Abstract
Introduction
The purpose of this study is to explore treatment preferences and identify patient characteristics in young bio-naive adults with moderate to severe psoriasis in the Nordic countries (Norway, Finland, Sweden, and Denmark).
Methods
Patients were 18–45 years old and bio-naive but referred for biologic treatment of moderate to severe psoriasis. Patients were included at eight Nordic dermatology clinics. Patients with significant comorbidity or psoriatic arthritis were excluded. The Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI) were assessed along with basic patient information.
A semistructured interview guide was used in individual qualitative interviews, asking patients about their treatment preferences and reasons, disease journey, and disease management. The interviews were analyzed using thematic content analysis. Twenty-four patients sufficed to reach saturation in this qualitative study.
Results
The patient sample characteristics represented a qualitative variation in age, sex, symptoms, duration of disease, and country. We included a total of 12 male and 12 female patients. The mean age was 34 years (range 18–45 years), the mean age at diagnosis was 20 years (range 6–34 years), the mean ± standard deviation (SD) time since diagnosis was 13 ± 8 years, PASI was 9.5 ± 4.7, and DLQI was 15.2 ± 6.4.
Interviews suggested that both the burden of disease as well as the burden of treatment influenced patient preferences regarding treatment attributes, hence getting alleviation from symptoms did not alone influence patient preferences. Time, effort, and inconvenience related to psoriasis treatments also influenced patient preferences.
Conclusions
This first in-depth, qualitative study in young bio-naive adults with psoriasis suggests that patient preferences are focusing not only on symptom relief but also on alleviating the burden of psoriasis treatment. Understanding the reasons for patient preferences and the perspectives of young adults is needed to guide individual shared decision-making in psoriasis management.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-023-00973-5.
Keywords: Disease burden, Patient preference, Semistructured interviews, Treatment attributes, Treatment burden
Plain Language Summary
Not much research has been done on understanding the disease burden and treatment needs of young adults suffering from psoriasis. This is an interview study with young adults from Nordic countries suffering from moderate to severe psoriasis with an active lifestyle. The adult patients were all referred for biologic treatment of psoriasis but had not yet started treatment when they were interviewed. The aim was to explore treatment preferences in this group.
The study showed that treatment goals depended upon both alleviation of symptoms and obtaining a low treatment burden. The most influential symptoms were scaling, itching, and visible plaques. The most important treatment burden features were efficacy, durability, speed of response, safety, and convenience. Understanding the reasons behind these different treatment preferences is essential to help shared-decision psoriasis management that matches individual needs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-023-00973-5.
Key Summary Points
| Why carry out this research? |
| Not much research has been done on understanding the needs of young adults suffering from psoriasis. |
| This is a qualitative study among young, bio-naive adults from Nordic countries suffering from moderate to severe psoriasis with an active lifestyle. The aim is to explore treatment preferences in this group qualitatively. |
| As part of a mixed-methods study, this study precedes a quantitative patient preference study. |
| What was learned from the study? |
| The treatment preferences varied greatly among participants. Understanding the reasons behind these diverse preferences is essential to shared-decision psoriasis management. |
| The analysis found that treatment goals depended upon two overall categories: alleviation of symptoms and low treatment burden. |
| The most influential symptoms were scaling, itching, and visible plaques. The most important treatment burden features were efficacy, durability, speed of response, safety, and convenience. |
Introduction
Psoriasis is a chronic immune-mediated disease associated with several comorbidities [1, 2]. While the global prevalence of psoriasis is 3%, the prevalence is higher in Denmark, Norway, and Sweden, ranging from 3.9% to 11.5% [3–6]. Signs of psoriasis include scaling, itching, pain, and bleeding, resulting in impaired health-related quality of life (HRQoL) and functioning. The Psoriasis Area and Severity Index (PASI) is used for assessment of extent and intensity of lesions for four body areas (head/neck, upper extremities, trunk, and lower extremities), ranging from 0 to 72 [7]. PASI only partly reflects the burden of disease as involvement of visible areas (such as the face, hands, the scalp, and nails) or genitals that tend to severely impact patients’ HRQoL through stigmatization, poor self-esteem, and reduced social and occupational functioning [8–12]. HRQoL may additionally be affected by treatment-related factors such as convenience, adverse effects, treatment success or failure, and costs [12]. Finally, comorbidities, coping skills, social support, and sociodemographic factors such as age, gender, and partner status reportedly affect HRQoL in psoriasis [12–18].
Favorable clinical properties, i.e., efficacy, safety, and tolerability, are required for a medicine to obtain regulatory approval. However, patient preferences may be influenced by other factors, such as speed of onset, cost, convenience of treatment, or durability of efficacy. This seems to be particularly important in situations where different treatment options have comparable efficacy and safety. Treatments for psoriasis include topicals, phototherapy, conventional systemic treatments (methotrexate, cyclosporin, fumaric acid, and retinoids), small molecules, and biologics. Different types of therapies have varying levels of efficacy, safety, speed of response, and durability of response as well as different processes related to the administration of treatment such as administration frequency, duration, and convenience, as well as different treatment costs. Among patients treated with tumor necrosis factor (TNF) inhibitors, some even achieve a PASI90 and PASI100 response but to a lower degree than patients treated with interleukin (IL)-17 or IL-23 inhibitors [19]. Taking individual patient preferences into account in treatment decisions leads to greater treatment satisfaction, adherence, and better long-term outcomes [20–22]. Factors associated with treatment preferences in psoriasis include symptoms, disease duration, treatment history, and sociodemographic factors such as age, gender, civil status, as well as HRQoL [4, 22]. As the panel of biologic treatment options for psoriasis has increased over the past years, it is relevant to investigate what patients expect from the treatment, and what their concerns are prior to starting biologics. The voice of patients reflected in qualitative interview studies is lacking, in particular for young bio-naive patients [23, 24]. Hence, this qualitative study aimed to explore the treatment preferences in young bio-naive patients with moderate to severe psoriasis who had no previous experience with biologics but were going to start such treatment. The impact of biologic treatment outcome is not explored. As part of a mixed-methods study, the findings of this qualitative study will be used to guide the design of a quantitative study on patient preferences for treatment of psoriasis.
Materials and Methods
The aim was to include the broadest and most diverse group of patients as possible within the in- and exclusion criteria. Inclusion criteria were moderate to severe psoriasis, age 18–45 years, that patients were bio-naive but referred for biologics, and that patients were cognitively and linguistically able to participate in an in-depth online or telephone interview. Exclusion criteria were significant comorbidities as assessed by the dermatologist, i.e., physical, cognitive, or psychological comorbidity that may affect patients’ preferences for biologics including cardiovascular disease, diabetes, and depression that are not well treated, or psoriatic arthritis. Patients with a diagnosis of psoriatic arthritis were excluded since they may have used therapies that are not used for patients purely with psoriasis and may therefore have had different treatment experiences compared with patients suffering from psoriasis alone.
Saturation was reached after interviewing 24 patients, comprising 6 patients from Denmark, Norway, Finland, and Sweden, respectively [25]. Patients were recruited from the following sites: Department of Dermatology, Stavanger University Hospital, Norway, Psoriasis Association Treatment Wards, Stockholm, Sweden, Department of Dermatology, Aarhus University Hospital, Denmark, Department of Dermatology, Turku University Hospital, Finland, Department of Dermatology and Venereology, Sahlgrenska University Hospital, Gothenburg, Sweden, Department of Dermatology, Tampere University Hospital, Finland, Department of Dermatology and Allergy, Herlev and Gentofte Hospital, University of Copenhagen, Denmark.
Eligible patients were invited to participate in an individual interview during prescheduled consultations ahead of initiation of biologic treatment. Patients received oral and written study information. If consenting, basic patient information (first name, contact information, age, current treatment for psoriasis, PASI, and DLQI) was recorded and transferred to four independent local interviewers, who contacted and arranged for an interview with the patient. Inclusion continued until six interviews had been carried out—with three men and three women to ensure equal gender representation—in each country, respectively.
Interviews
Interviews were carried out virtually from June 2021 to June 2022 by four native-speaking experienced patient interviewers. To ensure a uniform interview technique, all interviewers had received instructions by an external qualitative research specialist from AnthroConsult, Aarhus, Denmark. The semistructured interview guide was applied by the interviewers. They explored in depth the patients’ treatment preferences and the reasons for their preferences, using qualitative interview techniques. The interviewers included probing for details and examples, follow-up questions, and testing interpretations [26–29]. The following topics were discussed: demographic information and time since diagnosis, self-reported symptoms, impact of psoriasis on HRQoL, treatment experiences, treatment goals and preferences including a treatment attribute exercise, and an exercise on patient needs (see Supplementary Material for the full interview guide).
The interviewer in each country assigned a participant code to the individual patient (designating country, gender, and consecutive number of interviews) for pseudonymization. Interviews were recorded, transcribed verbatim, translated into English, and subsequently transferred to AnthroConsult for data analysis.
Ethics
This study was conducted in compliance with the international ethical standards. Data were protected according to the General Data Protection Regulation (GDPR). Ethics committee approval was obtained from the Ethical Review Authority in Sweden, the Regional Committees for Medical and Health Research Ethics in Norway, and the Ethics Committee for Human Sciences at the University of Turku in Finland. Ethics approval was not required in Denmark. All participants gave informed consent stating that their data would be anonymized before being used for research purpose and that the results would be published.
Analysis
Descriptive statistics (mean, range, and standard deviation) were used to characterize the sample.
The analyses focused on identifying the key characteristics of treatment for psoriasis. A combination of thematic content analysis and constructivist/narrative analysis was used through coding of interviews in key topics discussed (raised by either the interviewer or the interviewee) and condensation into the most important themes within the topics.
The translated transcripts were coded using NVivo (QSR International, Burlington, MA, USA) [30] by one person from AnthroConsult. Individual interview transcripts were imported as “cases/case nodes” and organized into country “sets.” The initial “coding tree” was created manually based on the questions and before the analysis (topics/”tree nodes”) in the question guide and references to potential influencing factors (e.g. gender, work situation, or having children) with a “case node attribute” per item. Each transcript was coded accordingly, i.e., marked up and assigned one or more relevant nodes (i.e., the topic(s) addressed in an utterance).
Within each node, the central meaning was identified (“condensation”). The coding framework was updated iteratively to reflect new themes that emerged during coding and regular discussions with the research team: Reading through each node led to the merging of some nodes while others were split up into different nodes or “branched out” into subthemes (“node trees”) within the node (thematic content analysis). Each node was examined for its relations to other nodes (associated factors or items), e.g., when describing the importance of effective treatment (a node), many patients stressed the importance of efficacy with regard to visual lesions (a symptom node in the tree node “symptoms”) and explained how this was key to social functioning (another node). References between nodes were made by using NodeLinks [30]. Interview participants were not invited to provide feedback on the coding.
Results
Patients aged between 18 and 45 years were included, and female and male patients were equally represented. Patient characteristics are presented in Table 1. There was a wide dispersion of age at diagnosis with psoriasis (6–34 years), time since diagnosis (2–34 years), PASI (3–18.3), and DLQI (2–27). Most participants (n = 21) had a partner, 3 were single, and 15 had children. Five of 14 patients working full-time had changed work functions due to psoriasis, e.g., by transferring from manual or technical to desk work. Two patients working reduced hours and one was in retraining owing to psoriasis. Two in five students/apprentices had changed their educational path owing to psoriasis, and one was in job training. Two patients had applied for disability pension, one partly because of psoriasis (Table 1).
Table 1.
Patient characteristics
| Patient population | Dermatologist reports | ||
|---|---|---|---|
| 24 young adults with moderate to severe psoriasis, 12 female and 12 male | Age, mean (range), years | 34 (18–45) | |
| Age at diagnosis, mean (range), years | 20.5 (6–34) | ||
| Time since diagnosis, mean (SD), years | 13.0 (8.3) | ||
| PASI, mean (SD) | 9.5 (4.7) | ||
| DLQI, mean (SD) | 15.2 (6.4) | ||
| Previous treatments (current treatments) | |||
| Phototherapy | 20 (2) | ||
| Topicals | 22 (8) | ||
| MTX oral | 17 (10) | ||
| MTX injectable | 9 (5) | ||
| Not known | 4 (2) | ||
PASI Psoriasis Area and Severity Index, DLQI Dermatology Life Quality Index, MTX methotrexate, SD standard deviation
The patients differed in PASI, DLQI, and self-reported current symptoms and affected areas with highest impact on HRQoL (Table 2). All patients were referred for and found eligible for biologic treatment by a dermatologist. Applying the rules of ten (PASI ≥ 10/DLQI ≥ 10) for initiation of biologics, a few patients may have presented with different issues, such as the localization of lesions or side effects from, e.g., methotrexate (Table 2).
Table 2.
Burden of symptoms evaluated by the dermatologist and the patient
| Dermatologist reported | Patient reported | ||||
|---|---|---|---|---|---|
| Age | Year of diagnosis | PASI | DLQI | Self-reported current involved areas with highest impact on quality of life (shown in bold) | Self-reported current symptoms with highest impact on quality of life (shown in bold) |
| 18 | 2011 | 12 | 16 | Neck, Ears, Nails, Chest, Shoulders, Legs | Itch, Scaling |
| 34 | 2017 | 11.4 | 21 | Everywhere including Face, Scalp, Nails, Genitals, Buttocks |
Itch, Scaling, Sleep disturbances |
| 41 | 1987 | 4.5 | 27 | Face, Scalp, Ears, Eyes, Nails, Genitals, Elbows, Knees | Joint pain |
| 40 | 2015 | 11 | 19 | Everywhere including genitals | Severe itch, Skin pain |
| 25 | 2002 | 17.6 | 16 | Everywhere including Genitals, Nails, Ears | Joint pain, Scaling |
| 31 | 2015 | 3.7 | 11 | Legs (severely affected), Scalp, Toenails, Back, Genitals |
Itch, Skin inflammation, Joint pain |
| 30 | 2019 | 1.8 | 15 | Neck, Scalp, Toenails | Itch, Scaling, Sleep disturbances |
| 36 | 1996 | 15 | 20 |
Everywhere on the entire body Legs severely affected |
Skin pain, Joint pain (neck and shoulders) |
| 31 | 2008 | 5.4 | 20 | Everywhere on the entire body | Itch, Joint pain, Painful plaques on palms and foot soles, Sleep disturbances |
| 37 | 2013–15 | 9.1 | 20 | Everywhere including Genitals, Head, Feet, Legs | Itch, Skin pain, Scaling |
| 45 | 1991 | 13.1 | 18 | Head, Ears, Hands (fingers, thumb, nails), Elbows, Legs, Feet, Genitals | Itch, Skin tightness/burning, Pain in one knee and elbow |
| 44 | 2008 | 12.1 | 17 | Under eye lids, Face, Scalp, Ears (inside and outside), Hands, Elbows, Genitals, Knees | Joint pain, sleep disturbances |
| 36 | 2012 | 10.4 | 10 | Everywhere including Scalp, Ears, Hands (worst), Arms, Torso, Genitals, Buttocks, Back, Feet | Scaling, Itch, Joint pain |
| 22 | 2005 | 7.6 | 9 |
Everywhere on the body, small and large spots currently mostly hands |
Scaling, Itch |
| 36 | 2013 | 7.9 | 10 | Everywhere including Scalp, Face, Arms, Stomach, Chest, Legs, Feet | Itch, Stinging on legs, Scaling |
| 34 | 2015 | 3 | 21 | Hands, Fingers, Scalp, Genitals | Pain, Itch |
| 36 | 2010 | 11.5 | 10 | Head, Ears, Face, Hands, Arms, Armpits, Neck, Feet, Ankle, Legs, Buttocks, Torso | Itch, Skin cracks, Pain |
| 35 | 2000 | 8.1 | 17 | Everywhere on the body from Head to Toes including Face, Scalp and Head | Scaling (scalp), Itch, Skin pain, Join pain, Sleep disturbances |
| 40 | 2003 | 3.9 | 2 | Elbows, Scalp | Itch, Scaling |
| 20 | 2017 | 12.9 | 23 | Everywhere on the body, including inside Ears, Face, Scalp | Painful cracks, Itch, Scratching, Sleep disturbances |
| 28 | 2007 | 15.6 | 6 | Arms, Ears, Legs, Nails | Itch, Join pain, Depression/self-loathing, Anxiety |
| 38 | 2014 | 6.6 | 22 | Everywhere on the body, including Scalp, Head, Hands (entirely covered), Elbows, Knees, Legs (lower part) | Itch, Skin cracks, Sleep disturbances, Mental health |
| 38 | 2007 | 18.3 | 9 |
Extent of lesions (large body parts affected), Scalp, Behind ears, Nails, Arms, Thighs, Calves, Genitals, Anus, Buttocks, Back, Navel |
Scaling, Itching, Joint pain |
| 41 | 2005 | 4.5 | 5 | Elbows, Legs | Itch |
PASI Psoriasis Area and Severity Index, DLQI Dermatology Life Quality Index
Treatment Goals
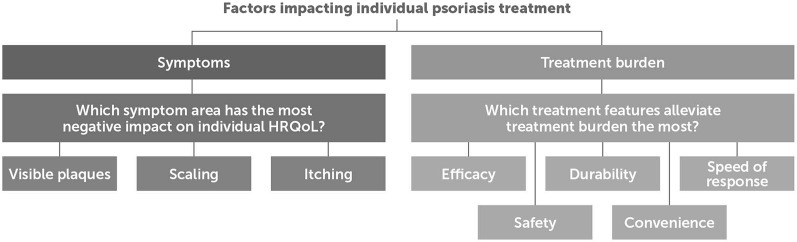
The analysis of interviews showed that the main goals of biologic treatments could be split into two main factors: obtaining symptom relief and alleviating the burden of psoriasis treatment (Fig. 1).
Fig. 1.
The two main factors impacting individual treatment of psoriasis in Nordic patients with moderate to severe psoriasis
Symptom Relief
Patients responded during interviews that visible signs and specific localizations of lesions had had impact on disease burden. Hence, severely affected body parts with scaling and itching, visible signs of disease of the face, hand, legs, or plantar involvement, and psoriasis in areas difficult to treat with topicals such as nails, scalp, or genitals added to the burden of symptoms. Visible signs of psoriasis and specific localization of lesions had impact on patients’ physical and mental well-being, indicated by relatively high DLQIs throughout the sample.
The preferences on symptom relief reported during interviews aimed at ameliorating physical symptoms such as pain, itching, flaking/scaling, sleep problems, and joint issues, and about improving psychosocial life quality: looking and feeling normal, e.g., “I hope the psoriasis disappears and that I get my life back.” (NOM1). Further, the treatment goal was to gain self-confidence and avoid the impact on intimacy, e.g., “Being free, not thinking about psoriasis all the time, free to touch and be intimate with my husband.” (DKF3) and of stigmatization, e.g., “People [are] distancing themselves because it looks terrible.” (DKF2).
Alleviation from the Burden of Psoriasis Treatment
The patients reported treatment-related goals revolved around being free from the inconvenience of phototherapy (time consuming and rigid to meet opening hours at the clinic), the inconvenience of daily topical treatment (time consuming and greasy), and avoiding side effects from methotrexate, e.g., nausea. The patients who were referred for biologic treatment had all gone through a series of ups and downs of short-lived treatment successes and failures, experienced lack of durability, slow onset of action, and adverse events (Table 3).
Table 3.
Themes and illustrative patient quotes
| Theme | Patient quotes on treatment preferences |
|---|---|
| Efficacy |
“It would be lovely to be able to go to the swimming pool with my children without having to worry about it. I do hold back now. It’s all about (my) appearance,” (DKM1) “It doesn’t matter if I still have a small spot here and there. It’s not about having immaculate skin, it’s about not having it in places that bother me, like my hands, nails, face, and joints.” (DKF3) “The most important thing for me is that the spots disappear, that I’ll become healthy. I want to look like an ordinary person” (FLF2) “If this [psoriasis] would go away completely and stay away for a long time, that would be extremely nice. As I said about my [affected] joints, when you’re into music and playing it yourself, it would be a pretty wild reason to have to give that up. And of course, not always itching and flaking skin everywhere, yes, that would be a great change to my everyday life.” (FLM2) |
| Durability |
“You hope that (biologic treatment) is effective for as long as possible. It’s hard that you always have to try out these drugs and then they say that this isn’t right for you after all.” (FLF3) “I thought a lot about whether I’ll have to try something new again in 2–3 years. It would mean a lot to me if you just found that one thing that works for you rather than going through lots of different things.” (DKF1) “Metoject [methotrexate] was effective at first but then lost effect. I was very satisfied with it in the beginning. Once it started working, my life was so much easier to live… All my skin issues were gone. But then it stopped working. Everything came back and I was very depressed for a while.” (SEF2) “You get your hopes up every time, then further down than where you came from” |
| Speed of response |
“It’s important to see an effect quickly. You sometimes hear of people who started a treatment [for psoriasis] and simply lost faith in it because they couldn’t see an effect… The first couple of times I used all those creams, I really felt it took a long time [approximately a month]. I felt like nothing happened at all and I had long discussions with my mom about whether I should take it or not… I quickly got tired of it, that’s for sure.” (DKF1) “[Speed of response] is important because it encourages you to continue. It gives meaning to the treatment and motivates you to go forward” … “A quick effect would be in one month and two months would be ok. Because of the motivation, it would be nice to see some effect in a couple of months.” (FLF1) “[How fast it works] is very important, to feel that it works and that it is worth having these injections. … If it works well, then you’ll see fast results. Then you are motivated to continue.” (SEF1) |
| Safety | “I’m scared now because I’ve had so many problems with MTX. I’m not sure what biologic treatment means. I do not know what it is, and I fear that I will get the same kind of side effects that I got with MTX. What will it do to my body? Imagine if I get even more problems than I have today. Right now, I’m unsure, and think that maybe light therapy is what I should take. At least then I do not get side effects.” (NOM2) |
| Convenience |
Speaking of self-injecting biologic treatment every two weeks: “then I almost don’t have to think about it anymore. I want for it [treatment] to do so that I don’t notice this disease anymore at all.” (DKM1) “It takes a long time for the skin to absorb [topicals] and I always had a whole ritual around it. I couldn’t just take a shower and go to bed like a regular person. Instead, I needed to lotion my entire body which might take an hour. And it was never absorbed, so I had to sleep with a plastic wrapper or a robe because the towels and bed linen got bleached by the lotion. It [biological treatment] is not something I have to spend time on every day, keep up and be reminded of. I’ll take this injection pen and then it’s over and done with. It’s once a week or every two weeks.” (SEF2) “The fact that you administer it at home by yourself once a week, I mean, it doesn’t affect everything else, that sounds very good. I don’t need to make appointments several times a week. It’ll be less work for me.” (SEM3) “If you could get rid of applying cream all over that would be very positive.” (FLM2) “An injection is so much faster compared with those two hours a day applying cream. In that sense, it’s surely acceptable compared with topical treatment.” (FLM3) |
Based on the two overarching goals of treatment, viz. symptom relief and alleviation from the burden of psoriasis treatment, five specific treatment goals were identified: efficacy, durability, speed of response, safety, and convenience. Efficacy was understood as how well the treatment improved symptoms. Durability was understood as the span of time the treatment worked for the patient without needing to change treatments. Speed of response time was understood as the speed with which a treatment worked. Avoiding side effects was understood as not having to face potential adverse effects from the treatment. Convenience was understood as the ease of treatment, including time for administration and the option to do self-treatment.
Efficacy was the treatment attribute mentioned as the top priority, e.g., “I'm insanely happy if the [biological] medicine can make me get rid of it [psoriasis] completely.” (NOM3) and “That the symptoms, that pain, itching and flaking would stop.” (FLF3). Patients could accept less than completely clear skin if treatment was effective regarding visible signs of disease, itching, joint symptoms, or disabling localizations (especially the palms and the soles of the feet), e.g., “I will never get rid of it [psoriasis] but that it’ll be visibly gone.” (SEM3). Freedom from symptoms was important to look and feel normal, e.g., “You're hoping this would at least diminish. It would make my life easier if it took away the itching and visible symptoms. That I would have a normal life.” (FLM1). In addition, effective biologic treatment was considered a relief of the burden of other treatments, especially topicals. Some referred to positive – if short-lived – effects from previous treatments setting the standard for what they aimed to achieve again.
Durability Having experienced failed attempts at disease control with previous treatments, patients reported that obtaining a durable treatment response was a preferred treatment attribute, though less outspoken in Sweden and Denmark. Four Danish patients knew there are several biologics available and were confident that one would work for them, while lacking knowledge about biologics worried several Norwegian patients, who all considered durability as important. Most patients were frustrated by earlier treatment failures, and some felt that biologic treatment may be their last chance of treatment success, e.g., a durable effect: “This back-and-forth is pretty racking” (FLF1).
Speed of treatment response Rapid onset of treatment response was mentioned as an important treatment attribute. A rapid treatment response was linked to hope, regarding the efficacy of treatment and improved well-being. Some took a fast treatment response as a sign of effectiveness and considered it to be meaningful and motivating for adherence. Perceptions of what constitutes a fast treatment response varied, ranging from immediately to 3 months. Overall, 1–2 months was considered a fast response of biologics.
Avoiding side effects Seven patients, primarily Norwegian and Swedish, reported low/no risk of severe side effects as a main treatment preference. Some, but not all, patients said their concerns about side effects were due to a lack of knowledge about biologics. All patients stressing the importance of avoiding side effects had children. Safety concerns mentioned were dying while having young children and increased risk of infections due to exposure to infections from having small children. Finally, while the routine blood work and vaccinations ahead of initiating biologics were reassuring to some, others interpreted this as a sign of biologics being dangerous.
Convenience Considering the hassle of phototherapy and topical treatments, convenience was the third most preferred attribute of biologics. Most patients believed that self-administering biologics with an injection pen would be comparably easy. Many had used a pen before with methotrexate. Few patients worried about self-injecting, but in turn believed that high efficacy and low treatment frequency (once per week or fortnight) would make it worthwhile and convenient compared with phototherapy and daily topical treatments. Patients with widespread lesions, full-time employment, and children particularly longed for less time-consuming treatment, e.g., “I have some routines with treatment, but it doesn't feel like it's part of normal life… That would be one big thing you would not need to think about.” (FLM3), rather than being constantly reminded of disease by “intrusive” treatments—meaning intrusive of their private life and time. They hoped that biologics would allow them to feel normal and healthy, and have more time for other meaningful things in life (family, work, and social life). It was important that treatment was flexible, and administration could be fitted into daily routines, rather than the other way around, e.g., “Not to spend time on treatment every day, maintain and be reminded of it. I’ll take this injection pen and then it’s over and done with.” (SEF3).
Discussion
This study suggests that young adult patients’ preferences for future biologic treatment are shaped not only by the burden of symptoms but also by the burden of treatment. Furthermore, it shows that the impact of psoriasis symptoms is highly individual. Some patients may have a highly affected DLQI with a PASI < 10. While the study provides qualitative input to an improved understanding of patients’ treatment preferences for moderate to severe psoriasis, the small sample size does not allow for ranking of the treatment preferences, i.e., efficacy, durability, convenience, side effects, and speed of response. This study adds to the understanding of the diversity of young bio-naive patients’ symptoms and treatment preferences and the reasons behind them and gives a voice to a group of patients in whom qualitative data are scarce [23, 24]. The patients reported on treatment attributes such as efficacy—obtaining clear or almost clear skin, relief from visible lesions and all or the most bothersome symptoms—and a durable treatment response following several failed attempts at disease control. In addition, another treatment attribute was convenience, i.e., quick, easy, and infrequent self-administration, which was important to relieve the burden of intensive phototherapy courses and daily topical treatment. In accordance with other studies, dissatisfaction and poor treatment adherence were particularly high with topicals [31]. To some patients, low risk of side effects and rapid treatment response were treatment attributes that were considered important, the latter to keep up hope and motivation for treatment. Stigmatization associated with psoriasis and impact on self-esteem are well known [12, 32, 33]. This study adds to an understanding of why effective treatment targeting visible disease is particularly important and challenges the treatment goal of PASI75 as sufficient for the patient to feel adequate relief. Appearance may be even more important in young adults in search for a partner. This study reports on the feeling of stigmatization, impact on self-esteem, and social isolation. Previous studies have shown that clear or almost clear skin is the ultimate preference of patients and that they mainly need relief from itch, visible symptoms, and genital lesions [20, 32]. This study furthermore adds that joint problems, hand, foot, scalp, nail, or genital involvement are highly disabling.
Age, gender, safety, and disease severity have previously been shown to affect treatment preferences [20, 21, 34]. When focusing on young, working adults, treatment attributes are concentrated around efficacy, speed of response, and convenience [20, 21, 34]. This study points to the reasons behind the treatment preferences with a need to fit treatment into a busy work life and family schedules, and a longing for normalization and social acceptance. This may be particularly relevant in bio-naive patients, who typically have years of experience with nonbiologics. Patients must allocate time and energy to manage topical treatment on a daily basis or take time to go to the dermatology clinic for phototherapy in balance with family life, work, and social functioning.
Young Nordic adults with psoriasis reportedly have high expectations of biologics [5, 21, 32, 35]. As treatment satisfaction is closely linked to knowledge and expectations of treatment [6], this study underlines the importance of communicating about the expected outcomes, i.e., efficacy, durability, speed of response, and risk of side effects as well as convenience.
Alignment of the patients’ and physicians’ treatment goal aims to improve patients’ HRQoL, increase treatment satisfaction and adherence and hence improve the outcomes of treatment [22, 32, 36]. It has been suggested to support shared decision-making and elicit patients’ individual treatment preferences directly [21, 35, 37].
Limitations
The limitations have been mitigated by including patients with a wide age range and from several countries. The sample included presumably professionally active patients per design. Still, as all eligible patients were invited to participate, there is a risk of selection bias as higher functioning patients may be more likely to volunteer. The sample included a high number of persons with a partner, family, and employment, which may be higher than the background populations in the Nordic countries [5]. Being a more heterogeneous group, patients with a diagnosis of psoriatic arthritis were excluded from this study, which may not fully reflect real life. Biologics are indicated in moderate to severe psoriasis, and as all patients included were bio-naive, the sample may represent patients with poor treatment experiences in the past, e.g., having experienced side effects or treatment failures, knowing they were starting biologics and therefore had a lot of expectation regarding the treatment. This may have affected their answers during interviews. There is a risk of recall bias, but as the interviews captured subjective disease experiences that influenced treatment preferences, this limitation is considered to be less important. Treatment preferences are related to the healthcare system [38, 39], but with socialized healthcare systems in the Nordic countries, the issue of cost was not critical in this Nordic setting. Overall, the Nordic countries constitute a similar sociocultural setting, yet some differences do exist. Finally, while 24 interviews are an adequate number to obtain saturation in a qualitative analysis, the sample is inadequate for statistical generalization and analysis of correlations or value ranking of attributes.
Conclusions
For the treatment of a chronic disease such as psoriasis, patients may have very different and diverse treatment preferences. Understanding the reasons for patient preferences and knowing the perspectives of young adults is needed to guide individual shared decision-making in psoriasis management. This first in-depth, qualitative study in young bio-naive adults with psoriasis suggests that treatment preferences focus not only on symptom relief but also on alleviating the burden of psoriasis treatment. The patient preferences regarding treatment attributes of biologics covered efficacy, durability, speed of response, safety, and convenience.
Qualitative studies should highlight the relative importance and weighing of these attributes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all patients for taking the time to participate in the study and share their experiences with psoriasis and treatment preferences.
Funding
This study was funded by UCB. AnthroConsult delivered the protocol, semi-structured interview guide, and provided data analysis on pseudonymized data; services that were financially supported by UCB. UCB paid for editorial assistance, review management, and rapid service fee.
Editorial Assistance
Charlotte Strøm, MD, Ph.D. at SharPen, provided editorial assistance with the manuscript. This service was financially supported by UCB. The authors thank AnthroConsult for providing qualitative research services and consultancy. The authors thank Costello Medical for review management.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, enrolling and assessing participants were performed by all clinical authors at their respective medical facilities. All authors reviewed draft versions, read, and approved the final manuscript.
List of Investigators
Flora Balieva‡, Department of Dermatology, Stavanger University Hospital, Norway; Department of Public Health, Faculty of Health Sciences University of Stavanger, Norway. Birgitta W. Claréus, Psoriasis Association Treatment Wards, Stockholm, Sweden. Kjersti Danielsen, Department of Community Medicine, UiT, The Arctic University of Norway, Tromsø, Norway. Lars Iversen, Department of Dermatology, Aarhus University Hospital, Denmark. Leena Koulu‡, Department of Dermatology, Turku University Hospital and University of Turku, Finland. Amra Osmanecevic‡, Department of Dermatology and Venereology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Sweden, Department of Dermatology and Venereology, Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden. Rafael Pasternack, Department of Dermatology, Tampere University Hospital, Finland. Lone Skov‡, Department of Dermatology and Allergy, Herlev and Gentofte Hospital, University of Copenhagen, Denmark. ‡Country Principal Investigator.
Prior Presentation
Poster presentation P1529 at EADV 2022, Milan, Italy, 7–10 September 2022: Treatment preferences in young bio-naïve patients, with moderate to severe psoriasis. Preliminary results from a mixed-methods study across the Nordic countries.
Disclosures
Flora Balieva has consultancy services for advisory board/lectures: Eli Lilly, Takeda, UCB. Kjersti Danielsen has served as a consultant, lecturer or participated in sponsored events/meetings by Novartis, Abbvie, LEO Pharma, UCB Pharma, Almirall, Meda Pharma, Bristol Myers Squibb, Galderma and Celgene. Rafael Pasternack has received educational grants from AbbVie and Janssen; honoraria from AbbVie, BMS, Eli Lilly, Janssen, Novartis, Sanofi Genzyme and UCB for consulting and/or speaking; participated in clinical studies sponsored by AbbVie, BMS, Novartis, Eli Lilly, Pfizer, and Regeneron. Amra Osmancevic has consultancy services for: Advisory board/lectures: AbbVie, Almirall, Amgen, Janssen, Eli Lilly, UCB, LEO Pharma, Pfizer, Novartis, Kiowa Kirin, Sanofi, BMS, Meda, Boehringer Ingelheim, Therakos and Recordati Rare Diseases. Clinical trials: AbbVie, Novartis, LEO Pharma, Janssen, UCB, Pfizer, Boehringer Ingelheim. Brigitta W Clareus has consultancy services for: Advisory board/lectures: AbbVie, Actavis, Almirall, Amgen, BMS, Janssen, Eli Lilly, LEO Pharma, Novartis, Oripharm, UCB. Clinical trials: LEO Pharma. Lars Iversen has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by: AbbVie, Almirall, Amgen, Astra Zeneca, BMS, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Janssen Cilag, Kyowa, Leo Pharma, Micreos Human Health, MSD, Novartis, Pfizer, Regranion, Samsung, Union Therapeutics, UCB. LI is also employed by MC2 Therapeutics. Leena Koulu has served an advisory board member or a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Leo Pharma, Perrigo, Pfizer, Sanofi, UCB Pharma. Lone Skov: Paid speaker and consultancy services concerning advisory board/lectures: AbbVie, Almirall, Amgen, Janssen, Eli Lilly, UCB, LEO Pharma, Pfizer, Novartis, Kiowa Kirin, Sanofi, BMS, Meda, Boehringer Ingelheim, Therakos and LEO Pharma, and has been a consultant or served on Advisory Boards with Recordati Rare Diseases; Clinical trials: AbbVie, Janssen Cilag, Novartis, Eli Lilly, LEO Pharma, UCB, BMS, Boehringer Ingelheim, Almirall and Sanofi; Investigator for AbbVie, Janssen Cilag, UCB, Pfizer, Boehringer Ingelheim, Eli Lilly, Novartis, and LEO Pharma and received research and educational grants from Almirall, Novartis, Sanofi, BMS, Janssen Cilag, and Leo Pharma. Louise Catton and Frederik Fierens are employees at UCB.
Compliance with Ethics Guidelines
This study was conducted in compliance with the international ethical standards. Data were protected according to the General Data Protection Regulation (GDPR). Ethics committee approval was obtained from the Ethical Review Authority in Sweden, the Regional Committees for Medical and Health Research Ethics in Norway, and the Ethics Committee for Human Sciences at the University of Turku in Finland. Ethics approval was not required in Denmark. All participants gave informed consent stating that their data will be anonymized before being used for research purpose and that the results will be published.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
The members of the Country Principal Investigators are mentioned in the Acknowledgements section.
References
- 1.Augustin M, Reich K, Glaeske G, Schaefer I, Radtke M. Co-morbidity and age-related prevalence of psoriasis: Analysis of health insurance data in Germany. Acta Derm Venereol. 2010;90(2):147–151. doi: 10.2340/00015555-0770. [DOI] [PubMed] [Google Scholar]
- 2.Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377–390. doi: 10.1016/j.jaad.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danielsen K, Duvetorp A, Iversen L, Østergaard M, Seifert O, Tveit KS, et al. Prevalence of psoriasis and psoriatic arthritis and patient perceptions of severity in Sweden, Norway and Denmark: Results from the Nordic Patient Survey of Psoriasis and Psoriatic Arthritis. Acta Derm Venereol. 2019;99(1):18–25. doi: 10.2340/00015555-3017. [DOI] [PubMed] [Google Scholar]
- 4.Ragnarson Tennvall G, Hjortsberg C, Bjarnason A, Gniadecki R, Heikkilä H, Jemec GB, et al. Treatment patterns, treatment satisfaction, severity of disease problems, and quality of life in patients with psoriasis in three Nordic countries. Acta Derm Venereol. 2013;93(4):442–445. doi: 10.2340/00015555-1485. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen MK, Enger M, Dahlborn AK, Juvik S, Fagerhed L, Dodge R, et al. The importance of achieving clear or almost clear skin for patients: results from the Nordic countries of the global. Acta Derm Venereol. 2019;99(2):158–163. doi: 10.2340/00015555-3048. [DOI] [PubMed] [Google Scholar]
- 6.Tveit KS, Duvetorp A, Østergaard M, Skov L, Danielsen K, Iversen L, et al. Treatment use and satisfaction among patients with psoriasis and psoriatic arthritis: results from the NORdic PAtient survey of Psoriasis and Psoriatic arthritis (NORPAPP) J Eur Acad Dermatol Venereol. 2019;33(2):340–354. doi: 10.1111/jdv.15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–244. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 8.Bożek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area and severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26(5):851–856. doi: 10.17219/acem/69804. [DOI] [PubMed] [Google Scholar]
- 9.Jankowiak B, Kowalewska B, Krajewska-Kułak E, Khvorik DF. Stigmatization and quality of life in patients with psoriasis. Dermatol Ther (Heidelb) 2020;10(2):285–296. doi: 10.1007/s13555-020-00363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jankowiak B, Kowalewska B, Krajewska-Kułak E, Khvorik DF, Niczyporuk W. Relationship between self-esteem and stigmatization in psoriasis patients. Postepy Dermatol Alergol. 2020;37(4):597–602. doi: 10.5114/ada.2020.93242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sojević Timotijević Z, Majcan P, Trajković G, Relić M, Novaković T, Mirković M, et al. The impact of changes in psoriasis area and severity index by body region on quality of life in patients with psoriasis. Acta Dermatovenerol Croat. 2017;25(3):215–222. [PubMed] [Google Scholar]
- 12.Obradors M, Blanch C, Comellas M, Figueras M, Lizan L. Health-related quality of life in patients with psoriasis: a systematic review of the European literature. Qual Life Res. 2016;25(11):2739–2754. doi: 10.1007/s11136-016-1321-7. [DOI] [PubMed] [Google Scholar]
- 13.Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014;20(8):13030/qt48r4w8h2. [PubMed]
- 14.Hesselvig JH, Egeberg A, Loft ND, Zachariae C, Kofoed K, Skov L. Correlation between dermatology life quality index and psoriasis area and severity index in patients with psoriasis treated with ustekinumab. Acta Derm Venereol. 2018;98(3):335–339. doi: 10.2340/00015555-2833. [DOI] [PubMed] [Google Scholar]
- 15.Norlin JM, Nilsson K, Persson U, Schmitt-Egenolf M. Complete skin clearance and Psoriasis Area and Severity Index response rates in clinical practice: predictors, health-related quality of life improvements and implications for treatment goals. Br J Dermatol. 2020;182(4):965–973. doi: 10.1111/bjd.18361. [DOI] [PubMed] [Google Scholar]
- 16.Pistone G, Gurreri R, Tilotta G, Caruso P, Curiale S, Bongiorno MR. Timing of quality of life improvements in psoriatic patients treated with different systemic therapies. Dermatol Ther. 2019;32(5):e13021. doi: 10.1111/dth.13021. [DOI] [PubMed] [Google Scholar]
- 17.Randa H, Lomholt JJ, Skov L, Zachariae R. Health-related quality of life in adolescents with psoriasis: an interview-based study. Br J Dermatol. 2018;178(6):1404–1411. doi: 10.1111/bjd.16326. [DOI] [PubMed] [Google Scholar]
- 18.Raval K, Lofland JH, Waters H, Piech CT. Disease and treatment burden of psoriasis: examining the impact of biologics. J Drugs Dermatol. 2011;10(2):189–196. [PubMed] [Google Scholar]
- 19.EADV Euroguiderm guideline for the treatment of psoriasis vulgaris. Syst Treat. 2021;35:281–317. doi: 10.1111/jdv.16926. [DOI] [PubMed] [Google Scholar]
- 20.Florek AG, Wang CJ, Armstrong AW. Treatment preferences and treatment satisfaction among psoriasis patients: a systematic review. Arch Dermatol Res. 2018;310(4):271–319. doi: 10.1007/s00403-018-1808-x. [DOI] [PubMed] [Google Scholar]
- 21.Maul JT, Navarini AA, Sommer R, Anzengruber F, Sorbe C, Mrowietz U, et al. Gender and age significantly determine patient needs and treatment goals in psoriasis—a lesson for practice. J Eur Acad Dermatol Venereol. 2019;33(4):700–708. doi: 10.1111/jdv.15324. [DOI] [PubMed] [Google Scholar]
- 22.Wolf P, Weger W, Legat F, Painsi C, Saxinger W, Müllegger R, et al. Quality of life and treatment goals in psoriasis from the patient perspective: results of an Austrian cross-sectional survey. J Dtsch Dermatol Ges. 2018;16(8):981–990. doi: 10.1111/ddg.13613. [DOI] [PubMed] [Google Scholar]
- 23.de Vere Hunt IJ, McNiven A, Roberts A, Parmar H, McPherson T. 'Not just a piece of skin in front of you'-a qualitative exploration of the experiences of adolescents with eczema and psoriasis with healthcare professionals. BMJ Open. 2021;11(1):e041108. doi: 10.1136/bmjopen-2020-041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newi AL, Tsianakas A, von Martial S, Sommer R, Blome C. How important is subjective well-being for patients? A qualitative interview study of people with psoriasis. Qual Life Res. 2022;31(12):3355–3363. doi: 10.1007/s11136-022-03189-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders B, Sim J, Kingstone T, Baker S, Waterfield J, Bartlam B, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52(4):1893–1907. doi: 10.1007/s11135-017-0574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Britten N. Qualitative interviews in medical research. BMJ. 1995;311(6999):251–253. doi: 10.1136/bmj.311.6999.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dicicco-Bloom B, Crabtree BF. The qualitative research interview. Med Educ. 2006;40(4):314–321. doi: 10.1111/j.1365-2929.2006.02418.x. [DOI] [PubMed] [Google Scholar]
- 28.Blome C, von Usslar K, Augustin M. Feasibility of using qualitative interviews to explore patients' treatment goals: experience from dermatology. Patient. 2016;9(3):261–269. doi: 10.1007/s40271-015-0149-5. [DOI] [PubMed] [Google Scholar]
- 29.Miles MBHAM. Qualitative data analysis: an expanded sourcebook. Thousand Oaks: Sage Publications; 1994. [Google Scholar]
- 30.Dhakal K. NVivo. J Med Libr Assoc. 2022;110(2):270–272. doi: 10.5195/jmla.2022.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaarschmidt ML, Umar N, Schmieder A, Terris DD, Goebeler M, Goerdt S, et al. Patient preferences for psoriasis treatments: impact of treatment experience. J Eur Acad Dermatol Venereol. 2013;27(2):187–198. doi: 10.1111/j.1468-3083.2011.04440.x. [DOI] [PubMed] [Google Scholar]
- 32.Belinchón I, Rivera R, Blanch C, Comellas M, Lizán L. Adherence, satisfaction and preferences for treatment in patients with psoriasis in the European Union: a systematic review of the literature. Patient Prefer Adherence. 2016;10:2357–2367. doi: 10.2147/PPA.S117006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puig L, Thom H, Mollon P, Tian H, Ramakrishna GS. Clear or almost clear skin improves the quality of life in patients with moderate-to-severe psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2017;31(2):213–220. doi: 10.1111/jdv.14007. [DOI] [PubMed] [Google Scholar]
- 34.Schaarschmidt ML, Herr R, Gutknecht M, Wroblewska K, Gerdes S, Sticherling M, et al. Patients' and physicians' preferences for systemic psoriasis treatments: a Nationwide Comparative Discrete Choice Experiment (PsoCompare) Acta Derm Venereol. 2018;98(2):200–205. doi: 10.2340/00015555-2834. [DOI] [PubMed] [Google Scholar]
- 35.Blome C, Gosau R, Radtke MA, Reich K, Rustenbach SJ, Spehr C, et al. Patient-relevant treatment goals in psoriasis. Arch Dermatol Res. 2016;308(2):69–78. doi: 10.1007/s00403-015-1613-8. [DOI] [PubMed] [Google Scholar]
- 36.Gorelick J, Shrom D, Sikand K, Renda L, Burge R, Dworkin C, et al. Understanding treatment preferences in patients with moderate to severe plaque psoriasis in the USA: results from a Cross-Sectional Patient Survey. Dermatol Ther (Heidelb) 2019;9(4):785–797. doi: 10.1007/s13555-019-00334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strohal R, Prinz JC, Girolomoni G, Nast A. A patient-centred approach to biological treatment decision making for psoriasis: an expert consensus. J Eur Acad Dermatol Venereol. 2015;29(12):2390–2398. doi: 10.1111/jdv.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rigopoulos D, Ioannides D, Chaidemenos G, Kallidis P, Voultsidou A, Matekovits A, et al. Patient preference study for different characteristics of systemic psoriasis treatments (Protimisis) Dermatol Ther. 2018;31(3):e12592. doi: 10.1111/dth.12592. [DOI] [PubMed] [Google Scholar]
- 39.Torbica A, Fattore G, Ayala F. Eliciting preferences to inform patient-centred policies: the case of psoriasis. Pharmacoeconomics. 2014;32(2):209–223. doi: 10.1007/s40273-013-0126-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.