Abstract
Background
The prevalence of asthma in Taiwan was increasing in the past 30 years, causing a great impact on adolescent health. This study aimed to investigate the current prevalence, impact, and associated factors of asthma in Taiwanese adolescents.
Material and methods
Parents or guardians provided passive consent at home prior to the survey. Adolescents aged 13–14 years completed a questionnaire survey in 2017 in Taipei, Taiwan. The prevalence, impact, and associated factors of asthma were analyzed. We also compared the asthma prevalence with the prevalence in 1995 and 2001.
Results
We analyzed 3474 validated questionnaires. The prevalence of physician-diagnosed asthma was 12.4%. The prevalence of current wheezing was 9.2% in 2017, which was 5.2% in 1995 and 7.0% in 2001. 3.3% of 13–14-year-old adolescents had severe asthma symptoms. Asthma significantly impacted the lives of adolescents. Of the students with asthma, 10.9% had school absenteeism, 16.5% urgently needed to see a doctor, 9.5% went to the emergency department, and 3.5% were admitted to hospitals within the preceding 12 months. The associated factors for physician-diagnosed asthma in Taiwanese adolescents were male (prevalence ratio [PR], 1.38; 95% confidence interval [CI], 1.05–1.83; p = 0.02), maternal history of asthma (PR, 2.61; 95% CI, 1.69–4.02; p < 0.01), and recent paracetamol use at least once per month (PR, 2.60; 95% CI, 1.24–5.42; p = 0.01). The associated factors for school absenteeism were nocturnal cough (PR, 1.99; 95% CI, 1.16–3.41; p = 0.01), current wheezing (PR, 7.52; 95% CI, 4.39–12.9; p < 0.01), and recent paracetamol use (at least once per month, PR, 3.16; 95% CI, 1.10–9.06; p = 0.03; at least once per year, PR, 2.19; 95% CI, 1.25–3.83; p < 0.01).
Conclusions
The prevalence of physician-diagnosed asthma was 12.4%. Asthma substantially impacted the lives of adolescents. Reducing nocturnal cough, wheezing frequency, and paracetamol usage might help decrease school absenteeism.
Keywords: Taiwan, Asthma, Asthma/epidemiology, Cross-sectional studies, Epidemiology/asthma/prevalence, Adolescent, Disease attributes, Humans
Introduction
Asthma is a chronic respiratory disease, with a prevalence in adolescents that varies widely from 0.9% to 21.3% in different countries.1 Asthma increased rapidly in recent decades in Taiwan. The prevalence of 13–14-year-old adolescents with asthma increased from 0.86% in 1974 to 4.1% in 1985, 5.2% in 1995, and 7.0% in 2001.2,3 Urbanization, westernized lifestyles, and air pollution have all contributed to the rapid increase of asthma in Taiwan.2,4,5
Approximately 358 million individuals are affected by asthma globally,6 placing a tremendous disease burden worldwide and impacting asthmatic individuals.7 A total of 26.2 million disability-adjusted life years are attributed to asthma, and 0.4 million people per year die from asthma globally.6 In Taiwan, the annual cost of each asthma patient is more than 7000 USD, equaling 215 million USD as the total expenditure per year.8,9 Unscheduled clinic or emergency department visits can result in asthma-associated school absenteeism.10 Asthma is responsible for 2.3 missed school days per person annually, which is a great impact on asthmatic students and their parents.11
The International Study of Asthma and Allergies in Childhood (ISAAC) is a well-known standardized asthma survey that has provided longitudinal asthma prevalence data since 1995.12,13 Based on the success of ISAAC, the Global Asthma Network (GAN) survey was initiated in 2012, performing global surveillance of asthma prevalence, severity, associated factors, and management in both pediatric and adult populations.14 Fifty-three centers from 20 countries completed the GAN Phase 1 survey by May 2020.15 Our group had conducted all three phases of the ISAAC survey in Taipei, Taiwan. We continued to conduct the GAN Phase 1 survey in Taipei to investigate current asthma epidemiology and monitor the increasing trend of asthma. We also investigated the associated factors and the impact on students’ lives associated with asthma in 13–14 year-old students in this survey.
Methods
Study design and study population
Following the GAN phase 1 protocol,14,16 we randomly selected 24 junior high schools from 12 districts in Taipei to reach a sample size of 3000 in this age group. All students and their parents/guardians from the eighth grade (13–14 years old) of each school were invited to participate. The questionnaires of 13–14-year-old students were answered by the students themselves, and the adults’ questionnaires were answered by both their parents or guardians.
The questionnaires were distributed to each school in October 2017. The questionnaires were sent back by teachers in November 2017. Questionnaires without complete information were excluded. Students' and parents’ questionnaires were matched using serial numbers. The Chinese version of the questionnaire we used was validated by the GAN Global Center through forward and back-translation processes. The study was approved by the Human Research Ethics Committee of Chang Gung Memorial Hospital (Protocol No. 201700105B0). Because of the coronavirus disease 2019 pandemic, the GAN worldwide survey and the data checking by the global data centers were not finalized until 2020.
Disease and associated factors definition
During the GAN survey, asthma symptoms were inquired, including current wheezing, wheezing attack frequency, sleep disturbance due to wheezing, and speech limitation to only 1 or 2 words at a time between breaths due to severe wheezing in the preceding 12 months.16 In the GAN and ISAAC surveys,16,17 the current prevalence of asthma was estimated from participants with a positive answer to “current wheezing”. “Current wheezing” was defined as a positive answer to the question “Have you had wheezing or whistling in the chest in the preceding 12 months?” Severe asthma symptoms were defined as participants with current wheezing and a positive response to 1 of the following in the preceding 12 months: (a) 4 or more attacks of wheezing, (b) sleep disturbance due to wheezing on 1 or more nights per week, and (c) wheezing severe enough to limit speech to only 1 or 2 words at a time between breaths. We also asked about the wheezing sound during or after exercise and dry cough at night, apart from a cough associated with a cold or chest infection.
“Asthma ever” was defined as a positive response to the question “Have you ever had asthma?” while “physician-diagnosed asthma” was defined as a positive response to the question “Was asthma confirmed by a doctor?” The frequency of urgent doctor and emergency department visits, as well as hospital admissions, were used as indicators of the clinical burden. Missed school days were an indicator of the burden on life. Exercise-induced wheezing and nocturnal cough were compared because they indicated troublesome symptoms to students.
Allergic rhinitis was defined as a positive response to the question, “Was your allergic rhinitis confirmed by a doctor?” Atopic dermatitis was defined as a positive response to the question, “Was your atopic dermatitis confirmed by a doctor?” In the GAN survey in Taipei, a food allergy questionnaire was modified from the nationwide food allergy survey in Singapore.18 Food allergy was defined as a positive response to the question, “Was your food allergy confirmed by a doctor?”
Associated factors used for Poisson regression analysis included sex, twin, older and younger sibling numbers, birthplace, truck frequency passing house (as an indicator of air pollution), exercise, diet habit, paracetamol intake, dog and cat at home, current and ever cigarette smoking, television watching time, and computer or cell phone usage time.
The students' and parents’ questionnaires were matched by serial numbers. Associated factors identified in parental questionnaires were also used for Poisson regression analysis, including parental physician-diagnosed asthma; allergic rhinitis; atopic dermatitis; food allergy; damp and mold spots at this moment, during pregnancy, and in the first year of life; and fuel types used for cooking and heating.
Comparison of asthma prevalence between GAN and ISAAC
To investigate the change in asthma prevalence, we compared the GAN data with the ISAAC Phase 1 and Phase 3 data. Our group conducted the ISAAC Phase 1 survey in October 1995 and the ISAAC Phase 3 survey from December 2001 to January 2002.3 using similar questionnaires in the same schools as those used for the GAN. All parameters related to asthma symptoms were compared.
Statistical analysis
The ninety-five percent confidence interval (CI) was calculated using an online binomial CI calculator with asymptotic assumption on the GitHub platform.19 The comparison between the prevalence of asthma symptoms between GAN, ISAAC phase 1, and phase 3 was analyzed by the Chi-square test. The Venn diagram demonstrating the relationship between asthma and other allergic diseases was illustrated using the VennDiagram R package (version 1.7.0).20 To evaluate the correlation between asthma and allergic rhinitis, atopic dermatitis, and food allergy, we performed Spearman correlation and Poisson regression analyses. Given that our study is cross-sectional and the prevalence of physician-diagnosed asthma is greater than 10%, we used prevalence ratios (PR) instead of odds ratios to avoid overestimating the impact of associated factors on physician-diagnosed asthma and asthma-related school absenteeism.21,22 Unadjusted and adjusted Poisson regression analyses were performed to analyze the associated factors for physician-diagnosed asthma and school absenteeism. During unadjusted Poisson regression, variables with a p-value less than 0.1 were included in the subsequent adjusted Poisson regression. Poisson regression was performed using IBM SPSS Statistics for Windows, version 20 (IBM Corp., Armonk, NY, USA). All hypothesis tests were two-sided, with a priori levels of significance set at p < 0.05.
Results
The questionnaire survey process
A total of 3734 questionnaires were delivered to the selected junior high schools in October 2017. The schools returned 3550 questionnaires in November 2017, and 3474 questionnaires were validated for the final analyses.
Asthma prevalence and associated symptoms
In the preceding 12 months, 321 (9.2%) students had current wheezing. A total of 494 (14.2%) 13–14-year-old students reported ever having asthma, and 431 (12.4%) of them had physician-diagnosed asthma. Exercise-induced wheezing and nocturnal coughing were common. A total of 870 (25%) students claimed ever having wheezing during or after exercise, while 963 (27.7%) students complained of nocturnal coughing. The prevalence of asthma and its associated symptoms are summarized in Table 1.
Table 1.
Asthma prevalence and associated symptoms.
| Items | Total |
Male |
Female |
P-value |
||||
|---|---|---|---|---|---|---|---|---|
| Number | Percentage | (95% CI) | Number | Percentage | Number | Percentage | (male vs. female) | |
| Total | 3474 | 100% | 1762b | 1601b | ||||
| Asthma symptoms in the past 12 months | ||||||||
| Wheezing, current | 321 | 9.2% | (8.2%–10.2%) | 172 | 9.8% | 135 | 8.4% | 0.16 |
| Wheezing attacks | 0.02 | |||||||
| 1 to 3 | 227 | 6.5% | (5.7%–7.3%) | 112 | 6.4% | 107 | 6.7% | |
| 4 to 12 | 62 | 1.8% | (1.4%–2.2%) | 43 | 2.4% | 18 | 1.1% | |
| More than 12 | 21 | 0.6% | (0.3%–0.9%) | 13 | 0.7% | 8 | 0.5% | |
| Wheezing that disturbed sleep | 0.51 | |||||||
| <1 per week | 79 | 2.3% | (1.8%–2.8%) | 39 | 2.2% | 34 | 2.1% | |
| ≥1 per week | 16 | 0.5% | (0.3%–0.7%) | 10 | 0.6% | 6 | 0.4% | |
| Severe wheezing that limited speech | 74 | 2.1% | (1.6%–2.6%) | 49 | 2.8% | 24 | 1.5% | 0.01 |
| Severe asthma symptomsa | 115 | 3.3% | (2.7%–3.9%) | 94 | 5.3% | 47 | 2.9% | <0.01 |
| Asthma ever | 494 | 14.2% | (13.0%–15.4%) | 284 | 16.1% | 195 | 12.2% | <0.01 |
| Asthma diagnosed by physicians | 431 | 12.4% | (11.3%–13.5%) | 253 | 14.4% | 164 | 10.2% | <0.01 |
| Exercise-induced wheezing | 870 | 25.0% | (23.6%–26.4%) | 477 | 27.1% | 364 | 22.7% | <0.01 |
| Nocturnal cough | 963 | 27.7% | (26.2%–29.2%) | 470 | 26.7% | 462 | 28.9% | 0.15 |
Severe asthma symptoms definition: cases with current wheezing have one or more of the following symptoms: (1) four or more attacks of wheezing; (2) woken by wheezing on one or more nights per week or; (3) wheezing severe enough to limit speech to only one or two words at a time, between breaths.
111 students didn't provide their gender in the questionnaires
One hundred forty-three (3.3%) students had experienced severe asthma symptoms. Among those with severe asthma symptoms, 83 (58%) students had 4 or more episodes of wheezing, 16 (11.2%) had sleep disturbance from wheezing more than once per week, and 74 (51.7%) had speech disturbance caused by wheezing.
We also examined the prevalence of asthma symptoms across genders. It was observed that males had a higher probability of experiencing more wheezing episodes in the preceding 12 months, as well as severe wheezing that limited speech. Additionally, a greater proportion of male adolescents reported physician-diagnosed asthma and exercise-induced wheezing compared to females.
The trend of prevalence of asthma
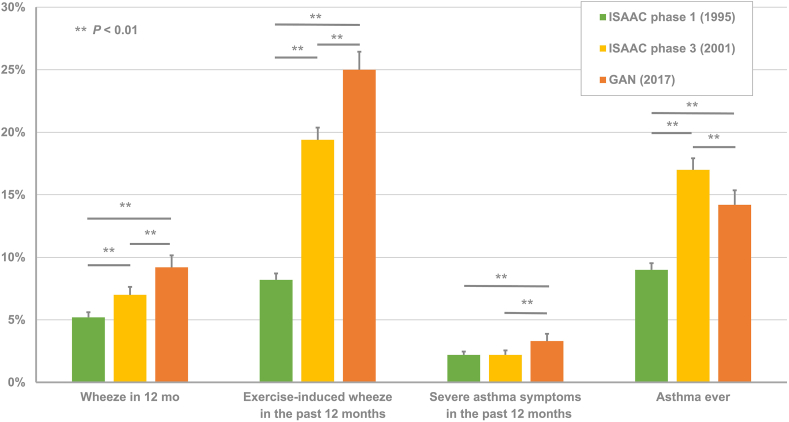
When we compared the prevalence of asthma in ISAAC Phase 1 and ISAAC Phase 2,2,3 the prevalence of current wheezing was 9.2% in 2017, higher than 5.2% in 1995 and 7.0% in 2001 (p < 0.01). Regarding exercise-induced wheezing, the prevalence was 8.2% in 1995 and 19.4% in 2001, lower than 25.0% in 2017 (p < 0.01). While 9.0% of 13–14-year-old students in 1995 and 17.0% in 2001 reported ever having asthma, the prevalence of ever-asthma was slightly lower at 14.2% in 2017.
The prevalence of severe asthma symptoms in the preceding 12 months has not been consistently elevated. In 1995 and 2001, the percentage of 13–14-year-old students with severe asthma symptoms was the same (2.2%). The prevalence was 3.3% in 2017. The data are compared in Fig. 1.
Fig. 1.
Asthma prevalence and associated symptoms changed from 1995 to 2017. The percentages of asthma-associated symptoms are compared between surveys performed in 1995, 2001, and 2017. (ISAAC: The International Study of Asthma and Allergies in Childhood; GAN: Global Asthma Network)
The impact of asthma
Asthma has several impacts on those 13–14-year-old students with physician-diagnosed asthma. Forty-seven (10.9%) of 431 students with physician-diagnosed asthma ever missed school because of breathing problems, and 5 (1.2%) even missed more than 12 days in the preceding 12 months. Of the 431 students with physician-diagnosed asthma, a total of 71 (16.5%) urgently needed to see a doctor, 41 (9.5%) ever went to the emergency department, and 15 (3.5%) were ever admitted to the hospital within the preceding 12 months. The detailed results are summarized in Table 2.
Table 2.
The impact of asthma
| Items in the past 12 months | Physician-diagnosed asthma |
||
|---|---|---|---|
| Number | Percentage | (95% CI) | |
| Total case number | 431 | ||
| School days missed | |||
| 1 to 3 | 37 | 8.6% | (6.0%–11.2%) |
| 4 to 12 | 5 | 1.2% | (0.2%–2.2%) |
| More than 12 | 5 | 1.2% | (0.2%–2.2%) |
| Urgent doctor visit | |||
| 1 to 3 | 56 | 13.0% | (9.8%–16.2%) |
| 4 to 12 | 14 | 3.2% | (1.5%–4.9%) |
| More than 12 | 1 | 0.2% | (0%–0.6%) |
| Urgent emergency room visit | |||
| 1 to 3 | 39 | 9.0% | (6.3%–11.7%) |
| 4 to 12 | 2 | 0.5% | (0%–1.2%) |
| More than 12 | 0 | 0.0% | (0%–0%) |
| Hospital admission | |||
| 1 | 12 | 2.8% | (1.2%–4.4%) |
| 2 | 1 | 0.2% | (0%–0.6%) |
| More than 2 | 2 | 0.5% | (0%–1.2%) |
The association between asthma and other allergic diseases
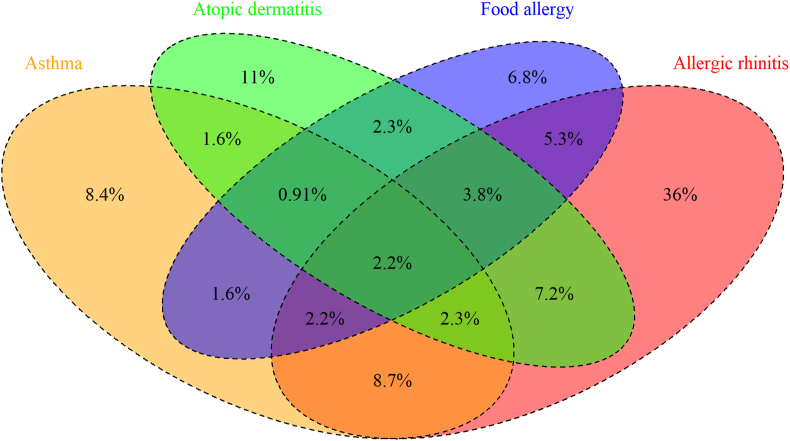
Physician-diagnosed asthma was correlated with other allergic diseases. Out of the total 13-14-year-old students, 1038 (29.9%) had physician-diagnosed allergic rhinitis, 484 (13.9%) had atopic dermatitis, and 386 (11.1%) had food allergy. According to Spearman correlation analysis, the rho coefficients between physician-diagnosed asthma and allergic rhinitis, atopic dermatitis, and food allergy were 0.21, 0.12, and 0.16, respectively (all p < 0.01). Students with allergic rhinitis (PR, 2.89; 95% CI, 2.39–3.50; p < 0.01), atopic dermatitis (PR, 2.09; 95% CI, 1.68–2.60; p < 0.01), and food allergies (PR, 2.61; 95% CI, 2.10–3.25; p < 0.01) had a higher prevalence of physician-diagnosed asthma. The Venn diagram (Fig. 2) showed the overlap between different allergic diseases. Among students with physician-diagnosed asthma, 30.1% had pure asthma, 31.2% had concomitant allergic rhinitis, 5.73% had atopic dermatitis, and 5.73% had food allergies. Additionally, 19.4% of the asthma patients had 2 allergic comorbidities, and 7.9% had all 3 different kinds of allergic diseases, including allergic rhinitis, atopic dermatitis, and food allergy.
Fig. 2.
Overlap between four different allergic diseases. The overlap between physician-diagnosed asthma, allergic rhinitis, atopic dermatitis, and food allergy is demonstrated in terms of percentages on a Venn diagram
The associated factors for physician-diagnosed asthma and asthma-associated school absenteeism
According to adjusted Poisson analysis, male adolescents were found to have a higher probability of physician-diagnosed asthma (PR 1.38; 95% CI, 1.05–1.83; p = 0.02). Additionally, students who reported using paracetamol at least once per month in the preceding year had a higher prevalence of physician-diagnosed asthma (PR, 2.60; 95% CI, 1.24–5.42; p = 0.01). Furthermore, students with a maternal history of asthma also had a higher prevalence of physician-diagnosed asthma (PR, 2.61; 95% CI, 1.69–4.02; p < 0.01) (Table 3).
Table 3.
The factors associated with physician-diagnosed asthma and school absenteeism in 13–14-year-old students.
| Variables | Adjusted analysis |
|
|---|---|---|
| PR (95% CI) | P-value | |
| Associated factors for physician-diagnosed asthma | ||
| Male | 1.38 (1.05–1.83) | 0.02 |
| Maternal history of asthma | 2.61 (1.69–4.02) | <0.01 |
| Paracetamol usage in the past 12 months (at least once per month) |
2.60 (1.24–5.42) | 0.01 |
| Associated factors for school absenteeism | ||
| Nocturnal cough | 1.99 (1.16–3.41) | 0.01 |
| Current wheezing (wheezing in the past 12 months) | 7.52 (4.39–12.9) | <0.01 |
| Paracetamol usage in the past 12 months (at least once per month) | 3.16 (1.10–9.06) | 0.03 |
| Paracetamol usage in the past 12 months (at least once per year) | 2.19 (1.25–3.83) | <0.01 |
Adjusted analysis for physician-diagnosed asthma is adjusted by body matrix index, fast food intake, and parental histories with asthma, allergic rhinitis, and food allergy (the factors with p < 0.1 at unadjusted Poisson regression). Adjusted analysis for school absenteeism is adjusted by night wakening, speech-limiting wheezing, exercise-induced wheezing, frequent truck passage, cooked vegetable ingestion, current cat exposure, and current tobacco smoking (the factors with p < 0.1 at unadjusted Poisson regression)
In terms of factors associated with school absenteeism, nocturnal cough was found to be associated with a higher probability of missing school (PR, 1.99; 95% CI, 1.16–3.41; p = 0.01), as were wheezing episodes in the preceding 12 months (PR, 7.52; 95% CI, 4.39–12.9; p < 0.01). Additionally, recent paracetamol use, at least once per month, was associated with a PR of 3.16 (95% CI, 1.10–9.06; p = 0.03), while the PR for at least once per year use was 2.19 (95% CI, 1.25–3.83; p < 0.01). The results of the adjusted analysis were summarized in Table 3.
Discussion
The global prevalence of current wheezing was diverse from 0.9% (New Delhi, India) to 21.3% (Cape Town, South Africa).1,23,24 The prevalence of current wheezing was 9.2% in Taiwan in 2017, approaching the global average (10.4%).1,23,24 In Taiwan, 3.3% of adolescents had severe asthma symptoms, slightly lower than the global average (4.9%, range 0.5%–12.0%).1 Out of the 26 centers that provided longitudinal current asthma data from ISAAC 1 to GAN, the prevalence of asthma significantly increased in 7 centers, including Taiwan.1
The prevalence of asthma in Taiwan is still raised along with rapid urbanization. In 1974, the asthma prevalence in Taipei was only 0.86% among 13-year-old students.2 Economic growth dramatically increased in the 1980s in Taiwan. Gross domestic product per capita increased from 2720 USD in 1981 to 28 371 USD in 2020.25 The prevalence of asthma increased to 4.1% in 1985, 5.2% in 1995, and 7.0% in 2001. The GAN survey demonstrated that the asthma prevalence in Taipei was still rising in 2017, similar to the trend in Spain.1,3 The reasons for this rise are multifactorial, including hygiene improvement, indoor lifestyle, vitamin D deficiency, and air pollution.4,26, 27, 28 Fortunately, the number of students with severe asthma symptoms has not increased rapidly in the past 22 years, only having increased from 2.2% to 3.3%. Medical progress and patient education improvement were factors decelerating the increasing trend of students with severe asthma symptoms.29, 30, 31
Asthma substantially impacts students' health and learning, and heavily burdens their families.7,10 In our GAN survey, 8.6% of students with physician-diagnosed asthma missed 1 to 3 school days in the preceding 12 months, 1.2% missed 4 to 12 days, and 1.2% even missed more than 12 days. Asthma-related school absenteeism causes low academic performance,32 and uncontrolled asthma was the major risk factor leading to school absenteeism.10 Nocturnal cough was one of the factors associated with school absenteeism and more than one-quarter of the students had nocturnal cough in the survey. Therefore, how to reduce nocturnal cough will be an important issue to decrease school absenteeism. Several school-based asthma-controlling interventions have been investigated to reduce asthma-related school absenteeism.33,34 More research will be required to find out the best strategy to prevent asthma-related school absenteeism and improve asthmatic adolescents’ academic performance in Taiwan.35
Asthma also imposed a significant burden on families with adolescents who had asthma. Because of asthma, 16.4% of students had to see a doctor urgently, 9.5% had to visit the emergency room, and 3.5% even required hospital admissions in our GAN survey. Their parents or family members had to accompany them during unscheduled medical emergencies, leading to work absenteeism and economic loss.11 Therefore, reducing medical emergencies caused by asthma will effectively reduce the burden on parents.
Current use and perinatal exposure to paracetamol have been associated with asthma.36,37 In this survey, the use of paracetamol over the preceding 12 months was associated with physician-diagnosed asthma and asthma-related school absenteeism. Several mechanisms have been proposed to explain the association between the use of paracetamol and asthma. Most of the mechanisms are associated with glutathione and impaired antioxidant activities.38, 39, 40 Some researchers have challenged the association between paracetamol and asthma.41 More well-designed studies will be required to clarify this association.
This study had several limitations and strengths. A major limitation was that the diagnosis of asthma was based on questionnaires. In addition, adolescents often underreport their associated factors, such as cigarette smoking,42 which might cause cigarette smoking to be not a risk factor for asthma in this survey. Another limitation is that the definition of severe asthma symptoms in the GAN protocol differs from that of severe asthma in the international guideline.1,43 Therefore, a different study is required to investigate the current status of severe asthma in Taiwan. The major strength of this study was that the GAN survey is an international project conducted with a similar validated core questionnaire used in the ISAAC studies in Taiwan.1,3 Therefore, the increasing trend of asthma in Taiwan is convincing. The government should invest more resources to slow down this increasing trend.
In conclusion, asthma prevalence in Taiwan is still increasing. Asthmatic students and their families spent more time seeking medical help, leading to school absenteeism. Reducing nocturnal cough, wheezing frequency, and paracetamol usage might help decrease school absenteeism. Policymakers and healthcare providers should work together to improve asthma care and halt the increase in asthma in Taiwan.
Funding source
The survey is supported by research grants (CMRPG3K1361-2, CMRPG2K0331-2, and CORPG2M0041) from Chang Gung Memorial Hospital, Chang Gung Medical Foundation.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, J.L.H., upon reasonable request.
Author contributions
All authors participated in the study design. K.W.S., L.L.L., C.Y.W., and S.J.H. translated, prepared, and distributed the questionnaires. K.W.S., D.C.Y, L.L.L., and S.J.H. collected and analyzed the data. D.C.Y, K.W.Y, and J.L.H. supervised the study. K.W.S. prepared the manuscript. D.C.Y, K.W.Y, and J.L.H. critically reviewed the manuscript. All authors approved the manuscript and consented to publication.
Ethics
Prior to conducting the questionnaire survey at the school, an information letter was sent home to parents or guardians, explaining the survey procedure in detail. Since the survey was conducted anonymously, passive consent was granted by the Human Research Ethics Committee. Parents or guardians were allowed to decline their children's participation, either through a written response or by verbally expressing their refusal. The survey was approved by the Human Research Ethics Committee of Chang Gung Memorial Hospital (Protocol No. 201700105B0).
Declaration of competing interest
All authors have no conflict of interest.
Acknowledgments
This study was sponsored by a research grant (CMRPG2K0331-2, CMRPG3K1361-2, and CORPG2M0041) from Chang Gung Memorial Hospital, Chang Gung Medical Foundation.
Footnotes
Full list of author information is available at the end of the article
Contributor Information
Kuo-Wei Yeh, Email: kwyeh@adm.cgmh.org.tw.
Jing-Long Huang, Email: long@adm.cgmh.org.tw.
References
- 1.Asher M.I., Rutter C.E., Bissell K., et al. Worldwide trends in the burden of asthma symptoms in school-aged children: global Asthma Network Phase I cross-sectional study. Lancet. 2021;398:1569–1580. doi: 10.1016/S0140-6736(21)01450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsieh K.H., Shen J.J. Prevalence of childhood asthma in Taipei, Taiwan, and other Asian Pacific countries. J Asthma. 1988;25:73–82. doi: 10.3109/02770908809071357. [DOI] [PubMed] [Google Scholar]
- 3.Yan D.C., Ou L.S., Tsai T.L., Wu W.F., Huang J.L. Prevalence and severity of symptoms of asthma, rhinitis, and eczema in 13- to 14-year-old children in Taipei, Taiwan. Ann Allergy Asthma Immunol. 2005;95:579–585. doi: 10.1016/S1081-1206(10)61022-8. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh C.Y., Jung C.R., Lin C.Y., Hwang B.F. Combined exposure to heavy metals in PM2.5 and pediatric asthma. J Allergy Clin Immunol. 2021;147:2171–2180 e13. doi: 10.1016/j.jaci.2020.12.634. [DOI] [PubMed] [Google Scholar]
- 5.Yeh K.W., Chiang L.C., Huang J.L. Epidemiology and current status of asthma and associated allergic diseases in Taiwan- ARIA Asia-Pacific Workshop report. Asian Pac J Allergy Immunol. 2008;26:257–264. [PubMed] [Google Scholar]
- 6.Pearce N., Ait-Khaled N., Beasley R., et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the international study of asthma and allergies in childhood (ISAAC) Thorax. 2007;62:758–766. doi: 10.1136/thx.2006.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaborators GBDCRD Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin H.C., Cho S.H., Ghoshal A.G., et al. Respiratory diseases and the impact of cough in Taiwan: results from the APBORD observational study. Medicine (Baltim) 2016;95 doi: 10.1097/MD.0000000000003854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu L.S., Sjakste T., Sakalauskas R., et al. The burden of allergic asthma in children: a landscape comparison based on data from Lithuanian, Latvian, and Taiwanese populations. Pediatr Neonatol. 2012;53:276–282. doi: 10.1016/j.pedneo.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Hsu J., Qin X., Beavers S.F., Mirabelli M.C. Asthma-related school absenteeism, morbidity, and modifiable factors. Am J Prev Med. 2016;51:23–32. doi: 10.1016/j.amepre.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nurmagambetov T., Kuwahara R., Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15:348–356. doi: 10.1513/AnnalsATS.201703-259OC. [DOI] [PubMed] [Google Scholar]
- 12.Asher M.I., Keil U., Anderson H.R., et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 13.Ellwood P., Asher M.I., Beasley R., Clayton T.O., Stewart A.W., Committee I.S. The international study of asthma and allergies in childhood (ISAAC): phase three rationale and methods. Int J Tubercul Lung Dis. 2005;9:10–16. [PubMed] [Google Scholar]
- 14.Ellwood P., Asher M.I., Billo N.E., et al. The Global Asthma Network rationale and methods for Phase I global surveillance: prevalence, severity, management and risk factors. Eur Respir J. 2017;49 doi: 10.1183/13993003.01605-2016. [DOI] [PubMed] [Google Scholar]
- 15.Ellwood P., Ellwood E., Rutter C., et al. Global asthma Network phase I surveillance: geographical coverage and response rates. J Clin Med. 2020;9:3688. doi: 10.3390/jcm9113688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Global Asthma Network Manual for Global Surveillance: Prevalence, Severity, Management and Risk Factors. 2016. http://globalasthmanetwork.org/surveillance/manual/manual.php Available from: [DOI] [PubMed] [Google Scholar]
- 17.Anderson H.R., Gupta R., Kapetanakis V., et al. International correlations between indicators of prevalence, hospital admissions and mortality for asthma in children. Int J Epidemiol. 2008;37:573–582. doi: 10.1093/ije/dyn039. [DOI] [PubMed] [Google Scholar]
- 18.Shek L.P., Cabrera-Morales E.A., Soh S.E., et al. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010;126:324–331. doi: 10.1016/j.jaci.2010.06.003. 31 e1-331. [DOI] [PubMed] [Google Scholar]
- 19.The online binomial confidence interval calculator on GitHub. Available from: https://pulipulichen.github.io/HTML5-Confidence-Intervals-Calculator/.
- 20.Chen H., Boutros P.C. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinf. 2011;12:35. doi: 10.1186/1471-2105-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez B.A.F., Leotti V.B., Silva G.S.E., Nunes L.N., Machado G., Corbellini L.G. Odds ratio or prevalence ratio? An overview of reported statistical methods and appropriateness of interpretations in cross-sectional studies with dichotomous outcomes in veterinary medicine. Front Vet Sci. 2017;4:193. doi: 10.3389/fvets.2017.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zocchetti C., Consonni D., Bertazzi P.A. Relationship between prevalence rate ratios and odds ratios in cross-sectional studies. Int J Epidemiol. 1997;26:220–223. doi: 10.1093/ije/26.1.220. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Marcos L., Asher M.I., Pearce N., et al. The burden of asthma, hay fever and eczema in children in 25 countries: GAN Phase I study. Eur Respir J. 2022;60 doi: 10.1183/13993003.02866-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortimer K., Lesosky M., Garcia-Marcos L., et al. The burden of asthma, hay fever and eczema in adults in 17 countries: GAN Phase I study. Eur Respir J. 2022;60 doi: 10.1183/13993003.02865-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.General situation of national income. 2020. https://eng.stat.gov.tw/public/data/dgbas03/bs2/yearbook_eng/y040.pdf Available from: [Google Scholar]
- 26.Platts-Mills T.A. The allergy epidemics: 1870-2010. J Allergy Clin Immunol. 2015;136:3–13. doi: 10.1016/j.jaci.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao T.C., Tu Y.L., Chang S.W., et al. Serum 25-hydroxyvitamin D levels in relation to lung function and exhaled nitric oxide in children. J Pediatr. 2014;165:1098–10103 e1. doi: 10.1016/j.jpeds.2014.08.048. [DOI] [PubMed] [Google Scholar]
- 28.Chen B.Y., Chen C.H., Chuang Y.C., Wu Y.H., Pan S.C., Guo Y.L. Changes in the relationship between childhood asthma and ambient air pollution in Taiwan: results from a nationwide survey repeated 5 years apart. Pediatr Allergy Immunol. 2019;30:188–194. doi: 10.1111/pai.12999. [DOI] [PubMed] [Google Scholar]
- 29.Yeh K.W., Chao S.Y., Chiang L.C., et al. Increasing asthma care knowledge and competence of public health nurses after a national asthma education program in Taiwan. Asian Pac J Allergy Immunol. 2006;24:183–189. [PubMed] [Google Scholar]
- 30.Liang H.J., Wu M.J., Jerng J.S., Yang C.H. Reinforcement of tobacco control and reduction in medical utilization for asthma in Taiwan: a population-based study. Int J Environ Res Publ Health. 2019;16 doi: 10.3390/ijerph16203950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhar R., Ip M., Kulkarni T., et al. Challenges faced in managing adult asthma: a perspective from Asian countries. Respirology. 2020;25:1235–1242. doi: 10.1111/resp.13935. [DOI] [PubMed] [Google Scholar]
- 32.Koinis-Mitchell D., Kopel S.J., Farrow M.L., McQuaid E.L., Nassau J.H. Asthma and academic performance in urban children. Ann Allergy Asthma Immunol. 2019;122:471–477. doi: 10.1016/j.anai.2019.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cicutto L., Gleason M., Szefler S.J. Establishing school-centered asthma programs. J Allergy Clin Immunol. 2014;134:1223–1230. doi: 10.1016/j.jaci.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Levy M., Heffner B., Stewart T., Beeman G. The efficacy of asthma case management in an urban school district in reducing school absences and hospitalizations for asthma. J Sch Health. 2006;76:320–324. doi: 10.1111/j.1746-1561.2006.00120.x. [DOI] [PubMed] [Google Scholar]
- 35.Chiang L.C., Huang J.L., Yeh K.W., Lu C.M. Effects of a self-management asthma educational program in Taiwan based on PRECEDE-PROCEED model for parents with asthmatic children. J Asthma. 2004;41:205–215. doi: 10.1081/jas-120026078. [DOI] [PubMed] [Google Scholar]
- 36.Beasley R., Clayton T., Crane J., et al. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6-7 years: analysis from Phase Three of the ISAAC programme. Lancet. 2008;372:1039–1048. doi: 10.1016/S0140-6736(08)61445-2. [DOI] [PubMed] [Google Scholar]
- 37.Sordillo J.E., Scirica C.V., Rifas-Shiman S.L., et al. Prenatal and infant exposure to acetaminophen and ibuprofen and the risk for wheeze and asthma in children. J Allergy Clin Immunol. 2015;135:441–448. doi: 10.1016/j.jaci.2014.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozer E., Evans S., Barr J., et al. Glutathione, glutathione-dependent enzymes and antioxidant status in erythrocytes from children treated with high-dose paracetamol. Br J Clin Pharmacol. 2003;55:234–240. doi: 10.1046/j.1365-2125.2003.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Micheli L., Cerretani D., Fiaschi A.I., Giorgi G., Romeo M.R., Runci F.M. Effect of acetaminophen on glutathione levels in rat testis and lung. Environ Health Perspect. 1994;102(Suppl 9):63–64. doi: 10.1289/ehp.94102s963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson J.D., Herzenberg L.A., Vasquez K., Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998;95:3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lourido-Cebreiro T., Salgado F.J., Valdes L., Gonzalez-Barcala F.J. The association between paracetamol and asthma is still under debate. J Asthma. 2017;54:32–38. doi: 10.1080/02770903.2016.1194431. [DOI] [PubMed] [Google Scholar]
- 42.Hwang J.H., Kim J.Y., Lee D.H., Jung H.G., Park S.W. Underestimation of self-reported smoking prevalence in Korean adolescents: evidence from gold standard by combined method. Int J Environ Res Publ Health. 2018;15 doi: 10.3390/ijerph15040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Chung Kian Fan, Wenzel Sally E., Brozek Jan L., et al. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. Eur Respir J. 2018;52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, J.L.H., upon reasonable request.