | ||||
|---|---|---|---|---|
| Drug | Mechanism of action |
Year of FDA approval for CRC |
Use in mCRC | Ref |
| Fluorouracil (5-FU) | Inhibits formation of thymidylate from uracil | 1962 | All lines of therapy | [97] |
| Irinotecan Hydrochloride | Topoisomerase I inhibitor | 1996 | All lines of therapy | [97] |
| Oxaliplatin | Forms intrastrand DNA adducts | 2002 | All lines of therapy | [97] |
| Capecitabine | Pro-drug of 5-FU; inhibits formation of thymidylate from uracil | 2005 | All lines of therapy | [97] |
| Trifluridine + Tipiracil (TAS-102) | Nucleoside analog + thymidine phosphorylase inhibitor | 2015 | Third-line therapy or beyond | [98] |
| Targeted therapy | ||||
| Bevacizumab | VEGF inhibitor | 2004 | Any line of therapy in combination with 5-FU, irinotecan, and/or oxaliplatin | [2] |
| Cetuximab | EGFR inhibitor | 2004 | In EGFR mutant, RAS/RAF wild-type cancers; any line of therapy in combination with 5-FU, irinotecan, and/or oxaliplatin | [2] |
| Panitumumab | EGFR inhibitor | 2006 | In EGFR mutant, RAS/RAF wild-type cancers; any line of therapy in combination with 5-FU, irinotecan, and/or oxaliplatin | [2] |
| Regorafenib | Multi-kinase inhibitor | 2012 | Second-line therapy or beyond | [2] |
| Aflibercept | VEGF-A, VEGF-B, PIGF inhibitor | 2012 | Second-line therapy or beyond in combination with irinotecan-based regimens | [2] |
| Ramucirumab | VEGFR2 inhibitor | 2015 | Second-line therapy or beyond in combination with irinotecan-based regimens | [2] |
| Encorafenib | BRAF inhibitor | 2020 | In BRAFV600E mutant cancers; second-line therapy in combination with cetuximab | [2] |
| Immunotherapy | ||||
| Nivolumab | PD-1 inhibitor | 2017 | In MSIhigh or dMMR cancers | [65] |
| Ipilimumab | CTLA-4 inhibitor | 2018 | In MSIhigh or dMMR cancers; in combination with nivolumab | [65] |
| Pembrolizumab | PD-1 inhibitor | 2020 | In MSIhigh or dMMR cancers | [99] |
Abbreviations: FDA, Food and Drug Administration; mCRC, metastatic colorectal cancer; VEGF, vascular endothelial growth factor; EGFR, epidermal growth factor receptor; PIGF, placental growth factor; VEGFR2, VEGF receptor 2; PD-1, programmed death protein 1; CTLA-4, cytotoxic T-lymphocyte-associated protein 4.
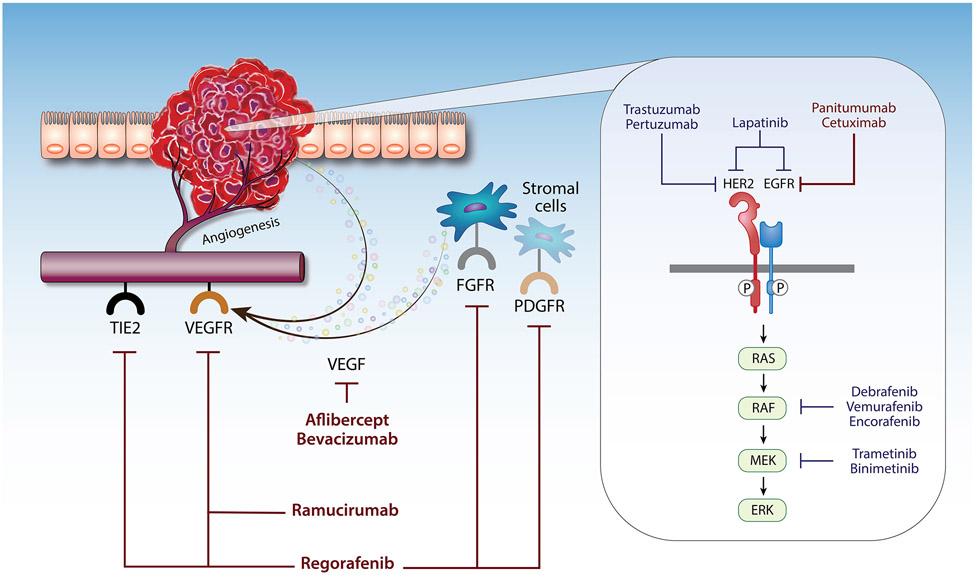
Figure 2. Targeted therapy for metastatic colorectal cancer.
Diagram illustrating the mechanism by which biological agents target the tumor cells or the tumor microenvironment (TME) to inhibit CRC metastasis. The EGFR encompasses four closely related receptor tyrosine kinases (i.e., EGFR, HER2-4). Dimerization of the receptors leads to their phosphorylation and subsequent activation of the effector proteins of the MAPK pathway (RAS, RAF, MEK, and ERK). Trastuzumab, pertuzumab, lapatinib, panitumumab, cetuximab, dabrafenib, vemurafenib, encorafenib, trametinib, and binimetinib target the HER2/EGFR-mediated MAPK pathway. Drugs such as bevacizumab, aflibercept, ramucirumab, and regorafenib target angiogenesis by inhibiting the VEGF receptors expressed on endothelial cells. Names of drugs in red indicate US FDA-approved therapy. Names of drugs in blue indicate drugs in clinical trials. Abbreviations: HER2, human epidermal growth factor receptor 2; EGFR, epidermal growth factor receptor; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; FGFR, fibroblast growth factor receptor; PDGFR, platelet-derived growth factor receptor; TIE2, tunica interna endothelial cell kinase 2.
Vascular endothelial growth factor (VEGF) inhibitors
VEGF is a signaling protein that promotes the growth of new blood vessels. Bevacizumab is a recombinant human monoclonal antibody against VEGF-A; therefore, it blocks tumor-mediated angiogenesis and interferes with the tumor’s blood supply. It was first approved by the FDA in 2004 for the treatment of mCRC and provides modest survival benefits when added to traditional chemotherapy. In 2012, Regorafenib, a small molecule multi-kinase inhibitor, was approved by the FDA for mCRC. It targets angiogenesis (VEGFR1-3, TIE2), growth factors for stromal cells (platelet-derived growth factor receptor (PDGFR)-β, fibroblast growth factor receptor (FGFR)), and oncogenic receptor tyrosine kinases (KIT, RET, RAF). The Phase III CORRECT trial showed modest survival benefits of regorafenib in patients whose cancer progressed despite standard chemotherapy [42].
Epidermal growth factor receptor (EGFR) inhibitors
EGFR encompasses four closely related receptor tyrosine kinases (i.e., EGFR, HER2-4). EGFR became an attractive target due to 60-80% of CRC cases having EGFR overexpression [43]. Cetuximab is a murine-human chimeric monoclonal antibody that competitively binds to EGFR with its ligands such as epidermal growth factor (EGF). The CRYSTAL trial showed that cetuximab reduced the risk of progression when added to chemotherapy [44]. Due to the immunogenic reactions potentially caused by murine-human chimeric antibodies, fully humanized antibodies targeting EGFR, such as Panitumumab, were also developed. Multiple trials found that Panitumumab showed similar efficacy and tolerability compared to cetuximab [45,46]. Of note, the benefits of EGFR-targeted therapies are limited to patients with RAS and RAF wild-type tumors since patients with mutations in these genes are resistant to anti-EGFR therapies [47]. Notably, left-sided tumors tend to have enriched EGFR expression compared to that of right-sided tumors. This “sidedness” leads to different clinical outcomes upon receiving anti-EGFR treatments. Several studies have demonstrated that patients with left-sided tumors have better clinical outcomes with anti-EGFR agents, while patients with right-sided tumors have improved outcomes with anti-VEGF agents, suggesting for the importance of tumor localization for effective therapy regimes [48].
HER2 is a member of the EGFR family and is overexpressed in multiple cancers including CRC [49]. The receptor is activated when it dimerizes with another EGFR member. In the preclinical setting, HER2-targeted monotherapy had limited activity against HER2-amplified colorectal tumors while dual-targeted therapy showed notable efficacy [50,51]. Based on this work, the HERACLES trial assessed the antitumor activity of dual-targeted therapy with trastuzumab (an anti-HER2 antibody) and lapatinib (a small molecular EGFR/HER2 dual inhibitor) in HER2+ mCRC and demonstrated that the combination therapy is active and well-tolerated [13]. Three years later, the MyPathway trial revealed a similar finding using trastuzumab and pertuzumab (an anti-HER2 antibody) [14]. In 2021, the DESTINY-CRC01 trial studied trastuzumab-deruxtecan (an antibody-drug conjugate linking trastuzumab to topoisomerase inhibitor deruxtecan) and found excellent response rates for mCRC patients who previously progressed on HER2-directed therapy [52]. However, HER2 mutations account for only 3-5% of CRC, and HER2-directed therapy is only indicated for patients with HER2-amplified tumors with wild-type RAS/RAF [49].
v-Raf murine sarcoma viral oncogene homolog B1 (BRAF) belongs to a family of proteins called RAF and plays a role in the EGFR-mediated MAPK pathway to regulate cell growth, differentiation, and proliferation. BRAFV600E mutations are found in approximately 10% of patients and are associated with poor prognosis when treated with standard chemotherapy [53]. Despite its efficacy in melanoma, single agent BRAF inhibitors did not show clinical activity in patients with BRAFV600E-mutant CRC [54]. Pre-clinical studies suggest that this is due to adaptive feedback activation of EGFR-mediated MAPK signaling [55]. To circumvent MAPK signaling reactivation, multiple clinical trials evaluating the efficacy and safety of BRAF inhibitors with EGFR and/or MEK inhibitors were conducted [56-58]. While many combinations showed promise, only the encorafenib (a BRAF inhibitor) and cetuximab combination is currently used due to its favorable combination of efficacy and tolerability based on results from the BEACON trial. [59].
Immunotherapy for MSIhigh colorectal cancer
Some tumor cells express inhibitory molecules, such as PD-L1, which binds to programmed death-1 (PD-1) receptor on T-cells and suppresses the T-cells, a process termed “immune checkpoint”. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) also functions as an immune checkpoint by binding to two surface protein B7 family members (CD80 and CD86) and suppressing immune cells. Over the past few decades, the success of immune checkpoint blockade targeting CTLA-4, PD-1 and PD-L1 have led to breakthroughs in cancer therapy (Table 1).
In 2015, a phase II study evaluated the clinical activity of pembrolizumab, an anti-PD-1 inhibitor, in patients with progressive metastatic cancers with or without MMR deficiency (dMMR). Their data demonstrated efficacy of pembrolizumab in all patients with dMMR, including those with mCRC [60]. To follow up with these findings, the phase III trial KEYNOTE-177 was conducted to compare the benefits of pembrolizumab monotherapy vs. standard chemotherapy in MSIhigh dMMR mCRC patients. Pembrolizumab was superior to chemotherapy and provided highly durable responses in dMMR CRC even in the first line setting [61]. A follow-up study revealed that pembrolizumab monotherapy leads to clinically meaningful improvements in health-related quality of life when compared with chemotherapy [62].
Immunotherapy is now the treatment of choice for treatment-naïve MSIhigh CRC patients with dMMR, which is found in approximately 14% of CRC [63]. A noteworthy phase II study showed a 100% success rate in 12 patients with dMMR advanced rectal cancer when given dostarlimab, a PD-1 inhibitor. All patients showed a complete clinical and pathological response after 6 months of therapy, with no evidence of residual tumor upon colonoscopy and tissue biopsy [64]. Studies are also being conducted to evaluate the efficacy of combining immunotherapy with chemotherapy, targeted therapy, or other immunotherapy drugs. The phase II CheckMate 142 clinical trial demonstrated efficacy of ipilimumab (CTLA-4 inhibitor) combined with nivolumab (PD-1 inhibitor) as a first line treatment option in MSIhigh dMMR mCRC [65]. The phase II AtezoTRIBE trial used atezolizumab, a PD-L1 inhibitor, along with FOLFOXIRI (a common chemotherapy combination regimen) and bevacizumab. This combination therapy improved survival in patients with previously untreated mCRC [66]. Additionally, toripalimab (PD-1 antibody) administered with regorafenib (multikinase inhibitor) improved response and overall survival in a phase II trial [67].
As clearly depicted by the recent progress in immunotherapy for MSIhigh tumors, the discovery of immune checkpoint inhibitors was an important advancement in the treatment of mCRC. However, a portion of MSIhigh tumors remain resistant to initial immunotherapy. Mutations that impair immune response, such as truncating mutations in β2 microglobulin (B2M), lead to failed antigen presentation and subsequent T-cell response, rendering immunotherapy ineffective. Furthermore, immunoediting resulting from constant interactions between the immune and cancer cells lead to the selection of tumor cells that lack expression of neoantigens, conferring resistance to immunotherapy. Overall, immunotherapy resistance of CRC may be related to insufficient tumor antigen presentation, T-cell exclusion, and immunosuppressive signaling in the TME. However, mechanisms of resistance that emerge in immunotherapy for MSIhigh CRC are still unclear, and further studies are required to elucidate this topic [68,69].
Emerging technologies and innovations for the treatment of metastatic colorectal cancer
Despite these advances in recent years, many patients still exhaust viable therapeutic options. To this date, a large proportion of CRC patients do not have actionable mutations or dMMR status; therefore, they would not benefit from the targeted and immunotherapies noted above. Cell manipulation technologies for immunotherapy, drug delivery systems using bacteria, and nanotechnology are emerging and promise viable therapeutic options for patients not benefitting from the current treatment paradigms. The following approaches may soon translate into clinical trials.
Chimeric antigen receptor-T (CAR-T) cell immunotherapy
Chimeric antigen receptor (CAR) T-cell therapy involves extraction of the patient’s T-cells and genetic modification of the cells to express chimeric antigen receptors (CARs) that recognize the cancer cells. The modified T-cells are infused back into the patient in a process called “adoptive cell transfer”. The engineered T-cells circulate in the bloodstream and effectively target the antigen-expressing cancer cells [70]. An emerging topic is the use of T-cells extracted from healthy donors. However, this can lead to severe toxicity such as graft-versus-host disease (GVHD) due to the host’s immune system recognizing the donor cell as foreign, constraining its clinical utility. Recent advancements in the use of CRISPR/Cas9 system may provide a means to circumvent this limitation. CRISPR/Cas9-mediated ablation of T cell receptor (TCR) from donor T-cells prevents toxicity induced by the allogeneic transplant, which has resulted in a pronounced success in preclinical studies [71].
While CAR T-cell therapy has been successful in the treatment of certain hematological malignancies, it is less efficacious in solid tumors with a response rate of only 9% [72]. This is due to a lack of a suitable target antigen that is uniformly and strongly expressed on solid tumors such as CRC but not expressed on non-cancerous cells [72]. Currently, there are no phase III clinical trials to investigate the efficacy of CAR T-cell therapy or adoptive cell transfer for advanced CRC. However, available data in early phases of clinical trials and pre-clinical data suggest that optimizing CAR T-cell therapy may be beneficial. Transfer of CAR T-cells using tumor associated glycoprotein (TAG)-72 as a target showed success but with limited persistence in a phase I trial [73]. Guanylyl cyclase C (GUCY2C), combination of carcinoembryonic antigen (CEA) and CD30, and HER2 all showed promise as targets of CAR T-cell therapy in pre-clinical trials using mouse models of mCRC [74-76].
Bacterial delivery systems
Fecal microbiota transplantation (FMT) is the administration of a fecal matter from a donor into the gastrointestinal tract of a recipient in order to directly change the recipient’s gut microbial composition and confer a health benefit [77]. Current evidence suggests that there may be benefits of FMT in cancer patients treated with immune checkpoint inhibitors. A phase I clinical trial in immunotherapy-refractory melanoma patients showed clinical responses in 30% of patients administered FMT along with anti-PD-1 immunotherapy [78]. Albeit preliminary, data favoring the addition of FMT to treatment regimen also exist for mCRC. A phase II trial testing the efficacy of FMT along with either pembrolizumab or nivolumab (PD-1 inhibitors) in mCRC patients who do not respond to anti-PD-1 therapy is currently ongoing (NCT04729322). As anti-PD-1 immunotherapy only works on 10-15% of CRC patients, combinatorial treatment regimen to enhance the success rate of PD-1 inhibition is a welcome addition. As FMT results in engraftment of donor microbiota within the host’s microbiome, it risks transferring pathogens and other unknown genes which may trigger chronic diseases. Therefore, clinical use of FMT will require identification of antitumor bacteria and highly defined consortia of microbiota-based products.
Applications of nanotechnology to deliver therapeutic agents
In 2018, a preclinical study showed effective delivery of 5-FU by encapsulating it into nanoparticles in solid breast cancer-bearing mice. It enhanced the anti-cancer activity and ameliorated the side effects of 5-FU chemotherapy [79]. Over the past few years, studies have also demonstrated successful delivery of anticancer drugs to target mCRC using nanosystems. A study conducted in 2021 revealed successful synthesis and characterization of a nanocarrier that recognizes metastatic CRC cells in secondary organs. Guanylyl cyclase C receptor (GCC) is a membrane protein expressed by the enterocytes of the intestines and therefore by CRC cells. Because of its lack of expression in other tissues such as the liver and lung, injection of a nanocarrier encapsulating the chemotherapy drug etoposide (Topoisomerase II inhibitor) into the tumor site reduced tumor growth with great specificity in a murine xenograft model [80]. Nanoparticles can also be used to deliver microRNAs. miR-122 is a liver-specific miRNA that regulates diverse hepatic functions. A recent study revealed effective development of a nanoformulation of miR-122 which was used in multiple murine models of liver mCRC models. Treatment with miR-122 effectively prevented liver metastases formation and prolonged survival by increasing antitumor T-cells in the liver [81]. Combination of immunotherapy or other targeted therapies with nanotechnology as a means to deliver drugs that are otherwise non-selective and have systemic toxicity may be a novel strategic venue for treating mCRC.
Concluding remarks and future perspectives
Metastasis to distant organs is the predominant basis for CRC mortality and poses challenges with effective therapies. Remarkable progress has been achieved in deciphering the various ways to target cancer cells, as reflected by the growing number of FDA-approved drugs for CRC and many ongoing clinical trials. However, despite the advent of targeted and immunotherapies, there is a compelling need to further unravel the mechanistic basis for metastasis and utilize state-of-the-art technologies in order to better target cancers that are resistant to immune checkpoint blockade or are microsatellite stable. Emerging technologies in pre-clinical phase studies, such as microbial therapies, immunostimulatory cytokines, nanotechnology, or oncolytic viruses, bacteria, and peptides, offer significant possibilities for therapeutic discoveries, and their success in other types of cancers provide hope for their potential clinical use for mCRC. However, much remains to be done to understand precisely how a subset of tumor cells acquire and maintain metastatic properties (see Outstanding questions). Moreover, identifying and characterizing metastasis-initiating cells and their cancer cell-specific markers for their potential use in targeted and immunotherapies will be of utmost importance. Replacing systemic chemotherapy with a combination of drugs that induce tumor-specific cytotoxicity, enhance antitumor immunity, and target mutated signaling pathways may minimize the adverse effects and soon become a treatment regime. Future developments using such a multi-pronged approach will allow more precise therapies, enabling prolonged patient survival.
Outstanding Questions.
What clones in a primary CRC are destined to metastasize (i.e., invade the ECM, enter the bloodstream, survive in the circulation, and colonize distant organs)?
What are the molecular mechanisms underlying metastatic organotropism?
What are the features of the premetastatic niche in different distant organs and how do such properties compare with the primary tumor niche?
How do the cell-intrinsic properties of the primary tumor compare with those of the metastatic tumor?
How much intratumoral versus intertumoral heterogeneity is there in metastatic lesions in one organ versus another organ?
How do the preceding questions impact therapeutic discoveries and implementation, especially in clinical trials?
Figure I. The invasion-metastasis cascade in colorectal cancer.
Step 1: tumor cells detach from one another. Step 2: tumor cells invade into the ECM by degrading the basement membrane. Step 3: tumor cells induce angiogenesis and enter the circulation. Step 4: tumor cells survive within the circulation by clustering with each other, leukocytes, and platelets. Step 5: tumor cells that have survived the circulation exit the bloodstream into the stroma of the target organ. Step 6: tumor cells seed into the secondary site. Step 7: tumor cells enter a dormant state, characterized by exit from the cell cycle and activation of survival pathways. Step 8: tumor cells are reactivated, allowing for their outgrowth and colonization at the secondary site. Abbreviations: MMP, matrix metalloproteinase; u-PA, urokinase plasminogen activator; VEGF, vascular endothelial growth factor.
Figure II. Metastasis-initiating cells (MICs) in colorectal cancer.
Metastases arise from a subset of tumor cells that have acquired invasiveness due to the activation of the NOTCH, Wnt/β-catenin, and TGF-β signaling pathways. Interaction between JAGGED (expressed by endothelial cells) and NOTCH (expressed by the tumor cells) allow for survival of the tumor cells around tumor vessels. Loss of APC or Axin promotes Wnt/β-catenin signaling and induce dedifferentiation and stemness of the MICs. MICs are resistant to the cytostatic effects of TGF-β signaling, which can also create an immune-suppressive microenvironment and further promote invasion of MICs. Abbreviations: TGF-β, transforming growth factor-β; β-cat, β-catenin; NICD, Notch intracellular domain; APC, adenomatous polyposis coli; T-reg, regulatory T-cell; MDSC, myeloid-derived suppressor cell; CAF, cancer-associated fibroblast.
ACKNOWLEDGEMENTS
This work was supported by NIH 7R01CA277795-22, NIH 5P30CA0113696 and the American Cancer Society. Figures were made with BioRender.com.
GLOSSARY
- Angiogenesis
The physiological process through which new blood vessels form from pre-existing vessels.
- Cancer-associated fibroblasts (CAFs)
A heterogeneous group of activated fibroblasts that build up and remodel the extracellular matrix in the tumor.
- Chemotherapy
A type of cancer treatment that targets rapidly dividing cells by directly or indirectly inducing DNA damage.
- Chimeric antigen receptor (CAR) T-cell therapy
A type of immunotherapy in which T-cells are isolated and genetically modified to target cancer cells ex vivo. The T-cells are re-introduced back into the patient.
- DNA mismatch repair (MMR)
A highly conserved system for recognizing and repairing the errors during DNA replication and recombination.
- Epithelial-mesenchymal transition (EMT)
A cellular process during which epithelial cells acquire mesenchymal phenotypes and behavior following the downregulation of epithelial features.
- Extracellular matrix (ECM)
A large network of proteins and other molecules that surround, support, and give structure to surrounding cells.
- Fecal microbiota transplantation (FMT)
Administration of a fecal matter from a donor into the GI tract of a recipient in order to directly change the recipient’s gut microbial composition and confer a health benefit.
- Immune checkpoint
A plethora of inhibitory pathways of the immune system that are crucial for maintaining self-tolerance and to avoid immune injury.
- Immunotherapy
A type of cancer treatment that boosts the patient’s own immune system to target cancer cells.
- Microbiome
The community of microorganisms (i.e., bacteria, fungi, and viruses) that exists in a particular environment.
- Microsatellite instability (MSI)
The condition of genetic hypermutability that results from impaired DNA mismatch repair (MMR).
- Myeloid-derived suppressor cells (MDSCs)
Immature neutrophils and monocytes that are characterized by the ability to suppress immune responses and are pathologically activated during cancer, infection, and inflammatory diseases.
- Partial EMT
A range of hybrid intermediate EMT state in which cancer cells exhibit both mesenchymal and epithelial characteristics; also called “hybrid EMT”.
- Regulatory T-cells (T-regs)
A specialized subpopulation of T-cells that suppress immune response, thereby maintaining homeostasis and self-tolerance.
- Targeted therapy
A type of precision cancer treatment that targets specific genes and proteins required for cancer cells to grow, divide, and spread.
- Tumor microenvironment (TME)
The environment around a tumor, including the surrounding blood vessels, immune cells, fibroblasts, signaling molecules and the extracellular matrix (ECM).
Footnotes
DECLARATION OF INTEREST
The authors declare no competing interests.
REFERENCES
- 1.Rumpold H et al. (2020) Prediction of mortality in metastatic colorectal cancer in a real-life population: a multicenter explorative analysis. BMC Cancer 20, 1149. 10.1186/s12885-020-07656-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie YH et al. (2020) Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther 5, 22. 10.1038/s41392-020-0116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganesh K et al. (2019) Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 16, 361–375. 10.1038/s41575-019-0126-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagao K et al. (2022) The Complete Loss of p53 Expression Uniquely Predicts Worse Prognosis in Colorectal Cancer. Int J Mol Sci 23. 10.3390/ijms23063252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassin O et al. (2022) Different hotspot p53 mutants exert distinct phenotypes and predict outcome of colorectal cancer patients. Nat Commun 13, 2800. 10.1038/s41467-022-30481-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon H et al. (2018) Mutant p53 gain of function underlies high expression levels of colorectal cancer stem cells markers. Oncogene 37, 1669–1684. 10.1038/s41388-017-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel M et al. (2021) The Role of p53 Dysfunction in Colorectal Cancer and Its Implication for Therapy. Cancers (Basel) 13. 10.3390/cancers13102296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang WW et al. (2018) The First Approved Gene Therapy Product for Cancer Ad-p53 (Gendicine): 12 Years in the Clinic. Hum Gene Ther 29, 160–179. 10.1089/hum.2017.218 [DOI] [PubMed] [Google Scholar]
- 9.Zehir A et al. (2017) Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23, 703–713. 10.1038/nm.4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaeger R et al. (2018) Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 33, 125–136 e123. 10.1016/j.ccell.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Priestley P et al. (2019) Pan-cancer whole-genome analyses of metastatic solid tumours. Nature 575, 210–216. 10.1038/s41586-019-1689-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendelaar PAJ et al. (2021) Whole genome sequencing of metastatic colorectal cancer reveals prior treatment effects and specific metastasis features. Nat Commun 12, 574. 10.1038/s41467-020-20887-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sartore-Bianchi A et al. (2016) Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 17, 738–746. 10.1016/S1470-2045(16)00150-9 [DOI] [PubMed] [Google Scholar]
- 14.Meric-Bernstam F et al. (2019) Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 20, 518–530. 10.1016/S1470-2045(18)30904-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker N et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- 16.Fumagalli A et al. (2020) Plasticity of Lgr5-Negative Cancer Cells Drives Metastasis in Colorectal Cancer. Cell Stem Cell 26, 569–578 e567. 10.1016/j.stem.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganesh K et al. (2020) L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat Cancer 1, 28–45. 10.1038/s43018-019-0006-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canellas-Socias A et al. (2022) Metastatic recurrence in colorectal cancer arises from residual EMP1(+) cells. Nature. 10.1038/s41586-022-05402-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakir B et al. (2020) EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol 30, 764–776. 10.1016/j.tcb.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aiello NM et al. (2018) EMT Subtype Influences Epithelial Plasticity and Mode of Cell Migration. Dev Cell 45, 681–695 e684. 10.1016/j.devcel.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang N et al. (2021) Novel therapeutic strategies: targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol 22, e358–e368. 10.1016/S1470-2045(21)00343-0 [DOI] [PubMed] [Google Scholar]
- 22.Sahai E et al. (2020) A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 20, 174–186. 10.1038/s41568-019-0238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tommelein J et al. (2015) Cancer-associated fibroblasts connect metastasis-promoting communication in colorectal cancer. Front Oncol 5, 63. 10.3389/fonc.2015.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi H et al. (2022) The Origin and Contribution of Cancer-Associated Fibroblasts in Colorectal Carcinogenesis. Gastroenterology 162, 890–906. 10.1053/j.gastro.2021.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong B et al. (2021) Colorectal cancer-associated fibroblasts promote metastasis by up-regulating LRG1 through stromal IL-6/STAT3 signaling. Cell Death Dis 13, 16. 10.1038/s41419-021-04461-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J et al. (2022) Cancer-Associated Stromal Fibroblast-Derived Transcriptomes Predict Poor Clinical Outcomes and Immunosuppression in Colon Cancer. Pathol Oncol Res 28, 1610350. 10.3389/pore.2022.1610350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanley CJ and Thomas GJ (2021) Targeting cancer associated fibroblasts to enhance immunotherapy: emerging strategies and future perspectives. Oncotarget 12, 1427–1433. 10.18632/oncotarget.27936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantovani A et al. (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23, 549–555. 10.1016/s1471-4906(02)02302-5 [DOI] [PubMed] [Google Scholar]
- 29.Murray PJ et al. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20. 10.1016/j.immuni.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y et al. (2022) Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell 40, 424–437 e425. 10.1016/j.ccell.2022.02.013 [DOI] [PubMed] [Google Scholar]
- 31.Xu C et al. (2017) SPP1, analyzed by bioinformatics methods, promotes the metastasis in colorectal cancer by activating EMT pathway. Biomed Pharmacother 91, 1167–1177. 10.1016/j.biopha.2017.05.056 [DOI] [PubMed] [Google Scholar]
- 32.Wu Y et al. (2022) Spatiotemporal Immune Landscape of Colorectal Cancer Liver Metastasis at Single-Cell Level. Cancer Discov 12, 134–153. 10.1158/2159-8290.CD-21-0316 [DOI] [PubMed] [Google Scholar]
- 33.Sieminska I and Baran J (2020) Myeloid-Derived Suppressor Cells in Colorectal Cancer. Front Immunol 11, 1526. 10.3389/fimmu.2020.01526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Limagne E et al. (2016) Accumulation of MDSC and Th17 Cells in Patients with Metastatic Colorectal Cancer Predicts the Efficacy of a FOLFOX-Bevacizumab Drug Treatment Regimen. Cancer Res 76, 5241–5252. 10.1158/0008-5472.CAN-15-3164 [DOI] [PubMed] [Google Scholar]
- 35.Tallon de Lara P et al. (2022) Antimetastatic defense by CD8(+) T cells. Trends Cancer 8, 145–157. 10.1016/j.trecan.2021.10.006 [DOI] [PubMed] [Google Scholar]
- 36.Lalos A et al. (2021) Prognostic significance of CD8+ T-cells density in stage III colorectal cancer depends on SDF-1 expression. Sci Rep 11, 775. 10.1038/s41598-020-80382-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tay RE et al. (2021) Revisiting the role of CD4(+) T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther 28, 5–17. 10.1038/s41417-020-0183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huppert LA et al. (2022) Tissue-specific Tregs in cancer metastasis: opportunities for precision immunotherapy. Cell Mol Immunol 19, 33–45. 10.1038/s41423-021-00742-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji D et al. (2020) Combination of radiotherapy and suppression of Tregs enhances abscopal antitumor effect and inhibits metastasis in rectal cancer. J Immunother Cancer 8. 10.1136/jitc-2020-000826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oshi M et al. (2022) Intratumoral density of regulatory T cells is a predictor of host immune response and chemotherapy response in colorectal cancer. Am J Cancer Res 12, 490–503 [PMC free article] [PubMed] [Google Scholar]
- 41.Yan B et al. (2022) Correlation and prognostic implications of intratumor and tumor draining lymph node Foxp3(+) T regulatory cells in colorectal cancer. BMC Gastroenterol 22, 122. 10.1186/s12876-022-02205-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grothey A et al. (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381, 303–312. 10.1016/S0140-6736(12)61900-X [DOI] [PubMed] [Google Scholar]
- 43.Pabla B et al. (2015) Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention. World J Clin Oncol 6, 133–141. 10.5306/wjco.v6.i5.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Cutsem E et al. (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360, 1408–1417. 10.1056/NEJMoa0805019 [DOI] [PubMed] [Google Scholar]
- 45.Douillard JY et al. (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28, 4697–4705. 10.1200/JCO.2009.27.4860 [DOI] [PubMed] [Google Scholar]
- 46.Price TJ et al. (2014) Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol 15, 569–579. 10.1016/S1470-2045(14)70118-4 [DOI] [PubMed] [Google Scholar]
- 47.Karapetis CS et al. (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359, 1757–1765. 10.1056/NEJMoa0804385 [DOI] [PubMed] [Google Scholar]
- 48.Creasy JM et al. (2018) The Impact of Primary Tumor Location on Long-Term Survival in Patients Undergoing Hepatic Resection for Metastatic Colon Cancer. Ann Surg Oncol 25, 431–438. 10.1245/s10434-017-6264-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahcene Djaballah S et al. (2022) HER2 in Colorectal Cancer: The Long and Winding Road From Negative Predictive Factor to Positive Actionable Target. Am Soc Clin Oncol Educ Book 42, 1–14. 10.1200/EDBK_351354 [DOI] [PubMed] [Google Scholar]
- 50.Bertotti A et al. (2011) A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 1, 508–523. 10.1158/2159-8290.CD-11-0109 [DOI] [PubMed] [Google Scholar]
- 51.Leto SM et al. (2015) Sustained Inhibition of HER3 and EGFR Is Necessary to Induce Regression of HER2-Amplified Gastrointestinal Carcinomas. Clin Cancer Res 21, 5519–5531. 10.1158/1078-0432.CCR-14-3066 [DOI] [PubMed] [Google Scholar]
- 52.Siena S et al. (2021) Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol 22, 779–789. 10.1016/S1470-2045(21)00086-3 [DOI] [PubMed] [Google Scholar]
- 53.Davies H et al. (2002) Mutations of the BRAF gene in human cancer. Nature 417, 949–954. 10.1038/nature00766 [DOI] [PubMed] [Google Scholar]
- 54.Kopetz S et al. (2015) Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J Clin Oncol 33, 4032–4038. 10.1200/JCO.2015.63.2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prahallad A et al. (2012) Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483, 100–103. 10.1038/nature10868 [DOI] [PubMed] [Google Scholar]
- 56.Kopetz S et al. (2021) Randomized Trial of Irinotecan and Cetuximab With or Without Vemurafenib in BRAF-Mutant Metastatic Colorectal Cancer (SWOG S1406). J Clin Oncol 39, 285–294. 10.1200/JCO.20.01994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z et al. (2022) Cetuximab and vemurafenib plus FOLFIRI (5-fluorouracil/leucovorin/irinotecan) for BRAF V600E-mutated advanced colorectal cancer (IMPROVEMENT): An open-label, single-arm, phase II trial. Eur J Cancer 163, 152–162. 10.1016/j.ejca.2021.12.028 [DOI] [PubMed] [Google Scholar]
- 58.Corcoran RB et al. (2015) Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J Clin Oncol 33, 4023–4031. 10.1200/JCO.2015.63.2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kopetz S et al. (2019) Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med 381, 1632–1643. 10.1056/NEJMoa1908075 [DOI] [PubMed] [Google Scholar]
- 60.Le DT et al. (2015) PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 372, 2509–2520. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andre T et al. (2020) Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med 383, 2207–2218. 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 62.Andre T et al. (2021) Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): an open-label, randomised, phase 3 trial. Lancet Oncol 22, 665–677. 10.1016/S1470-2045(21)00064-4 [DOI] [PubMed] [Google Scholar]
- 63.Sawayama H et al. (2020) Investigation of colorectal cancer in accordance with consensus molecular subtype classification. Ann Gastroenterol Surg 4, 528–539. 10.1002/ags3.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cercek A et al. (2022) PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med 386, 2363–2376. 10.1056/NEJMoa2201445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lenz HJ et al. (2022) First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J Clin Oncol 40, 161–170. 10.1200/JCO.21.01015 [DOI] [PubMed] [Google Scholar]
- 66.Antoniotti C et al. (2022) Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol 23, 876–887. 10.1016/S1470-2045(22)00274-1 [DOI] [PubMed] [Google Scholar]
- 67.Wang F et al. (2021) Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med 2, 100383. 10.1016/j.xcrm.2021.100383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Motta R et al. (2021) Immunotherapy in microsatellite instability metastatic colorectal cancer: Current status and future perspectives. J Clin Transl Res 7, 511–522 [PMC free article] [PubMed] [Google Scholar]
- 69.Weng J et al. (2022) Exploring immunotherapy in colorectal cancer. J Hematol Oncol 15, 95. 10.1186/s13045-022-01294-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gross G et al. (1989) Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 86, 10024–10028. 10.1073/pnas.86.24.10024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Razeghian E et al. (2021) A deep insight into CRISPR/Cas9 application in CAR-T cell-based tumor immunotherapies. Stem Cell Res Ther 12, 428. 10.1186/s13287-021-02510-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hou B et al. (2019) Efficiency of CAR-T Therapy for Treatment of Solid Tumor in Clinical Trials: A Meta-Analysis. Dis Markers 2019, 3425291. 10.1155/2019/3425291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hege KM et al. (2017) Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J Immunother Cancer 5, 22. 10.1186/s40425-017-0222-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Magee MS et al. (2018) Human GUCY2C-Targeted Chimeric Antigen Receptor (CAR)-Expressing T Cells Eliminate Colorectal Cancer Metastases. Cancer Immunol Res 6, 509–516. 10.1158/2326-6066.CIR-16-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hombach AA et al. (2019) Blocking CD30 on T Cells by a Dual Specific CAR for CD30 and Colon Cancer Antigens Improves the CAR T Cell Response against CD30(−) Tumors. Mol Ther 27, 1825–1835. 10.1016/j.ymthe.2019.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu J et al. (2021) HER2-specific chimeric antigen receptor-T cells for targeted therapy of metastatic colorectal cancer. Cell Death Dis 12, 1109. 10.1038/s41419-021-04100-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta S et al. (2016) Fecal microbiota transplantation: in perspective. Therap Adv Gastroenterol 9, 229–239. 10.1177/1756283X15607414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baruch EN et al. (2021) Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371, 602–609. 10.1126/science.abb5920 [DOI] [PubMed] [Google Scholar]
- 79.Haggag YA et al. (2018) Polymeric nano-encapsulation of 5-fluorouracil enhances anti-cancer activity and ameliorates side effects in solid Ehrlich Carcinoma-bearing mice. Biomed Pharmacother 105, 215–224. 10.1016/j.biopha.2018.05.124 [DOI] [PubMed] [Google Scholar]
- 80.Bouzo BL et al. (2021) Sphingomyelin nanosystems loaded with uroguanylin and etoposide for treating metastatic colorectal cancer. Sci Rep 11, 17213. 10.1038/s41598-021-96578-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sendi H et al. (2022) Nanoparticle Delivery of miR-122 Inhibits Colorectal Cancer Liver Metastasis. Cancer Res 82, 105–113. 10.1158/0008-5472.CAN-21-2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mendonsa AM et al. (2018) E-cadherin in contact inhibition and cancer. Oncogene 37, 4769–4780. 10.1038/s41388-018-0304-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cooper J and Giancotti FG (2019) Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 35, 347–367. 10.1016/j.ccell.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Apte RS et al. (2019) VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 176, 1248–1264. 10.1016/j.cell.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aceto N et al. (2015) En Route to Metastasis: Circulating Tumor Cell Clusters and Epithelial-to-Mesenchymal Transition. Trends Cancer 1, 44–52. 10.1016/j.trecan.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 86.Valastyan S and Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292. 10.1016/j.cell.2011.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sosa MS et al. (2014) Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 14, 611–622. 10.1038/nrc3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peinado H et al. (2017) Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer 17, 302–317. 10.1038/nrc.2017.6 [DOI] [PubMed] [Google Scholar]
- 89.Giancotti FG (2013) Mechanisms governing metastatic dormancy and reactivation. Cell 155, 750–764. 10.1016/j.cell.2013.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Massague J and Ganesh K (2021) Metastasis-Initiating Cells and Ecosystems. Cancer Discov 11, 971–994. 10.1158/2159-8290.CD-21-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tauriello DVF and Batlle E (2016) Targeting the Microenvironment in Advanced Colorectal Cancer. Trends Cancer 2, 495–504. 10.1016/j.trecan.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 92.Nusse R and Clevers H (2017) Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 169, 985–999. 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 93.Nixon BG et al. (2022) TGFbeta control of immune responses in cancer: a holistic immuno-oncology perspective. Nat Rev Immunol. 10.1038/s41577-022-00796-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lambert AW and Weinberg RA (2021) Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer 21, 325–338. 10.1038/s41568-021-00332-6 [DOI] [PubMed] [Google Scholar]
- 95.Kastenhuber ER and Lowe SW (2017) Putting p53 in Context. Cell 170, 1062–1078. 10.1016/j.cell.2017.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang Q et al. (2020) Mutant p53 on the Path to Metastasis. Trends Cancer 6, 62–73. 10.1016/j.trecan.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goodwin RA and Asmis TR (2009) Overview of systemic therapy for colorectal cancer. Clin Colon Rectal Surg 22, 251–256. 10.1055/s-0029-1242465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cleghorn S (2015) TAS-102 for metastatic refractory colorectal cancer. Lancet Oncol 16, e314. 10.1016/S1470-2045(15)70246-9 [DOI] [PubMed] [Google Scholar]
- 99.Casak SJ et al. (2021) FDA Approval Summary: Pembrolizumab for the First-line Treatment of Patients with MSI-H/dMMR Advanced Unresectable or Metastatic Colorectal Carcinoma. Clin Cancer Res 27, 4680–4684. 10.1158/1078-0432.CCR-21-0557 [DOI] [PMC free article] [PubMed] [Google Scholar]