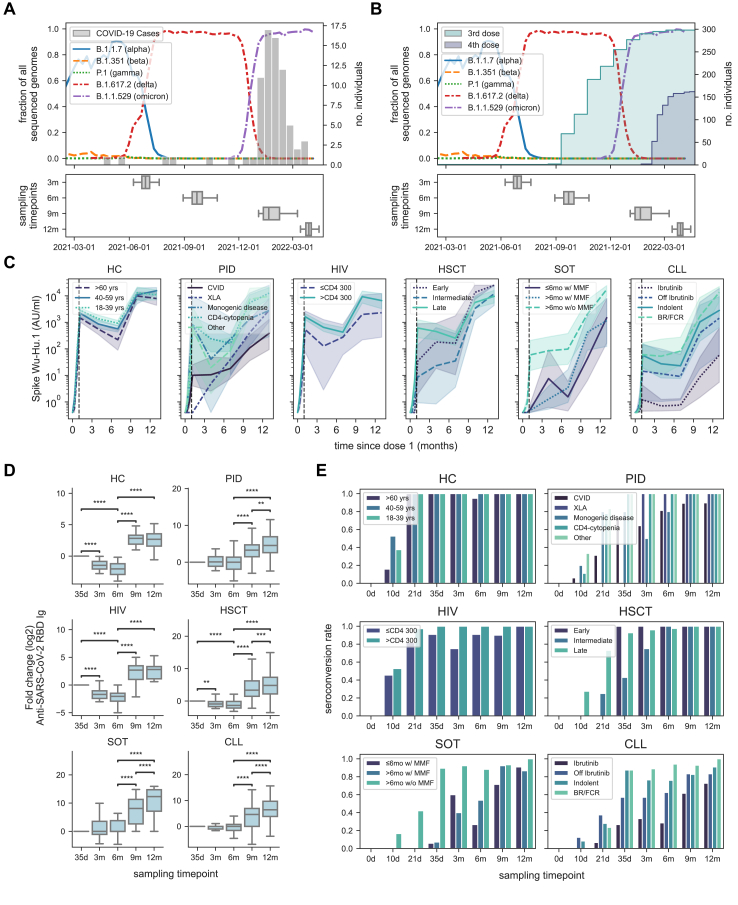
Fig. 1.
Dynamics of antibody titres of the COVAXID cohort. Epidemiology of prevailing SARS-CoV-2 subvariants in Sweden during the study period in relation to (A) number of verified SARS-CoV-2 infections (histogram) among study participants and (B) administration of third (shaded green cumulative histogram) and fourth (shaded blue cumulative histogram) mRNA vaccine doses. Below each graph, boxplots show the distribution of sample dates for each sampling timepoint. (C) Dynamics of Spike Ig RBD Ab titres (geometric mean with 95% CI) (shaded range) for each subgroup. The vertical dotted line represents the timepoint for the primary endpoint of the original clinical trial (35-day timepoint). (D) Fold change of Spike-RBD titres at each timepoint at a study group level. Values are normalized to the day 35-timepoint. Statistical tests were performed on paired Spike-RBD titres using Wilcoxon, and Bonferroni correction for multiple comparisons. (E) Seroconversion rates over time in each subgroup as defined by Spike RBD titres ≥0·8 AU/ml in the entire COVAXID cohort. Ab titres were quantified using the Roche-Elecsys platform. The star annotation (∗) indicates statistical significance at a p-value threshold of 0.05 (or ∗∗ for p < 0.01, ∗∗∗ for p < 0.001, ∗∗∗∗ for p < 0.0001). For sample sizes, please see Table 1. Whiskers for all box plots represents 1.5× IQR.