Abstract
Oats (Avena sativa L.) are a popular functional cereal grain due to their numerous health benefits. This review article summarized the information on the chemical composition and phytonutrients of oats grown in different countries. It also reviewed recently developed fermented oat products to highlight their potential for human health. Oats have an interesting nutritional profile that includes high-quality protein, unsaturated fats, soluble fiber, polyphenolic compounds, and micronutrients. Oat grain has a unique protein composition, with globulins serving as the primary storage protein, in contrast to other cereals, where prolamins are the main storage proteins. Oats have the highest fat content of any cereal, with low saturated fatty acids and high essential unsaturated fatty acid content, which can help reduce the risk of cardiovascular diseases. Oats are a good source of soluble dietary fiber, particularly β-glucan, which has outstanding functional properties and is extremely important in human nutrition. β-Glucan has been shown to lower blood cholesterol and glucose absorption in the intestine, thereby preventing diseases such as cardiovascular injury, dyslipidemia, hypertension, inflammatory state, and type 2 diabetes. Oats also contain high concentration of antioxidant compounds. Avenanthramides, which are unique to oats, are powerful antioxidants with high antioxidative activity in humans. Recognizing the nutritional benefits of oats, oat-based fermented food products are gaining popularity as functional foods with high probiotic potential.
1. Introduction
Oat (Avena sativa) is a cereal grain from the Poaceae family cultivated for cattle feed (70%) and human consumption (30%) [1]. Some studies suggest that oats can be traced back to around 2000 BC. [2]. However, the exact origins of the various Avena spp. are unknown. After many years of growing wheat and barley, oats became known and cultivated [3]. The genus Avena contains a polyploid collection of wild, weedy, and cultivated species [4]. Avena sativa is a hexaploid species that is the world's most widely grown and popular oat cultivar today [5].
Oats' nutritional composition differs significantly from that of other cereals, with high protein content and an ample amount of essential amino acids [6, 7]. Oats have a higher fat content (6–10%) than wheat and most other cereals (2-3%) [8]. It had the highest fat content of any cereal, with a high percentage of unsaturated fats [9]. The high nutritional value of oats is also due to their high β-glucan content [10]. Beta-glucan is a vital functional component in various food industries [11]. Furthermore, oats possess more than 20 unique polyphenolic compounds known as avenanthramides [12]. The antioxidant activity of avenanthramides is 10 to 30 times higher than that of other cereals' polyphenolic compounds such as ferulic acid, gentisic acid, p-hydroxybenzoic acid, protocatechuic acid, syringic acid, vanillic acid, and vanillin [13].
Oats are used for food in various forms, including whole grains, rolled oats, crushed oatmeal, and oat flour [14, 15]. Oats are best known as a breakfast cereal food, whether eaten whole or in the form of rolled oats. Oatmeal is primarily used for porridge and to prepare several baked goods such as oatcakes, oatmeal cookies, and oat bread. Several novel products using oats have been developed [16–18].
Oat-based foods have recently gained popularity due to their health benefits. Consumption of oat products has been associated with a reduced serum cholesterol level, a lower risk of cardiovascular disease (CVD), and a lower risk of obesity, hypertension, cancer, diabetes, and gastrointestinal disorders [19]. The European Food and Safety Authority and the US Food and Drug Administration (FDA) have accredited health claims for oat foods containing oat β-glucan to lower serum cholesterol and the risk of CVD [20]. Furthermore, the potential benefits of oats have been related to various other bioactive components in addition to β-glucan. Avenanthramides (AVAs), a unique oat antioxidant, help prevent free radicals from damaging low-density lipoprotein (LDL) cholesterol, and AVA-enriched oat extract combined with vitamin C inhibits LDL oxidation synergistically. AVAs have anti-inflammatory, antiproliferative, vasodilation, anti-itch, cytoprotective, and anticancer properties [21–23]. The purpose of this article is to review the nutritional potential of oat grains and oat-based fermented food products.
2. Nutritional Composition of Oats
2.1. Carbohydrate, Dietary Fiber, and β-Glucan
Oats contain fewer carbohydrates but more protein and lipids than other cereals [24]. However, starch remains the most abundant component like in other cereal grains, comprising approximately 60% of oat grains [25]. Amylose and amylopectin make up 98-99% of the carbohydrate constituents of oat starch granules. Oat starch has different characteristics such as short amylose, relatively high crystallinity, and a well-developed and small granule surface [26]. These special characteristics of oat starch make it unique from other cereal starches.
Oats have a well-balanced profile of soluble and insoluble dietary fibers [6]. Dietary fibers, also known as roughage, are edible plant parts that are essential components of human nutrition. Dietary fiber enters the large intestine and is partially or completely fermented by gut bacteria [27]. Fermentation produces various types of by-products, including gases and short-chain fatty acids. The combined action of the fermentation process and products contribute to the beneficial effects of dietary fiber on health [28]. The proximate composition of oats is summarized in Table 1.
Table 1.
Summary of the proximate composition of oat grains.
| Type of oat sample | Place | Moist. (%) | C. Prot. (%) | C. Fat (%) | C. Fib. (%) | Ash (%) | Total CHO (%) | Source |
|---|---|---|---|---|---|---|---|---|
| Hulled | — | 8.2 | 16.9 | 6.9 | 10.6 | 1.7 | 66.3 | [29] |
| Dehulled | India | — | 12.5 | 5.9 | 2.2 | 6.2 | 69.4 | [30] |
| Dehulled | Poland | 9.7 | 16.3 | 8.6 | 3.0 | 2.4 | 69.6 | [31] |
| Hulled | India | 6.7–8.2 | 12.9–14.4 | 4.2–5.1 | 12.6–13.1 | 2.6–3.9 | 55.7–59.9 | [32] |
| Dehulled | Morocco | 11.6–13.0 | 11.3–17.2 | 2.1–3.6 | — | 2.7–3.6 | 56.6–65.0 | [33] |
| Dehulled | India | 8.7 | 13.6 | 7.8 | 3.5 | 1.8 | 64.7 | [34] |
| Dehulled | Pakistan | 7.9–8.7 | 13.1–13.7 | 7.7–8.3 | 2.6–2.8 | 3.2–3.7 | 63.7–64.6 | [35] |
| Dehulled | Finland | 8.6 | 13.4 | 8.5 | — | 2.2 | — | [36] |
| Hulled | Pakistan | 9.6 | 14.5 | 6.3 | 15.4 | 5.7 | 47.9 | [37] |
| Hulled | Pakistan | — | 10 | 6.3 | 14.4 | 4.8 | 57.1 | [38] |
| Dehulled | Egypt | 10.0–10.5 | 11.6–13.6 | 7.2–8.9 | 3.5–5.9 | 2.0–2.2 | 69.4–75.6 | [39] |
| Hulled | Poland | — | 11.5 | 4.8 | 13.6 | 2.3 | 67.8 | [40] |
| Dehulled | Ethiopia | 8.5–9.8 | 11.9–15.8 | 6.7–10.3 | 2.1–3.5 | 1.2–1.3 | 72.6–74.3 | [41] |
“—” represent the values that the authors did not report. Moist.: moisture; C. Prot.: crude protein; C. Fat: crude fat; C. Fib.: crude fiber; CHO: carbohydrate.
Oat fibers from whole grains are nearly 60% insoluble and 40% soluble [42]. Mixed-linkage (1–3), (1–4)-β-D-glucans or β-glucans and arabinoxylans are significant sources of soluble and insoluble dietary fibers [43]. Oats have higher soluble fiber content than other cereals [44]. Soluble β-glucans found in subaleurone cell walls are one of the most extensively researched oat constituents [45, 46]. β-Glucan is a polysaccharide with a D-glucose unit linkage. Oat β-glucan is unique in that; it is composed of a group of linear polymers of glucose molecules linked by roughly 30% −(1–3) and 70% −(1–4) linkages [47]. These linkages are not arranged randomly, with (1–4) links appearing in groups of two to four and (1–3) links appearing singly [46]. This leads to molecules composed of −β (1–3) linked units, with the cellotriosyl: cellotetraosyl ratio being about 2.2 in oats [48]. The presence of β-(1–3) links breaks up the regularity of β-(1–4) link sequences, and the resulting increased flexibility allows water to penetrate the molecular chains and solubilize the fiber [49]. However, adjacent β-(1–4) links may exhibit interchain aggregation via strong hydrogen bonds, reducing β-glucan solubility. The β-glucan contents of oats are summarized in Table 2.
Table 2.
Summary of β-glucan content of dehulled oat grains.
Oats were first found to have cholesterol-lowering properties in 1997, and the active ingredient was identified as β-glucans [59]. After reviewing 42 clinical trials, the FDA acknowledged the cholesterol-lowering features of oats [60]. The recommended intake for a cholesterol-lowering effect is 3 g of oat β-glucan per day [19]. According to recent research, doses of 3–13 g/day resulted in total cholesterol reduction of 8.2–15.1 mg/dL and LDL reduction of 7.8–13.2 mg/dL [61]. These changes may appear insignificant compared with those obtained through drug therapy. However, a 1% reduction in blood cholesterol can reduce the risk by 2–4% [62].
In general, scientific studies have shown that eating oats, like other fiber-rich cereal grains, helps lose weight, lowers blood cholesterol levels, and improves postprandial glycemic and insulinemic responses in noninsulin-dependent diabetes mellitus and healthy subjects. Oatmeal reduces the risk of colon cancer, regulates blood pressure, and prevents cardiovascular disease. Oat-based foods also strengthen the immune system's defenses against parasites, bacteria, fungi, and viruses [63] (Figure 1).
Figure 1.
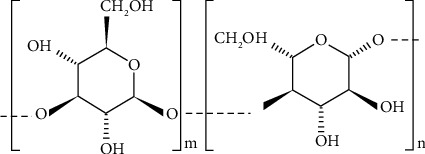
Structure of oat β -(1–3) (1–4)-glucan.
2.2. Protein and Amino Acid Profile of Oats
Oat grain has high protein content and a distinctive protein composition [64, 65]. Most cereals (including barley, wheat, and rye) rely heavily on prolamins as their main storage proteins, but oats are an exceptional case. The main storage proteins in oats are globulins (which are salt-water-soluble and account for roughly 55% of the total Osborn protein solubility classification), with prolamins accounting for a minor percentage [66]. Avenins also serve as protein storage for oats, accounting for 10 to 13% of the total protein content [44]. The oat protein consists of more limiting amino acids such as glutamine, lysine, and threonine and less proline compared to other cereal grains [44]. The protein content of oat groats ranges from 12.4 to 24.5% [66]. The embryonic axis and the scutellum contain greater quantities of amino acids than other parts of the kernel.
Enzymes are the most essential metabolically active proteins in the oats. Oats, like other cereal grains, contain a lot of enzymes. Previous research identified maltase, proteases, phenoxyacetylase, hydroxylase, α-amylase, lichenase, tyrosinase, phosphatase, and lipase as oat enzymes [67]. Table 3 shows the amino acid composition of oats from various sources.
Table 3.
Summary of the amino acid composition of dehulled oat grains.
| Place | Amino acids | Source | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Try | Thr | Ile | Leu | Lys | Met | Cys | Phe | Tyr | ||
| — | 0.23 | 0.58 | 0.69 | 1.28 | 0.70 | 0.31 | 0.41 | 0.90 | 0.57 | [29] |
| Latvia | 0.00 | 0.48 | 0.44 | 1.02 | 0.49 | 0.22 | 0.00 | 0.65 | 0.43 | [68] |
| China | 0.00 | 0.39 | 0.45 | 0.96 | 0.53 | 0.16 | 0.32 | 0.70 | 0.47 | [69] |
| Pakistan | 0.00 | 0.39–0.42 | 0.39–0.42 | 0.88–0.91 | 0.34–0.36 | 0.11–0.16 | 0.00 | 0.39–0.53 | 0.31–0.34 | [38] |
| Poland | 0.00 | 3.02∗ | 3.02∗ | 6.25∗ | 3.33∗ | 1.41∗ | 2.29∗ | 4.55∗ | 3.03∗ | [31] |
| Poland | 1.15∗ | 2.46∗ | 2.32∗ | 5.26∗ | 2.73∗ | 4.30∗ | 2.74∗ | 5.88∗ | 2.26∗ | [40] |
|
| ||||||||||
| Place | Val | Arg | His | Ala | Asp | Glu | Gly | Pro | Ser | |
|
| ||||||||||
| — | 0.94 | 1.19 | 0.41 | 0.88 | 1.45 | 3.71 | 0.84 | 0.93 | 0.75 | [29] |
| Latvia | 0.70 | 1.09 | 0.43 | 0.63 | 1.02 | 2.97 | 0.66 | 0.82 | 0.64 | [68] |
| China | 0.65 | 0.90 | 0.28 | 0.62 | 0.94 | 2.89 | 0.64 | 0.61 | 0.84 | [69] |
| Pakistan | 0.41–0.59 | 0.91–0.96 | 0.28–0.36 | 0.49–0.53 | 0.79–0.81 | 2.24–2.40 | 0.52–0.57 | 0.66–0.68 | 0.47–0.51 | [38] |
| Poland | 4.16∗ | 7.01∗ | 1.99∗ | 4.24∗ | 8.24∗ | 21.20∗ | 4.44∗ | 4.68∗ | 4.02∗ | [31] |
| Poland | 3.20∗ | 5.79∗ | 1.74∗ | 3.59∗ | 7.37∗ | 19.10∗ | 3.81∗ | 4.54∗ | 3.86∗ | [40] |
∗ Amino acid values are reported in g/16 g·N, whereas for other amino acids, values are reported in g/100 g. “—” represents the values that the authors did not report. Try: tyrosine; Thr: threonine; Ile: isoleucine; Leu: leucine; Lys: lysine; Met: methionine; Cys: cysteine; Phe: phenylalanine; Tyr: tyrosine; Val: valine; Arg: arginine; His: histidine; Ala: alanine; Asp: asparagine; Glu: glutamic acid; Gly: glycine; Pro: proline; Ser: serine.
2.3. Crude Fat and Fatty Acid Composition of Oats
Oats have the highest fat content of any cereal [44]. They are high in linoleic acid and low in saturated fat, which can help reduce the risk of heart and vascular diseases [70]. Monounsaturated fatty acids (MUFA, C18 : 1) and polyunsaturated fatty acids (PUFA, C18 : 2) are the most abundant fatty acids in oats, followed by saturated fatty acids (C16 : 0) [44]. Triglycerides also constitute the main component of lipids and phospholipids; glycolipids and sterols are also present in considerable amounts [67]. The high lipid content makes them a valuable functional food ingredient in a wide range of industries [9]. According to Van Den Broeck et al. [44], the fatty acid content in mg/100 g of the oat flour sample was 193.5–292.9 (C16 : 0), 11.5–33.3 (C18 : 0), 385.0–718.0 (C18 : 1), 532.3–748.9 (C18 : 2), and 12.3–16.1 (C18 : 3). The relative proportion of fatty acids of oat grains is summarized in Table 4.
Table 4.
Summary of the relative proportion of fatty acids in dehulled oat grains.
| Place | Saturated fatty acids | Monounsaturated fatty acids | Polyunsaturated fatty acids | Source | |||||
|---|---|---|---|---|---|---|---|---|---|
| C12 : 0 | C14 : 0 | C16 : 0 | C18 : 0 | C16 : 1 | C18 : 1 | C18 : 2 | C18 : 3 | ||
| — | 0.02 | 0.02 | 1.03 | 0.07 | 0.01 | 2.17 | 2.42 | 0.11 | [29] |
| Turkey | 0.00–0.51 | 0.08–4.38 | 10.80–22.4 | 1.30–4.80 | 0.00–5.30 | 19.60–37.90 | 18.90–54.00 | 2.40–8.30 | [71] |
| Poland | 0.00 | 0.27 | 18.60 | 1.90 | 0.19 | 43.1 | 32.80 | 0.90 | [58] |
| Czech Republic | 0.00 | 0.32–0.39 | 19.30–20.40 | 1.04–1.32 | 0.23–0.28 | 27.8–29.90 | 39.60–41.20 | 1.50–1.70 | [72] |
| Morocco | 0.00 | 0.18–0.27 | 15.30 - 16.40 | 2.80–3.50 | 0.17–0.57 | 41.30–44.50 | 33.00–35.10 | 0.81–1.86 | [33] |
| Poland | 0.00 | 0.29–0.35 | 21.30–23.90 | 1.12–1.68 | 0.20–0.31 | 36.10–38.00 | 34.90–36.00 | 0.97–1.39 | [73] |
“—” represents the values that the authors did not report.
2.4. Micronutrients of Oats
Micronutrients are minerals and vitamins that the body needs in minute amounts. On the other hand, they cannot be compromised, and deficiencies in any of them can result in life-threatening conditions [74]. Vitamins and minerals are essential for proper metabolism and tissue maintenance Micronutrients must be obtained through the diet because the body does not produce them. To achieve the best results from the diet, a proper balance of micronutrients and macronutrients is required [75].
Minerals are classified into two types: major and minor. Sodium, potassium, magnesium, calcium, phosphorus, chlorine, and sulfur are examples of major minerals with daily requirements greater than 100 mg for adults [76]. On the other hand, minor or trace elements are minerals with daily requirements of less than 100 mg, such as iron, zinc, copper, chromium, cobalt, molybdenum, selenium, nickel, manganese, fluorine, iodine, silicon, tin, and vanadium [76]. As with other cereal grains, the mineral content of oats ranged from 2 to 3%. According to Bhardwaj et al. [56], the iron and zinc contents of 43 oats ranged from 1.8 to 6.8 mg/100 g and 6.5 to 10.2 mg/100 g, respectively. Butt et al. [6] reported that the mineral content of oats per 100 g of flour sample was 60 mg of calcium, 372 mg of phosphorus, 3.8 mg of iron, and 3.9 mg of zinc. Table 5 updates the mineral content of the oat grains.
Table 5.
Summary of mineral composition (mg/100 g) of dehulled oat grains.
| Place | Ca | Fe | Mg | P | K | Na | Zn | Cu | Mn | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| — | 54.0 | 4.7 | 177.0 | 523.0 | 429.0 | 2.0 | 4.0 | 0.6 | 4.9 | [29] |
| Czech Republic | 99.0 | 4.1 | 139.0 | 502.0 | 575.0 | — | 2.1 | 0.4 | 3.9 | [77] |
| Pakistan | 43.2–69.4 | 3.0–4.1 | 129.3–171.4 | — | 289.6–315.3 | 8.3–9.1 | 2.9–3.4 | 0.4–0.5 | — | [35] |
| Morocco | 42.1–86.0 | 8.5–43.9 | 49.5–75.5 | 162.8–254.2 | 214.6–395.6 | 24.1–61.5 | 6.9–8.2 | — | 5.2–19.0 | [33] |
| Turkey | 56.9–127.0 | 3.0–8.1 | 125.3–202.5 | 242.9–455.7 | 305.6–562.1 | — | 1.5–3.8 | 1.8–8.7 | 2.6–6.3 | [78] |
| Egypt | 54.7–71.7 | 13.8–24.2 | 112.3–120.7 | 469.6–472.6 | 350.0–362.0 | 5.3–7.0 | 3.4–3.6 | 1.2–1.3 | 3.7–4.4 | [39] |
| Ethiopia | 44.0–102.7 | 2.5–3.0 | 62.4–89.1 | — | 241.7–258.3 | — | 1.6–2.1 | 0.2–0.4 | — | [41] |
| Brazil | — | 3.9–6.3 | — | — | — | — | 2.7–5.8 | 0.4–0.8 | 5.9–10.6 | [76] |
“—” represents the values that the authors did not report.
Oats' micronutrients include not only trace minerals but also vitamins, which are organic compounds required in microgram or milligram amounts. Oat grains contain significant amounts (mg/100 g) of vitamins such as thiamine (0.76), riboflavin (0.14), niacin (0.96), pantothenic acid (1.35), vitamin B-6 (0.12), and total folate (56) [29]. On the other hand, Youssef et al. [39] reported in mg/100 g of oats' vitamin C (0.1), thiamine (0.44–0.53), riboflavin (0.40–0.60), and vitamin E (0.13–0.87), while Gabrovska et al. [77] also reported vitamin C (0.1), niacin (0.68), vitamin B-6 (0.18), and vitamin E (1.32).
3. Bioactive Components and Health Benefits of Oats
Oat has gained popularity as a healthy food in recent years due to its high content of bioactive compounds that can benefit human health, such as β-glucan, avenanthramides, tocols, sterols, and avenacosides [20]. These compounds help prevent gastrointestinal disorders, type 2 diabetes (T2DM), CVD, and cancer [24].
In human cells, radicals are formed from normal metabolism and environmental radiation. These radicals can cause deoxyribonucleic acid (DNA) changes, which may produce cancerous cells or diseases like atherosclerosis [79, 80]. Free radicals are also known to oxidize LDL cholesterol, contributing to heart disease and stroke [81]. Thus, free radicals are key causal factors in many chronic diseases. The human body has a natural defense system against these reactions, but dietary antioxidants also contribute to body defense [82]. Some phenolic compounds found in oats have free radical scavenging activities with potential health-beneficial properties [83].
Anthranilic acid amides aid in the prevention of free radical damage to LDL cholesterol [84]. Anthranilic acid amide-enriched oat extract combined with vitamin C inhibits LDL oxidation synergistically in vitro [84, 85]. Oats contain a unique group of approximately 40 different anthranilic acid amides consisting of anthranilic acid derivatives and hydroxycinnamic acid derivatives [84, 86]. Oat antioxidants have been shown in animal and human studies to reduce CVD risk by inhibiting LDL cholesterol oxidation and peroxidation and reducing serum cholesterol [84]. As a result, eating oats and other foods is recommended as part of a healthy diet.
3.1. Phenolic Compounds of Oats
The importance of phenolics stems primarily from their high antioxidant capacity and health benefits. Oat products have recently gained popularity as bioactive ingredients for industries such as pharmaceuticals, food, and cosmetics [87]. Oats' primary antioxidants are polyphenolic compounds, flavonoids, and sterols [24]. The total phenolic content (TPC) and total flavonoid content (TFC) of oats are summarized in Table 6.
Table 6.
Summary of TPC and TFC of dehulled oat grains.
| Place | TPC | TFC | Source |
|---|---|---|---|
| Pakistan | 160.2–191.6 mg GAE/100 g | 70.8–128.0 mg QE/100 g | [35] |
| Czech | 772.9–890.6 mg GAE/kg | — | [88] |
| Morocco | 17.2–23.5 mg GAE/g | 6.6–22.5 mg rutin equivalent/g | [33] |
| India | 1,688.0–2,016.0 μg GAE/g | — | [89] |
| Turkey | 577.7 mg GAE/100 g | 346.9 mg QE/100 g | [90] |
| India | 1744.0–2687.0 μg GAE/g | 433.0–612.0 μg CE/g | [32] |
| Australia | 19.5–52.5 mg GAE/100 g | — | [91] |
| India | 75.2–79.5 mg GAE/100 g | 201.6–244.9 μg rutin equivalent/g | [51] |
| Pakistan | 36.1–101.6 mg GAE/100 g | 754.2–1147.1 mg GAE/100 g | [38] |
| China | 52.8–64.6 mg GAE/100 g | — | [92] |
| Ethiopia | 1.6–1.9 mg GAE/100 g | 0.5–0.8 mg CE/100 g | [93] |
“—” represents the values that the authors did not report. TE: Trolox equivalent; QE: quercetin equivalent.
Phenolics serve as potent antioxidants by scavenging reactive oxygen and nitrogen species and chelating transition minerals [84]. Oats contain 2.3 mg/100 g of tocopherols [94] and 12.4 to 586.6 mg/kg of total avenanthramides [95]. The total avenanthramide content varies depending on the milling fractions. Hitayezu et al. [96] reported 323.7 to 775.5 μg/g of avenanthramides in different oat milling fractions such as medium bran, fine bran, low bran, and whole oat groats flour. Avenanthramides are unique to oat grains and are not found in other cereal grains [85, 97]. Pioneering work in the identification of avenanthramide structures has been performed by Collins [98], Collins [86], and Collins et al. [99]. Avenanthramides have been reported to improve health parameters in animal and human studies. Avenanthramides have antioxidant, antiproliferative, antiatherogenic, and anti-inflammatory properties [100, 101]. Table 7 presents the total avenanthramides content in dehulled oat from different geographies.
Table 7.
Summary of total avenanthramide content of dehulled oat grains.
Avenanthramides are heat-stable under commercial processing conditions [109, 110]. Magee et al. [111] developed an anti-inflammatory oat-based product that contains a sufficient amount of avenanthramide (0.05–100.00 ppm) combined with hydrocortisone (0.1–1.0% w/w, based on the total weight of the composition). This patent was received under the category “Compositions for inhibiting or reducing skin inflammation.” Yang et al. [112] conducted an extensive study on the antioxidant capacity of avenanthramides (AVAs). The report showed that the antioxidant activity of AVA was 10–30 times higher than that of antioxidants of typical cereal grain components such as ferulic acid, gentisic acid, p-hydroxybenzoic acid, protocatechuic acid, syringic acid, vanillic acid, and vanillin.
Flavonoids are a type of polyphenolic unit that has a C6-C3-C6 skeleton and contains over 4,000 phenolic complexes. Flavonols, flavones, flavanols, flavanones, flavans, and anthocyanins are the most common flavonoids [113]. Collins [114] identified the major flavones present in oat flour as apigenin, luteolin, and tricin. Table 6 summarizes the TFC of oats. Flavonoids have generated interest because of their broad human health-promoting effects, which are related to their antioxidant properties and synergistic effects with other antioxidants and metals [115]. The synergistic effects of the antioxidant properties increased after interaction with iron and copper [116].
Natural antioxidants obtained from oat extracts, particularly avenanthramides, are currently the focus of intense research and interest among food scientists and health professionals. Several studies reveal a positive correlation between antioxidant-rich oat-based foods and a reduced risk of diseases associated with oxidative stress, such as cancer, cardiovascular, and neurodegenerative diseases [12, 23]. Saltzman et al. [117] compared the effects of an oat diet (oatmeal, oat muffin, spice cookies with oats, chocolate cookies with oats, custard with oats, and berry drink with oatmeal) with that of a control diet (cream of wheat, wheat muffin, spice cookies, chocolate cookies, custard, and berry drink with wheat bran) on cardiovascular risk and weight loss in 43 adults (men and women) for two weeks. The findings revealed that an oat-containing hypocaloric diet significantly lowers systolic blood pressure and promotes weight loss in both men and women. Ji et al. [118] reported that supplementing rats' diet with avenanthramide-enriched oat extract at 100 mg/kg diet (providing about 20 mg avenanthramides (Avns)/kg) increases superoxide dismutase (SOD) activity in skeletal muscle, liver, and kidneys, as well as glutathione peroxidase activity in heart and skeletal muscles. Table 8 reviews in vivo and in vitro studies on the positive health outcomes of oat extract and avenanthramides and how these properties were demonstrated. Many authors have reported multiple antioxidative and bioactive molecules of oats with a significant positive health outcome [22, 85, 92, 96, 105, 124].
Table 8.
Summary of in vivo and in vitro studies on the positive health outcomes of oat extracts and avenanthramides.
| Place | Potential bioactive compounds/polyphenol extract of oats | Study type | Observed health outcomes | Sources |
|---|---|---|---|---|
| Canada | Avenanthramide (AV-A, AV-B, and AV-C)-enriched oat extracts | In vivo: human blood samples were collected at 15, 30, and 45 min after drinking the test sample (oat beverage) and at 1, 2, 3, 5, and 10 hrs, and plasma GSH (glutathione or -glutamyl-cysteinyl-glycine tripeptide) status was assessed | Increasing plasma GSH (glutathione or -glutamyl-cysteinyl-glycine tripeptide) status and acting in synergy with other antioxidants such as vitamin E | [119] |
| Turkey | Organic solvent oat extracts | In vivo: test ointments (oat extracts) were applied topically to the wounded site of rats immediately after the wound was created with a surgical blade | Possessing a wound healing effect | [120] |
| Canada | Oat groats flour extract | In vitro: cells were cultured at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, L-glutamine, and penicillin-streptomycin. Cells were then incubated for additional 24 hrs in a new medium containing varying concentrations of oat extracts. The inhibition of nuclear factor kappa beta (NF-kB) was measured using human 293T cells. A TransAM™ NF-kB ELISA kit was used to measure NF-kB binding activity to its consensus binding site | Inhibition of NF-kB, indicating anti-inflammatory activity | [13] |
| India | Ethanol extract of oats flour | In vitro: Fenton's reagent was made by combining ferric chloride, hydrogen peroxide, and ascorbic acid in a 1 : 1 : 1 ratio. Oat extract, Fenton's reagent, DNA, and nuclease-free double-distilled water were used in the reaction. The protective effect of oat extracts was calculated using the retention percentage of normalized supercoiled DNA | DNA damage protection activity | [121] |
| — | Avenanthramides (AV-1p, AV-1c, AV-1f, AV-1s, AV-2p, AV-2c, AV-2f, and AV-2s) | In vitro: the comet assay (single-cell gel electrophoresis) was used to evaluate the test compounds' potential protective effects against DNA damage in cells stressed with hydrogen peroxide. For 24 hrs, HT-29 cells (human colon adenocarcinoma cells) were incubated in a medium containing the test compounds | Antigenotoxic effects | [122] |
| — | Avenanthramide (AV-2c) | In vitro: human aortic smooth muscle cells (SMC) were cultured in the SMBM medium (Cambrex) containing 10% fetal bovine serum (FBS), and the cell culture was kept at 37°C in a humidified incubator supplied with a 95% air and 5% CO2 atmosphere | Inhibition of vascular smooth muscle cell proliferation | [123] |
| — | Avenanthramides (isolated from oats) | In vitro: normal human epidermal neonatal keratinocytes were maintained in the serum-free Epilife medium supplemented with 0.2% (v/v) bovine pituitary extract (BPE), 5 μg/mL bovine insulin, 0.18 μg/mL hydrocortisone, 5 μg/mL bovine transferrin, and 0.2 ng/mL human epidermal growth factor | Anti-inflammatory and anti-itch activity | [22] |
Several studies indicate that oat phenolics serve as potent antioxidants by scavenging reactive oxygen and nitrogen species and chelating transition minerals [84, 112, 125, 126]. Many assays are used to determine the antioxidant activity of cereal grains. Table 9 summarizes the antioxidant activity of oats using ferric reducing antioxidant power (FRAP), oxygen radical absorbance capacity (ORAC), 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) free radical, and 1, 1-diphenyl-2-picrylhydrazyl (DPPH) free radical.
Table 9.
The antioxidant activity of oats using FRAP, ORAC, ABTS+, and DPPH assays.
| FRAP | Source | ORAC | Source | ABTS+ | Source | DPPH | Source |
|---|---|---|---|---|---|---|---|
| 7.4–11.1 mg/g | [127] | 27.7–31.8 µM·TE/g | [96] | 12.1 mg·TE/g | [128] | 76.92–237.14% | [129] |
| 110.5–212.6 µmol Fe2+/g | [129] | 11.0–28.0 µmol·TE/g | [13] | 1.7–3.0 µmol·TE/g | [131] | 506.8–532.8 mg·TE/kg | [88] |
| 104.5–298.8 µmol·TE/100 g | [130] | 32.9–117.9 µmol·TE/g | [131] | 0.8–3.5 mg Trolox/g | [133] | 152.4–280.1 µmol·TE/100 g | [91] |
| 160.8–262.2 µmol·TE/100 g | [91] | 17.1–25.6 µmol TE/g | [92] | IC50 (6.9–8.4 µg/ml) | [83] | 24.3–55.9% | [38] |
| 12.7–21.3 mg AAE/g | [33] | 88.4–99.5% | [121] | 11.2–18.3% | [93] |
“—” represents the values that the authors did not report. TE: Trolox equivalent; QE: quercetin equivalent; IC50: half maximal concentration; AAE/g: ascorbic acid equivalent; FRAP: ferric ions (Fe3+) reducing antioxidant power; ORAC: oxygen radical absorbance capacity; ABTS+: 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) free radical; DPPH: 1, 1-diphenyl-2-picrylhydrazyl.
4. Oat-Based Processed Food Products
The demand for oat-based products has increased in recent years as people have become more aware of the numerous nutritional and health benefits of oats [67]. In 1997, the Food and Drug Administration (FDA) officially recognized the importance of oat fiber with at least 0.75 g of beta-glucan per serving size as a functional food [16]. Furthermore, subsequent studies on the health implications of oat constituents like avenanthramides have raised hopes that the nutritional benefits of oats in human diets may extend far beyond those currently recognized [67]. There are many nonfermented and fermented oat-based products available in the market. Oat is used to make oat-based breakfast cereals, oatmeal, flakes, porridge, granola bars, muesli, oat bread, cookies, biscuits, oatrim, oat milk, infant food, and an oat-based fermented probiotic drink [134]. Table 10 presents some recent fermented oat-based products.
Table 10.
Fermented oat-based products.
| Type of products | Method of preparation | Strains used | Source |
|---|---|---|---|
| Probiotic foods | Made by mixing oat flour (18% w/v) and distilled water for 10 min in a water bath with shaking, fermented for 16 hrs at 37°C with a starter culture of 8 × 108 CFU/g, and stored at 4°C for 21 days | Lactobacillus plantarum UFG9 and its roseoflavin-resistant derivative Lp B2, Lactobacillus plantarum Lp90 and its isogenic Lp90Dcps2 mutant | [135] |
| Functional beverage | An oat-banana matrix with PromOat additive (OBPromOat) containing 353 g/kg of beta-glucan inoculated with 6 log of colony-forming units (CFU/g) of active culture and fermented at 37°C | Streptococcus thermophilus TKM3 KKP 2030p | [136] |
| Functional beverage | After combining oat flour, sugar, and water, the mixture is heated to 95°C for 10 min, cooled to 37°C, and inoculated with a starter culture using orbital shaking at 150 rpm for 8 hrs | Lactobacillus plantarum ATCC 8014 | [137] |
| Beverage (yogurt-like oat beverage) | Made by fermenting oat flakes flour with a starter culture (5 × 107 CFU/mL) at 30°C under stirring (100 rpm). After fermentation, the beverages were pasteurized at 63°C for 30 min and stored at 4°C for 30 days | Lactobacillus plantarum LP01, LP06, LP09, LP32, LP39, LP40, LP48, and LP51; Lactobacillus casei LC10, LC11 and LC03; and Lactobacillus paracasei LPC02 and LPC16 | [138] |
| Nutritionally enhanced food | After sterilizing oat flour (7.5% w/v), rice flour (7.5% w/v), and distilled water (or water +2% w/v of glucose), it was inoculated with a starter culture at 37°C | Lactobacillus paracasei CBA-L74 (Heinz Italia SpA) | [139] |
| Symbiotic oat-based beverage (SOB) | Whole oat flour (10% w/w) was mixed, gelatinized for 1 hr at 80°C, sterilized, cooled to 40°C, and incubated with a starter culture (0.003% w/w). The inoculated oat mixture was fermented at 30°C for 12 hrs with sugar, stabilizers (pectin and carrageenan), vitamin C, and citric acid added | ABY-3 (Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus, with Bifidobacterium BB-12 and Lactobacillus acidophilus LA-5), Lactobacillus helveticus, L. plantarum Vege-Start 60, GIN696265 | [140] |
| Probiotic drink | The mixture of oat mash (containing oat prebiotic beta-glucan), sucrose, and sweeteners (Huxol and sodium cyclamate) was inoculated with a starter culture at 37°C for 6–10 hrs and then stored at 4–6°C for 24 days | Lactobacillus plantarum B28 | [16] |
| Probiotic drink | After sterilizing, a suspension of oat flour (8% w/v) in distilled water, pasteurized honey (3% w/v), and a starter culture (1% v/v) were inoculated to achieve an initial cell count of 109/mL and allowed to ferment for 48 hrs at 37°C with shaking (180 rpm) | Lactobacillus plantarum M-13 | [141] |
| Probiotic drink | Made by fermenting oat flour with mixed strains and enriching it with isoflavones. The mixture solution was incubated anaerobically at 37°C for 48 hrs and then stored for 4 weeks at 4°C | Streptococcus thermophilus (TH-4®, strain number DSM15957) and probiotic strain Lactobacillus acidophilus (LA-5®, strain number: DSM13241) | [142] |
| Gruel (with properties that increase nonhaem iron absorption) | Wholegrain oatmeal (Kungso rnen AB, Jarna, Sweden) and water were mixed with different enzymes and heated to form oat gruel. The selected culture was inoculated into the heat-treated gruel. The organic acids DL-lactic acid and acetic acid were also added after fermentation | Lactobacillus plantarum 299v | [143] |
| Probiotic drink (oat milk) | The mixture of oat and water (8 : 100 w/v) was agitated for 20 min to produce oat milk, which was then homogenized for 3 min at 13,500 r/min and sterilized. A starter culture (1 : 1 volume ratio of L. reuteri and S. thermophilus PBS buffer suspensions) was incubated at 40°C. The fermentation process was stopped at a pH of 4.4–4.6, and the drink was finally stored at 4°C | Lactobacillus reuteri ATCC 55730 and Streptococcus thermophilus CECT 986 | [144] |
| Probiotic food | Whole grain oat flour and tap water were mixed in a 5.5% (w/v) ratio. Sucrose 1.5% (w/v) was added to the slurry, sterilized, and cooled to 37°C. The oat mash was inoculated with 5% (v/v) pure culture or mixed cultures. The cell count of the yeast cultures used was 109 CFU/mL and that of the LAB cultures was 1011 CFU/mL. Fermentation lasted 8–10 hrs at 37°C. The fermented oat was then kept at 4–6°C for 24 days | Lactobacillus plantarum B28, L casei spp paracasei B29, Candida rugosa Y28, C. lambica Y30 | [145] |
| Functional food | The whey protein concentrate (WPC) was developed by sterilizing distilled water with 5% whey protein concentrate (WPC70, 70% w/w protein). WPC medium was fermented for 10 hrs at 37°C. Fermented WPC was diluted (1 : 1, 2 : 1, 3 : 1) with mango pulp of the variety Ataulfo (Mangifera indica), pasteurized, and finally stored at 4°C | Lactobacillus acidophilus NCDC 291, Lactobacillus bulgaricus NCDC304 | [146] |
| Nutritionally enhanced food | The following ingredients were combined and homogenized: whole oat flour, moringa leaves, sugar, and tap water. Each probiotic bacteria-activated culture (108 CFU/mL) was added to the previous oat mixtures separately at a concentration of 1% (v/w) and incubated for 24 hrs at 37°C for L. plantarum fermentation and 8 hrs for L. delbrueckii ssp. bulgaricus fermentation. The fermented oat products were kept at 4°C for 21 days | Lactobacillus plantaram ATCC 14,917, Lactobacillus delbrueckii ssp. bulgaricus EMCC 11,102 | [147] |
| Nutritional and sensory-optimized beverage | Made by fermenting the flours of toasted oat (60–70% w/w), boiled stinging nettle leaves (5–15% w/w), roasted and soaked debittered white lupine (10–25% w/w), and 10% w/w premix. The premix was made by mixing flour of 2.8% w/w toasted black cardamom, 2.8% w/w malted wheat, 2.6% w/w pumpkin pulp, 1.1% w/w spiced chili pepper, and 0.7% table salt | Spontaneous fermentation | [148] |
The incorporation of oats has been shown to improve the overall quality of food [134]. Sanchez-Pardo et al. [149] found that pound cake made with 25% (w/w) oat fiber had better textural characteristics than the conventional product. Bread is an integral part of the daily diet of a large part of the world's population. According to Flander et al. [150], oat-based bread has a mild nutty and pleasant flavor. Because oats retain moisture well, bread stays fresher for longer [151]. Adding oat starch or oat lecithin to wheat bread was found to slow the bread's staling rate [152]. Oat starches and their modified products were organoleptically comparable to conventional ones used in pasta products [153]. Boukid [154] identified oat proteins as an emerging ingredient for food formulation.
Khanna and Mohan [155] discussed the suitability of the incorporation of oats into Indian diets such as bread (chapattis and missi rotis), breakfast items (upma and poha), snacks (biscuits), and beverages (smoothies and shakes). With a partial substitution of up to 50% of oats, the modified traditional products had acceptable sensory and textural characteristics. Chauhan et al. [151] also demonstrated that the incorporation of oat flour in the preparation of value-added functional foods such as bread and noodles was successful. Oats and their resistant starches make low-calorie, low-fat, high-fiber granola bars and cereals [26]. Breakfast cereals made with oats have received a lot of attention recently. These are high in functional ingredients such as β-glucan and bioactive components (high polyphenol and antioxidant content), which are known to lower serum and plasma cholesterol levels and postprandial glycemic response [156].
Another advantage of oats as a food ingredient is that they do not contain gluten; their storage proteins are avenins [157, 158]. As a result, they can be used in gluten-free food formulations for coeliac patients, as avenins are less likely to cause allergies [158]. Oat fiber with a minimum of 0.75 g of beta-glucan per serving size was used as a functional food in 1997 [16]. Oats have desirable properties for incorporation into various food formulations, although there are limitations to using them in bakery products for it lacks gluten which provides the elasticity and structure required for bread dough. As a result, most oat bread still contains wheat flour to make the dough rise and have a pleasing appearance [159].
5. Conclusion
This review shows that oats are significant sources of valuable nutrients, particularly protein and fat, with a significant concentration of healthy mono- and polyunsaturated fatty acids and a balanced amino acid composition. They are a good source of minerals that our bodies require. Oat grains are also an important source of natural antioxidants, which benefit human health by reducing the risk of various diseases. Oats are now used in many functional food formulations around the world, and they are important component of a healthy diet. Therefore, with respect to their nutritional, medicinal, and therapeutic properties, oats are credited as a good plant food for the future and are recommended for a healthier world.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Barcchiya J., Meena R. K., Lal N. Oat is a multifunctional cereal crop. Innovative Farming . 2017;2:114–116. [Google Scholar]
- 2.Webster F. Oats Cereal Qrain Quality . Berlin, Germany: Springer; 1996. [Google Scholar]
- 3.Stevens E. J., Armstrong K. W., Bezar H. J., Griffin W. B., Hampton J. G. Fodder oats an overview. Fodder oats: A World Overview . 2004;33:11–18. [Google Scholar]
- 4.Loskutov I. G., Rines H. W. Wild Crop Relatives: Genomic and Breeding Resources . Berlin, Germany: Springer; 2011. Avena. [Google Scholar]
- 5.Köse Ö. E., Mut Z., Akay H. Assessment of grain yield and quality traits of diverse oat (Avena sativa L.) Genotypes. Annali di Botanica . 2021;11:55–66. doi: 10.13133/2239-3129/16777. [DOI] [Google Scholar]
- 6.Butt M. S., Tahir-Nadeem M., Khan M. K. I., Shabir R., Butt M. S. Oat: Unique among the cereals. European Journal of Nutrition . 2008;47(2):68–79. doi: 10.1007/s00394-008-0698-7. [DOI] [PubMed] [Google Scholar]
- 7.Kumar L., Sehrawat R., Kong Y. Oat proteins: A perspective on functional properties. LWT--Food Science and Technology . 2021;152 doi: 10.1016/j.lwt.2021.112307.112307 [DOI] [Google Scholar]
- 8.Singh R., De S., Belkheir A. Avena sativa (Oat), A potential neutraceutical and therapeutic agent: an overview. Critical Reviews in Food Science and Nutrition . 2013;53(2):126–144. doi: 10.1080/10408398.2010.526725. [DOI] [PubMed] [Google Scholar]
- 9.Banaś A., Debski H., Banaś W., et al. Lipids in grain tissues of oat (Avena sativa): differences in content, time of deposition, and fatty acid composition. Journal of Experimental Botany . 2007;58(10):2463–2470. doi: 10.1093/jxb/erm125. [DOI] [PubMed] [Google Scholar]
- 10.Kivelä R., Henniges U., Sontag-Strohm T., Potthast A. Oxidation of oat β-glucan in aqueous solutions during processing. Carbohydrate Polymers . 2012;87(1):589–597. doi: 10.1016/j.carbpol.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad A., Anjum F. M., Zahoor T., Nawaz H., Ahmed Z. Extraction and characterization of β-d-glucan from oat for industrial utilization. International Journal of Biological Macromolecules . 2010;46(3):304–309. doi: 10.1016/j.ijbiomac.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Peterson D. M. Oat antioxidants. Journal of Cereal Science . 2001;33(2):115–129. doi: 10.1006/jcrs.2000.0349. [DOI] [Google Scholar]
- 13.Chu Y.-F., Wise M. L., Gulvady A. A., et al. In vitro antioxidant capacity and anti-inflammatory activity of seven common oats. Food Chemistry . 2013;139(1-4):426–431. doi: 10.1016/j.foodchem.2013.01.104. [DOI] [PubMed] [Google Scholar]
- 14.Getaneh F., Forsido S., Yetenayet B., Addisu A., Minbale A., Endale A. Traditional food processing practices of oats (Avena sativa) and its contribution to food security in Gozamin district of northwest Ethiopia. African Journal of Food, Agriculture, Nutrition and Development . 2021;21(5):18083–18100. doi: 10.18697/ajfand.100.19810. [DOI] [Google Scholar]
- 15.Lim T. Avena Sativa. Edible Medicinal and Non-medicinal Plants . Berlin, Germany: Springer; 2013. [Google Scholar]
- 16.Angelov A., Gotcheva V., Kuncheva R., Hristozova T. Development of a new oat-based probiotic drink. International Journal of Food Microbiology . 2006;112(1):75–80. doi: 10.1016/j.ijfoodmicro.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Angelov A., Yaneva-Marinova T., Gotcheva V. Oats as a matrix of choice for developing fermented functional beverages. Journal of Food Science and Technology . 2018;55(7):2351–2360. doi: 10.1007/s13197-018-3186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L., Wu D., Schlundt J., Conway P. L. Development of a dairy-free fermented oat-based beverage with enhanced probiotic and bioactive properties. Frontiers in Microbiology . 2020;11 doi: 10.3389/fmicb.2020.609734.609734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joyce S. A., Kamil A., Fleige L., Gahan C. G. The cholesterol-lowering effect of oats and oat beta glucan: modes of action and potential role of bile acids and the microbiome. Frontiers in Nutrition . 2019;6:171–215. doi: 10.3389/fnut.2019.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Villaluenga C., Penas E. Health benefits of oat: current evidence and molecular mechanisms. Current Opinion in Food Science . 2017;14:26–31. doi: 10.1016/j.cofs.2017.01.004. [DOI] [Google Scholar]
- 21.Ratnasari N., Walters M., Tsopmo A. Antioxidant and lipoxygenase activities of polyphenol extracts from oat brans treated with polysaccharide degrading enzymes. Heliyon . 2017;3(7) doi: 10.1016/j.heliyon.2017.e00351.e00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sur R., Nigam A., Grote D., Liebel F., Southall M. D. Avenanthramides, polyphenols from oats, exhibit anti-inflammatory and anti-itch activity. Archives of Dermatological Research . 2008;300(10):569–574. doi: 10.1007/s00403-008-0858-x. [DOI] [PubMed] [Google Scholar]
- 23.Tripathi V., Singh A., Ashraf M. Avenanthramides of oats: medicinal importance and future perspectives. Pharmacognosy Reviews . 2018;12(23):66–71. doi: 10.4103/phrev.phrev_34_17. [DOI] [Google Scholar]
- 24.Ryan D., Kendall M., Robards K. Bioactivity of oats as it relates to cardiovascular disease. Nutrition Research Reviews . 2007;20(2):147–162. doi: 10.1017/S0954422407782884. [DOI] [PubMed] [Google Scholar]
- 25.Zhu F. Structures, properties, modifications, and uses of oat starch. Food Chemistry . 2017;229:329–340. doi: 10.1016/j.foodchem.2017.02.064. [DOI] [PubMed] [Google Scholar]
- 26.Punia S., Sandhu K. S., Dhull S. B., et al. Oat starch: physico-chemical, morphological, rheological characteristics and its applications-A review. International Journal of Biological Macromolecules . 2020;154:493–498. doi: 10.1016/j.ijbiomac.2020.03.083. [DOI] [PubMed] [Google Scholar]
- 27.Rezende E. S. V., Lima G. C., Naves M. M. V. Dietary fibers as beneficial microbiota modulators: a proposed classification by prebiotic categories. Nutrition . 2021;89 doi: 10.1016/j.nut.2021.111217.111217 [DOI] [PubMed] [Google Scholar]
- 28.Rebello C. J., O’Neil C. E., Greenway F. L. Dietary fiber and satiety: the effects of oats on satiety. Nutrition Reviews . 2016;74(2):131–147. doi: 10.1093/nutrit/nuv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.USDA. U.S. Department of agriculture, agricultural research service. FoodData central, oat. 2019. https://fdc.nal.usda.gov/fdc-app.html#/food-details/1101825/nutrients .
- 30.Rani M., Bandral J. D., Sood M., Sharma S., Gupta S., Chand G. Effect of germination on physico-chemical and antinutritional factors of oats flour. The Pharma Innovation Journal . 2022;11:1424–1428. [Google Scholar]
- 31.Biel W., Maciorowski R., Bobko K., Jaskowska I. Chemical composition and energy value of dwarf oats grain. Italian Journal of Food Science . 2011;23:180–187. [Google Scholar]
- 32.Sandhu K. S., Godara P., Kaur M., Punia S. Effect of toasting on physical, functional and antioxidant properties of flour from oat (Avena sativa L.) cultivars. Journal of the Saudi Society of Agricultural Sciences . 2017;16(2):197–203. doi: 10.1016/j.jssas.2015.06.004. [DOI] [Google Scholar]
- 33.Marmouzi I., Saidi N., Meddah B., et al. Nutritional characteristics, biochemical composition and antioxidant activities of Moroccan Oat varieties. Journal of Food Measurement and Characterization . 2016;10(1):156–165. doi: 10.1007/s11694-015-9289-5. [DOI] [Google Scholar]
- 34.Kudake D., Pawar A., Muley A., Parate V., Talib M. V., Talib M. Enrichment of wheat flour noodles with oat flour: effect on physical, nutritional, antioxidant and sensory properties. International Journal of Current Microbiology and Applied Sciences . 2017;6(12):204–213. doi: 10.20546/ijcmas.2017.612.026. [DOI] [Google Scholar]
- 35.Manzoor M., Pasha I., Shehzad A., Zia M., Zhu M. Antioxidant profiling of indigenous oat cultivars with special reference to avenanthramides. International Food Research Journal . 2020;27:261–269. [Google Scholar]
- 36.Virkki L., Johansson L., Ylinen M., Maunu S., Ekholm P. Structural characterization of water-insoluble nonstarchy polysaccharides of oats and barley. Carbohydrate Polymers . 2005;59(3):357–366. doi: 10.1016/j.carbpol.2004.10.006. [DOI] [Google Scholar]
- 37.Ihsan M., Nisar M., Nazir N., et al. Genetic diversity in nutritional composition of oat (Avena sativa L.) germplasm reported from Pakistan. Saudi Journal of Biological Sciences . 2022;29(3):1487–1500. doi: 10.1016/j.sjbs.2021.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ibrahim M. S., Ahmad A., Sohail A., Asad M. J. Nutritional and functional characterization of different oat (Avena sativa L.) cultivars. International Journal of Food Properties . 2020;23(1):1373–1385. doi: 10.1080/10942912.2020.1806297. [DOI] [Google Scholar]
- 39.Youssef M., Nassar A., El–Fishawy F., Mostafa M. Assessment of proximate chemical composition and nutritional status of wheat biscuits fortified with oat powder. Assiut Journal of Agricultural Sciences . 2016;47:83–94. [Google Scholar]
- 40.Biel W., Bobko K., Maciorowski R. Chemical composition and nutritive value of husked and naked oats grain. Journal of Cereal Science . 2009;49(3):413–418. doi: 10.1016/j.jcs.2009.01.009. [DOI] [Google Scholar]
- 41.Alemayehu G. F., Forsido S. F., Tola Y. B., Teshager M. A., Assegie A. A., Amare E. Proximate, mineral and anti-nutrient compositions of oat grains (Avena sativa) cultivated in Ethiopia: implications for nutrition and mineral bioavailability. Heliyon . 2021;7(8) doi: 10.1016/j.heliyon.2021.e07722.e07722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menon R., Gonzalez T., Ferruzzi M., Jackson E., Winderl D., Watson J. Oats-from farm to fork. Advances in Food & Nutrition Research . 2016;77:1–55. doi: 10.1016/bs.afnr.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad A., Khalid N. Biopolymers for Food Design . Amsterdam, The Netherland: Elsevier; 2018. Dietary fibers in modern food production: A special perspective with β-glucans. [Google Scholar]
- 44.Van Den Broeck H. C., Londono D. M., Timmer R., Smulders M. J., Gilissen L. J., Van Der Meer I. M. Profiling of nutritional and health-related compounds in oat varieties. Foods . 2015;5(4):2–11. doi: 10.3390/foods5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sikora P., Tosh S. M., Brummer Y., Olsson O. Identification of high β-glucan oat lines and localization and chemical characterization of their seed kernel β-glucans. Food Chemistry . 2013;137(1):83–91. doi: 10.1016/j.foodchem.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Skendi A., Biliaderis C., Lazaridou A., Izydorczyk M. Structure and rheological properties of water soluble β-glucans from oat cultivars of Avena sativa and Avena bysantina. Journal of Cereal Science . 2003;38(1):15–31. doi: 10.1016/S0733-5210(02)00137-6. [DOI] [Google Scholar]
- 47.Cui S., Liu R. H. Oats Nutrition and Technology . Chichester, England: John Wiley and Sons, Ltd.; 2013. Health benefits of oat phytochemicals; pp. 171–194. [DOI] [Google Scholar]
- 48.Wood P. J. Advanced dietary fibre technology . Hoboken, NJ, USA: Blackwell Science Ltd; 2008. 28 cereal β-glucans: structure, properties and health claims; p. p. 315. [Google Scholar]
- 49.Yoo H.-U., Ko M.-J., Chung M.-S. Hydrolysis of beta-glucan in oat flour during subcritical-water extraction. Food Chemistry . 2020;308 doi: 10.1016/j.foodchem.2019.125670.125670 [DOI] [PubMed] [Google Scholar]
- 50.Antonini E., Lombardi F., Alfieri M., Diamantini G., Redaelli R., Ninfali P. Nutritional characterization of naked and dehulled oat cultivar samples at harvest and after storage. Journal of Cereal Science . 2016;72:46–53. doi: 10.1016/j.jcs.2016.09.016. [DOI] [Google Scholar]
- 51.Shah A., Masoodi F., Gani A., Ashwar B. A. Newly released oat varieties of himalayan region-techno-functional, rheological, and nutraceutical properties of flour. LWT-Food Science and Technology . 2016;70:111–118. doi: 10.1016/j.lwt.2016.02.033. [DOI] [Google Scholar]
- 52.Poonia A., Phogat D., Nagar S., Nagar S., Sharma P., Kumar V. Biochemical assessment of oat genotypes revealed variability in grain quality with nutrition and crop improvement implications. Food Chemistry . 2022;377 doi: 10.1016/j.foodchem.2021.131982.131982 [DOI] [PubMed] [Google Scholar]
- 53.Zhang K., Li X., Ma Z., Hu X. Solvent retention capacity of oat flour: relationship with oat β-glucan content and molecular weight. Food Hydrocolloids . 2019;93:19–23. doi: 10.1016/j.foodhyd.2019.02.001. [DOI] [Google Scholar]
- 54.Biel W., Kazimierska K., Bashutska U. Nutritional value of wheat, triticale, barley and oat grains. Acta Scientiarum Polonorum Zootechnica . 2020;19(2):19–28. doi: 10.21005/asp.2020.19.2.03. [DOI] [Google Scholar]
- 55.Németh R., Turóczi F., Csernus D., Solymos F., Jaksics E., Tömösközi S. Characterization of chemical composition and techno-functional properties of oat cultivars. Cereal Chemistry . 2021;98(6):1183–1192. doi: 10.1002/cche.10470. [DOI] [Google Scholar]
- 56.kaur S., Bhardwaj R. D., Kapoor R., Grewal S. K. Biochemical characterization of oat (Avena sativa L.) genotypes with high nutritional potential. LWT-Food Science and Technology . 2019;110:32–39. doi: 10.1016/j.lwt.2019.04.063. [DOI] [Google Scholar]
- 57.Menkovska M., Damjanovski D., Levkov V., et al. Content of Β–glucan in cereals grown by organic and conventional farming. Banat’s Journal of Biotechnology . 2017;8(16):39–47. doi: 10.7904/2068-4738-VIII(16)-39. [DOI] [Google Scholar]
- 58.Biel W., Jacyno E., Kawęcka M. Chemical composition of hulled, dehulled and naked oat grains. South African Journal of Animal Science . 2014;44(2):p. 189. doi: 10.4314/sajas.v44i2.12. [DOI] [Google Scholar]
- 59.Wood P. J. Cereal β-glucans in diet and health. Journal of Cereal Science . 2007;46(3):230–238. doi: 10.1016/j.jcs.2007.06.012. [DOI] [Google Scholar]
- 60.Othman R. A., Moghadasian M. H., Jones P. J. Cholesterol-lowering effects of oat β-glucan. Nutrition Reviews . 2011;69(6):299–309. doi: 10.1111/j.1753-4887.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- 61.Jalili T., Mah E., Medeiros D. M., Wildman R. E. Handbook of Nutraceuticals and Functional Foods . Boca Raton, FL, USA: CRC Press; 2019. Dietary fiber and coronary heart disease. [Google Scholar]
- 62.NCEP National Cholesterol Education Program (US) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2nd. College Park, MD, USA: NCEP National Cholesterol Education Program (US); 2002. Expert panel on detection, & treatment of high blood cholesterol in adults. [Google Scholar]
- 63.Zhu Y., Dong L., Huang L., et al. Effects of oat β-glucan, oat resistant starch, and the whole oat flour on insulin resistance, inflammation, and gut microbiota in high-fat-diet-induced type 2 diabetic rats. Journal of Functional Foods . 2020;69 doi: 10.1016/j.jff.2020.103939.103939 [DOI] [Google Scholar]
- 64.Chen O., Mah E., Dioum E., et al. The role of oat nutrients in the immune system: a narrative review. Nutrients . 2021;13(4):p. 1048. doi: 10.3390/nu13041048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kriger O., Kashirskikh E., Babich O., Noskova S. Oat protein concentrate production. Foods and Raw Materials . 2018;6(1):47–55. doi: 10.21603/2308-4057-2018-1-47-55. [DOI] [Google Scholar]
- 66.Klose C., Arendt E. K. Proteins in oats; their synthesis and changes during germination: A review. Critical Reviews in Food Science and Nutrition . 2012;52(7):629–639. doi: 10.1080/10408398.2010.504902. [DOI] [PubMed] [Google Scholar]
- 67.Sangwan S., Singh R., Tomar S. K. Nutritional and functional properties of oats: An update. Journal of Innovative Biology . 2014;1:3–14. [Google Scholar]
- 68.Sterna V., Zute S., Brunava L. Oat grain composition and its nutrition benefice. Agriculture and Agricultural Science Procedia . 2016;8:252–256. doi: 10.1016/j.aaspro.2016.02.100. [DOI] [Google Scholar]
- 69.Liu G., Li J., Shi K., et al. Composition, secondary structure, and self-assembly of oat protein isolate. Journal of Agricultural and Food Chemistry . 2009;57(11):4552–4558. doi: 10.1021/jf900135e. [DOI] [PubMed] [Google Scholar]
- 70.Kouřimská L., Sabolová M., Horčička P., Rys S., Božik M. Lipid content, fatty acid profile, and nutritional value of new oat cultivars. Journal of Cereal Science . 2018;84:44–48. doi: 10.1016/j.jcs.2018.09.012. [DOI] [Google Scholar]
- 71.Ahmet B., Ümit G., Musa Ö. M., Ziya D., Nurhan U. Oil contents and fatty acid composition of oat (Avena sativa) Seed and oils. Journal of Agroalimentary Processes and Technologies . 2019;25:182–186. [Google Scholar]
- 72.Kouřimská L., Pokhrel K., Božik M., Tilami S. K., Horčička P. Fat content and fatty acid profiles of recently registered varieties of naked and hulled oats with and without husks. Journal of Cereal Science . 2021;99 doi: 10.1016/j.jcs.2021.103216.103216 [DOI] [Google Scholar]
- 73.Biel W., Jacyno E. Chemical composition and nutritive value of protein in hulled dwarf oat lines and the effect on serum lipid profile in rats. Italian Journal of Food Science . 2014;26:p. 203. [Google Scholar]
- 74.Godswill A. G., Somtochukwu I. V., Ikechukwu A. O., Kate E. C. Health benefits of micronutrients (vitamins and minerals) and their associated deficiency diseases: a systematic review. International Journal of Food Sciences . 2020;3:1–32. doi: 10.47604/ijf.1024. [DOI] [Google Scholar]
- 75.Shenkin A. The key role of micronutrients. Clinical Nutrition . 2006;25:1–13. doi: 10.1016/j.clnu.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 76.De Oliveira Maximino J. V., Barros L. M., Pereira R. M., et al. Mineral and fatty acid content variation in white oat genotypes grown in Brazil. Biological Trace Element Research . 2021;199(3):1194–1206. doi: 10.1007/s12011-020-02229-1. [DOI] [PubMed] [Google Scholar]
- 77.Gabrovska D., Fiedlerová V., Holasová M., et al. Nutritional changes of common oat (Avena sativa L.) and naked oat (Avena nuda L.) during germination. Czech Journal of Food Sciences . 2004;22(5):317–320. doi: 10.17221/10691-cjfs. [DOI] [Google Scholar]
- 78.Özcan M., Bağcı A., Dursun N., et al. Macro and micro element contents of several oat (Avena sativa L.) genotype and variety grains. Iranian Journal of Chemistry and Chemical Engineering (International English Edition) . 2017;36:73–79. [Google Scholar]
- 79.Devasagayam T., Tilak J., Boloor K., Sane K. S., Ghaskadbi S. S., Lele R. Free radicals and antioxidants in human health: current status and future prospects. Journal of the Association of Physicians of India . 2004;52:794–804. [PubMed] [Google Scholar]
- 80.Phaniendra A., Jestadi D. B., Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian Journal of Clinical Biochemistry . 2015;30(1):11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jaleel A. Ageing and health: free radicals and oxidative stress. Journal of the College of Physicians and Surgeons-Pakistan: JCPSP . 2008;18(8):465–466. [PubMed] [Google Scholar]
- 82.A Puertollano M., Puertollano E., Alvarez De Cienfuegos G., A De Pablo M. Dietary antioxidants: immunity and host defense. Current Topics in Medicinal Chemistry . 2011;11(14):1752–1766. doi: 10.2174/156802611796235107. [DOI] [PubMed] [Google Scholar]
- 83.Tong L.-T., Liu L.-Y., Zhong K., Wang Y., Guo L.-N., Zhou S.-M. Effects of cultivar on phenolic content and antioxidant activity of naked oat in China. Journal of Integrative Agriculture . 2014;13(8):1809–1816. doi: 10.1016/S2095-3119(13)60626-7. [DOI] [Google Scholar]
- 84.Boz H. Phenolic amides (avenanthramides) in oats-a review. Czech Journal of Food Sciences . 2015;33(5):399–404. doi: 10.17221/696/2014-cjfs. [DOI] [Google Scholar]
- 85.Meydani M. Potential health benefits of avenanthramides of oats. Nutrition Reviews . 2009;67(12):731–735. doi: 10.1111/j.1753-4887.2009.00256.x. [DOI] [PubMed] [Google Scholar]
- 86.Collins F. W. Oat phenolics: avenanthramides, novel substituted N-cinnamoylanthranilate alkaloids from oat groats and hulls. Journal of Agricultural and Food Chemistry . 1989;37(1):60–66. doi: 10.1021/jf00085a015. [DOI] [Google Scholar]
- 87.Orozco-Mena R., Salmerón-Ochoa I., Ortega-Rivas E., Perez-Vega S. Development of a sustainable process for the solid-liquid extraction of antioxidants from oat. Sustainability . 2014;6(3):1504–1520. doi: 10.3390/su6031504. [DOI] [Google Scholar]
- 88.Capouchová I., Burešová B., Paznocht L., et al. Antioxidant activity and content of selected antioxidant compounds in grain of different oat cultivars. Plant Soil and Environment . 2020;66(7):327–333. doi: 10.17221/212/2020-PSE. [DOI] [Google Scholar]
- 89.Kaur M., Singh S. Physical characteristics of different oat cultivars:Influence on pasting, functional and antioxidant properties. Quality Assurance and Safety of Crops & Foods . 2017;9(3):285–293. doi: 10.3920/QAS2016.0987. [DOI] [Google Scholar]
- 90.Altuner F., Tuncturk R., Oral E., Tuncturk M. Evaluation of pigment, antioxidant capacity and bioactive compounds in microgreens of wheat landraces and cereals. Chilean Journal of Agricultural Research . 2021;81(4):643–654. doi: 10.4067/S0718-58392021000400643. [DOI] [Google Scholar]
- 91.Rao S., Santhakumar A. B., Chinkwo K. A., Blanchard C. L. Characterization of phenolic compound antioxidant activity in oat varieties using UHPLC–online ABTS and LC Q-TOF. Cereal Chemistry . 2019;96(5):958–966. doi: 10.1002/cche.10200. [DOI] [Google Scholar]
- 92.Chen C., Wang L., Wang R., et al. Phenolic contents, cellular antioxidant activity and antiproliferative capacity of different varieties of oats. Food Chemistry . 2018;239:260–267. doi: 10.1016/j.foodchem.2017.06.104. [DOI] [PubMed] [Google Scholar]
- 93.Alemayehu G. F., Forsido S. F., Tola Y. B., Teshager M. A., Assegie A. A., Amare E. International Conference on Advances of Science and Technology . Berlin, Germany: Springer; 2021. Effect of toasting and natural fermentation on the phytochemical and functional properties of oats grown in Ethiopia. [Google Scholar]
- 94.Lásztity R. Oat grain: a wonderful reservoir of natural nutrients and biologically active substances. Food Reviews International . 1998;14(1):99–119. doi: 10.1080/87559129809541150. [DOI] [Google Scholar]
- 95.Leonova S., Gnutikov A., Loskutov I., Blinova E., Gustafsson K.-E., Olsson O. Diversity of avenanthramide content in wild and cultivated oats. Proceedings on applied botany, genetics and breeding . 2020;181(1):30–47. doi: 10.30901/2227-8834-2020-1-30-47. [DOI] [Google Scholar]
- 96.Hitayezu R., Baakdah M. M., Kinnin J., Henderson K., Tsopmo A. Antioxidant activity, avenanthramide and phenolic acid contents of oat milling fractions. Journal of Cereal Science . 2015;63:35–40. doi: 10.1016/j.jcs.2015.02.005. [DOI] [Google Scholar]
- 97.Liu L., Zubik L., Collins F. W., Marko M., Meydani M. The antiatherogenic potential of oat phenolic compounds. Atherosclerosis . 2004;175(1):39–49. doi: 10.1016/j.atherosclerosis.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 98.Collins F. Oat phenolics: isolation and structural elucidation of substituted benzox-azinones. Cereal Foods World . 1983;28:p. 560. [Google Scholar]
- 99.Collins F. W., Mclachlan D., Blackwell B. Oat phenolics: avenalumic acids, a new group of bound phenolic acids from oat groats and hulls. Cereal Chemistry . 1991;68:184–189. [Google Scholar]
- 100.Peterson D. M., Hahn M. J., Emmons C. L. Oat avenanthramides exhibit antioxidant activities in vitro. Food Chemistry . 2002;79(4):473–478. doi: 10.1016/S0308-8146(02)00219-4. [DOI] [Google Scholar]
- 101.Raguindin P. F., Adam Itodo O., Stoyanov J., et al. A systematic review of phytochemicals in oat and buckwheat. Food Chemistry . 2021;338 doi: 10.1016/j.foodchem.2020.127982.127982 [DOI] [PubMed] [Google Scholar]
- 102.Li X.-P., Li M.-Y., Ling A.-J., et al. Effects of genotype and environment on avenanthramides and antioxidant activity of oats grown in northwestern China. Journal of Cereal Science . 2017;73:130–137. doi: 10.1016/j.jcs.2016.12.005. [DOI] [Google Scholar]
- 103.Pridal A. A., Böttger W., Ross A. B. Analysis of avenanthramides in oat products and estimation of avenanthramide intake in humans. Food Chemistry . 2018;253:93–100. doi: 10.1016/j.foodchem.2018.01.138. [DOI] [PubMed] [Google Scholar]
- 104.Michels D. K., Chatham L. A., Butts-Wilmsmeyer C. J., Juvik J. A., Kolb F. L. Variation in avenanthramide content in spring oat over multiple environments. Journal of Cereal Science . 2020;91 doi: 10.1016/j.jcs.2019.102886.102886 [DOI] [Google Scholar]
- 105.Hernandez-Hernandez O., Pereira-Caro G., Borges G., Crozier A., Olsson O. Characterization and antioxidant activity of avenanthramides from selected oat lines developed by mutagenesis technique. Food Chemistry . 2021;343 doi: 10.1016/j.foodchem.2020.128408.128408 [DOI] [PubMed] [Google Scholar]
- 106.Dvořáček V., Jágr M., Kotrbová Kozak A., et al. Avenanthramides: unique bioactive substances of oat grain in the context of cultivar, cropping system, weather conditions and other grain parameters. Plants . 2021;10(11):p. 2485. doi: 10.3390/plants10112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Woolman M., Liu K. Simplified analysis and expanded profiles of avenanthramides in oat grains. Foods . 2022;11(4):p. 560. doi: 10.3390/foods11040560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xie Z., Mui T., Sintara M., et al. Rapid quantitation of avenanthramides in oat-containing products by high-performance liquid chromatography coupled with triple quadrupole mass spectrometry (HPLC-TQMS) Food Chemistry . 2017;224:280–288. doi: 10.1016/j.foodchem.2016.12.079. [DOI] [PubMed] [Google Scholar]
- 109.Bryngelsson S., Dimberg L. H., Kamal-Eldin A. Effects of commercial processing on levels of antioxidants in oats (Avena sativa L.) Journal of Agricultural and Food Chemistry . 2002;50(7):1890–1896. doi: 10.1021/jf011222z. [DOI] [PubMed] [Google Scholar]
- 110.Dimberg L. H., Sunnerheim K., Sundberg B., Walsh K. Stability of oat avenanthramides. Cereal Chemistry Journal . 2001;78(3):278–281. doi: 10.1094/CCHEM.2001.78.3.278. [DOI] [Google Scholar]
- 111.Magee L., Liebel F., Southall M. Compositions for inhibiting or reducing inflammation of skin. U.S. Patent Application . 2007;11:p. 888. [Google Scholar]
- 112.Yang J., Ou B., Wise M. L., Chu Y. In vitro total antioxidant capacity and anti-inflammatory activity of three common oat-derived avenanthramides. Food Chemistry . 2014;160:338–345. doi: 10.1016/j.foodchem.2014.03.059. [DOI] [PubMed] [Google Scholar]
- 113.Tanwar B., Modgil R. Flavonoids: dietary occurrence and health benefits. Spatula DD . 2012;2(1):59–68. doi: 10.5455/spatula.20120328100506. [DOI] [Google Scholar]
- 114.Collins F. W. Oats: Chemistry and technology . St. Paul, MN, USA: American Association of Cereal Chemists, Inc; 1986. Oat phenolics: structure, occurrence, and function; pp. 227–295. [Google Scholar]
- 115.Yao L. H., Jiang Y.-M., Shi J., et al. Flavonoids in food and their health benefits. Plant Foods for Human Nutrition . 2004;59(3):113–122. doi: 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]
- 116.Mira L., Tereza Fernandez M., Santos M., Rocha R., Helena Florêncio M., Jennings K. R. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radical Research . 2002;36(11):1199–1208. doi: 10.1080/1071576021000016463. [DOI] [PubMed] [Google Scholar]
- 117.Saltzman E., Das S. K., Lichtenstein A. H., et al. An oat-containing hypocaloric diet reduces systolic blood pressure and improves lipid profile beyond effects of weight loss in men and women. The Journal of Nutrition . 2001;131(5):1465–1470. doi: 10.1093/jn/131.5.1465. [DOI] [PubMed] [Google Scholar]
- 118.Ji L., Lay D., Chung E., Fu Y., Peterson D. M. Effects of avenanthramides on oxidant generation and antioxidant enzyme activity in exercised rats. Nutrition Research . 2003;23(11):1579–1590. doi: 10.1016/s0271-5317(03)00165-9. [DOI] [Google Scholar]
- 119.Chen C.-Y. O., Milbury P. E., Collins F. W., Blumberg J. B. Avenanthramides are bioavailable and have antioxidant activity in humans after acute consumption of an enriched mixture from oats. The Journal of Nutrition . 2007;137(6):1375–1382. doi: 10.1093/jn/137.6.1375. [DOI] [PubMed] [Google Scholar]
- 120.Akkol E. K., Süntar I., Orhan I. E., Keles H., Kan A., Çoksari G. Assessment of dermal wound healing and in vitro antioxidant properties of Avena sativa L. Journal of Cereal Science . 2011;53(3):285–290. doi: 10.1016/j.jcs.2011.01.009. [DOI] [Google Scholar]
- 121.Singh S., Kaur M., Sogi D. S., Purewal S. S. A comparative study of phytochemicals, antioxidant potential and in-vitro DNA damage protection activity of different oat (Avena sativa) cultivars from India. Journal of Food Measurement and Characterization . 2019;13(1):347–356. doi: 10.1007/s11694-018-9950-x. [DOI] [Google Scholar]
- 122.Lee-Manion A. M., Price R. K., Strain J., Dimberg L. H., Sunnerheim K., Welch R. W. In vitro antioxidant activity and antigenotoxic effects of avenanthramides and related compounds. Journal of Agricultural and Food Chemistry . 2009;57(22):10619–10624. doi: 10.1021/jf9024739. [DOI] [PubMed] [Google Scholar]
- 123.Nie L., Wise M. L., Peterson D. M., Meydani M. Avenanthramide, a polyphenol from oats, inhibits vascular smooth muscle cell proliferation and enhances nitric oxide production. Atherosclerosis . 2006;186(2):260–266. doi: 10.1016/j.atherosclerosis.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 124.Kim I.-S., Hwang C.-W., Yang W.-S., Kim C.-H. Multiple antioxidative and bioactive molecules of oats (Avena sativa L.) in human health. Antioxidants . 2021;10(9):p. 1454. doi: 10.3390/antiox10091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen C.-Y., Milbury P. E., Kwak H.-K., Blumberg J. B., Collins F. W., Samuel P. Avenanthramides and phenolic acids from oats are bioavailable and act synergistically with vitamin C to enhance hamster and human LDL resistance to oxidation. The Journal of Nutrition . 2004;134(6):1459–1466. doi: 10.1093/jn/134.6.1459. [DOI] [PubMed] [Google Scholar]
- 126.Handelman G. J., Cao G., Walter M. F., et al. Antioxidant capacity of oat (Avena sativa L.) extracts. Inhibition of low-density lipoprotein oxidation and oxygen radical absorbance capacity. Journal of Agricultural and Food Chemistry . 1999;47(12):4888–4893. doi: 10.1021/jf990529j. [DOI] [PubMed] [Google Scholar]
- 127.Goyal M., Kaur H., Kaur A. Intra-genotypic variability for antioxidant and bioactive potential in oat under a dual-purpose system. Cereal Research Communications . 2022;50(4):941–952. doi: 10.1007/s42976-022-00256-3. [DOI] [Google Scholar]
- 128.Ham H., Woo K. S., Park J.-Y., et al. Antioxidant and anti-proliferative activities of oats under different solvent extraction conditions. Journal of the Korean Society of Food Science and Nutrition . 2016;45(6):918–922. doi: 10.3746/jkfn.2016.45.6.918. [DOI] [Google Scholar]
- 129.Rakić S., Janković S., Marčetić M., Živković D., Kuzevski J. The impact of storage on the primary and secondary metabolites, antioxidant activity and digestibility of oat grains (Avena sativa) Journal of Functional Foods . 2014;7:373–380. doi: 10.1016/j.jff.2014.01.022. [DOI] [Google Scholar]
- 130.Rao S., Santhakumar A. B., Chinkwo K. A., Blanchard C. L. Investigation of phenolic compounds with antioxidant activity in barley and oats affected by variation in growing location. Cereal Chemistry . 2020;97(4):772–782. doi: 10.1002/cche.10291. [DOI] [Google Scholar]
- 131.Escobedo-Flores Y., Chavez-Flores D., Salmeron I., Molina-Guerrero C., Perez-Vega S. Optimization of supercritical fluid extraction of polyphenols from oats (Avena sativa L.) and their antioxidant activities. Journal of Cereal Science . 2018;80:198–204. doi: 10.1016/j.jcs.2018.03.002. [DOI] [Google Scholar]
- 132.Boeck T., D’Amico S., Zechner E., Jaegar H., Schoenlechner R. Journal of Land Management . Vol. 69. Food and Environment; 2018. Nutritional properties of various oat and naked oat cultivars. Die Bodenkultur; pp. 215–226. [Google Scholar]
- 133.Brindzova L., Čertík M., Rapta P., Zalibera M., Mikulajova A., Takacsova M. Antioxidant activity, β-glucan and lipid contents of oat varieties. Czech Journal of Food Sciences . 2008;26(3):163–173. doi: 10.17221/2564-cjfs. [DOI] [Google Scholar]
- 134.Rasane P., Jha A., Sabikhi L., Kumar A., Unnikrishnan V. Nutritional advantages of oats and opportunities for its processing as value added foods-a review. Journal of Food Science and Technology . 2015;52(2):662–675. doi: 10.1007/s13197-013-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Russo P., De Chiara M. L. V., Capozzi V., et al. Lactobacillus plantarum strains for multifunctional oat-based foods. LWT--Food Science and Technology . 2016;68:288–294. doi: 10.1016/j.lwt.2015.12.040. [DOI] [Google Scholar]
- 136.Goncerzewicz A., Misiewicz A., Owczarek L., Jasińska U., Skąpska S. The effect of a newly developed oat-banana fermented beverage with a beta-glucan additive on ldhL gene expression in Streptococcus thermophilus TKM3 KKP 2030p. Current Microbiology . 2016;73(6):773–780. doi: 10.1007/s00284-016-1126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gupta S., Cox S., Abu-Ghannam N. Process optimization for the development of a functional beverage based on lactic acid fermentation of oats. Biochemical Engineering Journal . 2010;52(2-3):199–204. doi: 10.1016/j.bej.2010.08.008. [DOI] [Google Scholar]
- 138.Luana N., Rossana C., Curiel J. A., Kaisa P., Marco G., Rizzello C. G. Manufacture and characterization of a yogurt-like beverage made with oat flakes fermented by selected lactic acid bacteria. International Journal of Food Microbiology . 2014;185:17–26. doi: 10.1016/j.ijfoodmicro.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 139.Gallo M., Passannanti F., Colucci Cante R., et al. Lactic fermentation of cereals aqueous mixture of oat and rice flours with and without glucose addition. Heliyon . 2020;6(9) doi: 10.1016/j.heliyon.2020.e04920.e04920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang C., Liang S., Wang H., Guo M. Physiochemical properties and probiotic survivability of symbiotic oat-based beverage. Food Science and Biotechnology . 2018;27(3):735–743. doi: 10.1007/s10068-017-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gupta M., Bajaj B. K. Development of fermented oat flour beverage as a potential probiotic vehicle. Food Bioscience . 2017;20:104–109. doi: 10.1016/j.fbio.2017.08.007. [DOI] [Google Scholar]
- 142.Duru K. C., Kovaleva E., Danilova I., Belousova A. Production and assessment of novel probiotic fermented oat flour enriched with isoflavones. Food Science and Technology . 2019;111:9–15. doi: 10.1016/j.lwt.2019.04.102. [DOI] [Google Scholar]
- 143.Bering S., Suchdev S., Sjøltov L., Berggren A., Tetens I., Bukhave K. A lactic acid-fermented oat gruel increases non-haem iron absorption from a phytate-rich meal in healthy women of childbearing age. British Journal of Nutrition . 2006;96(1):80–85. doi: 10.1079/bjn20061683. [DOI] [PubMed] [Google Scholar]
- 144.Bernat N., Cháfer M., González-Martínez C., Rodríguez-García J., Chiralt A. Optimisation of oat milk formulation to obtain fermented derivatives by using probiotic Lactobacillus reuteri microorganisms. Food Science and Technology International . 2015;21(2):145–157. doi: 10.1177/1082013213518936. [DOI] [PubMed] [Google Scholar]
- 145.Angelov A., Gotcheva V., Hristozova T., Gargova S. Application of pure and mixed probiotic lactic acid bacteria and yeast cultures for oat fermentation. Journal of the Science of Food and Agriculture . 2005;85(12):2134–2141. doi: 10.1002/jsfa.2223. [DOI] [Google Scholar]
- 146.Sharma P., Trivedi N., Gat Y. Development of functional fermented whey–oat-based product using probiotic bacteria. Biotech . 2017;7(4):272–278. doi: 10.1007/s13205-017-0906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abdelshafy A. M., El-Naggar E. A., Kenawi M. N. Morenga leaves for promotion the healthy benefits of oat fermented by probiotic bacteria: the first investigation. Applied Food Research . 2022;2 doi: 10.1016/j.afres.2022.100166.100166 [DOI] [Google Scholar]
- 148.Alemayehu G. F., Forsido S. F., Tola Y. B., Amare E. Optimization of nutritional and sensory properties of fermented oat-based composite beverage. Heliyon . 2022;8(10) doi: 10.1016/j.heliyon.2022.e10771.e10771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sanchez-Pardo M., Jiménez-García E., González-García I. Study about the addition of chemically modified starches (cross-linked cornstarches), dextrins, and oats fiber in baked pound cake. Journal of Biotechnology . 2010;150:316–325. doi: 10.1016/J.JBIOTEC.2010.09.298. [DOI] [Google Scholar]
- 150.Flander L., Salmenkallio-Marttila M., Suortti T., Autio K. Optimization of ingredients and baking process for improved wholemeal oat bread quality. LWT--Food Science and Technology . 2007;40(5):860–870. doi: 10.1016/j.lwt.2006.05.004. [DOI] [Google Scholar]
- 151.Chauhan D., Kumar K., Kumar S., Kumar H. Effect of incorporation of oat flour on nutritional and organoleptic characteristics of bread and noodles. Current Research in Nutrition and Food Science Journal . 2018;6(1):148–156. doi: 10.12944/CRNFSJ.6.1.17. [DOI] [Google Scholar]
- 152.Gray J., Bemiller J. Bread staling: molecular basis and control. Comprehensive Reviews in Food Science and Food Safety . 2003;2:1–21. doi: 10.1111/j.1541-4337.2003.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 153.Hager A.-S., Czerny M., Bez J., Zannini E., Arendt E. K. Starch properties, in vitro digestibility and sensory evaluation of fresh egg pasta produced from oat, teff and wheat flour. Journal of Cereal Science . 2013;58(1):156–163. doi: 10.1016/j.jcs.2013.03.004. [DOI] [Google Scholar]
- 154.Boukid F. Oat proteins as emerging ingredients for food formulation: where we stand? European Food Research and Technology . 2021;247(3):535–544. doi: 10.1007/s00217-020-03661-2. [DOI] [Google Scholar]
- 155.Khanna P., Mohan S. Acceptability & incorporation of oats in the Indian diet. International Journal of Food Science and Nutrition . 2017;2:124–131. [Google Scholar]
- 156.Ryan L., Thondre P. S., Henry C. J. K. Oat-based breakfast cereals are a rich source of polyphenols and high in antioxidant potential. Journal of Food Composition and Analysis . 2011;24(7):929–934. doi: 10.1016/j.jfca.2011.02.002. [DOI] [Google Scholar]
- 157.Decker E. A., Rose D. J., Stewart D. Processing of oats and the impact of processing operations on nutrition and health benefits. British Journal of Nutrition . 2014;112(S2):S58–S64. doi: 10.1017/S000711451400227X. [DOI] [PubMed] [Google Scholar]
- 158.Smulders M. J., van de Wiel C. C., van den Broeck H. C., et al. Oats in healthy gluten-free and regular diets: a perspective. Food Research International . 2018;110:3–10. doi: 10.1016/j.foodres.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 159.Zhou B. L., Zhu F., Shan F., Cai Y. Z., Corke H. Gluten enhances cooking, textural, and sensory properties of oat noodles. Cereal Chemistry Journal . 2011;88(3):228–233. doi: 10.1094/CCHEM-01-11-0006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


