Abstract
Swyer syndrome—a rare syndrome associated with complete gonadal dysgenesis—is seen in phenotypically female patients with 46-XY karyotype. They usually present with primary amenorrhea or delayed puberty. The dysgenetic gonad, which is nonfunctional, is prone to undergo malignant transformation such as dysgerminoma, gonadoblastoma, etc. Timely diagnosis helps in deciding appropriate management strategies for the patient such as hormone replacement therapy and gonadectomy. Thirty-year-old patient with a female external phenotype presented to us with complaints of primary amenorrhea. There was no similar family history of infertility, amenorrhea, abnormal external genitalia development, or cryptorchidism. On physical examination, the breast development of the patient was within normal limits for her age (Tanner stage 5), however; the axillary and pubic hair were underdeveloped (Tanner stage 2). Pelvic and inguinal ultrasound of the patient showed a hypoplastic uterus along with a cystic structure in left pelvis with no evidence of any testes like structure in inguinal region, pelvis, or abdomen. The patient was further evaluated with MRI of pelvis which confirmed the ultrasound findings of a hypoplastic uterus along with a dysplastic cystic left gonad with no evidence of any ovary or ovary-like structure/testes/testes-like structure in abdomen. Possibility of complete gonadal dysgenesis was given which was further confirmed by the hormonal assay that showed hypergonadotropic-hypogonadism with raised serum follicular stimulating hormone (FSH) and serum luteinizing hormone (LH) levels and a low estradiol, low testosterone, and low anti-Mullerian hormone (AMH) levels. Serum prolactin (PRL), serum thyroid stimulating hormone (TSH), and serum beta human chorionic gonadotropin (beta hCG) levels were within normal range. The cytogenetic report of the patient showed a 46-XY karyotype confirming our diagnosis. The patient was advised to undergo prophylactic gonadectomy for the left gonad. Swyer syndrome is a rare disorder of sexual development which needs vigorous clinical, laboratory, and radiological evaluation. Ultrasound is the primary investigation of choice whereas MRI is used as a problem-solving tool in localizing the streak gonads. Early diagnosis is crucial in these patients since prophylactic gonadectomy reduces the risk of developing germ cell tumor.
Keywords: Primary amenorrhea, Swyer syndrome, Gonadal dysgenesis
Introduction
Swyer syndrome is a rare syndrome associated with complete gonadal dysgenesis and is seen in phenotypically female patients with 46-XY karyotype [1,2]. It occurs due to mutation in the SRY gene present on Y chromosome which helps in testicular differentiation [3]. Therefore, these patients show the absence of Wolffian structures. Due to the absence of anti-Mullerian hormone that is released by the testes, there may be variable degrees of development of Mullerian structures. The gonads are nonfunctioning, dysgenetic, and steak [2]. These patients usually present with primary amenorrhea or delayed puberty with usually a normal female external phenotype making the diagnosis difficult just on the basis of clinical examination.
Imaging plays an important role in accurately identifying the internal genitalia and to see degree of development of Mullerian structures. There can be variable degrees of development of Mullerian structures in these patients. The dysgenetic gonad which is nonfunctional is prone to undergo malignant transformation such as dysgerminoma, gonadoblastoma, etc. [2,3]. Timely diagnosis helps in deciding appropriate management strategies for the patient—such as hormone replacement therapy and gonadectomy.
Case presentation
A 30-year-old young lady came to our department with complaints of primary amenorrhea. There was no similar family history of infertility, amenorrhea, abnormal external genitalia development, or cryptorchidism. She was phenotypically female with normal female external genitalia and showed normal breast development on clinical examination. However, the axillary and pubic hair were underdeveloped for her age (Fig. 1).
Fig. 1.
A and B showing normal breast development (A) with scanty development of axillary hairs (B).
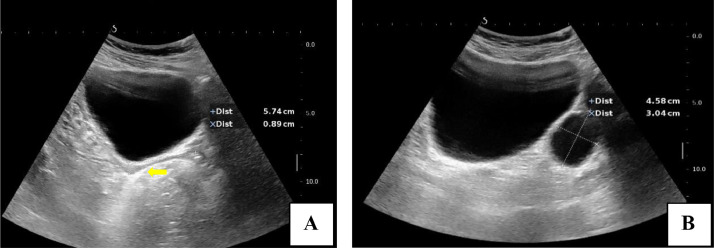
The patient was then taken up for ultrasound. On ultrasound, a hypoplastic uterus was seen just posterior to urinary bladder, measuring 5.7 × 0.8 cm in size. Along with this, there was a well-circumscribed anechoic structure of size 4.5 × 3 cm noted in left hemipelvis just anterior to the left internal iliac vessels with no demonstrable follicle-like structure. Right ovary was also not seen. There was no evidence of any ectopic abdominal or inguinal testes on ultrasound of pelvic and inguinal regions (Fig. 2).
Fig. 2.
(A and B) Gray scale ultrasound images of pelvis revealed a hypoplastic uterus (arrow in A) with anechoic cystic structure in left hemipelvis (B).
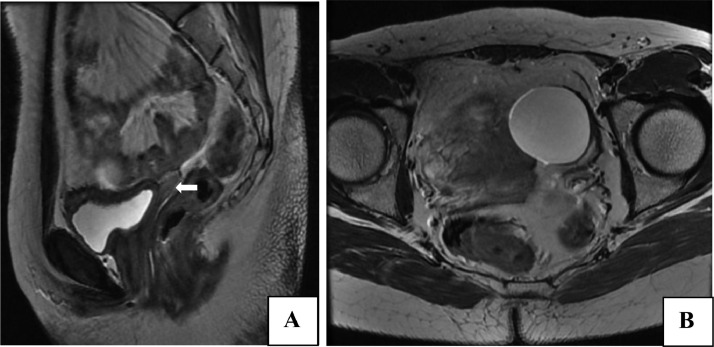
The patient underwent MRI of abdomen and pelvis for further evaluation—to look for any ovarian or testes like structure not well seen on ultrasound. MRI confirmed the findings on ultrasound and showed a hypoplastic uterus and cystic lesion in left hemipelvis in the designated position of left ovary with nonvisualization of ovary on right side. A provisional diagnosis of complete gonadal dysgenesis with hypoplastic uterus was given on the basis of clinical examination and imaging findings (Fig. 3).
Fig. 3.
(A and B) MRI confirming the ultrasound findings of a hypoplastic uterus (white arrow in A) and the cystic lesion in left hemipelvis (B).
Her blood investigations and hormonal assays revealed hypergonadotropic-hypogonadism with high levels of serum follicular stimulating hormone (FSH), serum luteinizing hormone (LH), and low estradiol and testosterone levels{FSH: 91 IU/mL, LH: 54 mIU/mL, estradiol: 12 pg/mL, testosterone: 1.07 ng/mL}. Serum prolactin (PRL), serum thyroid stimulating hormone (TSH), and serum beta human chorionic gonadotropin (beta hCG) levels were within normal range.
She was then referred to the department of cytogenetics for karyotyping which revealed a 46-XY karyotype—favoring our imaging diagnosis of complete gonadal dysgenesis or Swyer syndrome. The patient was advised by the clinician to undergo surgery for removal of the left dysplastic gonad because of the risk of development of malignancy (Fig. 4).
Fig. 4.
Cytogenetic analysis showing a 46-XY karyotype.
Discussion
Swyer syndrome—a rare syndrome associated with complete gonadal dysgenesis—is seen in phenotypically female patients with 46-XY karyotype [1]. They usually present with primary amenorrhea or delayed puberty. The pathogenesis of this condition includes mutation in the DNA binding site of SRY gene present on Y chromosome, which plays a crucial role in the development of gonads. The dysgenetic gonad, which is nonfunctional, is prone to undergo malignant transformation such as dysgerminoma, gonadoblastoma, etc. [6], [7], [8], [9], [10]. Timely diagnosis helps in deciding appropriate management strategies for the patient such as hormone replacement therapy and gonadectomy.
The closest differential of Swyer syndrome is complete androgen insensitivity syndrome, which is X-linked genetic disorder in which a phenotypically female patient having a 46-XY karyotype has resistance to androgen [4], [5].
The given table (Table 1) summarizes the main differences between the 2 conditions.
Table 1.
Differences between Swyer syndrome and androgen insensitivity syndrome.
| Feature | Swyer syndrome | Androgen insensitivity syndrome |
|---|---|---|
| Karyotype | 46-XY | 46-XY |
| Pathology | Lack of SRY gene on Y chromosome | X-linked mutation in androgen receptor gene |
| Lack of testicular development | Resistance to androgens | |
| Hormonal assay | Low testosterone, low AMH | High testosterone, normal AMH |
| Phenotype and external genitalia | Female | Female |
| Internal genitalia | Streak gonads | Testes |
| Mullerian structures | Present to variable degrees | Absent |
| Testes | Absent | Present |
AMH, anti-Mullerian hormone.
Conclusion
Adult female patients with complaints of primary amenorrhea should undergo vigorous diagnostic work-up after a thorough clinical examination of breasts, axilla, pubic region, and external genitalia followed by imaging, hormonal assay, and karyotype if needed. Ultrasound is usually the first imaging modality which is used in assessing these patients. MRI can be used as a problem-solving tool and also to localize streak gonads accurately.
Patient consent
Written detailed informed consent was obtained by the author from the patient included in the case report.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chand MT, Turner S, Solomon LA, Jay A, Rabah R, Misra VK. A case of 45, X/46, XY mosaicism presenting as Swyer syndrome. J Pediatr Adolescent Gynecol. 2020;33(5):577–580. doi: 10.1016/j.jpag.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Korkmaz H, Özkaya M, Akarsu E. Swyer syndrome: a case report. Turk J Endocrinol Metab. 2014;18(2) [Google Scholar]
- 3.Mayur P, Parikshaa G, Anil B, Shalini G, Arvind R. ‘Size does matter’: prophylactic gonadectomy in a case of Swyer syndrome. J Gynecol Obstet Hum Reprod. 2019;48(4):283–286. doi: 10.1016/j.jogoh.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Da Silva Rios S, Monteiro IC, Braz Dos Santos LG, Caldas NG, Chen AC, Chen JR, et al. A case of Swyer syndrome associated with advanced gonadal dysgerminoma involving long survival. Case Rep Oncol. 2015;8(1):179–184. doi: 10.1159/000381451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunes E, Rodrigues C, Geraldes F, Águas F. Differentiating Swyer syndrome and complete androgen insensitivity syndrome: a diagnostic dilemma. J Pediatr Adolesc Gynecol. 2014;27(3):e67–e68. doi: 10.1016/j.jpag.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Yadav P, Khaladkar S, Gujrati A. Imaging findings in dysgerminoma in a case of 46 XY, complete gonadal dysgenesis (Swyer syndrome) J Clin Diagn Res: JCDR. 2016;10(9):TD10. doi: 10.7860/JCDR/2016/19488.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culha C, Ozkaya M, Serter R, Sahin I, Aydin B, Aral Y. Swyer's syndrome: in a fifty-year-old female. J Obstet Gynecol India. 2012;62:571–574. doi: 10.1007/s13224-011-0100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piazza MJ, Urbanetz AA. Germ cell tumors in dysgenetic gonads. Clinics. 2019;74 doi: 10.6061/clinics/2019/e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raafey MA, Abdulwaasey M, Fatima SS, Uddin Z, Tariq MU. Bilateral gonadoblastoma with dysgerminoma in a phenotypically normal female with 46xx karyotype: report of a rare case and literature review. Cureus. 2020;12(7) doi: 10.7759/cureus.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han Y, Wang Y, Li Q, Dai S, He A, Wang E. Dysgerminoma in a case of 46, XY pure gonadal dysgenesis (Swyer syndrome): a case report. Diagn Pathol. 2011;6:1–6. doi: 10.1186/1746-1596-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]