Abstract
Objective
To investigate the impact of frailty-originated, evidence-based early activity training on postoperative delirium in patients who have undergone brain tumor resection.
Methods
A randomized controlled trial was conducted at the Second Affiliated Hospital of Zhejiang University School of Medicine, from July 2019 to June 2020. Data on the patients' general information, incidence and duration of delirium, duration of hospital stay, and activities of daily living were collected. From the first day after surgery, the patients were randomly assigned to either the traditional care group or the frailty-originated rehabilitation towards intracranial tumors using distinct evidence (FORTITUDE) group. Non-parametric, chi-square, and log-rank tests were used to compare the onset time and duration of postoperative delirium and activities of daily living performed by the participants between the two groups.
Results
In total, 291 patients, 150 and 141 in the control group and FORTITUDE group, respectively, participated in the study. Patients in the FORTITUDE group had a lower incidence of postoperative delirium (15.6% vs. 28.7%, P = 0.007), delayed onset of delirium (Z = −2.108, P = 0.035), shorter duration of postoperative delirium (χ2 = 26.67, P < 0.001), shorter hospital stay (Z = −2.037, P = 0.042), and higher scores in the activities of daily living one week (Z = −2.304, P = 0.021) and one month (Z = −2.724, P = 0.006) after surgery than in the control group.
Conclusions
The FORTITUDE program was safe and effective in reducing the incidence and duration of postoperative delirium and improving the quality of life of patients who underwent brain tumor resection.
Keywords: Postoperative delirium, Frailty, Rehabilitation, Activity of daily living, Brain tumor
Introduction
Postoperative delirium is a common manifestation of an acute disturbance in the central nervous system function that occurs in the aftermath of craniotomy procedures.1 It is characterized by fluctuations in consciousness, disruptions in attention and concentration, alterations in thinking processes, and changes in the level of consciousness. Epidemiological studies have demonstrated that the frequency of postoperative delirium in surgical patient population ranges from 11% to 51%,2 with the onset typically observed within 1–3 days after surgery.
The postoperative delirium has considerable clinical implications and has been associated with impairments in memory,3 decreases in long-term cognitive function, increased complications, and prolonged hospital stays, affecting health care costs4,5 and the well-being of patients caregivers.6
The risk factors for the development of postoperative delirium include anesthesia, advanced age, cognitive impairment, and presence of multiple comorbid conditions.7,8 Frailty has also been identified as a potent risk factor for postoperative delirium, with a three-to eight-fold increase in incidence9,10 observed in frail patients who also experience increased risks of falls, disability, hospitalization, and death.11 It is noteworthy that frailty is bidirectional, and with appropriate interventions, it can be reversed, and exercise is likely the medicine that will reverse frailty.12
In this study, we proposed an innovative early rehabilitation program for patients undergoing intracranial tumor surgery that was premised on mitigating the risk factors contributing to the development of postoperative delirium. This study aimed to evaluate the effect of evidence-based early activity training on postoperative delirium in patients with brain tumor resection.
Methods
Study design and ethical considerations
We conducted a single-center, prospective, randomized, open-label, blinded-end point trial to assess the efficacy of our proposed early rehabilitation program compared to a control group. The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the Human Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (IRB No. 2019 Yan No. 117). All participants provided written informed consent.
Patient inclusion and exclusion criteria
Patients who underwent craniotomies for brain tumors at the Second Affiliated Hospital of Zhejiang University School of Medicine between July 2019 and June 2020 were considered potential participants for this study. The inclusion criteria were as follows: (1) a Glasgow coma scale score of 14 or higher, (2) a muscle strength grade of 4 or higher, (3) age between 18 and 75 years, (4) stable patients who did not require emergency surgery, and (5) signed informed consent forms. The exclusion criteria were as follows: (1) patients with mental disorders or cognitive impairment, (2) drug or alcohol dependence, (3) frontal lobe tumors, and (4) patients requiring bed rest.
Randomized allocation of participants
Written informed consent was obtained from the included patients with postoperative brain tumors, and simple randomization was performed using a computer-generated cluster randomization method. The trial had two groups: the traditional care group (the control group) and the frailty-originated rehabilitation towards intracranial tumors using distinct evidence (FORTITUDE) group (the FORTITUDE group).
FORTITUDE program
Based on routine nursing practices, the FORTITUDE group carried out a systematic early rehabilitation program (FORTITUDE) for patients after intracranial tumor surgery. The program was informed through a comprehensive literature review, current guidelines, and expert consensus on “fast track surgery,” “enhanced recovery after surgery,” “early mobilization,” “enhanced recovery,” and “enhanced rehabilitation.” Six references13, 14, 15, 16, 17, 18 were carefully selected from databases such as PubMed, EBSCO, the Cochrane Library, and related websites. The systematic FORTITUDE program included the following components (Table 1).
-
(1)
Day 0 (Surgery Day): Once vital signs were stable, patients were encouraged to turn over 2 h after the operation, change positions every 2–3 h, and had the head of their bed elevated by 15–30° after 6 h.13, 14, 15, 16
-
(2)
Day 1 (the first day after neurosurgery): Patients' head of the bed was elevated to 45°, and the patient was gradually transitioned to a sitting position. Early rehabilitation exercise training began 24 h after the operation and continued for 5 consecutive days, with each session lasting 60 min.14,17 The training activities included Bobath exercise for joint and muscle strength for 10 min, muscle group strength training with a rubber band for 10 min, functional training for upper limb and hand fine motor skills for 15 min, balance training for 10 min, and walking with assistance and surveillance protection for 15 min.
-
(3)
Day 2 (the second day after neurosurgery): Daily living skills training, including washing, eating, and dressing, was started.18 The patients wore a portable fingertip oxygen saturation monitor during the activities and were accompanied by medical staff throughout for safety.
-
(4)
Days 3–5 (the third to fifth day after neurosurgery): Early rehabilitation exercise and daily living skills training for 60 min.
-
(5)
Safety: It was important to monitor the patient's vital signs and conditions during the rehabilitation program, and the early activities outlined in the FORTITUDE program should be terminated immediately if the following situations arose13: heart rate exceeded 130 beats per min or drop below 40 beats per min; respiratory rate was less than 5 times per min; exceeded 40 times per min; oxygen saturation dropped below 88%; or the patient experienced severe headaches, nausea, vomiting, or any other events that impacted their ability to participate in the activities.
Table 1.
Frailty-originated rehabilitation towards intracranial tumor using distinct evidence (FORTITUDE) program for patients following intracranial tumor surgery.
| Day | Activity |
|---|---|
| 0 | Turn over 2 h after the operation |
| Change positions every 2–3 h | |
| Elevate head of bed by 15°–30° after 6 h | |
| 1 | Raise head of bed to 45° |
| Transition to sitting position gradually | |
| Early exercise training (lasts 60 min): | |
| |
| |
| |
| |
| |
| 2–5 | Early exercise training (lasts 60 min) |
| Daily living skills training: washing, eating, and dressing | |
| Wear portable fingertip oxygen saturation monitor and accompanied by medical staff during activity |
Day 0: Surgery day; Day 1: The first day after neurosurgery; Day 2–5: The second to fifth day after neurosurgery.
To reduce heterogeneity in nursing care, a specialized research nurse and doctor team provided coaching to clinical bedside nurses over a period of 6 weeks prior to the implementation of this program at our center. Once the patient's condition was permitted, trained bedside nurses and family members jointly assisted patients in performing activities under the FORTITUDE program.
Standard care
The control group received standard care, which included: (1) elevating the head of the bed to 30° 6 h after surgery, (2) implementing multimodal analgesia using oral, intravenous, and physical methods to manage pain, (3) removing the catheter on the second postoperative day, and (4) allowing general mobility and self-directed movements based on the patients' condition; however, structured rehabilitation exercises under professional guidance were not arranged until they were transferred to a rehabilitation hospital after discharge.
Measurements
-
1)
General data collection: The following demographic information was collected from each participant: age, gender, body mass index (BMI), history of disease, level of education, smoking status, history of alcohol consumption, living situation, and frailty status.
-
2)
Frailty assessment: The Fried frailty phenotype is a commonly used clinical tool for frailty assessment. In 2001, Fried et al19 proposed five diagnostic criteria: (1) Unintentional weight loss: ≥ 10 pounds in a previous year or at least 5% of the previous year's body weight; (2) Exhaustion: based on the Center for Epidemiologic Studies Depression Scale (CES–D), patients in depression mode for three or more days in the last week; (3) Weakness: grip strength in the lowest 20% at baseline, adjusted for gender and BMI; (4) Slow walking speed: Based on time to walk 15 feet, 20% of the population was defined at baseline, adjusted for gender and standing height (male height > 173 cm and female height > 159 cm, 15 feet's walk ≥ 6 s and male height ≤ 173 cm and female height ≤ 159 cm, 15 feet's walk ≥ 7 s); (5) Low physical activity: males with Kcals of physical activity per week < 383 and females with Kcals per week < 270 were frail. The detailed criteria could be found in the Appendix. In this study, we defined frailty as meeting three or more of these criteria and non-frailty as meeting fewer than three criteria.
-
3)
Nursing delirium screening scale (Nu-DESC)20: Delirium status was monitored throughout the hospital stay using the Nu-DESC, which consists of five characteristics: disorientation, inappropriate behavior, inappropriate communication, hallucinations, and psychomotor retardation. Due to its high sensitivity, specificity, and ease of use, the Nu-DESC was the preferred delirium assessment tool among non-ICU nurses. Assessments were performed by two separate research team nurses at three time points each day (8:00, 12:00, and 16:00) after the patients returned to the wards following surgery. In the event of disagreement between the two raters, an experienced senior nurse, independent of the research teams, was involved in the final determination. Inter-rater reliability analysis using the Kappa statistic was performed to determine the consistency among the raters. A score of 0, 1, and 2 indicated the absence of delirium, mild symptoms, and moderate-to-severe symptoms, respectively. The total possible score was 10, and a score equal to or greater than 2 was considered positive.
-
4)
Follow-up activities of daily living (ADL) using the Barthel Index (BI)21: The BI was used to assess ADL at the time of discharge through a face-to-face interview by research team nurses and 7 and 30 days after discharge through a structured telephone interview. The BI comprised 10 personal activities: feeding, personal toileting, bathing, dressing and undressing, getting on and off the toilet, controlling the bladder and bowel, moving from a wheelchair to a bed and returning, walking on a level surface (or propelling a wheelchair if unable to walk), and ascending and descending stairs. A higher BI score indicated a greater ability to function independently following hospital discharge.
Outcome
Outcome assessment was performed by the research team nurses, who were independent of the treatment team and unaware of the patients' treatment allocation. Moreover, they had no access to the patients' medical records or any information that might have revealed their treatment assignments, allowing an unbiased comparison of the study results. The primary outcome was the incidence of delirium in patients who underwent intracranial tumor resection during hospitalization. The secondary outcomes included: (1) the duration of delirium, (2) the onset time of postoperative delirium (the period until the onset of delirium after surgery), (3) BI scores at discharge, 7 days, and 30 days post-discharge, (4) the duration of postoperative hospital stay, and (5) the hospitalization costs. Additionally, safety outcomes, including adverse events such as falls from bed, unplanned extubation, intracranial hemorrhage, and brain herniation that occurred during the hospital stay, were monitored.
Risk management
Immediate measures, such as maintaining a quiet environment, comforting the patient, using soothing music to divert attention, allowing family members to accompany the patient, and administering sedatives whenever necessary, should be taken during the acute delirium. Additionally, close monitoring of patients' vital signs and changes in their delirium status was essential. If a patient experienced a fall or signs of cerebral herniation, a prompt computed tomography scan should be performed, and surgical intervention or conservative treatment should be conducted based on the specific circumstances. In the event of accidental extubation, reintubation or close clinical observation should be performed based on the importance of the removed tube.
Sample size calculation
Based on pre-experimental results, the incidence of delirium was estimated to be approximately 30% in the control group and 15% in the FORTITUDE group within one month of brain tumor surgery. With a bilateral alpha set at 0.05 and a power level of 0.8, the sample size for each group was calculated to be 118 using PASS 2021 software, with a 1:1 ratio between the two groups. To account for an acceptable loss-to-follow-up rate of 15%, the minimum sample size for each group was established at 139.
Data analysis
Continuous data that did not meet the criteria for normality and homogeneity of variance were reported as medians (interquartile ranges). Non-parametric tests were used to compare the differences between the two groups. Categorical data were reported as n (%). The chi-square test, continuity correction, Fisher's exact test, and log-rank test were used to analyze the differences between the groups. All statistical analyses were performed using SPSS 26 statistical software (SPSS Inc., Chicago, IL, USA), and figures were generated using GraphPad Prism (version 9.3.1, GraphPad Software, LLC). Statistical significance was set at P < 0.05.
Results
Baseline characteristics of the enrolled participants
A flow diagram for study participant selection is presented in Fig. 1. A total of 150 and 141 participants were enrolled in the control group and FORTITUDE group, respectively. The median age of patients in the FORTITUDE group was 53.00 years, which did not statistically differ from that of the control group (median age 55.00 years). Both groups had similar sex ratios (χ2 = 1.478, P = 0.224) and median BMI values (FORTITUDE group median BMI: 23.14 kg/m2; control group median BMI: 23.53 kg/m2, Z = ˗0.678, P = 0.498). Additionally, there were no statistically significant differences between the two groups for past medical history (χ2 = 2.322, P = 0.128), smoking status (χ2 = 1.727, P = 0.189), drinking status (χ2 = 2.337, P = 0.126), educational level (χ2 = 3.753, P = 0.153), tumor type (χ2 = 4.623, P = 0.206), surgical sites (χ2 = 5.629, P = 0.133), frailty status (FORTITUDE group: 45 patients (31.9%); control group: 55 patients (36.7%), χ2 = 0.728, P = 0.394), bedridden status (χ2 = 2.245, P = 0.134), and living status (χ2 = 0.466, P = 0.495) (Table 2).
Fig. 1.
Flow diagram for study participants. FORTITUDE, Frailty Originated Rehabilitation Towards Intracranial Tumor Using Distinct Evidence.
Table 2.
The baseline characteristics of the enrolled patients.
| The FORTITUDE group |
The control group |
Statistics | P value | |
|---|---|---|---|---|
| (n = 141) | (n = 150) | |||
| Age [years, median (IQR)] | 53.00 (46.50–59.50) | 55.00 (49.00–60.00) | Z = −1.448 | 0.147 |
| Gender [n (%)] | χ2 = 1.478 | 0.224 | ||
| Male | 54 (38.3) | 68 (45.3) | ||
| Female | 87 (61.7) | 82 (54.7) | ||
| BMI [kg/m2, median (IQR)] | 23.14 (20.74–25.59) | 23.53 (21.32–25.39) | Z = −0.678 | 0.498 |
| With past disease history [n (%)] | 94 (66.7) | 87 (58.0) | χ2 = 2.322 | 0.128 |
| Smoking status [n (%)] | χ2 = 1.727 | 0.189 | ||
| Current smoker | 20 (14.2) | 30 (20.0) | ||
| Never smoked or former smoker | 121 (85.8) | 120 (80.0) | ||
| Drinking status [n (%)] | χ2 = 2.337 | 0.126 | ||
| Current drink | 11 (7.8) | 20 (13.3) | ||
| Never or former drink | 130 (92.2) | 130 (86.7) | ||
| Education [n (%)] | χ2 = 3.753 | 0.153 | ||
| Illiteracy or elementary school | 62 (44.0) | 59 (39.3) | ||
| Middle or high school | 61 (43.3) | 59 (39.3) | ||
| University | 18 (12.8) | 32 (21.3) | ||
| Tumor type [n (%)] | χ2 = 4.623 | 0.206 | ||
| Meningioma | 56 (39.7) | 57 (38.0) | ||
| Glioma | 45 (31.9) | 52 (34.7) | ||
| Acoustic neurinoma | 32 (22.7) | 24 (16.0) | ||
| Others | 8 (5.7) | 17 (11.3) | ||
| Surgical sites [n (%)] | χ2 = 5.629 | 0.133 | ||
| Parietal | 49 (34.8) | 47 (31.3) | ||
| Temporal | 45 (31.9) | 50 (33.3) | ||
| Occipital | 15 (10.6) | 29 (19.3) | ||
| Cerebellopontine angle | 32 (22.7) | 24 (16.0) | ||
| Frailty before admission [n (%)] | χ2 = 0.728 | 0.394 | ||
| Yes | 45 (31.9) | 55 (36.7) | ||
| No | 96 (68.1) | 95 (63.3) | ||
| Bedridden [n (%)] | χ2 = 2.245 | 0.134 | ||
| Yes | 23 (16.3) | 35 (23.3) | ||
| No | 118 (83.7) | 115 (76.7) | ||
| Live alone [n (%)] | χ2 = 0.466 | 0.495 | ||
| Yes | 2 (1.4) | 5 (3.3) | ||
| No | 139 (98.6) | 145 (96.7) |
BMI, body Mass Index; FORTITUDE, Frailty Originated Rehabilitation Towards Intracranial Tumor Using Distinct Evidence; IQR, interquartile Range.
Incidence, onset time, and duration of postoperative delirium
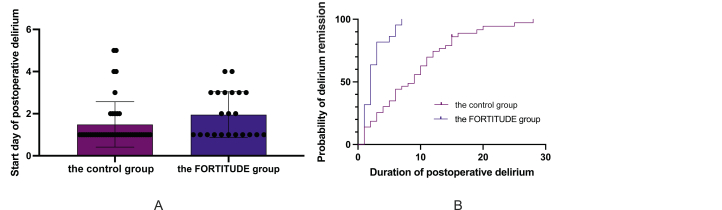
Of 150 patients in the control group, 43 patients developed delirium after neurosurgery, whereas only 22 patients (comprising 15.6%) experienced postoperative delirium in the FORTITUDE group (χ2 = 7.150, P = 0.007) (Table 3). The interrater reliability for the raters was kappa = 0.945 (P = 0.005) (Supplement Table 2). Furthermore, the implementation of FORTITUDE care resulted in a delayed onset time of postoperative delirium compared to the control group (1.95 ± 1.09 days vs. 1.49 ± 1.08 days, Z = ˗2.108, P = 0.035) (Fig. 2A). Moreover, the duration of delirium in the FORTITUDE group was significantly shorter than that in the control group, as determined by survival analysis (χ2 = 26.67, P < 0.001) (Fig. 2B).
Table 3.
The comparison of postoperative delirium, hospitalization, and prognosis of the enrolled patients.
| The FORTITUDE group |
The control group |
Statistics | P value | |
|---|---|---|---|---|
| (n = 141) | (n = 150) | |||
| Postoperative delirium [n (%)] | χ2 = 7.150 | 0.007 | ||
| Yes | 22 (15.6) | 43 (28.7) | ||
| No | 119 (84.4) | 107 (71.3) | ||
| Frailty at discharge [n (%)] | χ2 = 4.478 | 0.034 | ||
| Yes | 28 (19.9) | 46 (30.7) | ||
| No | 113 (80.1) | 104 (69.3) | ||
| Postoperative hospital stay [(days, median (IQR)] | 7.00 (6.00–11.00) | 8.00 (6.00–11.25) | Z = −2.037 | 0.042 |
| Total cost [(yuan, median (IQR)] | 51,821.00 (43,849.00–61,521.00) | 51,967.00 (44,211.25–74,923.75) | Z = −1.529 | 0.126 |
| ADL at admission [median (IQR)] | 100.00 (80.00–100.00) | 95.00 (78.75–100.00) | Z = −1.581 | 0.114 |
| ADL at 1 week after discharge [median (IQR)] | 90.00 (85.00–90.00) | 90.00 (70.00–90.00) | Z = −2.304 | 0.021 |
| ADL at 1 month after discharge [median (Range)] | 100.00 (80.00–100.00) | 100.00 (40.00–100.00) | Z = −2.724 | 0.006 |
ADL, activities of daily living; FORTITUDE, Frailty Originated Rehabilitation Towards Intracranial Tumor Using Distinct Evidence; IQR, interquartile Range.
Fig. 2.
Comparison of postoperative delirium onset time and duration between the control group and the FORTITUDE group. (A) Postoperative delirium onset time comparison; (B) Postoperative delirium duration comparison. FORTITUDE, Frailty Originated Rehabilitation Towards Intracranial Tumor Using Distinct Evidence.
Hospitalization and prognosis
Before admission, the frailty percentage in the FORTITUDE group was not significantly different from that in the control group (χ2 = 0.728, P = 0.394). Conversely, at discharge, the frailty percentage in the FORTITUDE group was much lower than that in the control group (19.9% vs. 30.7%, χ2 = 4.478, P = 0.034) (Fig. 3). Although the average postoperative hospital stay was slightly longer in the control group than in the FORTITUDE group (Z = −2.037, P = 0.042), the overall hospitalization costs were similar in the two groups (Z = −1.529, P = 0.126). At the time of admission, ADL scores were comparable in both groups (Z = −1.581, P = 0.114). However, patients in the FORTITUDE group exhibited improved functional prognoses, as evidenced by higher ADL scores at one week (Z = −2.304, P = 0.021) and one month (Z = −2.724, P = 0.006) after hospital discharge (Table 3).
Fig. 3.
Comparison of frailty percentage before admission and at discharge between the FORTITUDE group and the control group. (A) Frailty before admission; (B) Frailty at discharge. FORTITUDE, Frailty Originated Rehabilitation Towards Intracranial Tumor Using Distinct Evidence.
Complications
Safety outcomes, including adverse events such as falls from bed, unplanned extubation, intracranial hemorrhage, and brain herniation during the hospital stay, did not significantly differ between the two groups (P > 0.05).
Discussion
Delirium is a common neurosurgical complication that manifests increased speech, emotional agitation, and heightened physical activity. The onset of delirium can lead to accidents, pose a threat to patient safety, and cause significant psychological stress to the patients' families and health care staff. Factors contributing to postoperative delirium include advanced age, infection, pain, anesthesia, medications, and tube irritation. Frailty is also a significant contributing factor characterized by a decrease in physiological reserve and increased vulnerability to stressors, which can increase the risk of adverse health outcomes, including postoperative delirium.22 Previous studies have reported a significant association between preoperative frailty and postoperative delirium.9,23 Frailty can increase the risk of postoperative delirium in several ways.22 First, frailty impairs the physiological reserve and reduces the body's ability to cope with stressors, including surgery and anesthesia, making patients more susceptible to postoperative delirium. Second, frailty is associated with a chronic low-grade inflammatory state, which could lead to an increased risk of postoperative delirium. Third, frailty is often associated with polypharmacy, which increases the risk of adverse drug reactions, including delirium. Fourth, frailty is often associated with malnutrition, which could lead to electrolyte imbalance and other metabolic disturbances that increase the risk of postoperative delirium. Frailty can be reversed through appropriate functional exercise,24 nutritional support,25 and comprehensive nursing intervention.26 We designed an advanced rehabilitation program aimed at reducing and managing preventable risk factors, especially frailty, to mitigate the occurrence of delirium.
In addition to sufficient nutritional support, the process of motor relearning following a central nervous system injury is a key component in improving frailty and promoting rehabilitation. The central nervous system can be reorganized to promote nerve function through motor learning and training.27 With advancements in medical technology, accelerated rehabilitation is widely practiced in clinical settings. Early rehabilitation has gained considerable attention in nursing as a crucial component of accelerated rehabilitation. Early rehabilitation activities have been applied to various disease areas.28,29 For instance, a randomized controlled trial30 conducted in China showed that fast-track surgery significantly reduced postoperative stress reactions and accelerated the rehabilitation of patients who underwent gastric cancer surgery. In another systematic review and meta-analysis,31 early rehabilitation after total knee arthroplasty resulted in reduced postoperative pain, improved mobility and function, and a shorter duration of hospital stay. Similarly, a study32 conducted in South Korea reported that preoperative rehabilitation was effective in reducing postoperative pulmonary complications and the duration of hospital stay in lung cancer surgery. However, some studies have reported the opposite effects of early rehabilitation on patient outcomes. For instance, an Italian study by Tedesco et al.33 found that early rehabilitation in older adults who underwent surgery for hip fractures was associated with longer hospital stays and higher rehospitalization rates. This discrepancy in results might be due to differences in patient populations or variations in rehabilitation programs across studies.
Early rehabilitation programs in clinical research often exhibit significant individual differences and lack measurable standards, making it challenging to guide clinical practice. Therefore, the development of an evidence-based, safe, and practical activity plan that considers the unique characteristics of the disease is urgently needed. In this study, an evidence-based team was established to formulate an appropriate activity plan for patients after brain tumor resection. For this, the FORTITUDE program was implemented to reduce postoperative delirium in patients who underwent craniotomy. The evidence-based FORTITUDE program standardizes rehabilitation exercises and integrates daily living skills training to aid patients in returning to daily activities. The incidence of delirium was significantly lower in the FORTITUDE group than in the control group, with a delayed onset and shorter duration of delirium. Our findings suggest that early mobilization has a positive effect on reducing postoperative delirium. The FORTITUDE program was well tolerated and was a safe and feasible option for patients.
This study aimed to provide recommendations for postoperative rehabilitation after brain tumor surgery by conducting a systematic review of the available literature. Six publications13, 14, 15, 16, 17, 18 containing consensus reports, reviews, and clinical recommendations were included in this study. The FORTITUDE program was conducted according to the available evidence of the clinical course chronology in postoperative rehabilitation for up to three days and from a new angle by improving frailty. Additional high-quality clinical studies are required to further investigate the relationship between an extended rehabilitation period and prognosis. Our study had several limitations that should be considered. First, this was a single-center study focused on patients who underwent neurosurgical craniotomy. Although the homogeneity of the nursing intervention was ensured to observe the impact of early activities on delirium, a subgroup analysis of different types of tumors was not performed. Therefore, multicenter studies focusing on specific types of neurosurgical diseases should be conducted in the future. Second, this prospective randomized open-blind end point study might have introduced measurement bias and subjective bias for both researchers and participants; however, it was worth mentioning that it was much closer to real clinical practice. The average cost of implementing the FORTITUDE program was approximately ¥2000, while the standard care group incurred costs of around ¥1000. Relative to the overall hospitalization costs of over ¥50,000 for patients, these expenses were minimal. Notably, the average costs for patients in the FORTITUDE group were slightly lower than those for patients in the control group, although this difference was not statistically significant. Furthermore, the FORTITUDE group experienced positive outcomes, such as a lower incidence and delayed onset of postoperative delirium, shorter duration of delirium, shorter hospital stay, and better functional scores in daily activities. Therefore, the FORTITUDE program demonstrated a higher level of cost-effectiveness than standard care. Third, this study explored the effect of improving physical function through early mobilization on postoperative delirium by changing the patient's frailty state. Although it has been confirmed that a systematic early rehabilitation program can improve physical function and reduce the occurrence of postoperative delirium, the mediating effect of frailty between early mobilization and postoperative delirium has not yet been studied in depth, and further studies are required to fully understand these relationships.
Conclusions
Our study successfully implemented an evidence-based FORTITUDE program that demonstrated both efficacy and safety. Compared to the control group, patients in the FORTITUDE group exhibited a reduced incidence of postoperative delirium, a delayed onset time of postoperative delirium, a shorter duration of postoperative delirium and hospital stay, and an improved functional prognosis after neurosurgery.
Acknowledgments
We would like to thank Dr. Xiaobo Yu for assisting us in improving this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.apjon.2023.100263.
CRediT author statement
Data collection, Qiuping Gu and Minglan Zhu; Data validation, Yuan Yuan and Yuping Zhang; Methodology and analysis, Yuping Zhang; Writing – Original Draft Preparation, Yuan Yuan; Writing – Review & Editing, Qiuping Gu and Meijuan Lan; Supervision, Meijuan Lan and Minglan Zhu. All authors had full access to all the data in the study, and the corresponding author had final responsibility for the decision to submit for publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Declaration of competing interest
All authors have none to declare.
Funding
The study was funded by the Zhejiang Provincial Department of Education Project (Grant No. Y201839036). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the Human Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (IRB No. 2019 Yan No. 117). All participants provided written informed consent.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, ML. The data are not publicly available due to sensitive nature of the data and the need to protect participant confidentiality.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Jin Z., Hu J., Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125:492–504. doi: 10.1016/j.bja.2020.06.063. [DOI] [PubMed] [Google Scholar]
- 2.Inouye S.K., Westendorp R.G., Saczynski J.S. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maldonado J.R. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33:461–519. doi: 10.1016/j.ccc.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Austin C.A., O'Gorman T., Stern E., et al. Association between postoperative delirium and long-term cognitive function after major nonemergent surgery. JAMA Surg. 2019;154:328–334. doi: 10.1001/jamasurg.2018.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudolph J.L., Marcantonio E.R. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg. 2011;112:1202–1211. doi: 10.1213/ANE.0b013e3182147f6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martins S., Pinho E., Correia R., et al. What effect does delirium have on family and nurses of older adult patients? Aging Ment Health. 2018;22:903–911. doi: 10.1080/13607863.2017.1393794. [DOI] [PubMed] [Google Scholar]
- 7.Bramley P., McArthur K., Blayney A., McCullagh I. Risk factors for postoperative delirium: an umbrella review of systematic reviews. Int J Surg. 2021;93 doi: 10.1016/j.ijsu.2021.106063. [DOI] [PubMed] [Google Scholar]
- 8.Wittmann M., Kirfel A., Jossen D., Mayr A., Menzenbach J. The impact of perioperative and predisposing risk factors on the development of postoperative delirium and a possible gender difference. Geriatrics. 2022;7 doi: 10.3390/geriatrics7030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gracie T.J., Caufield-Noll C., Wang N.Y., Sieber F.E. The association of preoperative frailty and postoperative delirium: a meta-analysis. Anesth Analg. 2021;133:314–323. doi: 10.1213/ANE.0000000000005609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung P., Pereira M.A., Hiebert B., et al. The impact of frailty on postoperative delirium in cardiac surgery patients. J Thorac Cardiovasc Surg. 2015;149:869–875. doi: 10.1016/j.jtcvs.2014.10.118. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Garcia S., Garcia-Pena C., Salva A., et al. Frailty in community-dwelling older adults: association with adverse outcomes. Clin Interv Aging. 2017;12:1003–1011. doi: 10.2147/CIA.S139860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bray N.W., Smart R.R., Jakobi J.M., Jones G.R. Exercise prescription to reverse frailty. Appl Physiol Nutr Metab. 2016;41:1112–1116. doi: 10.1139/apnm-2016-0226. [DOI] [PubMed] [Google Scholar]
- 13.Sommers J., Engelbert R.H., Dettling-Ihnenfeldt D., et al. Physiotherapy in the intensive care unit: an evidence-based, expert driven, practical statement and rehabilitation recommendations. Clin Rehabil. 2015;29:1051–1063. doi: 10.1177/0269215514567156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damkliang J., Considine J., Kent B., Street M. Using an evidence-based care bundle to improve initial emergency nursing management of patients with severe traumatic brain injury. J Clin Nurs. 2015;24:3365–3373. doi: 10.1111/jocn.12923. [DOI] [PubMed] [Google Scholar]
- 15.Picetti E., Catena F., Abu-Zidan F., et al. Early management of isolated severe traumatic brain injury patients in a hospital without neurosurgical capabilities: a consensus and clinical recommendations of the World Society of Emergency Surgery (WSES) World J Emerg Surg. 2023;18:5. doi: 10.1186/s13017-022-00468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krasny-Pacini A., Chevignard M., Evans J. Goal management training for rehabilitation of executive functions: a systematic review of effectiveness in patients with acquired brain injury. Disabil Rehabil. 2014;36:105–116. doi: 10.3109/09638288.2013.777807. [DOI] [PubMed] [Google Scholar]
- 17.Hawryluk G.W.J., Rubiano A.M., Totten A.M., et al. Guidelines for the management of severe traumatic brain injury: 2020 update of the decompressive craniectomy recommendations. Neurosurgery. 2020;87:427–434. doi: 10.1093/neuros/nyaa278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asklid D., Segelman J., Gedda C., Hjern F., Pekkari K., Gustafsson U.O. The impact of perioperative fluid therapy on short-term outcomes and 5-year survival among patients undergoing colorectal cancer surgery – a prospective cohort study within an ERAS protocol. Eur J Surg Oncol. 2017;43:1433–1439. doi: 10.1016/j.ejso.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 20.Gaudreau J.D., Gagnon P., Harel F., Tremblay A., Roy M.A. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage. 2005;29:368–375. doi: 10.1016/j.jpainsymman.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Mahoney F.I., Barthel D.W. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 22.Dasgupta M., Dumbrell A.C. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54:1578–1589. doi: 10.1111/j.1532-5415.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu C.Y., Gong N., Liu W. The association between preoperative frailty and postoperative delirium: a systematic review and meta-analysis. J Perianesth Nurs. 2022;37:53–62 e1. doi: 10.1016/j.jopan.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Walston J., Buta B., Xue Q.L. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med. 2018;34:25–38. doi: 10.1016/j.cger.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smoliner C., Norman K., Scheufele R., Hartig W., Pirlich M., Lochs H. Effects of food fortification on nutritional and functional status in frail elderly nursing home residents at risk of malnutrition. Nutrition. 2008;24:1139–1144. doi: 10.1016/j.nut.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Cameron I.D., Fairhall N., Langron C., et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. doi: 10.1186/1741-7015-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dionisio A., Duarte I.C., Patricio M., Castelo-Branco M. The use of repetitive transcranial magnetic stimulation for stroke rehabilitation: a systematic review. J Stroke Cerebrovasc Dis. 2018;27:1–31. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Pan X.L. Efficacy of early rehabilitation therapy on movement ability of hemiplegic lower extremity in patients with acute cerebrovascular accident. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000009544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kernc D., Strojnik V., Vengust R. Early initiation of a strength training based rehabilitation after lumbar spine fusion improves core muscle strength: a randomized controlled trial. J Orthop Surg Res. 2018;13:151. doi: 10.1186/s13018-018-0853-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D., Kong Y., Zhong B., Zhou X., Zhou Y. Fast-track surgery improves postoperative recovery in patients with gastric cancer: a randomized comparison with conventional postoperative care. J Gastrointest Surg. 2010;14:620–627. doi: 10.1007/s11605-009-1139-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang L., Lee M., Zhang Z., Moodie J., Cheng D., Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-009857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X., Cheung D.S.T., Smith R., Lai A.Y.K., Lin C.C. The effectiveness of pre- and post-operative rehabilitation for lung cancer: a systematic review and meta-analysis on postoperative pulmonary complications and length of hospital stay. Clin Rehabil. 2022;36:172–189. doi: 10.1177/02692155211043267. [DOI] [PubMed] [Google Scholar]
- 33.Tedesco D., Gibertoni D., Rucci P., et al. Impact of rehabilitation on mortality and readmissions after surgery for hip fracture. BMC Health Serv Res. 2018;18:701. doi: 10.1186/s12913-018-3523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, ML. The data are not publicly available due to sensitive nature of the data and the need to protect participant confidentiality.