Abstract
Introduction
Alopecia areata (AA) is an autoimmune disease with an underlying immuno-inflammatory pathogenesis. Treatments can include systemic corticosteroids and immunomodulators (such as Janus kinase inhibitors); these medications may be associated with a risk of some adverse events. However, large-scale observational studies of baseline incidence rates (IRs) of infection, cardiovascular disease, malignancy, and thromboembolism in US patients with AA, including those with alopecia totalis or alopecia universalis (AT/AU), are limited. This real-world, US claims-based study aimed to estimate the incidence of events in patients with AA compared with matched patients without AA.
Methods
Patients aged ≥ 12 years enrolled in the Optum Clinformatics Data Mart database from 1 October 2016 to 30 September 2020, with ≥ 2 AA diagnosis codes were included in the AA cohort. Patients without AA were age-, sex-, and race-matched 3:1 to patients with AA. Baseline comorbidities were evaluated during the 12-month period pre-index date. Incident cases of serious/herpes infections, malignancies, major adverse cardiovascular events (MACE), and thromboembolic events were evaluated post-index date. Data are presented using descriptive statistics, proportional percentages, frequencies, and IRs (calculated with 95% CI).
Results
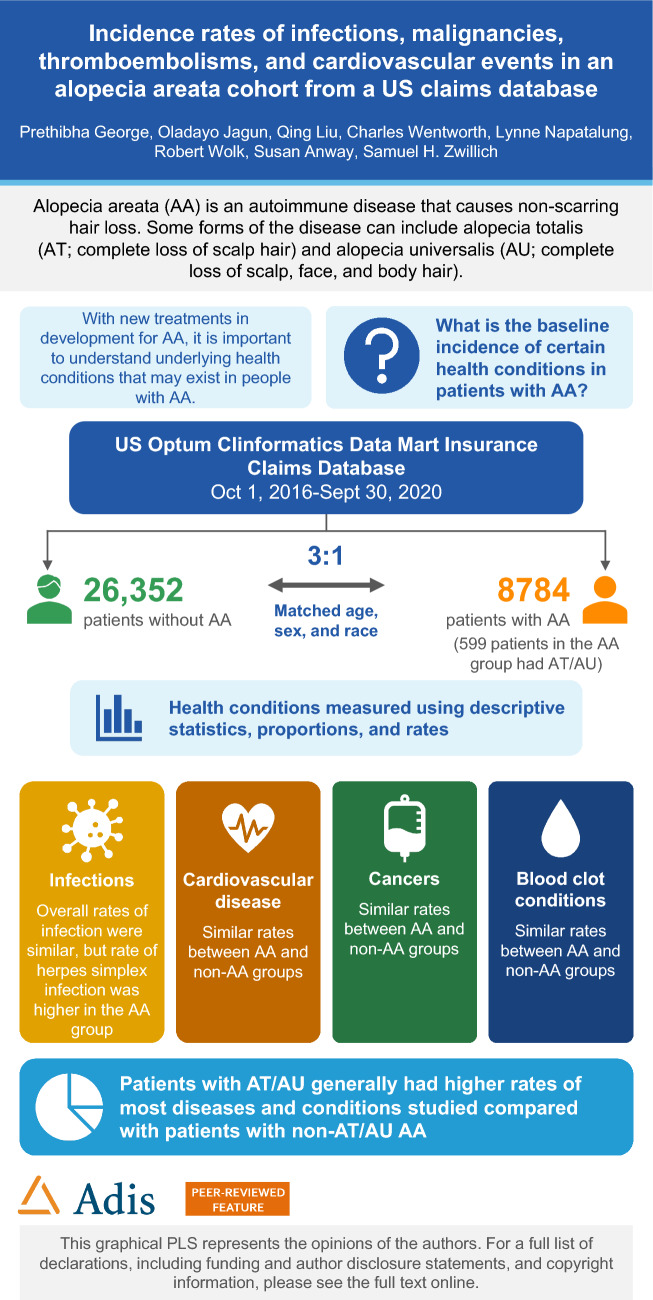
Overall, 8784 patients with AA, 599 of whom had AT/AU, were matched to 26,352 patients without AA. IRs per 1000 person-years among the AA and non-AA cohorts, respectively, were 18.5 and 20.6 for serious infections, 19.5 and 9.7 for herpes simplex infections, 7.8 and 7.6 for herpes zoster infections, 12.5 and 11.6 for primary malignancies, 16.0 and 18.1 for MACE, and 4.9 and 6.1 for venous thromboembolisms. Compared with patients with non-AT/AU AA, patients with AT/AU largely had higher IRs for most baseline comorbidities and outcome events evaluated.
Conclusion
Patients with AA had a higher IR of herpes simplex infection than the matched non-AA cohort. Patients with AT/AU generally had higher rates of outcome events than patients without AT/AU.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-023-00937-9.
Keywords: Alopecia areata, Alopecia totalis, Alopecia universalis, Infection, MACE, Malignancies, Safety, US claims
Key Summary Points
| Why carry out this study? |
| Alopecia areata (AA) can be treated with systemic corticosteroids and immunomodulators (such as Janus kinase inhibitors), and these medications may be associated with a risk of some adverse events, such as infections, cardiovascular disease, malignancies, and thromboembolisms. |
| Studies of the baseline incidence rates of infections, cardiovascular events, malignancies, and thromboembolic events in US patients with AA, including subtypes alopecia totalis or alopecia universalis (AT/AU), have been inconsistent or lacking. |
| This real-world, US claims-based study aimed to estimate the incidence of baseline comorbidities and outcome events in patients with AA compared with matched patients without AA. |
| What was learned from the study? |
| Patients with AA had a greater incidence of herpes simplex infection than those in the matched non-AA cohort, while other outcomes, including cardiovascular disease, malignancy, and thromboembolism, had overall similar rates between cohorts; patients with the AT/AU subtypes generally had higher rates of outcome events than those without the AT/AU subtypes. |
| These data may provide greater understanding of the comorbid disease burden in patients with AA. |
Digital Features
This article is published with digital features, including a graphical abstract to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.22843514.
Introduction
Alopecia areata (AA) is an autoimmune disease that has an underlying immuno-inflammatory pathogenesis leading to nonscarring loss of scalp, face, and/or body hair [1]. AA can be treated with systemic corticosteroids and immunomodulators (such as Janus kinase [JAK] inhibitors). These medications may be associated with a higher risk of some adverse events, including infection, cardiovascular disease, malignancy, and thromboembolism [2]. As such, understanding the baseline risk of these outcomes in a population with the disease is important [1]. Prior data are inconsistent or lacking regarding the incidence of infection, cardiovascular disease, malignancy, and thromboembolism among patients with AA [3–8].
Current off-label treatment options for AA (including subtypes alopecia totalis [AT; complete loss of scalp hair] or alopecia universalis [AU; complete loss of scalp, face, and body hair]) [9, 10] include topical, intralesional, and systemic corticosteroids; topical calcineurin inhibitors, prostaglandin analogs, and minoxidil; systemic cyclosporine, methotrexate, and azathioprine; and phototherapy [11, 12]. However, these are often ineffective or incur unwanted adverse effects, especially for long-term treatment of chronic AA or AT/AU [13]. Recently, baricitinib, an oral JAK1/2 inhibitor, received US Food and Drug Administration approval for AA treatment in adults [14]. Additional JAK inhibitors such as deuruxolitinib (JAK1/2 inhibitor), jaktinib (JAK1/2/3 inhibitor), and ivarmacitinib (JAK1 inhibitor), as well as ritlecitinib, an oral, selective inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) family kinases, are currently under investigation [2, 15–18]. Owing to their effects on the immune system, inhibitors of the JAK pathways have been associated with increased risks of certain adverse events, including infection, cardiovascular disease, malignancy, and thromboembolism [2]; however, it is unclear whether all JAK inhibitors carry these risks. To better understand the impact of AA treatments on safety profiles of treated patients, baseline rates of outcome events in large cohorts of patients with AA must be established. However, large-scale observational studies of the incidence rates (IRs) of infections, malignancies, cardiovascular events, and arterial and venous thromboembolic events associated with patients with AA (including the subtypes AT/AU) representative of the US population have been lacking, and comparisons with matched cohorts without AA are limited.
This study aimed to estimate the baseline IRs for outcome events of interest to the ritlecitinib AA clinical program (serious infections, herpes zoster [HZ] infections, herpes simplex infections, malignancies [excluding nonmelanoma skin cancer (NMSC)] and NMSC [defined as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC)], major adverse cardiovascular events [MACE], and thromboembolic events) in patients ≥ 12 years of age with AA in a real-world setting. The US Optum Clinformatics Data Mart (CDM) claims database was used to contextualize these events in a cohort of patients with AA (stratified by subtypes AT/AU and non-AT/AU) and a matched cohort of patients without AA.
Methods
Study Design and Data Source
This retrospective cohort study used medical and pharmaceutical claims from US administrative claims data in the Optum CDM from 1 October 2016 to 30 September 2020. The Optum CDM is a database comprising administrative health claims for Medicare Advantage and large commercial health plan members (with the exclusion of Medicare Fee for Service). All records were de-identified in compliance with the Health Insurance Portability and Accountability Act; therefore, informed consent from patients was not needed or obtained, and approval from an institutional review board was not required.
Patient Population
The inclusion criteria were enrollment in the database from 1 October 2016 to 30 September 2020; ≥ 12 years of age at date of cohort entry (defined as either the date when the 365 days of continuous enrollment was satisfied or 1 October 2016, whichever was later. The earliest possible cohort entry date was 1 October 2016); ≥ 365 continuous days of enrollment prior to cohort entry and ≥ 180 days of follow-up after index date (defined as date of first AA diagnosis for the AA cohort and date of cohort entry for the non-AA cohort); and no missing data for date of birth, sex, or race at cohort entry [19].
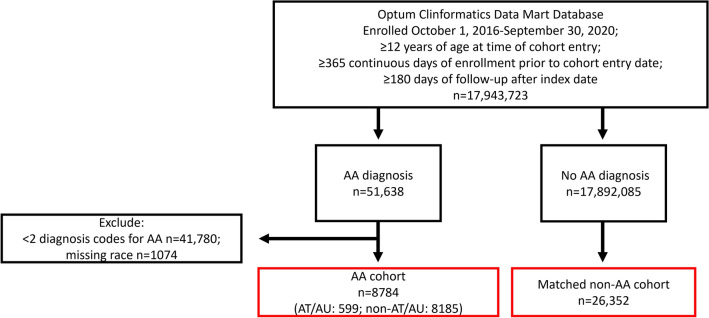
The study population consisted of patients with AA (AA cohort), who were identified as having ≥ 2 International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes for AA (L63.x) with separate visit dates of any length apart. Patients with AA with a diagnosis of alopecia of other etiology (traction and scarring alopecia ICD-10 codes such as L65 [other nonscarring hair loss] or L66 [cicatricial alopecia (scarring hair loss]) during the study period were excluded. Patients with AA were subdivided as either AT/AU (ICD-10 codes L63.0 or L63.1) or non-AT/AU AA (all other AA codes). Three patients without an AA diagnosis during the study period were age, sex, and race matched to one patient in the AA cohort. A diagnosis of androgenic alopecia was permitted in either cohort. Patients in both cohorts were followed up from the index date until the earliest of the following events: end of the study period (30 September 2020), death, dropout from the Optum CDM, or occurrence of endpoint of interest.
Study Variables
Demographic variables recorded at the index date were calendar year, sex, age, race and ethnicity, US geographic region, and insurance type. Baseline comorbidities and history of prescription medication use were ascertained if recorded during the 12-month pre-index period.
The outcome events of interest were estimated if they occurred after the index date. These included infections (serious, HZ, or herpes simplex infection); any primary malignancy (except NMSC), BCC, SCC, cervical carcinoma in situ, malignant melanoma, lymphoma, and female breast cancer; MACE, deep vein thrombosis (DVT), pulmonary embolism (PE), and arterial thromboembolism (ATE); and all-cause death. MACE were defined as a composite measure comprising cardiovascular death (based on cause-of-death data), acute myocardial infarction (MI), unstable angina, any stroke, hospitalization due to heart failure, or coronary revascularization (percutaneous intervention [PCI] or coronary artery bypass graft [CABG]).
Statistical Analysis
Descriptive statistics were used to define patient characteristics, while proportional percentages and frequencies were used to describe categorical variables. Data analysis was performed with statistical software SAS version 9.4 or higher. The incidence proportions (IPs) of outcome events were defined as the number of patients with an event divided by the total number of patients at risk for the respective cohort. The IRs of outcome events such as infections were defined as the number of incident event cases divided by patient-years at risk for the respective cohort and were calculated with 95% CIs.
When there were multiple records of the same outcome event, only the first record of a given event was used in computing IRs. Different outcome events did not censor each other’s follow-up. For MACE outcome analysis, known prior heart disease that is a component of the current definition of MACE or any type of stroke was excluded. This was done for each MACE component, thus ensuring the same population at risk for overall MACE events and all of its components.
Results
Study Population and Demographics
In total, 17,943,723 patients were enrolled in the Optum CDM database from 1 October 2016 to 30 September 2020; were ≥ 12 years of age at the date of the cohort; had ≥ 365 continuous days of enrollment prior to cohort entry and ≥ 180 days of follow-up after index date; and had no missing data for date of birth or sex at cohort entry. The study population consisted of 8784 patients with AA (6.8% AT/AU, 93.2% non-AT/AU) matched to 26,352 patients without AA (Fig. 1; Table 1) [19]. The mean age of the study population was 45.6 years, and 55.6% were female. A total of 59.2% of patients were White, 20.3% were Hispanic, 10.2% were Black, and 10.2% were Asian. Patient insurance status included either commercial insurance (80.9% versus 79.1% for the AA and non-AA cohorts, respectively) or Medicare (19.2% versus 20.9% for the AA and non-AA cohorts, respectively). The mean age for patients with AA in the AT/AU and non-AT/AU subtypes was 53.3 and 45.0 years, respectively. A total of 70.6% and 54.5% of patients in the AT/AU and non-AT/AU AA subtypes were female, respectively; 70.8% and 58.4% of patients in the AT/AU and non-AT/AU AA subtypes were White, respectively; and 65.4% of patients in the AT/AU subtype had commercial insurance, while 34.6% had Medicare coverage.
Fig. 1.
Study design flow chart. AA alopecia areata, AT alopecia totalis, AU alopecia universalis
Table 1.
Demographic characteristics at index date in the AA and matched non-AA cohorts
| Characteristic | AA cohort (n = 8784) | Non-AA cohort (n = 26,352) | AT/AU subtype (n = 599) | Non-AT/AU subtype (n = 8185) |
|---|---|---|---|---|
| AA subtype, n (%) | ||||
| AT/AU | 599 (6.8) | – | 599 (100) | – |
| Non-AT/AU | 8185 (93.2) | – | – | 8185 (100) |
| Age | ||||
| Mean (SD), y | 45.6 (17.9) | 45.6 (17.9) | 53.3 (18.8) | 45.0 (17.7) |
| Sex, n (%) | ||||
| Male | 3904 (44.4) | 11,712 (44.4) | 176 (29.4) | 3728 (45.5) |
| Female | 4880 (55.6) | 14,640 (55.6) | 423 (70.6) | 4457 (54.5) |
| Race/ethnicity, n (%) | ||||
| Asian | 900 (10.2) | 2700 (10.2) | 34 (5.7) | 866 (10.6) |
| Black | 894 (10.2) | 2682 (10.2) | 56 (9.3) | 838 (10.2) |
| Hispanic | 1787 (20.3) | 5361 (20.3) | 85 (14.2) | 1702 (20.8) |
| White | 5203 (59.2) | 15,609 (59.2) | 424 (70.8) | 4779 (58.4) |
| US region, n (%) | ||||
| Midwest | 1635 (18.6) | 5994 (22.7) | 116 (19.4) | 1519 (18.6) |
| Northeast | 1503 (17.1) | 2749 (10.4) | 83 (13.9) | 1420 (17.3) |
| South | 3668 (41.8) | 10,830 (41.1) | 280 (46.7) | 3388 (41.4) |
| West | 1947 (22.2) | 5913 (22.4) | 119 (19.9) | 1828 (22.3) |
| Unknown | 31 (0.4) | 866 (3.3) | 1 (0.2) | 30 (0.4) |
| Insurance type, n (%) | ||||
| Commercial | 7102 (80.9) | 20,836 (79.1) | 392 (65.4) | 6710 (82.0) |
| Medicare Advantage | 1685 (19.2) | 5497 (20.9) | 207 (34.6) | 1478 (18.1) |
AA alopecia areata, AT alopecia totalis, AU alopecia universalis
Baseline Comorbidities
The proportions of baseline comorbidities in the AA versus non-AA cohorts, respectively, were 25.4% versus 24.3% for hypertension, 9.1% versus 10.6% for diabetes mellitus, 26.9% versus 23.9% for hyperlipidemia, 4.7% versus 3.5% for having never smoked, and 11.4% versus 9.7% for obesity (Table 2). The proportions of baseline comorbidities in patients with AA in the AT/AU and non-AT/AU subtypes, respectively, were 39.1% versus 24.4% for hypertension, 13.5% versus 8.8% for diabetes mellitus, 39.2% versus 26.0% for hyperlipidemia, 4.3% versus 4.8% for having never smoked, and 17.2% versus 10.9% for obesity. The proportion of patients with any prior primary malignancy was 4.2% for the AA cohort and 3.5% for the matched non-AA cohort. The proportion of patients with any prior primary malignancy was 8.3% for patients with AT/AU and 3.9% for patients with non-AT/AU AA. The proportion of patients with a history of prescriptions for systemic steroids was 42.8% for patients with AA and 24.3% for the matched non-AA cohort (Table 3). The proportion of patients with a history of prescriptions for immunomodulators was 7.6% for the AA cohort and 4.1% for the non-AA cohort. The proportion of patients with a history of prescriptions for systemic steroids was 45.6% for patients with AT/AU and 42.6% for patients with non-AT/AU AA. The proportion of patients with a history of prescriptions for immunomodulators was 11.7% for patients with AT/AU and 7.3% for patients with non-AT/AU AA.
Table 2.
Baseline comorbidities and history of surgical procedures in the AA and matched non-AA cohorts
| History of comorbid conditions, n (%) | AA cohort (n = 8784) | Non-AA cohort (n = 26,352) | AT/AU subtype (n = 599) | Non-AT/AU subtype (n = 8185) |
|---|---|---|---|---|
| Hypertension | 2233 (25.4) | 6409 (24.3) | 234 (39.1) | 1999 (24.4) |
| Diabetes mellitus | 802 (9.1) | 2791 (10.6) | 81 (13.5) | 721 (8.8) |
| Hyperlipidemia | 2364 (26.9) | 6301 (23.9) | 235 (39.2) | 2129 (26.0) |
| Smoking | ||||
| Current | 754 (8.6) | 2269 (8.6) | 62 (10.4) | 692 (8.5) |
| Previous | 38 (0.4) | 157 (0.6) | 4 (0.7) | 34 (0.4) |
| Never | 416 (4.7) | 932 (3.5) | 26 (4.3) | 390 (4.8) |
| Unknown | 27 (0.3) | 48 (0.2) | 1 (0.2) | 26 (0.3) |
| Substance abuse | 228 (2.6) | 601 (2.3) | 18 (3.0) | 210 (2.6) |
| Obesity | ||||
| Yes | 999 (11.4) | 2556 (9.7) | 103 (17.2) | 896 (10.9) |
| COPD | 275 (3.1) | 832 (3.2) | 51 (8.5) | 224 (2.7) |
| Any primary malignancya | 373 (4.2) | 919 (3.5) | 50 (8.3) | 323 (3.9) |
| BCC | 87 (1.0) | 238 (0.9) | 8 (1.3) | 79 (1.0) |
| SCC | 52 (0.6) | 119 (0.5) | 10 (1.7) | 42 (0.5) |
| Cervical carcinoma in situ | 6 (0.1) | 20 (0.1) | 0 | 6 (0.1) |
| Malignant melanoma | 16 (0.2) | 47 (0.2) | 1 (0.2) | 15 (0.2) |
| Lymphoma | 29 (0.3) | 50 (0.2) | 3 (0.5) | 26 (0.3) |
| Female breast cancer | 78 (0.9) | 191 (0.7) | 14 (2.3) | 64 (0.8) |
| Acute myocardial infarction | 42 (0.5) | 108 (0.4) | 8 (1.3) | 34 (0.4) |
| Unstable angina | 15 (0.2) | 61 (0.2) | 2 (0.3) | 13 (0.2) |
| Ischemic stroke | 17 (0.2) | 42 (0.2) | 1 (0.2) | 16 (0.2) |
| Hemorrhagic stroke | 2 (< 0.1) | 6 (< 0.1) | 1 (0.2) | 1 (0) |
| Hospitalization due to heart failure | 64 (0.7) | 172 (0.7) | 9 (1.5) | 55 (0.7) |
| Coronary revascularization by PCI or CABG | 22 (0.3) | 76 (0.3) | 2 (0.3) | 20 (0.2) |
| DVT | 60 (0.7) | 133 (0.5) | 10 (1.7) | 50 (0.6) |
| PE | 28 (0.3) | 83 (0.3) | 4 (0.7) | 24 (0.3) |
| DVT or PE | 76 (0.9) | 188 (0.7) | 12 (2.0) | 64 (0.8) |
| ATE | 7 (0.1) | 27 (0.1) | 0 | 7 (0.1) |
| DVT or PE or ATE | 81 (0.9) | 208 (0.8) | 12 (2.0) | 69 (0.8) |
| History of any surgery in the last 6 months prior to index date | 117 (1.3) | 353 (1.3) | 17 (2.8) | 100 (1.2) |
AA alopecia areata, AT alopecia totalis, ATE arterial thromboembolism, AU alopecia universalis, BCC basal cell carcinoma, CABG coronary artery bypass graft, COPD chronic obstructive pulmonary disease, DVT deep vein thrombosis, PCI percutaneous coronary intervention, PE pulmonary embolism, SCC squamous cell carcinoma
aExcluding nonmelanoma skin cancer
Table 3.
History of prescription medicine use in the AA and matched non-AA cohortsa
| Characteristic, n (%) | AA cohort (n = 8784) | Non-AA cohort (n = 26,352) | AT/AU subtype (n = 599) | Non-AT/AU subtype (n = 8185) |
|---|---|---|---|---|
| Janus kinase inhibitorsb | 13 (0.1) | 9 (< 0.1) | 3 (0.5) | 10 (0.1) |
| Other drugs used in dermatologyc | 111 (1.3) | 112 (0.4) | 8 (1.3) | 103 (1.3) |
| Systemic steroids | 3761 (42.8) | 6414 (24.3) | 273 (45.6) | 3488 (42.6) |
| Immunomodulators | 666 (7.6) | 1075 (4.1) | 70 (11.7) | 596 (7.3) |
| Antidiabetic agents | 569 (6.5) | 2075 (7.9) | 52 (8.7) | 517 (6.3) |
| Lipid-modifying agents | 1328 (15.1) | 3912 (14.8) | 122 (20.4) | 1206 (14.7) |
| Antihypertensive agents | 2055 (23.4) | 5693 (21.6) | 199 (33.2) | 1856 (22.7) |
| Hormone replacement therapy | 79 (0.9) | 152 (0.6) | 5 (0.8) | 74 (0.9) |
| Oral contraceptives | 633 (7.2) | 1298 (4.9) | 44 (7.3) | 589 (7.2) |
AA alopecia areata, AT alopecia totalis, AU alopecia universalis
aHistory of prescription medications was determined during the 12 months prior to the index date
bTofacitinib, baricitinib, upadacitinib, filgotinib, ruxolitinib
cAlefacept, certolizumab, etanercept, adalimumab, infliximab, golimumab, ustekinumab, apremilast, secukinumab, tildrakizumab, brodalumab, risankizumab, ixekizumab, guselkumab, dupilumab
Outcome Events
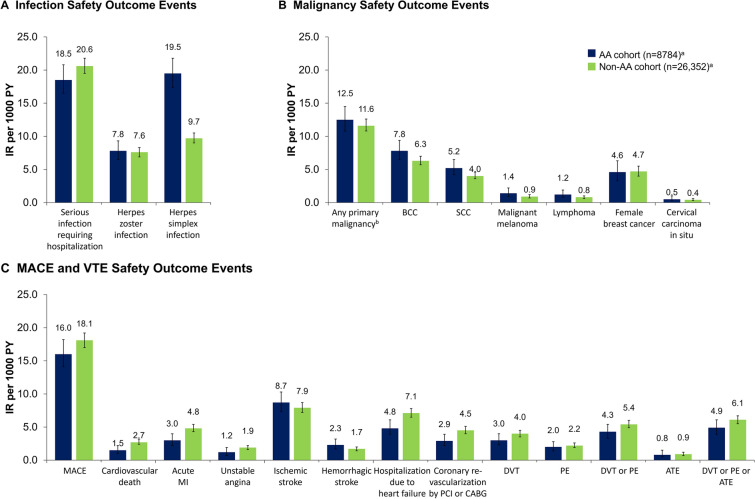
In the AA cohort, the IRs for infections ranged from 7.8 per 1000 person-years (PY) (95% CI 6.5–9.3; IP, 1.4%) for HZ infections to 19.5 (95% CI 17.4–21.8; IP, 3.3%) for herpes simplex infections. Similar rates of infections were seen in the non-AA cohorts, except for herpes simplex infection, which was lower than in the AA cohort (IR, 9.7; 95% CI 9.0–10.5; IP, 2.2%; Fig. 2A, Supplementary Table S1).
Fig. 2.
Incidence rates per 1000 PY for A infection, B malignancy, and C MACE/VTE outcome events of interest in the AA and matched non-AA cohorts. aThe total number of patients at risk (i.e., denominator) differed depending on whether the outcome event was acute or chronic (including all malignancy events, MACE, DVT, PE, and ATE). Therefore, the denominator was not always 8784 for the AA cohort or 26,352 for the non-AA cohort. bExcluding nonmelanoma skin cancer. AA alopecia areata, ATE arterial thromboembolism, BCC basal cell carcinoma, CABG coronary artery bypass graft, DVT deep vein thrombosis, IR incidence rate, MACE major adverse cardiovascular event, MI myocardial infarction, PCI percutaneous coronary intervention, PE pulmonary embolism, PY patient-years, SCC squamous cell carcinoma, VTE venous thromboembolic event
The IP of any primary malignancy (excluding NMSC) in the AA cohort was 2.2% (IR, 12.5; 95% CI 10.8–14.5); in the non-AA cohort, the IP was 2.7% (IR, 11.6; 95% CI 10.8–12.6; Fig. 2B, Supplementary Table S1). Specific cancer IRs in the AA cohort were 7.8 (95% CI 6.5–9.4; IP, 1.4%) and 5.2 (95% CI 4.2–6.5; IP, 0.9%) for BCC and SCC, respectively, while the other cancer IRs were 1.4 (95% CI 0.9–2.2; IP, 0.3%) for malignant melanoma, 1.2 (95% CI 0.8–1.9; IP, 0.2%) for lymphoma, and 4.6 (95% CI 3.3–6.3; IP, 0.8%) for female breast cancer. In the non-AA cohort, IRs for BCC and SCC were 6.3 (95% CI 5.7–7.0; IP, 1.5%) and 4.0 (95% CI 3.6–4.6; IP, 0.9%), respectively; 0.9 (95% CI 0.7–1.2, IP, 0.2%) for malignant melanoma; 0.8 (95% CI 0.6–1.0; IP, 0.2%) for lymphoma; and 4.7 (95% CI 4.0–5.5; IP, 1.1%) for female breast cancer.
The overall MACE IR was 16.0 (95% CI 14.1–18.2; IP, 2.8%) for the AA cohort and 18.1 (95% CI 17.0–19.2; IP, 4.1%) for the non-AA cohort (Fig. 2C, Supplementary Table S1). The IR for occurrence of any thromboembolic event was 4.9 (95% CI 3.9–6.1; IP, 0.9%) for the AA cohort and 6.1 (95% CI 5.5–6.7; IP, 1.4%) for the non-AA cohort.
When stratified by age, adolescent patients aged 12–17 years in the AA and non-AA cohorts showed few or no cases of most outcome events studied (Supplementary Table S2); however, the 398 adolescent patients in the AA cohort represented only 4.5% of the total AA cohort. Adult patients aged 18–50 years and ≥ 51 years in the AA cohorts and non-AA cohorts had IRs that were generally consistent with the results for the total cohorts (Supplementary Tables S3, S4).
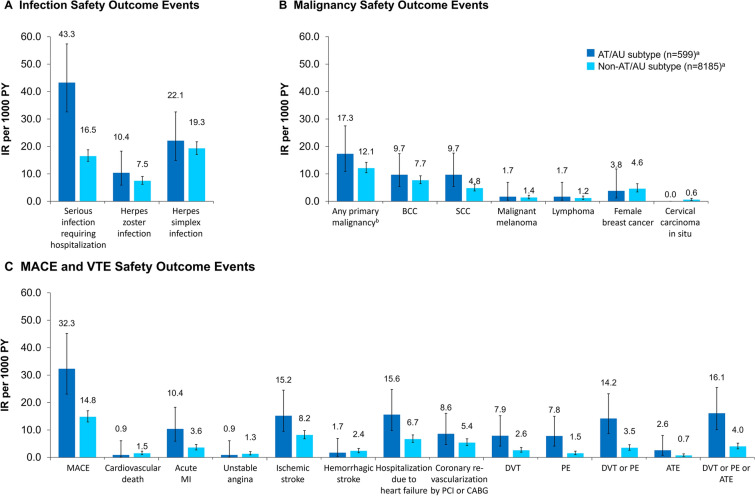
Generally, patients with AT/AU had higher IRs of events compared with patients with non-AT/AU AA, including serious infection requiring hospitalization, HZ infection, herpes simplex infection, any primary malignancy (except female breast cancer or cervical carcinoma in situ), BCC, SCC, malignant melanoma, lymphoma, MACE, acute MI, ischemic stroke, hospitalization due to heart failure, coronary revascularization by PCI or CABG, any thromboembolic event, and all-cause death (Fig. 3, Supplementary Table S5). Events with the highest IRs among patients with AT/AU were serious infection requiring hospitalization (43.3; 95% CI 32.6–57.4 per 1000 PY) and MACE (32.3; 95% CI 23.1–45.2 per 1000 PY).
Fig. 3.
Incidence rates per 1000 PY for A infection, B malignancy, and C MACE/VTE outcome events of interest in patients with AT/AU and non-AT/AU AA. aThe total number of patients at risk (i.e., denominator) differed depending on whether the outcome event was chronic or acute. Therefore, the denominator was not always 599 for the AT/AU subtype or 8185 for the non-AT/AU subtype cohort. bExcluding nonmelanoma skin cancer. AT alopecia totalis, ATE arterial thromboembolism, AU alopecia universalis, BCC basal cell carcinoma, CABG coronary artery bypass graft, DVT deep vein thrombosis, IR incidence rate, MACE major adverse cardiovascular event, MI myocardial infarction, PCI percutaneous coronary intervention, PE pulmonary embolism, PY patient-years, SCC squamous cell carcinoma, VTE venous thromboembolic event
Discussion
This large, retrospective, US-based cohort study found that, compared with age-, sex-, and race-matched non-AA patients, patients with AA aged ≥ 12 years generally had similar IRs of outcome events after diagnosis with AA, with the exception of higher IR for herpes simplex infections. Higher rates of herpes simplex infection in patients with AA may be due to a higher baseline usage of immunosuppressants.
IRs for overall and individual malignancies were low, and there were no differences in malignancy IRs between patients with AA and matched members of the non-AA cohort. Prior studies examining the IRs of malignancies in patients with AA have also generally shown an absence of difference in number of malignancies [20]. One study in Taiwan found significantly lower rates of uterine and cervical cancer but higher risk of breast cancer in patients with AA aged < 50 years compared with matched controls without AA [21].
The overall MACE IR was 16.0 (95% CI 14.1–18.2; IP, 2.8%) for the AA cohort and 18.1 (95% CI 17.0–19.2; IP, 4.1%) for the non-AA cohort. The IR for occurrence of any thromboembolic event was 4.9 (95% CI 3.9–6.1; IP, 0.9%) for the AA cohort and 6.1 (95% CI 5.5–6.7; IP, 1.4%) for the non-AA cohort. To our knowledge, this is the first study to compare the rates of MACE in patients with AA as a composite event of stroke, MI, and cardiovascular death. Compared with those in the matched non-AA cohort, patients with AA had similar IRs for components of MACE. Globally, the reported impact of AA on the individual components of MACE has been inconsistent. One study from Taiwan found that the IR for hemorrhagic stroke was 1.01 and 0.42 per 1000 PY for patients with AA and those in the matched cohort without AA, respectively, while rates of ischemic stroke were 2.89 versus 1.69 per 1000 PY, respectively [4]. In a US regional study, patients with AA had decreased odds for incident stroke (odds ratio, 0.39; 95% CI 0.18–0.87) and a trend toward decreased risk of incident acute MI (odds ratio, 0.91; 95% CI 0.59–1.39) [22]. Another US-based study found no significant increase in the risk of heart disease in patients with mild AA (odds ratio, 1.17; 95% CI 0.93–1.48) [8].
Compared with those in the matched non-AA cohort, patients with AA had similar IRs for VTEs. In the US general population, the annual IRs of DVT and PE have been reported as 45–117 and 45–58 cases per 100,000 PY, respectively [3, 23]. One US cohort study reported an IR of 94 per 100,000 PY for VTEs in patients with AA [7]. Differences in IRs for MACE and thromboembolic events between studies may reflect differences in patient populations between regions and varied inclusion criteria for patients with AA.
When stratified by age groups, few events were recorded for adolescent patients, but adolescent patients represented only < 5% of all patients in the AA cohort, thus limiting any interpretation of the results for this age group.
This study also evaluated the underlying differences in comorbidities and outcome events in patients with AT/AU versus those with non-AT/AU AA. Generally, patients with AT/AU had a higher incidence of baseline comorbidities, risk factors, and outcome events than patients with non-AT/AU AA, although it cannot be excluded that patients with AT/AU may see doctors more frequently due to the severity of their AA [24, 25] and thus have a greater probability of being diagnosed with other comorbidities. Future studies should examine a potential mechanistic link between more severe AA and comorbidities, as well as study larger cohorts of patients with AT/AU than the 599 examined in this study. The higher rates of certain baseline comorbidities and outcome events in patients with the AT/AU subtypes suggest that it may be beneficial to screen for such conditions upon AA diagnosis, which may allow opportunities for earlier treatment intervention.
This study had some limitations related to the inherent design of a retrospective database analysis. First, owing to the usage of the Optum CDM, this study was limited to patients with AA who had insurance through large commercial or Medicare Advantage health plans and may not reflect the underlying comorbidities of those covered under other types of insurance or those without coverage. Second, patients in the non-AT/AU AA cohort may have had total loss of hair on the scalp, face, and/or body but did not have AT/AU diagnostic codes recorded in their claims record. Third, there were differences in the age and sex distributions between the AT/AU and non-AT/AU AA subtype cohorts, which may have contributed to differences in the incidence of events of interest. Finally, descriptive statistics were used for this study, and future analyses will be needed to adjust for other potential confounding factors.
Regardless of these limitations, this study leveraged large cohorts, used a strict inclusion criterion of ≥ 2 AA diagnosis codes for cohort entry, and specifically examined a population of patients with AT/AU. In addition, this is the first study to evaluate the real-world burden of malignancies, cardiovascular events, and VTEs in US patients with AA compared with matched controls.
Conclusions
In this real-world, US claims-based, retrospective cohort study of outcome events, including infection, cardiovascular disease, malignancy, and thromboembolism, patients with AA had a greater incidence of herpes simplex infection than those in the matched non-AA cohort, while other outcomes, including cardiovascular disease, malignancies, and thromboembolism, had overall similar rates between cohorts. Patients with the AT/AU subtypes generally had higher rates of outcome events than those with AA without the AT/AU subtypes. These data may inform greater understanding of the comorbid disease burden in patients with AA. Future research is needed to explore the mechanisms behind increased risks for outcome events in patients with AT/AU.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study and the Rapid Service fees were funded by Pfizer.
Medical Writing and/or Editorial Assistance
Medical writing support for the development of this publication was provided by Carolyn Maskin, PhD, of Health Interactions, Inc., and was funded by Pfizer.
Author Contributions
Prethibha George, Oladayo Jagun, Qing Liu, Lynne Napatalung, Robert Wolk, Susan Anway, and Samuel H. Zwillich conceived and designed the study. Qing Liu and Charles Wentworth participated in acquisition of the data. Prethibha George, Oladayo Jagun, Qing Liu, Charles Wentworth, Lynne Napatalung, Robert Wolk, Susan Anway, and Samuel H. Zwillich drafted the manuscript, and revised it critically for important intellectual content. Qing Liu and Charles Wentworth performed statistical analysis of the data.
Disclosures
At the time of the study, Prethibha George, Charles Wentworth, and Susan Anway were employees of Pfizer, Inc., and may hold stock or stock options in Pfizer, Inc. Prethibha George, Charles Wentworth, and Susan Anway have no current affiliations. Oladayo Jagun, Qing Liu, Lynne Napatalung, Robert Wolk, and Samuel H. Zwillich are employees of and may hold stock or stock options in Pfizer Inc. The study population and inclusion criteria were similar to a previous analysis that investigated the prevalence of AA and the IRs of autoimmune and inflammatory diseases and mental health conditions (10.1111/1346-8138.16839).
Compliance with Ethics Guidelines
All database records were de-identified in compliance with the Health Insurance Portability and Accountability Act; therefore, informed consent from patients was not needed or obtained, and approval from an institutional review board was not required.
Data Availability
The data used and analyzed in the current study are available from OptumLabs through the OptumLabs Data Warehouse. Restrictions apply to the availability of these data, which were used under license for the current study and therefore are not publicly available. However, the datasets analyzed during the current study are available from the corresponding author upon reasonable request and with permission from OptumLabs.
References
- 1.Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366(16):1515–1525. doi: 10.1056/NEJMra1103442. [DOI] [PubMed] [Google Scholar]
- 2.Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76(4):736–744. doi: 10.1016/j.jaad.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Kang JH, Lin HC, Kao S, Tsai MC, Chung SD. Alopecia areata increases the risk of stroke: a 3-year follow-up study. Sci Rep. 2015;26(5):11718. doi: 10.1038/srep11718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang KP, Mullangi S, Guo Y, Qureshi AA. Autoimmune, atopic, and mental health comorbid conditions associated with alopecia areata in the United States. JAMA Dermatol. 2013;149(7):789–794. doi: 10.1001/jamadermatol.2013.3049. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Lee H, Lee CH, Lee WS. Comorbidities in alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80(2):466–477.e416. doi: 10.1016/j.jaad.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Schneeweiss MC, Kim SC, Wyss R, Jin Y, Chin K, Merola JF, et al. Incidence of venous thromboembolism in patients with dermatologist-diagnosed chronic inflammatory skin diseases. JAMA Dermatol. 2021;157(7):805–816. doi: 10.1001/jamadermatol.2021.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H, Kim YC, Choi JW. Alopecia areata is not a risk factor for heart diseases: A 10-year retrospective cohort study. PLoS ONE. 2021;16(5):e0250216. doi: 10.1371/journal.pone.0250216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hordinsky MK. Overview of alopecia areata. J Investig Dermatol Symp Proc. 2013;16(1):S13–15. doi: 10.1038/jidsymp.2013.4. [DOI] [PubMed] [Google Scholar]
- 10.Kassira S, Korta DZ, Chapman LW, Dann F. Review of treatment for alopecia totalis and alopecia universalis. Int J Dermatol. 2017;56(8):801–810. doi: 10.1111/ijd.13612. [DOI] [PubMed] [Google Scholar]
- 11.Gupta AK, Carviel JL, Foley KA, Shear NH, Piraccini BM, Piguet V, et al. Monotherapy for alopecia areata: a systematic review and network meta-analysis. Skin Appendage Disord. 2019;5(6):331–337. doi: 10.1159/000501940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cranwell WC, Lai VW, Photiou L, Meah N, Wall D, Rathnayake D, et al. Treatment of alopecia areata: an Australian expert consensus statement. Australas J Dermatol. 2019;60(2):163–170. doi: 10.1111/ajd.12941. [DOI] [PubMed] [Google Scholar]
- 13.Hussain ST, Mostaghimi A, Barr PJ, Brown JR, Joyce C, Huang KP. Utilization of mental health resources and complementary and alternative therapies for alopecia areata: a U.S. survey. Int J Trichol. 2017;9(4):160–164. doi: 10.4103/ijt.ijt_53_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration. FDA approves first systemic treatment for alopecia areata. 2022. News release. https://www.fda.gov/news-events/press-announcements/fda-approves-first-systemic-treatment-alopecia-areata#:~:text=Today%2C%20the%20U.S.%20Food%20and,in%20the%20U.S.%20each%20year. Accessed 1 July 2022.
- 15.Xu H, Jesson MI, Seneviratne UI, Lin TH, Sharif MN, Xue L, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14(6):1235–1242. doi: 10.1021/acschembio.9b00188. [DOI] [PubMed] [Google Scholar]
- 16.King B. Top-line results from THRIVE-AA1: a phase 3 clinical trial of CTP-543 (deuruxolitinib), an oral JAK inhibitor, in adult patients with moderate to severe alopecia areata. In: 31st Annual Meeting of the European Academy of Dermatology and Venereology (EADV). 2022.
- 17.Liu J, Lv B, Yin H, Zhu X, Wei H, Ding Y. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple ascending dose and food effect study to evaluate the tolerance, pharmacokinetics of jaktinib, a new selective janus kinase inhibitor in healthy Chinese volunteers. Front Pharmacol. 2020;11:604314. doi: 10.3389/fphar.2020.604314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reistone Biopharma announces positive topline phase 2 results for SHR0302, a selective JAK1 inhibitor, for treatment of patients with alopecia areata. 2021. News release. https://www.prnewswire.com/news-releases/reistone-announces-positive-topline-phase-2-results-for-shr0302-a-selective-jak1-inhibitor-for-treatment-of-patients-with-alopecia-areata-301360967.html. Accessed 14 Sept 2022.
- 19.George P, Jagun O, Liu Q, Wentworth C, Napatalung L, Wolk R, et al. Prevalence of autoimmune and inflammatory diseases and mental health conditions among an alopecia areata cohort from a US administrative claims database. J Dermatol. 2023 doi: 10.1111/1346-8138.16839. [DOI] [PubMed] [Google Scholar]
- 20.Miller R, Conic RZ, Bergfeld W, Mesinkovska NA. Prevalence of comorbid conditions and sun-induced skin cancers in patients with alopecia areata. J Investig Dermatol Symp Proc. 2015;17(2):61–62. doi: 10.1038/jidsymp.2015.44. [DOI] [PubMed] [Google Scholar]
- 21.Chen CC, Chang YT, Liu HN, Chen YJ. Cancer risk in patients with alopecia areata: a nationwide population-based matched cohort study. Cancer Med. 2018;7(5):2153–2159. doi: 10.1002/cam4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang KP, Joyce CJ, Topaz M, Guo Y, Mostaghimi A. Cardiovascular risk in patients with alopecia areata (AA): A propensity-matched retrospective analysis. J Am Acad Dermatol. 2016;75(1):151–154. doi: 10.1016/j.jaad.2016.02.1234. [DOI] [PubMed] [Google Scholar]
- 23.Akyea RK, Leonardi-Bee J, Asselbergs FW, Patel RS, Durrington P, Wierzbicki AS, et al. Predicting major adverse cardiovascular events for secondary prevention: protocol for a systematic review and meta-analysis of risk prediction models. BMJ Open. 2020;10(7):e034564. doi: 10.1136/bmjopen-2019-034564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray M, Swallow E, Gandhi K, Carley C, Sikirica V, Wang T, et al. Healthcare utilization and costs among US adolescents with alopecia areata. J Health Econ Outcomes Res. 2022;9(2):11–18. doi: 10.36469/jheor.2022.36229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostaghimi A, Meche A, Ray M, Gandhi K, Gruben D, Sikirica V. Comorbidities, healthcare utilization, and costs associated with alopecia totalis and alopecia universalis in the United States. SKIN J Cutan Med. 2022;6(6):511–517. doi: 10.25251/skin.6.6.9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used and analyzed in the current study are available from OptumLabs through the OptumLabs Data Warehouse. Restrictions apply to the availability of these data, which were used under license for the current study and therefore are not publicly available. However, the datasets analyzed during the current study are available from the corresponding author upon reasonable request and with permission from OptumLabs.