Abstract
Background
Longitudinal estimates of long COVID burden during Omicron remain limited. This study characterized long-term impacts of COVID-19 and booster vaccination on symptoms, Health-Related Quality of Life (HRQoL), and Work Productivity Activity Impairment (WPAI).
Methods
Outpatients with ≥ 1 self-reported symptom and positive SARS-CoV-2 test at CVS Health United States test sites were recruited between 01/31 and 04/30/2022. Symptoms, EQ-5D and WPAI were collected via online surveys until 6 months following infection. Both observed and model-based estimates were analyzed. Effect sizes based on Cohen’s d quantified the magnitude of outcome changes over time, within and between vaccination groups. Mixed models for repeated measures were conducted for multivariable analyses, adjusting for covariates. Logistic regression assessed odds ratio (OR) of long COVID between vaccination groups.
Results
At long COVID start (Week 4), 328 participants included 87 (27%) Boosted with BNT162b2, 86 (26%) with a BNT162b2 primary series (Primed), and 155 (47%) Unvaccinated. Mean age was 42.0 years, 73.8% were female, 26.5% had ≥ 1 comorbidity, 36.9% prior infection, and 39.6% reported ≥ 3 symptoms (mean: 3.1 symptoms). At Month 6, among 260 participants, Boosted reported a mean of 1.1 symptoms versus 3.4 and 2.8 in Unvaccinated and Primed, respectively (p < 0.001). Boosted had reduced risks of ≥ 3 symptoms versus Unvaccinated (observed: OR 0.22, 95% CI 0.10–0.47, p < 0.001; model-based: OR 0.36, 95% CI 0.15–0.87, p = 0.019) and Primed (observed: OR 0.29, 95% CI 0.13–0.67, p = 0.003; model-based: OR 0.59, 95% CI 0.21–1.65, p = 0.459). Results were consistent using ≥ 2 symptoms. Regarding HRQoL, among those with long COVID, Boosted had higher EQ-5D Utility Index (UI) than Unvaccinated (observed: 0.922 vs. 0.731, p = 0.014; model-based: 0.910 vs. 0.758, p-value = 0.038) and Primed (0.922 vs. 0.648, p = 0.014; model-based: 0.910 vs. 0.708, p-value = 0.008). Observed and model-based estimates for EQ-VAS and UI among Boosted were comparable with pre-COVID since Month 3. Subjects vaccinated generally reported better WPAI scores.
Conclusions
Long COVID negatively impacted HRQoL and WPAI. The BNT162b2 booster could have a beneficial effect in reducing the risk and burden of long COVID. Boosted participants reported fewer and less durable symptoms, which contributed to improve HRQoL and maintain WPAI levels. Limitations included self-reported data and small sample size for WPAI.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41687-023-00616-5.
Keywords: COVID-19, SARS-CoV-2, COVID-19 symptoms, Long COVID, BNT162b2, Booster vaccine, HRQoL, WPAI
Background
Long COVID is a broad array of health problems and complications of SARS-CoV-2 infection [1]. There is no single definition of long-COVID presently and estimates of people affected vary widely across studies and variants [1]. In the US, the Centers for Disease Control and Prevention (CDC) define long COVID as a multitude of symptoms and conditions persisting or emerging beyond four weeks after infection, and not explained by an alternative diagnosis [1]. Anyone who has been infected can experience long COVID [2]. While most individuals with long COVID are mildly affected and recover shortly after infection, some can suffer from persistent symptoms that can affect the HRQoL and substantially impact daily activities including social and professional activities [3].
Multiple studies assessed whether vaccinated individuals are associated with a lower risk of long COVID, several of them show that long COVID is less likely to occur following breakthrough infection in vaccinated compared with unvaccinated individuals [4–8]. However, numerous studies were conducted in selected populationsy [4, 5, 7], assessed a mix of COVID-19 vaccines, and the certainty of evidence was considered low when pooling studies [6, 8]. Moreover, there is limited data on the long-term effects of COVID-19 and the potential benefits of COVID-19 vaccination on symptoms, well-being, and ability to return to work.
We aimed to address these gaps using a prospective longitudinal study design assessing long COVID prevalence and levels of HRQoL and WPAI through 6 months following infection, and among groups defined by COVID-19 vaccination status. Our study focused on BNT162b2, the most used COVID-19 vaccine in the US [1].
Methods
Study design and cohorts
The study design was previously described [9] (clinicaltrials.gov NCT05160636). Briefly, this was a nationwide prospective survey-based patient-reported outcomes (PRO) study targeting adults ≥ 18 years old with a positive reverse transcription–polymerase chain reaction (RT-PCR) test result and self-reporting at least one symptom suggestive of acute SARS-CoV-2 infection at time of testing [9]. Recruitment of consenting participants was carried out between 01/31/2022 and 04/30/2022, with follow-up occurring through October 30, 2022. Interim results for the acute phase (up to 4 weeks after infection) were published [9]. This analysis presents the final results with outcomes until month 6 (Additional file 1: Fig. S1).
At enrollment, we categorized the participants based on their COVID-19 vaccination history. Immunocompetent participants were considered primed with BNT162b2 if they self-reported receipt of 2 doses ≥ 14 days prior to SARS-CoV-2 testing. They were considered partially vaccinated if reporting receipt of only one of the two primary series doses, and boosted if reporting receipt of at least one dose after the primary series. Participants self-reporting an immunocompromising condition and receipt of 3 doses were considered primed; if reporting 4 doses, they were considered boosted. Participants were considered unvaccinated if they did not report any COVID-19 vaccine dose prior to testing. The study population was classified in three mutually exclusive cohorts: (1) the “Primed” cohort of subjects who received primary series, (2) the “Boosted” cohort of subjects with at least one dose after primary series, and (3) the “Unvaccinated” cohort of subject with no evidence of vaccination. Heterologous schedules were excluded. To confirm vaccination status, participants’ subsequent responses to vaccination date questions were compared with their index responses (at time of registering for testing); if responses did not match, the information was queried and adjudicated, and the latest information was used (Additional file 1: Fig. S1).
Baseline characteristics and symptoms
Baseline characteristics were obtained via the CVS Health pre-test screening questionnaire. These included self-reported demographics, comorbidities, COVID-19 vaccination history, social determinants of health including the Social Vulnerability Index (SVI), work and/or residency in a high-risk or healthcare setting, and symptoms [10]. The list of COVID-19 symptoms came from the CDC’s definition [11].
Long COVID Symptoms
The presence of long COVID symptoms was assessed via a questionnaire including 20 symptoms based on the 2021 CDC list [1]. The questionnaire was administered at week 4, month 3 and 6 after enrollment. Week 4 was considered the start of long COVID, in alignment with CDC [1]. The list of symptoms included general symptoms (tiredness or fatigue, symptoms that get worse after physical or mental activities, fever, general pain/discomfort), respiratory and cardiac symptoms (difficulty breathing or shortness of breath, cough, chest or stomach pain, fast-beating or pounding heart (also known as heart palpitations)), neurological symptoms (change in smell or taste, headache, dizziness on standing (lightheadedness), difficulty thinking or concentrating (“brain fog”), pins-and-needles feeling, sleep problems, mood changes, memory loss), and other symptoms (diarrhea, joint or muscle pain, rash, changes in period cycles) (Additional file 1: Fig. S2).
Studies describing the prevalence of long COVID in the general population testing positive for COVID-19 have used different thresholds for duration and intensity of symptoms [12–14]. Interim data from the CDC-funded INSPIRE Registry reported long COVID among subjects who were symptomatic at time of testing using a cutoff of ≥ 3 symptoms at 3 months post infection, with follow-up surveys scheduled every three months until 18 months post-enrollment [15, 16]. Considering the similarities in the total number of symptoms assessed (~ 20), eligibility criteria and study design, this study used a similar cut-off of ≥ 3 symptoms, assessed 3- and 6-months following infection.
Health-related quality of Life
We used EQ-5D-5L to assess HRQoL [17]. Completion of the questionnaire was requested at enrollment, at month 1, 3 and 6 [9]. Five dimensions of EQ-5D-5L at each time point were converted into the Utility Index (UI) using the US-based weights by Pickard et al. [18]. UI and visual analogue scale (VAS) scores were compared among cohorts and across assessment times [17].
Work productivity and activity impairment
To measure impairments in both paid work and unpaid work, we used the Work Productivity and Activity Impairment General Health V2.0 (WPAI:GH) measure [19, 20]. Participants were asked to complete the survey seven days after their RT-PCR test, at month 1, 3 and 6. Only employed subjects were included for work productivity analyses. The WPAI results were compared across cohorts and assessment times.
Statistical methods
The statistical methods were previously described [9]. Descriptive statistics were used to analyze participant characteristics at baseline. Continuous variables were described using means and standard deviations (SDs). Categorical variables were summarized with frequency and proportions. For continuous variables, t-tests were used to test difference in means. Between-group differences in categorical variables were tested by using chi-square statistics. When expected cell frequency count was less than 5, Fisher’s exact tests were used for 2-by-2 tables and Freeman-Halton tests for r-by-c tables [21, 22]. Analysis of variance (ANOVA) was used to test differences in means. Tukey’s studentized range test was adopted for post ANOVA pairwise comparisons of means between study cohorts [21, 23]. P values were all two-sided. Logistic regression model [20] was used to assess the odds ratio of long COVID symptoms between study cohorts.
Mixed models for repeated measures (MMRM) were used to estimate the magnitude of COVID-19 impact on HRQoL and WPAI over time [24]. Assessment time was fitted as a categorical covariate and a repeated effect (repeated by subject). Least squares mean (LS mean) and standard errors of PRO scores for each time point of assessment were calculated. Per guidelines, no adjustment was made for missing data when scoring the EQ-5D-5L UI and WPAI [17, 20]. All available data were included in the analysis. Tukey’s adjustment was conducted for the comparisons of least-square means between study cohorts at each time point.
Cohen’s d, or a variation of it, was calculated to assess the difference in pre-COVID scores among Boosted, Primed and Unvaccinated, the magnitude of score change from baseline at Week 4, Month 3 and 6 within each cohort, as well as the differences between cohorts [25, 26]. Specifically, within-cohort effect size (ES) was calculated as mean change from baseline to follow-up, divided by the standard deviation of change scores from baseline to follow-up [9]. Between-cohort ES was calculated as the difference in mean score between cohorts, divided by the pooled SD of scores, or the difference in mean changes from baseline between cohorts, divided by the pooled SD of change scores [9]. Values of 0.2, 0.5, and 0.8 SD units were taken represent “small,” “medium,” and “large” effect sizes, respectively [9, 25]. All analyses were conducted with SAS Version 9.4 (SAS Institute, Cary, NC). The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline [27].
Results
Study population
At Week 4, 328 study participants completed the Week 4 survey (i.e., four weeks after infection). Table 1 describes the baseline characteristics of these participants. There were 87 (27%) Boosted, 86 (26%) Primed, and 155 (47%) Unvaccinated. There were no subjects partially vaccinated. Mean time from last dose of BNT162b2 to testing positive was 2.3 (SD: 1.9) months and 6.9 (SD: 3.0) months for Boosted and Primed, respectively. The population was 42.0 years old on average, 73.8% were female, 26.5% reported ≥ 1 comorbidity and 36.9% were previously infected.
Table 1.
Baseline demographic and clinical characteristics of study participants at week 4
| All | Boosted | Primed | Unvaccinated | ||
|---|---|---|---|---|---|
| Total, n (%) | 328 | 87 (26.5) | 86 (26.2) | 155 (47.3) | |
| Age, years | |||||
| Mean, SD | 42.0 (14.5) | 44.3 (17.0) | 41.7 (14.2) | 40.9 (13.0) | |
| 18–29 | 73 (22.3%) | 18 (20.7%) | 23 (26.7%) | 32 (20.6%) | |
| 30–49 | 160 (48.8%) | 40 (46.0%) | 34 (39.5%) | 86 (55.5%) | |
| 50–64 | 67 (20.4%) | 17 (19.5%) | 23 (26.7%) | 27 (17.4%) | |
| ≥ 65 | 28 (8.5%) | 12 (13.8%) | 6 (7.0%) | 10 (6.5%) | |
| Gender | |||||
| Female | 242 (73.8%) | 58 (66.7%) | 68 (79.1%) | 116 (74.8%) | |
| Male | 86 (26.2%) | 29 (33.3%) | 18 (20.9%) | 39 (25.2%) | |
| Race / Ethnicity | |||||
| White or Caucasian (not Hispanic or Latino) | 234 (71.3%) | 63 (72.4%) | 65 (75.6%) | 106 (68.4%) | |
| Black or African American | 13 (4.0%) | 2 (2.3%) | 2 (2.3%) | 9 (5.8%) | |
| Hispanic | 44 (13.4%) | 9 (10.3%) | 16 (18.6%) | 19 (12.3%) | |
| Asian | 16 (4.9%) | 7 (8.1%) | 2 (2.3%) | 7 (4.5%) | |
| Patient Refused | 9 (2.7%) | 4 (4.6%) | 0 (0.0%) | 5 (3.2%) | |
| Other | 12 (3.7%) | 2 (2.3%) | 1 (1.2%) | 9 (5.8%) | |
| CMS Geographic Region (n, %) | ** | ||||
| Region 1: ME, NH, VT, MA, CT, RI | 15 (4.6%) | 4 (4.6%) | 4 (4.7%) | 7 (4.5%) | |
| Region 2: NY, NJ, PR, VI | 9 (2.7%) | 4 (4.6%) | 2 (2.3%) | 3 (1.9%) | |
| Region 3: PA, DE, MD, DC, WV, VA | 31 (9.5%) | 11 (12.6%) | 8 (9.3%) | 12 (7.7%) | |
| Region 4: KY, TN, NC, SC, GA, MS, AL, FL | 116 (35.4%) | 29 (33.3%) | 26 (30.2%) | 61 (39.4%) | |
| Region 5: MN, WI, IL, MI, IN, OH | 47 (14.3%) | 10 (11.5%) | 17 (19.8%) | 20 (12.9%) | |
| Region 6: NM, OK, AR, TX, LA | 59 (18.0%) | 19 (21.8%) | 21 (24.4%) | 19 (12.3%) | |
| Region 7: NE, IA, KS, MO | 16 (4.9%) | 3 (3.5%) | 6 (7.0%) | 7 (4.5%) | |
| Region 8: MT, ND, SD, WY, UT, CO | 1 (0.3%) | 1 (1.2%) | 0 (0.0%) | 0 (0.0%) | |
| Region 9: CA, NV, AZ, GU | 33 (10.1%) | 5 (5.8%) | 2 (2.3%) | 26 (16.8%) | |
| Region 10: AK, WA, OR, ID | 1 (0.3%) | 1 (1.2%) | 0 (0.0%) | 0 (0.0%) | |
| US Geographic Region | * | ||||
| Northeast | 41 (12.5%) | 11 (12.6%) | 11 (12.8%) | 19 (12.3%) | |
| South | 188 (57.3%) | 56 (64.4%) | 50 (58.1%) | 82 (52.9%) | |
| Midwest | 63 (19.2%) | 13 (14.9%) | 23 (26.7%) | 27 (17.4%) | |
| West | 36 (11.0%) | 7 (8.1%) | 2 (2.3%) | 27 (17.4%) | |
| Previously Tested Positive | 121 (36.9%) | 32 (36.8%) | 28 (32.6%) | 61 (39.4%) | |
| Work in healthcare | 37 (11.3%) | 9 (10.3%) | 13 (15.1%) | 15 (9.7%) | |
| Work in high-risk setting | 33 (10.1%) | 8 (9.2%) | 14 (16.3%) | 11 (7.1%) | |
| Live in high-risk setting | 16 (4.9%) | 3 (3.5%) | 6 (7.0%) | 7 (4.5%) | |
| Social vulnerability index, Mean (SD) | 0.43 (0.22) | 0.36 (0.20) | 0.44 (0.22) | 0.47 (0.21) | *** |
| Self-Reported Comorbiditya | |||||
| Number of comorbidities, Mean (SD) | 0.35 (0.65) | 0.39 (0.62) | 0.41 (0.77) | 0.30 (0.59) | |
| Asthma or Chronic Lung Disease | 30 (9.2%) | 11 (12.6%) | 7 (8.1%) | 12 (7.7%) | |
| Cirrhosis of the liver | 1 (0.3%) | 0 (0.0%) | 1 (1.2%) | 0 (0.0%) | |
| Immunocompromised Conditions or Weakened Immune Systemb | 16 (4.9%) | 0 (0.0%) | 10 (11.6%) | 6 (3.9%) | *** |
| Diabetes | 11 (3.4%) | 4 (4.6%) | 3 (3.5%) | 4 (2.6%) | |
| Heart Conditions or Hypertension | 41 (12.5%) | 14 (16.1%) | 9 (10.5%) | 18 (11.6%) | |
| Overweight or obesity | 16 (4.9%) | 5 (5.8%) | 5 (5.8%) | 6 (3.9%) | |
| At least 1 comorbidity | 87 (26.5%) | 28 (32.2%) | 24 (27.9%) | 35 (22.6%) | |
| Index dayc acute COVID-19 symptoms | |||||
| Number of acute COVID-19 symptoms, Mean (SD) | 5.4 (2.6) | 4.7 (2.5) | 5.7 (2.5) | 5.6 (2.6) | ** |
| Systemic symptoms | |||||
| Fever | 127 (38.7%) | 22 (25.3%) | 35 (40.7%) | 70 (45.2%) | ** |
| Chills | 165 (50.3%) | 27 (31.0%) | 48 (55.8%) | 90 (58.1%) | *** |
| Muscle or Body Aches | 183 (55.8%) | 36 (41.4%) | 52 (60.5%) | 95 (61.3%) | ** |
| Headache | 224 (68.3%) | 51 (58.6%) | 62 (72.1%) | 111 (71.6%) | |
| Fatigue | 204 (62.2%) | 48 (55.2%) | 56 (65.1%) | 100 (64.5%) | |
| Respiratory symptoms | |||||
| Shortness of Breath or Difficulty Breathing | 42 (12.8%) | 6 (6.9%) | 13 (15.1%) | 23 (14.8%) | |
| Cough | 243 (74.1%) | 65 (74.7%) | 63 (73.3%) | 115 (74.2%) | |
| Sore Throat | 187 (57.0%) | 53 (60.9%) | 50 (58.1%) | 84 (54.2%) | |
| New/Recent Loss of Taste or Smell | 35 (10.7%) | 7 (8.1%) | 13 (15.1%) | 15 (9.7%) | |
| Congestion or Runny Nose | 247 (75.3%) | 69 (79.3%) | 72 (83.7%) | 106 (68.4%) | * |
| GI symptoms | |||||
| Nausea or Vomiting | 42 (12.8%) | 7 (8.1%) | 11 (12.8%) | 24 (15.5%) | |
| Diarrhea | 69 (21.0%) | 14 (16.1%) | 15 (17.4%) | 40 (25.8%) | |
SD Standard Deviation
*P < 0.05; **P < 0.01; ***P < 0.001 for the statistical tests of comparisons between Boosted cohort, Primed cohort, and Unvaccinated cohort
aSVI ranges from 0 to 1. A community with higher value is more socially vulnerable
bImmunocompromised conditions includes compromised immune system (such as from immuno-compromising drugs, solid organ or blood stem cell transplant, HIV, or other conditions), conditions that result in a weakened immune system, including cancer treatment, and kidney failure or end stage renal disease
cCOVID-19 test nasal swab day
The three groups generally looked similar in terms of age, gender, race, prior infection, number of comorbidities and risk levels in workplace and household settings. Boosted participants were associated with less social vulnerability, with a mean SVI of 0.36 compared with 0.44 in Primed and 0.47 in Unvaccinated (p < 0.001). Primed and Unvaccinated looked similar in terms of SVI, comorbidities, and acute COVID-19 symptoms. Boosted reported fewer acute COVID-19 symptoms, with a mean of 4.7 acute symptoms compared with 5.7 in Primed and 5.6 in Unvaccinated (p = 0.009). Systemic symptoms (fever, chills and muscle or body aches) and congestion/ runny nose among Boosted were significantly lower than Primed and Unvaccinated. By Month 6, 21% (68/328) of participants had dropped off.
Long COVID-19 risk and symptoms
Time trends through Month 6
Prevalence rates of long COVID-19 symptoms by assessment time and vaccination status are presented in Table 2 and Additional file 1: Fig. S3. Overall, 39.6%, 37.3% and 35.0% of participants experienced ≥ 3 symptoms at Week 4, Month 3 and 6, respectively. The most prevalent symptom was consistently reported to be “Fatigue or Tiredness”, experienced by 41.2%, 37.3% and 38.8% at Week 4, Month 3 and 6, respectively.
Table 2.
Summary of post-COVID-19 symptoms at 1-, 3- and 6-month follow-up
| All | Boosted | Primed | Unvaccinated | ||
|---|---|---|---|---|---|
| Month 1 | |||||
| n | 328 | 87 | 86 | 155 | |
| Mean (SD) | 3.1 (3.6) | 2.0 (2.3) | 3.1 (3.5) | 3.7 (4.1) | ** |
| ≥ 3 symptoms | 130 (39.6%) | 26 (29.9%) | 36 (41.9%) | 68 (43.9%) | |
| General symptoms | |||||
| ≥ 1 symptom | 154 (47.0%) | 35 (40.2%) | 44 (51.2%) | 75 (48.4%) | |
| Tiredness or fatigue | 135 (41.2%) | 28 (32.2%) | 42 (48.8%) | 65 (41.9%) | |
| Symptoms that get worse after physical or mental activities | 50 (15.2%) | 8 (9.2%) | 10 (11.6%) | 32 (20.6%) | * |
| Fever | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) | |
| General pain/discomfort | 50 (15.2%) | 5 (5.8%) | 15 (17.4%) | 30 (19.4%) | * |
| Respiratory and cardiac | |||||
| ≥ 1 symptom | 142 (43.3%) | 34 (39.1%) | 34 (39.5%) | 74 (47.7%) | |
| Difficulty breathing or shortness of breath | 58 (17.7%) | 13 (14.9%) | 13 (15.1%) | 32 (20.6%) | |
| Cough | 86 (26.2%) | 21 (24.1%) | 19 (22.1%) | 46 (29.7%) | |
| Chest or stomach pain | 32 (9.8%) | 4 (4.6%) | 10 (11.6%) | 18 (11.6%) | |
| Fast-beating or pounding heart (also known as heart palpitations) | 38 (11.6%) | 6 (6.9%) | 11 (12.8%) | 21 (13.5%) | |
| Neurologic | |||||
| ≥ 1 symptom | 161 (49.1%) | 38 (43.7%) | 45 (52.3%) | 78 (50.3%) | |
| Change in smell or taste | 51 (15.5%) | 5 (5.8%) | 14 (16.3%) | 32 (20.6%) | ** |
| Headache | 67 (20.4%) | 10 (11.5%) | 18 (20.9%) | 39 (25.2%) | * |
| Dizziness on standing (lightheadedness) | 45 (13.7%) | 7 (8.1%) | 13 (15.1%) | 25 (16.1%) | |
| Difficulty thinking or concentrating (sometimes referred to as “brain fog”) | 86 (26.2%) | 17 (19.5%) | 26 (30.2%) | 43 (27.7%) | |
| Pins-and-needles feeling | 24 (7.3%) | 3 (3.5%) | 7 (8.1%) | 14 (9.0%) | |
| Sleep problems | 81 (24.7%) | 14 (16.1%) | 21 (24.4%) | 46 (29.7%) | |
| Mood changes | 36 (11.0%) | 6 (6.9%) | 7 (8.1%) | 23 (14.8%) | |
| Memory loss | 38 (11.6%) | 4 (4.6%) | 7 (8.1%) | 27 (17.4%) | ** |
| Other | |||||
| ≥ 1 symptom | 106 (32.3%) | 19 (21.8%) | 28 (32.6%) | 59 (38.1%) | * |
| Diarrhea | 23 (7.0%) | 3 (3.5%) | 3 (3.5%) | 17 (11.0%) | * |
| Joint or muscle pain | 66 (20.1%) | 10 (11.5%) | 18 (20.9%) | 38 (24.5%) | |
| Rash | 11 (3.4%) | 2 (2.3%) | 1 (1.2%) | 8 (5.2%) | |
| Changes in period cycles | 28 (11.6%) | 4 (6.9%) | 8 (11.8%) | 16 (13.8%) | |
| Month 3 | |||||
| n | 292 | 73 | 77 | 142 | |
| Mean (SD) | 2.7 (3.5) | 1.4 (1.9) | 2.8 (3.5) | 3.3 (4.0) | ** |
| ≥ 3 symptoms | 109 (37.3%) | 17 (23.3%) | 32 (41.6%) | 60 (42.3%) | * |
| General symptoms | |||||
| ≥ 1 symptom | 121 (41.4%) | 24 (32.9%) | 32 (41.6%) | 65 (45.8%) | |
| Tiredness or fatigue | 108 (37.0%) | 21 (28.8%) | 31 (40.3%) | 56 (39.4%) | |
| Symptoms that get worse after physical or mental activities | 36 (12.3%) | 5 (6.9%) | 11 (14.3%) | 20 (14.1%) | |
| Fever | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) | |
| General pain/discomfort | 43 (14.7%) | 3 (4.1%) | 12 (15.6%) | 28 (19.7%) | ** |
| Respiratory and cardiac | |||||
| ≥ 1 symptom | 83 (28.4%) | 15 (20.5%) | 23 (29.9%) | 45 (31.7%) | |
| Difficulty breathing or shortness of breath | 42 (14.4%) | 5 (6.9%) | 15 (19.5%) | 22 (15.5%) | |
| Cough | 38 (13.0%) | 8 (11.0%) | 9 (11.7%) | 21 (14.8%) | |
| Chest or stomach pain | 17 (5.8%) | 2 (2.7%) | 5 (6.5%) | 10 (7.0%) | |
| Fast-beating or pounding heart (also known as heart palpitations) | 29 (9.9%) | 3 (4.1%) | 9 (11.7%) | 17 (12.0%) | |
| Neurologic | |||||
| ≥ 1 symptom | 140 (47.9%) | 26 (35.6%) | 39 (50.6%) | 75 (52.8%) | * |
| Change in smell or taste | 35 (12.0%) | 5 (6.9%) | 9 (11.7%) | 21 (14.8%) | |
| Headache | 51 (17.5%) | 6 (8.2%) | 14 (18.2%) | 31 (21.8%) | * |
| Dizziness on standing (lightheadedness) | 43 (14.7%) | 3 (4.1%) | 14 (18.2%) | 26 (18.3%) | ** |
| Difficulty thinking or concentrating (sometimes referred to as “brain fog”) | 70 (24.0%) | 11 (15.1%) | 15 (19.5%) | 44 (31.0%) | * |
| Pins-and-needles feeling | 28 (9.6%) | 4 (5.5%) | 9 (11.7%) | 15 (10.6%) | |
| Sleep problems | 63 (21.6%) | 8 (11.0%) | 17 (22.1%) | 38 (26.8%) | * |
| Mood changes | 31 (10.6%) | 2 (2.7%) | 6 (7.8%) | 23 (16.2%) | ** |
| Memory loss | 35 (12.0%) | 0 (0.0%) | 9 (11.7%) | 26 (18.3%) | *** |
| Other | |||||
| ≥ 1 symptom | 88 (30.1%) | 16 (21.9%) | 25 (32.5%) | 47 (33.1%) | |
| Diarrhea | 18 (6.2%) | 2 (2.7%) | 5 (6.5%) | 11 (7.8%) | |
| Joint or muscle pain | 53 (18.2%) | 7 (9.6%) | 15 (19.5%) | 31 (21.8%) | |
| Rash | 9 (3.1%) | 4 (5.5%) | 0 (0.0%) | 5 (3.5%) | |
| Changes in period cycles | 36 (16.7%) | 5 (10.4%) | 13 (22.0%) | 18 (16.7%) | |
| Month 6 | |||||
| n | 260 | 67 | 72 | 121 | |
| Mean (SD) | 2.7 (3.7) | 1.1 (1.8) | 2.8 (3.6) | 3.4 (4.2) | *** |
| ≥ 3 symptoms | 91 (35.0%) | 10 (14.9%) | 27 (37.5%) | 54 (44.6%) | *** |
| General symptoms | |||||
| ≥ 1 symptom | 112 (43.1%) | 16 (23.9%) | 34 (47.2%) | 62 (51.2%) | ** |
| Tiredness or fatigue | 101 (38.8%) | 15 (22.4%) | 32 (44.4%) | 54 (44.6%) | ** |
| Symptoms that get worse after physical or mental activities | 28 (10.8%) | 1 (1.5%) | 8 (11.1%) | 19 (15.7%) | ** |
| Fever | 3 (1.2%) | 0 (0.0%) | 1 (1.4%) | 2 (1.7%) | |
| General pain/discomfort | 36 (13.8%) | 2 (3.0%) | 10 (13.9%) | 24 (19.8%) | ** |
| Respiratory and cardiac | |||||
| ≥ 1 symptom | 68 (26.2%) | 4 (6.0%) | 18 (25.0%) | 46 (38.0%) | *** |
| Difficulty breathing or shortness of breath | 32 (12.3%) | 3 (4.5%) | 7 (9.7%) | 22 (18.2%) | * |
| Cough | 32 (12.3%) | 0 (0.0%) | 9 (12.5%) | 23 (19.0%) | *** |
| Chest or stomach pain | 17 (6.5%) | 1 (1.5%) | 4 (5.6%) | 12 (9.9%) | |
| Fast-beating or pounding heart (also known as heart palpitations) | 29 (11.2%) | 3 (4.5%) | 7 (9.7%) | 19 (15.7%) | |
| Neurologic | |||||
| ≥ 1 symptom | 121 (46.5%) | 21 (31.3%) | 34 (47.2%) | 66 (54.5%) | ** |
| Change in smell or taste | 27 (10.4%) | 4 (6.0%) | 8 (11.1%) | 15 (12.4%) | |
| Headache | 50 (19.2%) | 5 (7.5%) | 16 (22.2%) | 29 (24.0%) | * |
| Dizziness on standing (lightheadedness) | 38 (14.6%) | 7 (10.4%) | 7 (9.7%) | 24 (19.8%) | |
| Difficulty thinking or concentrating (sometimes referred to as “brain fog”) | 58 (22.3%) | 5 (7.5%) | 19 (26.4%) | 34 (28.1%) | ** |
| Pins-and-needles feeling | 27 (10.4%) | 3 (4.5%) | 8 (11.1%) | 16 (13.2%) | |
| Sleep problems | 52 (20.0%) | 3 (4.5%) | 16 (22.2%) | 33 (27.3%) | *** |
| Mood changes | 32 (12.3%) | 3 (4.5%) | 9 (12.5%) | 20 (16.5%) | * |
| Memory loss | 37 (14.2%) | 2 (3.0%) | 12 (16.7%) | 23 (19.0%) | ** |
| Other | |||||
| ≥ 1 symptom | 76 (29.2%) | 14 (20.9%) | 23 (31.9%) | 39 (32.2%) | |
| Diarrhea | 14 (5.4%) | 1 (1.5%) | 3 (4.2%) | 10 (8.3%) | |
| Joint or muscle pain | 38 (14.6%) | 6 (9.0%) | 12 (16.7%) | 20 (16.5%) | |
| Rash | 5 (1.9%) | 1 (1.5%) | 0 (0.0%) | 4 (3.3%) | |
| Changes in period cycles | 32 (16.8%) | 8 (18.6%) | 11 (20.0%) | 13 (14.0%) | |
*P < 0.05; **P < 0.01; ***P < 0.001 for the statistical tests of comparisons between Boosted cohort, Primed cohort, and Unvaccinated cohort
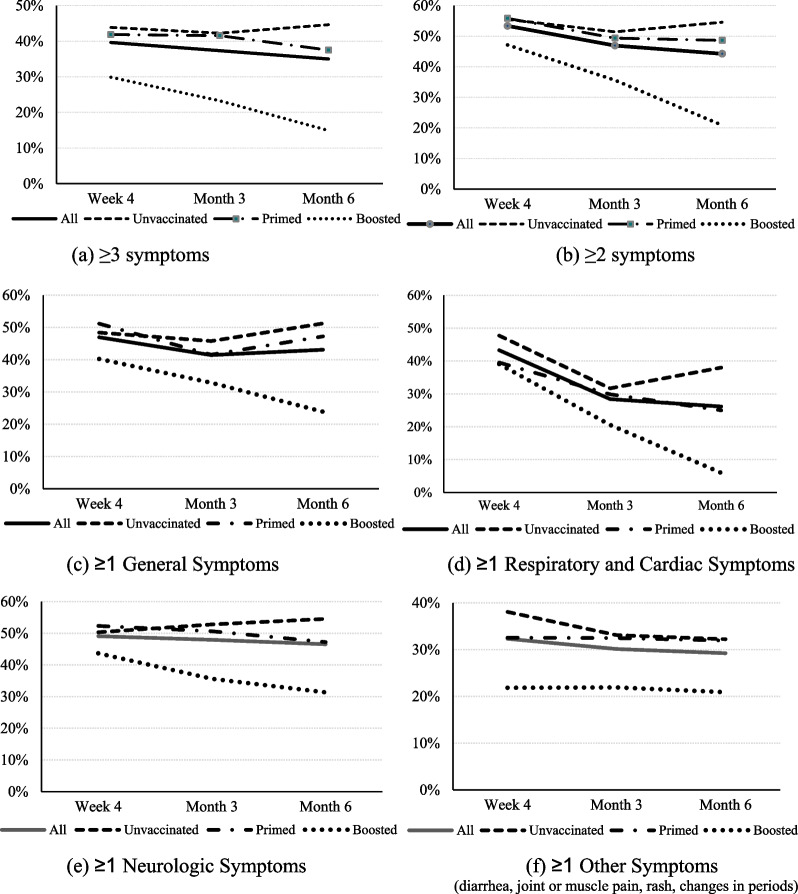
Figure 1 illustrates time trends of prevalence rates of long COVID symptoms by vaccination status. Across all time points, the Boosted line appeared below and clearly separated from the ones of Unvaccinated and Primed when using the base case definition of long COVID with a cut-off of ≥ 3 symptoms (Fig. 1a), as well as when using ≥ 2 symptoms (Fig. 1b). Figure 1c–f show the prevalence rates of long COVID by body system: across all time points and symptom type, the Boosted line appeared below and clearly separated from the ones of Unvaccinated and Primed. Among Boosted, declines were visible at Month 6 in Respiratory and Cardiac Symptoms, General Symptoms, and Neurologic Symptoms.
Fig. 1.
Trajectory of prevalence of long COVID over time, by vaccination status and type
From Week 4 to Month 6, noticeable improvements were observed among Boosted in several symptoms, such as Tiredness or Fatigue (32.2–22.4%), Cough (24.1–0.0%), Difficulty thinking or concentrating (19.5–7.5%), Difficulty breathing or shortness of breath (14.9–4.5%), Sleep problems (16.1–4.5%), and Symptoms that get worse after physical or mental activities (9.2–1.5%). Additional file 1: Figs. S4 and S5 illustrate, respectively, time trends of the mean numbers of symptoms by vaccination status, and the proportions of participants reporting no symptoms over time: the Boosted lines were clearly separated from the ones of Unvaccinated and Primed.
Week 4
At long COVID start, participants reported a mean of 3.1 symptoms. Boosted reported fewer symptoms: on average, 2.0 symptoms, significantly lower than 3.1 in Primed and 3.7 in Unvaccinated (p = 0.002). The prevalence of long COVID (≥ 3 symptoms) was 29.9%, 41.9%, and 43.9% among Boosted, Primed and Unvaccinated, respectively, being directionally lower in Boosted (p = 0.091). Relative to Unvaccinated, all symptoms’ prevalence rates were numerically lower in Boosted, and numerically lower or similar relative to Primed. Several symptoms were significantly lower: symptoms worsening after physical or mental activities (8.5%), General pain/discomfort (5.8%), Change in smell or taste (5.8%), Headache (11.5%), Memory loss (4.6%), and Diarrhea (3.5%) (Table 2).
Month 3
At Month 3, the prevalence of long-COVID was 23.3%, 41.6%, and 42.3% among Boosted, Primed and Unvaccinated, respectively, being lowest in Boosted (p = 0.016). Study participants reported a mean of 2.7 symptoms. Subjects Boosted reported on average 1.4 symptoms, significantly lower than 2.8 in Primed and 3.3 in Unvaccinated (p = 0.001) (Table 2). All symptoms’ prevalence rates were numerically lower in Boosted compared to Unvaccinated and Primed. Significantly lower rates were reported for Boosted for General pain/discomfort (4.1%), Headache (8.2%), Dizziness on standing (lightheadedness) (4.1%), Difficulty thinking or concentrating (15.1%), Sleep problems (11.0%), Mood changes (2.7%), and Memory loss (0.0%) (Table 2).
Month 6
At Month 6, the prevalence of long-COVID was 14.9%, 37.5%, and 44.6% among Boosted, Primary and Unvaccinated, respectively, being lowest among Boosted (p < 0.001). Odds Ratios (OR) calculated based on observed data showed that pre-COVID booster vaccination was associated with a 78% reduced risk of long COVID (expressed as ≥ 3 symptoms) versus Unvaccinated (OR 0.22, 95% CI 0.10–0.47, p < 0.001) and 71% versus Primed (OR 0.29, 95% CI 0.12–0.67, p = 0.003). Those Primed were associated with a non-significant reduction in the odds of long COVID against Unvaccinated (OR 0.74, 95% CI 0.41–1.35, p = 0.332). The logistic regression model provided a similar result of 64% reduced odds of long COVID for Boosted versus Unvaccinated (OR 0.36, 95% CI 0.15–0.87, p = 0.019), and a non-significant reduction in the odds of long COVID against Primed (OR 0.59, 95% CI 0.21–1.65, p = 0.459). Similarly, a non-significant reduction in the odds of long COVID was estimated for Primed versus Unvaccinated (OR 0.60, 95% CI 0.27–1.34, p = 0.296). Results were consistent when using an alternative definition of long COVID as ≥ 2 symptoms (Table 3).
Table 3.
Long COVID symptoms at month 6 by vaccination status: observed and model-based estimates
| Descriptive statistics | Observed | Logistic regression | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Base Case: ≥ 3 symptoms | |||||
| Boosted vs Unvaccinated | 10 (14.9%) versus 54 (44.6%) | 0.22 (0.10, 0.47) | < 0.001 | 0.36 (0.15, 0.87) | 0.019 |
| Primed vs. Unvaccinated | 27 (37.5%) versus 54 (44.6%) | 0.74 (0.41, 1.35) | 0.332 | 0.60 (0.27, 1.34) | 0.296 |
| Boosted vs Primed | 10 (14.9%) versus 27 (37.5%) | 0.29 (0.13, 0.67) | 0.003 | 0.59 (0.21, 1.65) | 0.459 |
| Sensitivity: ≥ 2 symptoms | |||||
| Boosted vs Unvaccinated | 14 (20.9%) versus 66 (54.5%) | 0.22 (0.11, 0.44) | < 0.001 | 0.30 (0.13, 0.70) | 0.003 |
| Primed vs. Unvaccinated | 35 (48.6%) versus 66 (54.5%) | 0.79 (0.44, 1.41) | 0.425 | 0.64 (0.30, 1.39) | 0.370 |
| Boosted vs Primed | 14 (20.9%) versus 35 (48.6%) | 0.28 (0.13, 0.59) | 0.001 | 0.46 (0.18, 1.20) | 0.140 |
Note for logistic regression model: the logistic regression model for number of post-COVID ≥ 3 versus < 3 used GEE estimation with unstructured correlation matrix. Covariates were variables for time, vaccination status and interaction of time by vaccination status, as well as covariates of participant pre-COVID-19 symptom onset score, sociodemographic characteristics (age, sex, regions, social vulnerability, race/ethnicity, high risk occupations), previously tested positive for COVID-19, severity of acute illness (number of symptoms reported on index date), and immunocompromised status
Study participants reported a mean of 2.7 symptoms at 6 months. The average number of symptoms was 1.1 in Boosted, significantly lower than 2.8 in Primed and 3.4 in Unvaccinated (p < 0.001) (Table 2). All symptoms’ prevalence rates were numerically lower or similar in Boosted compared with Unvaccinated and Primed. Significantly lower rates were reported in Boosted for Tiredness or Fatigue (22.4%), Symptoms that get worse after physical or mental activities, (1.5%), General pain/discomfort (3.0%), Difficulty breathing or shortness of breath (4.5%), Cough (0.0%), Fast-beating or pounding heart (4.5%), Headache (7.5%), Difficulty thinking or concentrating (7.5%), Sleep problems (4.5%), Mood changes (4.5%), Memory loss (3.0%) (Table 2).
EQ-5D-5L
The study participants with long COVID (≥ 3 symptoms at Week 4, N = 130) reported a pre-COVID mean EQ-VAS and Utility Index (UI) scores of, respectively, 84.9 and 0.879 (Table 4). Such values were not significantly different by vaccination status.
Table 4.
Summary of Observed EQ-5D-5L and WPAI scores by assessment time and vaccination status in long COVID subjects
| Score | Change from pre-COVID-19 | Relative to unvaccinated | Relative to primed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | Mean (SD) | P value | ES | Mean (SD) | P value | ES | Mean (SD) | P value | ES | ||
| EQ-VAS | ||||||||||||
| Pre-COVID-19 | All | 130 | 84.9 (12.2) | |||||||||
| Boosted | 26 | 84.6 (13.5) | − 1.1 (12.1) | 0.692 | − 0.09 | 0.8 (12.9) | 0.804 | 0.06 | ||||
| Primed | 36 | 83.8 (12.5) | − 1.9 (11.9) | 0.431 | − 0.16 | |||||||
| Unvaccinated | 68 | 85.7 (11.6) | ||||||||||
| Week 4 | All | 123 | 74.2 (16.2) | − 10.9 (15.6) | < 0.001 | − 0.67 | ||||||
| Boosted | 26 | 74.4 (18.1) | − 10.2 (16.4) | 0.004 | − 0.62 | 1.2 (16.8) | 0.751 | 0.07 | − 1.6 (16.4) | 0.713 | − 0.10 | |
| Primed | 33 | 76.0 (15.0) | − 7.7 (11.8) | 0.001 | − 0.65 | 2.8 (15.8) | 0.406 | 0.18 | ||||
| Unvaccinated | 64 | 73.1 (16.2) | − 12.9 (16.8) | < 0.001 | − 0.77 | |||||||
| Month 3 | All | 108 | 76.4 (16.0) | − 9.0 (15.2) | < 0.001 | − 0.56 | ||||||
| Boosted | 17 | 80.9 (10.4) | − 3.8 (13.6) | 0.266 | − 0.28 | 5.4 (16.1) | 0.223 | 0.34 | 5.2 (14.4) | 0.233 | 0.36 | |
| Primed | 32 | 75.7 (16.1) | − 9.7 (10.2) | < 0.001 | − 0.95 | 0.2 (16.9) | 0.955 | 0.01 | ||||
| Unvaccinated | 59 | 75.5 (17.3) | − 10.1 (17.7) | < 0.001 | − 0.57 | |||||||
| Month 6 | All | 89 | 74.5 (17.2) | − 10.0 (16.6) | < 0.001 | − 0.58 | ||||||
| Boosted | 10 | 80.4 (11.4) | − 6.3 (11.5) | 0.121 | − 0.55 | 5.5 (15.8) | 0.319 | 0.35 | 8.8 (18.1) | 0.200 | 0.48 | |
| Primed | 27 | 71.6 (20.0) | − 11.2 (17.4) | 0.003 | − 0.64 | − 3.3 (17.7) | 0.439 | − 0.18 | ||||
| Unvaccinated | 52 | 74.9 (16.5) | − 10.0 (17.2) | < 0.001 | − 0.58 | |||||||
| EQ-5D-5L UI | ||||||||||||
| Pre-COVID-19 | All | 130 | 0.879 (0.144) | |||||||||
| Boosted | 26 | 0.910 (0.109) | 0.038 (0.117) | 0.163 | 0.32 | 0.039 (0.167) | 0.369 | 0.23 | ||||
| Primed | 36 | 0.871 (0.199) | − 0.001 (0.152) | 0.975 | − 0.01 | |||||||
| Unvaccinated | 68 | 0.872 (0.120) | ||||||||||
| Week 4 | All | 130 | 0.744 (0.196) | − 0.135 (0.160) | < 0.001 | − 0.69 | ||||||
| Boosted | 26 | 0.827 (0.122) | − 0.083 (0.126) | 0.003 | − 0.66 | 0.081 (0.162) | 0.032 | 0.50 | 0.036 (0.139) | 0.319 | 0.26 | |
| Primed | 36 | 0.752 (0.198) | − 0.119 (0.148) | < 0.001 | − 0.80 | 0.045 (0.165) | 0.188 | 0.27 | ||||
| Unvaccinated | 68 | 0.708 (0.209) | − 0.164 (0.173) | < 0.001 | − 0.95 | |||||||
| Month 3 | All | 109 | 0.742 (0.236) | − 0.153 (0.188) | < 0.001 | − 0.65 | ||||||
| Boosted | 17 | 0.877 (0.114) | − 0.029 (0.114) | 0.311 | − 0.25 | 0.153 (0.177) | 0.002 | 0.86 | 0.136 (0.168) | 0.010 | 0.81 | |
| Primed | 32 | 0.725 (0.275) | − 0.165 (0.190) | < 0.001 | − 0.87 | 0.017 (0.191) | 0.685 | 0.09 | ||||
| Unvaccinated | 60 | 0.713 (0.230) | − 0.182 (0.191) | < 0.001 | − 0.95 | |||||||
| Month 6 | All | 91 | 0.727 (0.242) | − 0.156 (0.213) | < 0.001 | − 0.64 | ||||||
| Boosted | 10 | 0.922 (0.031) | 0.001 (0.092) | 0.973 | 0.01 | 0.156 (0.179) | 0.014 | 0.87 | 0.218 (0.228) | 0.014 | 0.96 | |
| Primed | 27 | 0.648 (0.273) | − 0.217 (0.259) | < 0.001 | − 0.84 | − 0.062 (0.215) | 0.225 | − 0.29 | ||||
| Unvaccinated | 54 | 0.731 (0.229) | − 0.155 (0.190) | < 0.001 | − 0.82 | |||||||
| Work productivity loss | ||||||||||||
| Pre-COVID-19 | All | 91 | 15.1 (24.7) | |||||||||
| Boosted | 19 | 6.6 (11.0) | − 10.5 (22.4) | 0.092 | − 0.47 | − 11.2 (23.5) | 0.117 | − 0.48 | ||||
| Primed | 27 | 17.9 (29.2) | 0.8 (27.0) | 0.907 | 0.03 | |||||||
| Unvaccinated | 45 | 17.1 (25.6) | ||||||||||
| Week 4 | All | 85 | 31.7 (25.4) | 15.1 (32.8) | < 0.001 | 0.59 | ||||||
| Boosted | 17 | 26.8 (24.1) | 19.4 (26.9) | 0.010 | 0.72 | − 12.6 (25.1) | 0.087 | − 0.50 | 4.3 (23.3) | 0.562 | 0.18 | |
| Primed | 26 | 22.5 (22.8) | 2.7 (30.1) | 0.649 | 0.09 | − 16.8 (24.5) | 0.008 | − 0.69 | ||||
| Unvaccinated | 42 | 39.3 (25.5) | 20.5 (35.4) | 0.001 | 0.58 | |||||||
| Month 3 | All | 74 | 26.1 (26.6) | 13.3 (35.2) | 0.002 | 0.50 | ||||||
| Boosted | 11 | 18.2 (23.1) | 11.2 (25.0) | 0.170 | 0.45 | − 12.1 (25.6) | 0.173 | − 0.47 | − 4.9 (26.8) | 0.616 | − 0.18 | |
| Primed | 25 | 23.1 (28.2) | 11.7 (40.1) | 0.157 | 0.29 | − 7.2 (27.0) | 0.305 | − 0.27 | ||||
| Unvaccinated | 38 | 30.3 (26.2) | 14.8 (35.5) | 0.014 | 0.42 | |||||||
| Month 6 | All | 60 | 33.9 (26.2) | 19.9 (38.0) | < 0.001 | 0.76 | ||||||
| Boosted | 6 | 43.1 (29.8) | 31.4 (29.1) | 0.057 | 1.08 | 6.5 (26.6) | 0.587 | 0.24 | 15.8 (26.2) | 0.203 | 0.60 | |
| Primed | 22 | 27.4 (25.3) | 11.5 (41.3) | 0.205 | 0.28 | − 9.3 (25.7) | 0.199 | − 0.36 | ||||
| Unvaccinated | 32 | 36.6 (26.1) | 22.5 (37.7) | 0.002 | 0.60 | |||||||
| Activity Impairment | ||||||||||||
| Pre-COVID-19 | All | 130 | 21.3 (29.3) | |||||||||
| Boosted | 26 | 14.2 (22.5) | − 8.9 (28.3) | 0.179 | − 0.31 | − 8.8 (28.2) | 0.229 | − 0.31 | ||||
| Primed | 36 | 23.1 (31.7) | 0.0 (30.7) | 0.996 | 0.00 | |||||||
| Unvaccinated | 68 | 23.1 (30.2) | ||||||||||
| Week 4 | All | 130 | 38.1 (25.7) | 16.8 (35.6) | < 0.001 | 0.65 | ||||||
| Boosted | 26 | 31.2 (19.7) | 16.9 (20.9) | < 0.001 | 0.81 | − 15.2 (24.4) | 0.008 | − 0.62 | 3.7 (22.3) | 0.528 | 0.16 | |
| Primed | 36 | 27.5 (24.1) | 4.4 (34.9) | 0.451 | 0.13 | − 18.8 (25.3) | < 0.001 | − 0.74 | ||||
| Unvaccinated | 68 | 46.3 (26.0) | 23.2 (39.0) | < 0.001 | 0.60 | |||||||
| Month 3 | All | 109 | 31.7 (28.3) | 12.9 (36.6) | < 0.001 | 0.46 | ||||||
| Boosted | 17 | 28.2 (24.6) | 14.1 (26.5) | 0.044 | 0.53 | − 4.3 (27.8) | 0.579 | − 0.15 | − 4.0 (28.5) | 0.646 | − 0.14 | |
| Primed | 32 | 32.2 (30.4) | 14.7 (37.6) | 0.035 | 0.39 | − 0.3 (29.2) | 0.961 | − 0.01 | ||||
| Unvaccinated | 60 | 32.5 (28.6) | 11.7 (38.9) | 0.024 | 0.30 | |||||||
| Month 6 | All | 91 | 37.5 (27.5) | 17.4 (39.2) | < 0.001 | 0.63 | ||||||
| Boosted | 10 | 31.0 (24.2) | 27.0 (25.4) | 0.010 | 1.06 | − 9.6 (27.5) | 0.316 | − 0.35 | − 2.7 (26.8) | 0.787 | − 0.10 | |
| Primed | 27 | 33.7 (27.6) | 13.3 (37.0) | 0.073 | 0.36 | − 6.9 (27.9) | 0.299 | − 0.25 | ||||
| Unvaccinated | 54 | 40.6 (28.0) | 17.6 (42.4) | 0.004 | 0.41 | |||||||
At Week 4, the observed (Table 4) and model-based (Table 5) EQ-VAS scores were lower than pre-COVID, regardless of vaccination status (not significantly for Primed according to the model-based estimates). The observed EQ-VAS scores for the entire long COVID population were numerically similar between Week 4 (74.2) and Month 6 (74.5), and, by the end of the study period, they did not return to pre-COVID levels. The observed EQ-VAS scores in Unvaccinated and Primed were still significantly lower than pre-COVID at Month 3 and 6; only Boosted reported EQ-VAS levels not different than pre-COVID at Month 3 (p = 0.266) and Month 6 (p = 0.121). The model-based EQ-VAS scores were numerically lower at Month 3 and 6 regardless of vaccination status, while not significantly different from pre-COVID, except for Primed at Month 6 (Table 5).
Table 5.
Least-Square Mean Estimates and 95% CI of EQ-5D-5L and WPAI-GH scores in long COVID subjects
| Score | Change from pre-COVID | Compared to Unvaccinated | Compared to Primed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LSE (95% CI) | LSE (95% CI) | P value | ES | LSE (95% CI) | P value | ES | LSE (95% CI) | P value | ES | ||
| EQ-5D-5L: Utility Index | |||||||||||
| Pre-COVID (Observed), Mean (SD) | Boosted | 0.910 (0.109) | |||||||||
| Primed | 0.871 (0.199) | ||||||||||
| Unvaccinated | 0.872 (0.120) | ||||||||||
| Week 4 | Boosted | 0.830 (0.748, 0.912) | − 0.056 (− 0.138, 0.027) | 0.183 | − 0.44 | 0.076 (− 0.012, 0.164) | 0.108 | 0.47 | 0.028 (− 0.069, 0.125) | 0.772 | 0.20 |
| Primed | 0.802 (0.722, 0.881) | − 0.084 (− 0.163, − 0.004) | 0.039 | − 0.57 | 0.048 (− 0.032, 0.127) | 0.337 | 0.29 | ||||
| Unvaccinated | 0.754 (0.689, 0.820) | − 0.131 (− 0.197, − 0.066) | < 0.001 | − 0.76 | |||||||
| Month 3 | Boosted | 0.910 (0.808, 1.011) | 0.024 (− 0.078, 0.125) | 0.644 | 0.21 | 0.161 (0.046, 0.275) | 0.003 | 0.91 | 0.137 (0.011, 0.263) | 0.030 | 0.81 |
| Primed | 0.773 (0.687, 0.859) | − 0.113 (− 0.199, − 0.027) | 0.011 | − 0.59 | 0.024 (− 0.070, 0.118) | 0.818 | 0.13 | ||||
| Unvaccinated | 0.749 (0.678, 0.819) | − 0.137 (− 0.207, − 0.067) | < 0.001 | − 0.72 | |||||||
| Month 6 | Boosted | 0.910 (0.790, 1.030) | 0.024 (− 0.096, 0.144) | 0.688 | 0.27 | 0.152 (0.007, 0.298) | 0.038 | 0.85 | 0.202 (0.045, 0.359) | 0.008 | 0.89 |
| Primed | 0.708 (0.617, 0.800) | − 0.178 (− 0.269, − 0.086) | < 0.001 | − 0.69 | − 0.050 (− 0.153, 0.054) | 0.497 | − 0.23 | ||||
| Unvaccinated | 0.758 (0.685, 0.831) | − 0.128 (− 0.201, − 0.055) | 0.001 | − 0.67 | |||||||
| EQ-5D-5L: VAS | |||||||||||
| Pre-COVID (Observed), Mean (SD) | Boosted | 84.6 (13.5) | |||||||||
| Primed | 83.8 (12.5) | ||||||||||
| Unvaccinated | 85.7 (11.6) | ||||||||||
| Week 4 | Boosted | 77.3 (70.2, 84.3) | − 7.8 (− 14.9, − 0.7) | 0.032 | − 0.43 | − 0.2 (− 8.0, 7.6) | 0.998 | − 0.01 | − 3.4 (− 12.1, 5.3) | 0.618 | − 0.21 |
| Primed | 80.7 (73.8, 87.6) | − 4.3 (− 11.3, 2.6) | 0.218 | − 0.29 | 3.2 (− 4.0, 10.5) | 0.544 | 0.20 | ||||
| Unvaccinated | 77.5 (71.8, 83.1) | − 7.6 (− 13.2, − 2.0) | 0.008 | − 0.47 | |||||||
| Month 3 | Boosted | 82.6 (74.5, 90.6) | − 2.5 (− 10.5, 5.6) | 0.545 | − 0.24 | 2.3 (− 6.5, 11.1) | 0.806 | 0.14 | 3.6 (− 6.2, 13.3) | 0.663 | 0.25 |
| Primed | 79.0 (72.1, 85.9) | − 6.0 (− 13.0, 0.9) | 0.087 | − 0.38 | − 1.2 (− 8.5, 6.0) | 0.914 | − 0.07 | ||||
| Unvaccinated | 80.2 (74.5, 86.0) | − 4.8 (− 10.5, 0.9) | 0.099 | − 0.28 | |||||||
| Month 6 | Boosted | 82.3 (72.3, 92.2) | − 2.8 (− 12.7, 7.2) | 0.584 | − 0.24 | 2.7 (− 9.6, 14.9) | 0.865 | 0.17 | 4.8 (− 8.3, 17.9) | 0.658 | 0.27 |
| Primed | 77.4 (69.8, 85.1) | − 7.6 (− 15.2, 0.0) | 0.051 | − 0.38 | − 2.2 (− 10.8, 6.4) | 0.820 | − 0.12 | ||||
| Unvaccinated | 79.6 (73.6, 85.7) | − 5.4 (− 11.5, 0.6) | 0.079 | − 0.33 | |||||||
| WPAI: Work Productivity Loss | |||||||||||
| Pre-COVID (Observed), Mean (SD) | Boosted | 6.6 (11.0) | |||||||||
| Primed | 17.9 (29.2) | ||||||||||
| Unvaccinated | 17.1 (25.6) | ||||||||||
| Week 4 | Boosted | 25.5 (9.4, 41.7) | 11.8 (− 4.3, 28.0) | 0.149 | 0.49 | − 8.2 (− 25.0, 8.7) | 0.484 | − 0.32 | 4.2 (− 14.1, 22.48) | 0.849 | 0.18 |
| Primed | 21.4 (6.6, 36.2) | 7.7 (− 7.1, 22.5) | 0.306 | 0.34 | − 12.3 (− 28.0, 3.3) | 0.151 | − 0.50 | ||||
| Unvaccinated | 33.7 (20.9, 46.5) | 20.0 (7.2, 32.8) | 0.003 | 0.78 | |||||||
| Month 3 | Boosted | 10.1 (0, 30.8) | − 3.6 (− 24.4, 17.1) | 0.728 | − 0.16 | − 18.5 (− 41.0, 4.0) | 0.129 | − 0.72 | − 12.8 (− 37.4, 11.9) | 0.437 | − 0.48 |
| Primed | 22.8 (7.0, 38.7) | 9.1 (− 6.7, 25.0) | 0.257 | 0.32 | − 5.7 (− 23.4, 12.1) | 0.725 | − 0.21 | ||||
| Unvaccinated | 28.5 (14.1, 43.0) | 14.8 (0.3, 29.3) | 0.045 | 0.57 | |||||||
| Month 6 | Boosted | 42.6 (18.2, 67.0) | 28.9 (4.5, 53.3) | 0.021 | 0.97 | 7.89 (− 21.3, 37.1) | 0.796 | 0.30 | 15.9 (− 15.1, 47.0) | 0.442 | 0.61 |
| Primed | 26.7 (9.7, 43.6) | 12.9 (− 4.0, 29.8) | 0.132 | 0.51 | − 8.1 (− 28.0, 11.9) | 0.604 | − 0.31 | ||||
| Unvaccinated | 34.7 (20.1, 49.3) | 21.0 (6.4, 35.6) | 0.005 | 0.81 | |||||||
| WPAI: Activity Impairment | |||||||||||
| Pre-COVID (Observed), Mean (SD) | Boosted | 14.2 (22.5) | |||||||||
| Primed | 23.1 (31.7) | ||||||||||
| Unvaccinated | 23.1 (30.2) | ||||||||||
| Week 4 | Boosted | 25.7 (14.1, 37.4) | 5.6 (− 6.1, 17.3) | 0.346 | 0.28 | − 13.0 (− 26.1, 0.1) | 0.053 | − 0.53 | 6.9 (− 7.4, 21.3) | 0.489 | 0.31 |
| Primed | 18.8 (7.7, 30.0) | − 1.3 (− 12.5, 9.8) | 0.813 | − 0.06 | − 19.9 (− 31.6, − 8.2) | < 0.001 | − 0.79 | ||||
| Unvaccinated | 38.7 (29.5, 47.9) | 18.6 (9.3, 27.8) | < 0.001 | 0.71 | |||||||
| Month 3 | Boosted | 17.8 (2.0, 33.6) | − 2.3 (− 18.1, 13.4) | 0.770 | − 0.10 | − 7.1 (− 25.6, 11.4) | 0.635 | − 0.26 | − 5.4 (− 25.8, 14.9) | 0.802 | − 0.19 |
| Primed | 23.3 (10.5, 36.0) | 3.1 (− 9.7, 15.9) | 0.632 | 0.10 | − 1.7 (− 16.6, 13.2) | 0.963 | − 0.06 | ||||
| Unvaccinated | 24.9 (14.6, 35.3) | 4.8 (− 5.6, 15.1) | 0.365 | 0.17 | |||||||
| Month 6 | Boosted | 29.8 (11.2, 48.4) | 9.6 (− 9.0, 28.3) | 0.308 | 0.40 | − 4.6 (− 27.9, 18.6) | 0.885 | − 0.17 | 5.3 (− 19.6, 30.2) | 0.870 | 0.20 |
| Primed | 24.5 (11.1, 37.9) | 4.4 (− 9.0, 17.7) | 0.521 | 0.16 | − 9.9 (− 25.7, 5.9) | 0.301 | − 0.36 | ||||
| Unvaccinated | 34.4 (24.0, 44.9) | 14.3 (3.8, 24.7) | 0.008 | 0.51 | |||||||
Mixed models for repeated measurements with unstructured correlation matrix include variables for time, vaccination status and interaction of time by vaccination status, as well as covariates of participant pre-COVID-19 symptom onset score, sociodemographic characteristics (age, sex, regions, social vulnerability, race/ethnicity, high risk occupations), previously tested positive for COVID-19, severity of acute illness (number of symptoms reported on index date), and immunocompromised status
Both observed (Table 4) and model-based (Table 5) UI scores were lower than pre-COVID for Unvaccinated and Primed for Month 3 and 6 while not different among Boosted. Consistently, the model-based UI scores of Boosted were not different from pre-COVID at Month 3, 6 and since Week 4, too (Table 5).
The observed UIs values for the entire long-COVID population were numerically similar between Week 4 (0.74), Month 3 (0.74) and 6 (0.73) and did not return to pre-COVID levels. Such effect was driven by the detrimental effect of COVID-19 reported by Unvaccinated and Primed. At Week 4, the mean UI change from pre-COVID in Boosted was significantly lower than in Unvaccinated based on observed data and numerically lower according to model-based estimates.
The mean changes in UIs from pre-COVID in Boosted were significantly lower versus Unvaccinated at Month 3 and 6 based on both observed and model estimates, with medium-to-high effect sizes. The mean changes from pre-COVID in UIs reported by Boosted were significantly lower than Primed at Month 3 and 6 based on both observed and model estimates, with medium-to-high effect sizes.
The impact of COVID-19 on the HRQoL was detrimental for participants with long COVID, but significantly less so for Boosted, the group with the highest UI and EQ-VAS scores during the 6-month study period.
WPAI
The WPAI analyses had a smaller eligible population and were impacted by small sample size.
A total of 85 long COVID study participants (66%) reported being currently employed at Week 4 and were eligible to complete the work productivity questions. Of those, 42 (49%) Unvaccinated, 26 (31%) Primed and 17 (20%) Boosted.
Participants reported a pre-COVID mean Work Productivity (WP) loss of 15.1% (Table 4). Such values were not significantly different by vaccination status. At Week 4, the observed WP loss increased substantially to 31.7%. The observed WP loss scores for the entire long-COVID population were numerically similar between Week 4 (31.7%), Month 3 (26.1%) and Month 6 (33.9%), and did not return to pre-COVID levels. Such effect was driven by the detrimental effect of COVID-19 reported by Unvaccinated. At Week 4, both observed and model-based WP scores were significantly lower than pre-COVID for the Unvaccinated. At Month 3 and 6, the observed and model-based mean change in WP levels were not significantly different than pre-COVID for Boosted and Primed (non-significant for model-based estimated for Boosted at Month 6), suggesting a return to pre-COVID levels for these vaccinated groups only.
The long COVID study participants (N = 130) reported a pre-COVID mean Activity Impairment (AI) of 21.3% (Table 4). Such values were not significantly different by vaccination status. At Week 4, the observed AI increased substantially to 38.1%. The observed AI scores for the entire long-COVID population were numerically similar between Week 4 (38.1%), Month 3 (31.7%) and Month 6 (37.5%), and did not return to pre-COVID levels. Such effect was driven by the detrimental effect of COVID-19 reported by Unvaccinated, who reported the highest AI across all time points. At Week 4, the mean change from pre-COVID was significantly lower than Unvaccinated for Boosted and Primed based on both the observed and model-based estimates. At Month 3 and Month 6, all groups were still highly impacted; subjects Boosted reported the lowest AI levels.
Discussion
In this national study conducted among US symptomatic outpatients with a documented SARS-CoV-2 infection, long COVID had a detrimental effect on well-being, work productivity and activity levels. Long COVID symptoms were persistent over 6 months in over a third of the study cohort, resulting in prolonged limitation of activities and work productivity. Compared with Primed and Unvaccinated, subjects Boosted with BNT162b2 prior to a breakthrough infection were associated with significantly lower likelihood of long COVID onset, fewer symptoms, and faster improvement over time. Positive trends in the outcomes assessed were observed for Primed versus Unvaccinated, although generally not statistically significant, most likely due to the relatively long mean time since last dose for Primed. The prevalence of enduring symptoms after a mild infection in this study was generally consistent with published literature [9, 28]. These findings support growing research that prior COVID-19 vaccination may have a protective effect against long COVID [9, 28].
This study has several strengths compared with prior research. It is one of a limited number assessing diverse PROs associated with long COVID in a nationwide real-world source population. As such, it contributes a holistic picture of the long-term humanistic outcomes of COVID-19, assessed directly from a patient’s perspective. From an internal validity perspective, the study enrolled patients within days from testing positive and prospectively collected survey-based data, potentially reducing recall bias risks. Moreover, the study leveraged widely used validated PRO instruments (EQ-5D-5L, WPAI) and a questionnaire capturing a comprehensive symptoms list aligned to CDC research on long COVID [1]. With asymptomatic infections excluded by design, our estimates can be interpreted exclusively as related to symptomatic disease, potentially reducing the risk of overestimating the prevalence of long COVID among symptomatic. Further, both observed and model-based analyses and the sensitivity analysis yielded consistent results. Finally, with all study activities carried out virtually, this study piloted an innovative approach to agile and digitally enabled research during a pandemic.
The study is subject to several limitations. As previously described [9], all data collected was self-reported, subject to missingness, errors, recall bias, social desirability bias and selection bias associated with survey drop-off. Out of 328 participants, 68 (21%) dropped off at Month 6, possibly due to response fatigue and/or survey burden. Such drop-off rate should be interpreted in the context of participants being asked not to skip surveys. Such a strict requirement allowed for a clean assessment of changes in outcomes prevalence over time, but at the cost of attrition. Other limitations included the female over-representation, the relatively healthy status of the source population, and the fact that the study included adults only [9]. Further, the long COVID definitions used were based on presence of symptoms, with no assessment of severity of symptoms. Despite adjusting for several covariates, risk of residual confounding remains. The findings may not be generalizable to prior or future variants, other countries, time periods and populations that were excluded. Finally, while the study groups had similar baseline characteristics, this study did not explore views, perceptions, and barriers to prevention. Literature explored determinants of COVID-19 vaccination hesitancy, including concerns on vaccine safety and perceived benefit, infringement on personal freedoms, institutional trust, and misinformation [29]. This is consistent with elements of the protection motivation theory, wherein people formulate their health-related responses based on their perceived susceptibility, severity, and efficacy of the response (in this case, COVID-19 vaccination) against their self-efficacy and ability to act on that assessment [30]. Future research could connect observed behaviors to such theories and further support the interpretation of findings.
Booster vaccination with BNT162b2 has been shown to be safe and effective at reducing the risk of infection and potentially protective against long COVID [6, 28]; this study shows its potential beneficial effect in preventing long COVID and attenuating its burden. While this study contributes to addressing knowledge gaps related to long COVID, the characterization of long COVID continues to evolve [9]. Future studies could corroborate these findings with different data collection methods, non-COVID comparator, longer follow-up times, use of COVID-19 specific validated instruments, and protective effects on other individuals.
Conclusions
Long COVID had a detrimental effect on well-being, work productivity and activity levels. The BNT162b2 booster was associated with less symptomatic infection and faster improvement, with boosted subjects experiencing the lowest number of symptoms over time. This, in turn, contributed to improved HRQoL and maintained productivity and activities. These findings support current recommendations for broad use of BNT162b2.
Supplementary Information
Additional file 1: Figure S1 Study Flow Chart. Figure S2 Questionnaire on long COVID symptoms. Figure S3 Prevalence of long COVID symptoms by vaccination status. Figure S4 Number of symptoms over time by vaccination status. Figure S5 Absence of long COVID symptoms by vaccination status.
Acknowledgements
The authors acknowledge Nancy Gifford (Pfizer employee), Joseph Ferenchick, Shiyu Lin and Shawn Edmonds (CVS Health employees) for specific contributions to this research project. Editorial support was provided by Laura Anatale-Tardiff and Leena Samuel at CVS Health and was funded by Pfizer.
Abbreviations
- ANOVA
Analysis of variance
- CDC
Centers for Disease Control and Prevention
- CI
Confidence interval
- ES
Effect size
- SD
Standard deviation
- SVI
Social vulnerability index
- UI
Utility index
- VAS
Visual analogue scale
- WPAI
Work productivity and impairment
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article. XS: Conceptualization, Methodology, Data curation, Formal analysis, Writing—original draft. MDF and YP: Investigation, Project administration, Conceptualization, Writing—Review and editing. MMM, HC, LP, MBA, and JMZ: Conceptualization, Writing—Review and editing. JCC: Conceptualization, Methodology, Formal analysis, Writing—Review and editing.
Funding
This study was sponsored by Pfizer Inc.
Availability of data and materials
Aggregated data that support the findings of this study are available upon reasonable request from the corresponding author MDF, subject to review. These data are not publicly available due to them containing information that could compromise research participant privacy/consent.
Declarations
Ethics approval and consent to participate
This study was approved by the Sterling IRB, Protocol #C4591034. Participation in the study was voluntary and anonymous. Consent was obtained electronically via the CVS Health E-Consent platform. Participants were informed of their right to refuse or withdraw from the study at any time. Participants were compensated for their time.
Consent for publication
All authors have given their approval for this manuscript version to be published.
Competing interests
MDF, MMM, JMZ, MBA, LP and JCC are employees of Pfizer and may hold stock or stock options of Pfizer. XS and HC are employees of CVS Health and may hold stock of CVS health. YPT was employee of CVS Health when current study was conducted.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention. Long COVID or Post-COVID Conditions. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html. Accessed May 5, 2023.
- 2.Ledford H. How common is long COVID? Why studies give different answers. Nature. 2022;606(7916):852–853. doi: 10.1038/d41586-022-01702-2. [DOI] [PubMed] [Google Scholar]
- 3.Robertson M, Qasmieh S, Kulkarni S, Teasdale CA, Jones HE, McNairy M, Borrell LN, Nash D (2022) The epidemiology of long COVID in US adults two years after the start of the US SARS-CoV-2 pandemic. medRxiv
- 4.Azzolini E, Levi R, Sarti R, Pozzi C, Mollura M, Mantovani A, Rescigno M. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. 2022;328(7):676–678. doi: 10.1001/jama.2022.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Aly Z, Bowe B, Xie Y (2022) Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 28(7):1471–1467 [DOI] [PMC free article] [PubMed]
- 6.Notarte KI, Catahay JA, Velasco JV, Pastrana A, Ver AT, Pangilinan FC, Peligro PJ, Casimiro M, Guerrero JJ, Gellaco MML. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. EClinicalMedicine. 2022;53:101624. doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ioannou GN, Baraff A, Fox A, Shahoumian T, Hickok A, O’Hare AM, Bohnert AS, Boyko EJ, Maciejewski ML, Bowling CB. Rates and factors associated with documentation of diagnostic codes for long COVID in the national Veterans Affairs health care system. JAMA Netw Open. 2022;5(7):e2224359. doi: 10.1001/jamanetworkopen.2022.24359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byambasuren O, Stehlik P, Clark J, Alcorn K, Glasziou P (2022) Impact of COVID-19 vaccination on long COVID: a systematic review and meta-analysis. medRxiv. https://www.medrxiv.org/content/medrxiv/early/2022/06/22/2022.06.20.22276621.full.pdf. [DOI] [PMC free article] [PubMed]
- 9.Di Fusco M, Sun X, Moran MM, Coetzer H, Zamparo JM, Puzniak L, Alvarez MB, Tabak YP, Cappelleri JC (2022) Impact of COVID-19 and effects of BNT162b2 on patient-reported outcomes: quality of life, symptoms, and work productivity among US adult outpatients. J Patient Rep Outcomes 6:123 [DOI] [PMC free article] [PubMed]
- 10.CDC/ATSDR Social Vulnerability Index. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html Accessed 24 May 2021
- 11.Centers for Disease Control and Prevention. Updates—Symptoms of COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed 5 May 2023.
- 12.Landry M, Bornstein S, Nagaraj N, Sardon Jr GA, Castel A, Vyas A, McDonnell K, Agneshwar M, Wilkinson A, Goldman L (2023) Postacute sequelae of SARS-CoV-2 in university setting. Emerg Infect Dis 29(3):519 [DOI] [PMC free article] [PubMed]
- 13.Perlis RH, Santillana M, Ognyanova K, Safarpour A, Trujillo KL, Simonson MD, Green J, Quintana A, Druckman J, Baum MA. Prevalence and correlates of long COVID symptoms among US adults. JAMA Netw Open. 2022;5(10):e2238804. doi: 10.1001/jamanetworkopen.2022.38804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, Karamchandani U, Simms-Williams N, Cassambai S, Ardavani A. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine. 2023;55:101762. doi: 10.1016/j.eclinm.2022.101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb M, Wang R, Yu H, Spatz ES, Montoy JC, Rodriguez R, Chang AM, Elmore JG, Hannikainen PA, Hill M (2023) Severe fatigue and persistent symptoms at three months following SARS-CoV-2 infections during the pre-delta, delta, and omicron time periods: a multicenter prospective cohort study. Clin Infect Dis 76(11):1930–1941 [DOI] [PMC free article] [PubMed]
- 16.Spatz ES, Gottlieb M, Wisk LE, Anderson J, Chang AM, Gentile NL, Hill MJ, Huebinger RM, Idris AH, Kinsman J (2022) Three-month symptom profiles among symptomatic adults with positive and negative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) tests: a prospective cohort study from the INSPIRE Group. Clin Infect Dis 75(9):1559–1566 [DOI] [PMC free article] [PubMed]
- 17.EuroQol Research Foundation. (2019) EQ-5D-5L User Guide, Version 3.0. https://euroqol.org/publications/user-guides. Accessed 5 Aug 2021
- 18.Pickard AS, Law EH, Jiang R, Pullenayegum E, Shaw JW, Xie F, Oppe M, Boye KS, Chapman RH, Gong CL, Balch A, Busschbach JJV. United States valuation of EQ-5D-5L health states using an international protocol. Value Health. 2019;22(8):931–941. doi: 10.1016/j.jval.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 20.Reilly Associates (2002) WPAI Scoring. http://www.reillyassociates.net/WPAI_Scoring.html. Accessed 5 Aug 2021
- 21.Rosner B. Fundamentals of biostatistics. 8. Boston, MA: Cengage learning; 2015. [Google Scholar]
- 22.Freeman G, Halton JH. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951;38(1/2):141–149. doi: 10.2307/2332323. [DOI] [PubMed] [Google Scholar]
- 23.Tukey J. Multiple comparisons. J Am Stat Assoc. 1953;48(263):624–625. [Google Scholar]
- 24.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. 2. Hoboken: Wiley; 2011. [Google Scholar]
- 25.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale: Lawrence Erlbaum Assoc; 1988. [Google Scholar]
- 26.McLeod LD, Cappelleri JC, Hays RD (2016) Best (but oft-forgotten) practices: expressing and interpreting associations and effect sizes in clinical outcome assessments. Am J Clin Nutr 103(3):685–93. Erratum: 2017; 105:241. 2016 10.3945/ajcn.115.120378. Erratum: 2017 10.3945/ajcn.116.148593 [DOI] [PMC free article] [PubMed]
- 27.STROBE Statement—Checklist of items that should be included in reports of cohort studies. https://www.strobe-statement.org/download/strobe-checklist-cohort-studies-pdf. Accessed 5 Aug 2022 [DOI] [PubMed]
- 28.Byambasuren O, Stehlik P, Clark J. Effect of covid-19 vaccination on long covid: systematic review. BMJ Med. 2023;2:e000385. doi: 10.1136/bmjmed-2022-000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stamm TA, Partheymüller J, Mosor E, Ritschl V, Kritzinger S, Alunno A, Eberl J-M. Determinants of COVID-19 vaccine fatigue. Nat Med. 2023;29(5):1164–1171. doi: 10.1038/s41591-023-02282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers RW (1983) Cognitive and psychological processes in fear appeals and attitude change: a revised theory of protection motivation. Social psychophysiology: a sourcebook. Guilford Press, New York, pp 153–176
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1 Study Flow Chart. Figure S2 Questionnaire on long COVID symptoms. Figure S3 Prevalence of long COVID symptoms by vaccination status. Figure S4 Number of symptoms over time by vaccination status. Figure S5 Absence of long COVID symptoms by vaccination status.
Data Availability Statement
Aggregated data that support the findings of this study are available upon reasonable request from the corresponding author MDF, subject to review. These data are not publicly available due to them containing information that could compromise research participant privacy/consent.