Abstract
Near isogenic F2 (NIF2) population frequently developed by conventional backcross has dramatically contributed to QTL identification in plants. Developing such a NIF2 population is time-consuming. Thus, it is urgent to rapidly produce a NIF2 population for QTL cloning. Here, we proposed a rapid QTL cloning strategy by generating a Pseudo-near isogenic F2 population (Pseudo-NIF2), which segregates at the target QTL but is fixed at other QTLs for the target trait. Nineteen QTLs for GL, GW, and TGW were detected in the F2 population from the cross between Zhenshan 97 and Egy316. To verify the efficiency of Pseudo-NIF2 in QTL quick cloning, the novel moderate QTL qGL10.1 which explained 9.1% and 5.6% of grain length variation in F2 and F2:3 populations was taken as an example. An F2 plant (F2-120), which segregated at qGL10.1 but fixed at other 8 QTLs for grain length, was screened to generate a Pseudo-NIF2 population by selfing cross. In the Pseudo-NIF2 population, the segregation ratio of plants with long grains to short grains fits 3:1, indicating that one gene controlled the variation of grain length. Based on the Pseudo-NIF2 and its progeny, qGL10.1 was fine mapped to a 19.3-kb region, where a gene OsMADS56 was verified as the candidate by functional polymorphism between parental alleles. Pseudo-NIF2 strategy is a rapid way for QTL cloning, which saves 3 to 4 cropping seasons compared to the conventional way. Applying the method for cloning QTL with moderate or major effects is promising.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11032-023-01408-x.
Keywords: Grain length, Primary QTL mapping, Pseudo-near isogenic F2, Rapid QTL cloning
Background
Rice grain size, represented by grain length (GL), grain width (GW), and thousand-grain weight (TGW), plays a crucial role in the final grain yield and grain appearance quality (Tang et al. 2021; Shi et al. 2022). Identifying the QTLs with negative or positive effects will facilitate grain size improvement by selecting the desired alleles (Zhang et al. 2020). Over the last two decades, hundreds of grain size QTLs were detected; QTLs for GL are often negative regulators, and QTLs for grain width are frequently positive regulators (http://www.gramene.org).
Currently, dozens of QTLs for grain size have been cloned in rice. For example, seven QTLs, GW2, GS5, qSW5/GW5, GS6, GLW7, GW8, and WG7, mainly control GW (Song et al. 2007; Shomura et al. 2008; Li et al. 2011; Wang et al. 2012; Sun et al. 2013; Si et al. 2016; Huang et al. 2020), and eight QTLs, GS2/GL2, GS3, qLGY3/OsLG3b, TGW3/GL3.3, GL3.1/qGL3, TGW6, GL7/GW7, and SG3, regulate GL (Fan et al. 2006; Hu et al. 2015; Ishimaru et al. 2013; Qi et al. 2012; Si et al. 2016; Wang et al. 2015a; Wang et al. 2012; Wang et al. 2015b; Li et al. 2020). Some QTLs, such as GW7 and GW6a, have pleiotropic effects on GL and GW; GW6a positively regulates GL and GW (Song et al. 2015); meanwhile, GL7/GW7 positively regulate GL and negatively regulate GW (Wang et al. 2015b). Most of these QTLs were identified by a forward genetic strategy with a near isogenic F2 (NIF2) population, except a few identified by an inverse genetic strategy with mutants.
It is a prerequisite for QTL cloning in a forward genetic method that only one target QTL segregates in a population. Therefore, the NIF2 population is ideal for QTL cloning because it blocks the genomic background noise due to a homogeneous genome except for the targeting QTL region. Two strategies were developed to generate a NIF2 population: the single segment introgression line (SSCLs) and the heterogeneous inbred family (HIFs); in both, the target trait segregates in a simple Mendelian way in the NIF2 population, and thus, the QTL will be easily cloned (Cheng et al. 2021). Most QTLs like GS3 and Ghd7 were cloned with the SSCL-derived NIF2 (Fan et al. 2006; Xue et al. 2008), and a few QTLs, such as Ghd8 and FZP, were cloned with the residual heterozygous line derived NIF2 (Yan et al. 2011; Bai et al. 2017).
The SSCL is developed by continuous backcross with marker-aided selection for the target chromosomal region. Likewise, the first complete set of NIF2 was introduced by Eshed et al. (1992) in Lycopersicon pennellii, each line carrying a single chromosomal segment in a homogeneous background of Lycopersicon esculentum, encompassing the whole wild tomato genome. Then, this method is popularly utilized in rice and other crops due to its directionally targeting (Cheng et al. 2021; Li et al. 2022). Moreover, Qiao et al. (2016) reported that more than twenty sets of introgression lines (ILs) and chromosome segment substitution lines (CSSLs) had been constructed in rice. The HIFs could be obtained in the process of developing a recombinant inbred line population because the heterozygote ratio would be decreased at one-half every selfing generation. However, HIF is hard to recover a target genome region without screening the whole-genome heterozygosity with markers. Tuinstra et al. (1997) introduce the HIFs approach to develop NIF2 that carry different QTLs through selfing and selection protocol. Alonso-Blanco et al. (2003) developed eleven NIF2 from a set of recombinant inbred lines (RILs), and detected 4 QTLs controlling germination in Arabidopsis thaliana.
Recently, Zhang et al. (2021) introduced a rapid mapping approach (RapMap) using the F2 gradient populations. They constructed 15 bi-parental populations, where their parents had a gradient increase in the grain shape traits. Furthermore, the RapMap hypothesis is that a few QTLs can be detected in a population from parents with significant phenotypic differences, but only one QTL can be detected using parents with minor phenotypic differences. However, this hypothesis is not always correct because sometimes the parents exhibit similar trait performance but with very different genome constitutions (Mao et al. 2011). Additionally, Feng et al. (2021) developed a single-gene resolution linkage map tool using a large F2 population of 3756 plants and successfully mapped three known major QTLs. The high-resolution mapping comes at the expense of increased population size that could cause a largely increased unaffordable cost.
In this study, we aim to quickly clone a QTL by coining a Pseudo-near isogenic F2 (Pseudo-NIF2) population at F3 generation in which the target QTL is segregating, but the other QTLs for the same target trait are fixed. Following this method, a moderate QTL qGL10.1 was identified at F3, which confirmed its rapid and high efficiency for QTL cloning.
Materials and methods
Population construction and phenotyping
A Chinese indica cultivar Zhenshan 97 (ZS97) was crossed with an Egyptian rice Egy316. The hybrids were identified and selfed to obtain an F2 population of 200 individuals. The population seeds were sown in the seedling bed on 17 May and then transplanted to the field on 8 June 2019. The corresponding F2:3 families were planted in Wuhan 2020; each family contains 16 plants in two rows. The planting density was 26.5*16.5 cm between rows and plants within a row. GL, GW, and TGW were investigated for the two parents, the F2 population and F2:3 families, using the phenotype scorer machine (Yang et al. 2014).
Extraction of high-quality DNA and SNPs
In this study, the CTAB (Cetyl Trimethyl Ammonium Bromide) method (Murray and Thompson 1980) was used to extract high-quality DNA. Young leaves were sampled from all F2 plants and two parents for DNA extraction. The TruePrep DNA Library Prep Kit V2 for Illumina (Novozen Biotechnology Co., Ltd., Nanjing, China) was used to construct the DNA sequencing library; Illumina 3000 is used for sequencing. The sequencing depth of each parent and each F2 individual is 10× and 1×, respectively.
Fastqc v0.11.9 software package is used to evaluate the quality of original sequencing data (Brown et al. 2017). The base mass value (Q) is used as a reference index, Q=[−10log10(E)], and E is the sequencing error rate. If the number of bases with Q>30 accounts for more than 80% of the total, the sequencing quality is qualified. Bases with unqualified quality will be rejected in subsequent steps. The reading length of the next-generation sequencers is concise, and if the inserted DNA fragment is too short, the linker sequence will be detected. The Trimomatic v0.32 software package was used to remove the adapter sequences and the low-quality base (Bolger et al. 2014). The specific method of removing low-quality bases is to set a sliding window of 4 bases, slide from the 5′ end of reads, and calculate the base quality (Q) in the window; if the average value is lower than 15, all bases will be removed from the window. Then, reads were compared to the Nipponbare reference genome (Goff et al. 2002) using MEM algorism implemented in the BWA v0.7.17 tool (Li 2013). For the convenience of subsequent data analysis, SAM files generated at this time were converted into BAM files using the SAMtools tool (Li et al. 2009). After that, the Picard tool was used to sort reads according to chromosome and physical location. Because the PCR process during the establishment of the database will result in many repetitive sequences, which is unfavorable for subsequent analysis, it is necessary to use the MarkDuplicates command in Picard software (https://github.com/broadinstitute/Picard) to eliminate such repetitive sequences. The Genome Analysis Toolkit (GATK) v4.1.6.0 software package extracted SNPs and InDels information (Van der Auwera and O’Connor 2020).
Linkage map construction and QTL mapping
The QTL IciMapping 4.2 software was used to construct the linkage map and identify QTL (Meng et al. 2015). The inclusive composite interval mapping (ICIM) method was used to detect a significant association between phenotypic traits and marker loci in the datasets, and a test of 1000 permutations was used to identify the LOD threshold that corresponded to a genome-wide false discovery rate of 5% (P < 0.05). Then, a LOD threshold of 2.5 was used to declare the presence of QTLs.
Developing Pseudo-near isogenic F2 population
We introduce a rapid strategy of developing Pseudo-NIF2 instead of the time-consuming continuous backcrosses method. The principle of this Pseudo-NIF2 method is that one F2 or F3 plant heterozygous only at one QTL but homozygous at the other QTLs for the same trait can be selected for developing a NIF2 population. Only one QTL is expected to control the variation of the target trait in the NIF2 population, which benefits QTL cloning. After QTL mapping in the F2 population, we focused on cloning the new grain length QTL, qGL10.1. An F2 plant (F2-120) with a heterozygous segment harboring qGL10.1 but homozygous at the other QTLs affecting grain length was chosen to generate a sizeable F3 population (Pseudo-NIF2). A set of insertion/deletion (InDel) markers previously developed by Hu et al. (2020) was used to verify the genotypes of QTLs. The remaining primers were designed based on the InDel information between the two parents by Primer Premier 6 software and listed in (Additional file 1: Table S1).
qGL10.1 cloning using the Pseudo-NIF2 population approach
For qGL10.1 fine mapping, 373 Pseudo-NIF2 plants of the F2:3-120 family were grown in Wuhan, 2021. The young leaves were collected for DNA extraction, and the plants were genotyped using the flanking markers of qGL10.1. The phenotyping and genotyping data were analyzed using the QTL IciMapping 4.2 (Meng et al. 2015). Then, 4505 Pseudo-NIF2 plants from one F3:4 family planted in Hainan 2021 for qGL10.1 cloning.
Results
The phenotypic variation for the parents, F2, and F2:3 populations
Egy316 displayed better performance in terms of grain shape traits than ZS97. Egy316 had a longer GL, heavier TGW but smaller GW than ZS97 (Fig. 1A–D). A continuous distribution pattern for the grain size traits was observed in F2 and F2:3 populations, indicating that they are typical quantitative traits controlled by polygenes (Table 1 and Fig. 1E–G). Moreover, significant positive correlations were detected between GL, GW, and TGW within the same generations and between the two generations except the case between GL in F2:3 and GW in F2 (Table 2). Moreover, all traits showed the highest correlation coefficients between generations.
Fig. 1.
Comparison of grain shape traits between ZS97 and Egy316 in 2019 and 2020. (A) Grain length, (B) grain width, and (C) thousand-grain weight. (D) Grain morphology of ZS97 and Egy316. Comparison of frequency distributions of grain shape traits in the F2 and F2:3 populations. (E) Grain length, (F) grain width, and (G) thousand-grain weight in both populations. *, **, and *** significance at P < 0.05, 0.01, and 0.001, respectively. The colors were indicated in the figures, yellow for ZS97, red for Egy316 in (A), (B), (C), and yellow for F2 population and red for F2:3 population in (E), (F), and (G)
Table 1.
Phenotypic variation of the grain size in the F2 and F2:3 generations from the cross ZS97/Egy316
| Traits | F2 | F2:3 | ||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| Grain length (mm) | 8.66 ± 0.5 | 7.3–10.1 | 8.3 ± 0.7 | 6.8–9.8 |
| Grain width (mm) | 3.11 ± 0.2 | 2.5–3.6 | 2.9 ± 0.3 | 2.2–3.5 |
| Thousand-grain weight (g) | 25.5 ± 4.6 | 12.6–37.1 | 25.8 ± 3.8 | 13.0–32.5 |
SD standard deviation
Table 2.
Correlation coefficient between grain size traits in the ZS97*Egy316 population
| GL-F2 | GW-F2 | TGW-F2 | GL-F2:3 | GW-F2:3 | |
|---|---|---|---|---|---|
| GW-F2 | 0.327** | ||||
| TGW-F2 | 0.460** | 0.598** | |||
| GL-F2:3 | 0.774** | 0.101 | 0.281** | ||
| GW-F2:3 | 0.162* | 0.622** | 0.274** | 0.357** | |
| TGW-F2:3 | 0.481** | 0.451** | 0.473** | 0.326** | 0.322** |
GL grain length, GW grain weight, TGW thousand-grain weight. * and ** significant at 0.05 and 0.01, respectively
Linkage map construction
1,143,008 high-quality SNPs were converted into 1478 bins (Additional file 1: Table S2) and assigned to the rice chromosomes to develop the linkage map (Fig. 2, Additional file 1: Table S3). In detail, the genetic map had a total length of 1630 cM with an average interval distance of 1.1 cM between bins with a slight variation of 0.93 cM on chromosome 10 to 1.39 cM on chromosome 12. Chromosome 1 has the most bin numbers (184), and chromosome 12 has the fewer bin numbers (81); moreover, the largest interval distance on each chromosome is less than 10 cM except for chromosomes 1, 6, and 12, which reflected the uniform and high-density map.
Fig. 2.
High-density linkage map showing the positions of known genes and QTLs for grain shape traits detected in the F2 and F2:3 populations. The y-axis indicates the genetic distance in centimorgan. Major genes controlling grain size in the ZS97*Egy316 population were presented in black color, the QTLs commonly detected in F2 and F2:3 populations indicated in red color, and the QTLs only detected in F2 or F2:3 population indicated in blue color or purple color, respectively
QTLs identified for grain size
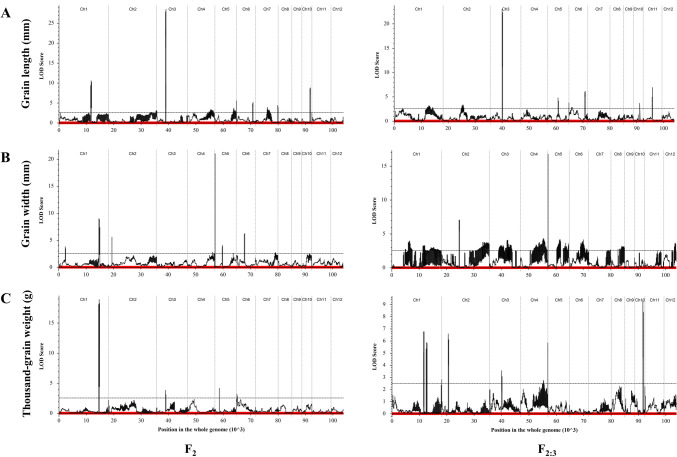
For GL, 9 QTLs were detected in the F2 population (Table 3 and Fig. 3A). All QTLs acted in dominance; moreover, Egy316 alleles at 6 QTLs increased grain length while ZS97 alleles decreased grain length at the other 3 QTLs. Only two QTLs, qGL1.2 and qGL3.1 on chromosomes 1 and 3, showed major effects with a contribution of 10.9% and 22.1% of grain length variation, respectively. Accordingly, 9 QTLs were identified in the F3 population (Table 3 and Fig. 3A). Only one QTL qGL3.1 showed a major effect that explained 19.2% of grain length variation. The parental allele of Egy316 increased grain length at 7 QTLs. Six QTLs were commonly detected in both populations, and qGL3.1 explained the highest contribution. qGL3.1 is likely identical to GS3 because it is closely linked to GS3. Moreover, ZS97 carries a functional GS3 allele, whereas Egy316 owns a non-functional gs3 allele due to the non-synonymous mutation in exon two (C165A) resulting in a stop codon. qGL10.1 showed a moderate effect that explained 9.1% and 5.6% in F2 and F3 generations, respectively (Table 3 and Fig. 3A); It is a new QTL because no QTL has been identified around the region.
Table 3.
Quantitative trait loci (QTL) for grain size in the F2 and F2:3 populations from the cross ZS97/Egy316
| Trait | QTL | Interval (bp) | F2 | F2:3 | Neighbor gene | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | A | D | PVE % | LOD | A | D | PVE % | ||||
| GL | qGL1.1 | chr01_6252160-6528362 | 2.7 | − 0.06 | −0.11 | 2.7 | 2.5 | −0.22 | −0.41 | 2.3 | |
| qGL1.2 | chr01_36420775-36829167 | 9.9 | 0.25 | 0.11 | 10.9 | 3.1 | 0.31 | −0.11 | 2.6 | ||
| qGL2.1 | chr02_24836231-25154458 | 3.3 | 0.11 | −0.50 | 2.4 | ||||||
| qGL3.1 | chr03_16285838-16886243 | 26.6 | 0.36 | −0.31 | 22.1 | 23 | 0.42 | −0.11 | 19.2 | −0.4 Mb to GS3 | |
| qGL4.1 | chr04_22263737-22639082 | 3.3 | 0.11 | −0.03 | 2.9 | ||||||
| qGL4.2 | chr04_33536150-33674756 | 2.5 | −0.07 | −0.09 | 2.6 | ||||||
| qGL5.1 | chr05_22523494-22643495 | 4.7 | 0.11 | −0.11 | 3.8 | ||||||
| qGL6.1 | chr06_1-721973 | 3.9 | 0.14 | −0.01 | 3.1 | 3.7 | 0.12 | −0.02 | 4.4 | ||
| qGL6.2 | chr06_28042873-28197236 | 5.7 | 0.15 | 0.01 | 4.6 | 6.1 | 0.11 | 0.12 | 6.8 | ||
| qGL7.1 | chr07_22710399-22852604 | 6.6 | 0.29 | 0.19 | 6.3 | ||||||
| qGL10.1 | chr10_22826960-23064487 | 7.9 | −0.21 | −0.01 | 9.1 | 3.6 | −0.11 | −0.04 | 5.6 | ||
| qGL11.1 | chr11_22000836-22382239 | 6.8 | 0.30 | 0.11 | 3.9 | ||||||
| GW | qGW1.1 | chr01_40061411-40232403 | 23 | 0.21 | 0.07 | 16.2 | 2.8 | 0.14 | 0.12 | 3.2 | |
| qGW2.1 | chr02_8624971-8785185 | 5.1 | 0.05 | 0.04 | 7.2 | 7.1 | 0.16 | 0.08 | 5 | +0.5 Mb to GW2 | |
| qGW2.2 | chr02_23227105-23395836 | 7.1 | 0.16 | 0.08 | 5.1 | ||||||
| qGW3.1 | chr03_27774267-28044025 | 2.6 | 0.06 | −0.15 | 3.5 | 4.1 | 0.06 | −0.29 | 6.2 | ||
| qGW4.1 | chr04_34347387-34635947 | 2.5 | −0.04 | 0.11 | 2 | 4.3 | −0.12 | 0.18 | 5.2 | ||
| qGW5.1 | chr05_4872672-5032093 | 19.1 | −0.15 | −0.02 | 16.3 | 16.7 | −0.19 | 0.11 | 10.2 | −0.4 Mb to GW5 | |
| qGW6.1 | chr06_4804633-4979917 | 4.1 | −0.01 | −0.11 | 3.3 | 10.7 | −0.19 | 0.11 | 8.3 | ||
| qGW6.2 | chr06_26495233-26725102 | 4.1 | 0.13 | 0.15 | 3.2 | ||||||
| qGW10.1 | chr10_23724974-23956998 | 2.8 | −0.06 | −0.12 | 2.8 | 6.7 | −1.11 | −0.18 | 3.1 | ||
| TGW | qTGW1.1 | chr01_39735532-40061411 | 16 | 3.41 | 2.81 | 7.5 | 5.8 | 0.15 | 1.51 | 2.5 | |
| qTGW2.1 | chr02_27175409-27295409 | 2.9 | 1.31 | 0.71 | 3.9 | 6.5 | 1.10 | 0.21 | 2.9 | ||
| qTGW3.1 | chr03_20243818-20710332 | 3.5 | 0.81 | −0.32 | 2.5 | ||||||
| qTGW4.1 | chr04_33674756-33842888 | 2.7 | −0.50 | −0.61 | 2.1 | ||||||
| qTGW5.1 | chr05_17173895-17293895 | 4.1 | −1.91 | 0.52 | 5.9 | 5.8 | −1.32 | 0.41 | 5.8 | ||
| qTGW10.1 | chr10_23064487-23314589 | 9.3 | −1.50 | 0.04 | 5.5 | ||||||
Traits: GL, grain length (mm); GW, grain width (mm); and TGW, thousand-grain weight (g). Interval, location of quantitative trait loci (QTLs) on chromosome by bp; LOD, logarithm of odds; A, additive effect; D, Dominance effect and PVE (%), the phenotypic variance explained by each QTL. Position of neighbor genes according to The Rice Annotation Project Database
Fig. 3.
Logarithm of odds (LOD) score curves for the grain shape traits QTLs for (A) grain length, (B) grain width, and (C) thousand-grain weight in F2 and F2:3 populations. Scattered dotted line indicates LOD threshold (2.5)
For GW, 7 QTLs were detected in the F2 population (Table 3). Moreover, ZS97 alleles at 3 QTLs increased GW; Egy316 alleles decreased GW at 4 QTLs. Besides, 9 QTLs were detected in the F3 population (Table 3). qGW5.1 contributed with the highest phenotypic variation of 16.3% and 10.2% in the F2 and F3 populations (Table 3 and Fig. 3B). qGW10.1 was commonly detected in both generations sharing a similar location with qGL10.1 and accounted for 2.8% and 3.1% of the phenotypic variation in the F2 and F3, respectively.
Regarding TGW, 3 QTLs were detected in the F2 populations. The Egy316 alleles increase the TGW for two QTLs, while the ZS97 allele decreases the TGW at one QTL. Furthermore, in the F3 population, 6 QTLs were detected. Three QTLs had positive additive effects and the other three QTLs had negative additive effects. qTGW10.1 was only detected in the F3 population and shared the same location with qGL10.1 and qGW10.1 (Table 3 and Fig. 3C).
In summary, 27 QTLs controlling grain shape traits were identified in the F2 and F2:3 populations and distributed on nine chromosomes except chromosomes 8, 9, and 12 (Table 3). Three QTLs showed a pleiotropic effect on the grain size traits (Fig. 2) and 14 QTLs were stable across the two generations.
qGL10.1 validation in the Pseudo-NIF2 population
According to the genome constitutions of 200 F2 plants and the putative QTLs identified in the F2 population (Table 3), the F2-120 plant carried heterozygous qGL10.1, but homozygous genomes in other grain length QTL regions were chosen to generate the Pseudo-NIF2 population. The genotypes of the F2-120 plant at grain length QTLs were validated with InDel markers (Fig. 4A). The Pseudo-NIF2:3 consisting of 373 plants showed a bimodal distribution for the grain length with the boundary of 7.8 mm (Fig. 4B). Among them, 86 plants had short grains of less than 7.8 mm, 287 plants had long grains of more than 7.8 mm. The ratio of the number of plants with long grains to the plants with short grains fits to 3:1 [χ2 (1, n = 373) P = 0.751], indicating one gene controlling grain length variation in the Pseudo-NIF2:3 population. Thus, qGL10.1 as a qualitative gene was limited to ~ a 1.8 Mb interval between markers RID10-25 and Indel10-2188 (Fig. 4C). Then, we planted 4505 plants from an F3:4 family, and qGL10.1 was fine mapped to a 19.3-kb region between markers InDel10-20589 and InDel10-20878, which contains only one open reading frame Os10g0536100 (Fig. 4D) encoding a MADS-box family protein (https://rapdb.dna.affrc.go.jp/), which is associated with flower organ (Klee 2017). Thus, it could be the candidate for qGL10.1.
Fig. 4.
Mapped-based cloning of qGL10.1. (A) The grain length QTLs in the F2-120 plant; three colors represent the genotypes composition; yellow = heterozygous, red = homozygous (ZS97), and blue = homozygous (Egy316). (B) Frequency distribution of grain length in Pseudo-NIF2 (F2:3-120 families). (C) qGL10.1 was convincingly mapped to the interval between markers RID10-25 and InDel10-2188 on chromosome 10 using 373 psudo-NIF2 individuals. (D) qGL10.1 was narrowed down to a 19.3 kb genomic DNA region between markers InDel10-20859 and InDel10-20878 using 4505 F3:4 individuals. The numbers above the bar in panel indicating the number of recombinants. The black and white colors indicate homozygous ZS97 and Egy316, respectively, while the downward diagonal line indicates heterozygous. (E) The structure and allelic variation of the candidate gene Os10g0536100 (qGL10.1), the only predicted open reading frame in the 19.3-kb region. The black and white vertical boxes indicate the exons and promotor region, respectively. The white horizontal box indicates the deletion region in Egy316
Os10g0536100 genomic DNA length is 15.67 kb which contains 7 exons and 6 introns and encodes a protein with 233 amino acids. To validate its candidate, comparative sequencing between parents for Os10g0536100 showed that a 1008-bp region covered the partial promoter (95 bp), the complete first exon (185 bp), and the partial second intron (728 bp) was deleted in Egy316, and 4 SNPs were located on exons 3, 4, and 5 (Fig. 4E). In detail, the SNP on exon 3 was a synonymous mutation. In contrast, the two SNPs on exons 4 and one SNP on exon 5 lead to amino acid changes (Cys119Phe and Asn137Lys, and Arg148Gln) (Additional file 1: Table S4). Based on the deletion, we coined a codominant marker (InDel10-20862) to genotype the Pseudo-NIF2 plants; cosegregating analysis showed that the ZS97 homozyogtes had short grains, and the Egy316 homozygotes and heterozygotes always have long grains (Additional file 2: Fig. S1), indicated that the MADS-box gene is the gene underlying qGL10.1.
Discussion
The success of QTL cloning needs an advanced population in which a quantitative trait is characterized with a qualitative trait pattern. Frequently, the NIF2 population is the ideal advanced population in which the target trait variation is contributed by the target QTL rather than other genetic factors. However, the conventional way to develop a NIF2 population takes 5–6 cropping seasons, including 4–5 backcrosses and one selfing cross after QTL mapping (Fig. 5A). More than 90% of the genome in the NIF2 population is fixed with the recurrent parent so that the genetic background noise is well blocked. However, the Pseudo-NIF2 population proposed in this study is immediately obtained after QTL mapping; thus, it saves at least 3–4 cropping seasons (Fig. 5B). It is noted that the genomic constitution of the Pseudo-NIF2 population is a random mixture of both parents, but the other detected QTL regions is fixed with female or male parents.
Fig. 5.
Comparison of the genome constitutions between (A) the conventionally developing NIF2 and (B) the Pseudo-NIF2. MAS, marker-assisted selection for the target trait. Three colors represent the genotypes composition; yellow = heterozygous, red = homozygous (Parent 1), and blue = homozygous (Parent 2)
Generally, a bi-parental mapping population can identify 6–8 QTLs for a trait with moderate or high heritability and 3–4 QTLs for a trait of low heritability (Liang et al. 2022; Wang et al. 2022). To screen a plant with the potential for developing a Pseudo-NIF2, the size of an F2 population for QTL mapping should be more than 200 plants because the possibility of homozygote at 5 loci is 1/32. In such an F2 population, 5–6 plants would be candidates for developing a Pseudo-NIF2. For example, qGL10.1 is a moderate QTL stably identified in both generations in this study. Two plants segregating at qGL10.1 but fixed at other QTLs were screened, and its candidate gene Os10g0536100 was identified at F3:4 generation. If the size of Pseudo-NIF2 is large enough, it would be identified at F2:3 generation, which saves 4 cropping seasons than the conventional method.
The latest study used 4000 BC5F3 individuals to map GL10 to an 18.5-kb region, which contains the same candidate gene Os10g0536100, and a complementation test determined its function in regulating grain length; furthermore, its pleiotropic effect on grain shape and grain weight was approved by Zhan et al. (2022). Furthermore, the study confirmed the correct candidate gene identified by our rapid QTL cloning method, indicated the Pseudo-NIF2 strategy is rapid, efficient, and promising for QTL cloning. To guarantee the successful cloning of a minor QTL following this strategy, the threshold for QTL claiming is recommended to decrease. Therefore, some marginal effects of minor QTLs will be identified and taken into consideration for screening the candidate F2 plant. Hence, the genetic background noise is well controlled, which is helpful for a minor QTL cloning at F3 or F4 generation.
A total of 27 QTLs were reported in this study. Of them, 16 QTLs were commonly identified in F2 and F2:3 populations indicating they were environmentally stable (Table 3). Three QTL clusters, each containing 3 QTLs, were located on chromosomes 1, 4, and 10 (Fig. 2). The QTL cluster based on primary mapping probably contains a pleiotropy QTL or a few linked QTLs. Some grain size QTLs such as GS3 and GW7 have pleiotropic effects on GL, GW, and TGW (Fan et al. 2006; Wang et al. 2015b). The cluster on chromosome 1 contained qGL1.2, qTGW1.1, and qGW1.1. qTGW1.1 and qGW1.1 were very closely linked to each other, indicating the possibility of one QTL with pleiotropic effects on GW and TGW. Nevertheless, this pleiotropy QTL is more than 3 Mb away from qGL1.2. The pleiotropy QTL qGL10.1 contributes to the QTL cluster on chromosome 10 because these three QTLs located within 1 Mb, and the pleiotropic effects of Os10g0536100 were validated by Zhan et al. (2022). Three QTLs qGL4.2, qGW4.1, and qTGW4.1 closely located in a small region of less than 1 Mb (Fig. 2) are likely a gene with minor effects on GL, GW, and TGW. Both linked QTLs and pleiotropy QTL could well explain the correlation between these grain size traits.
Here, we identified two moderate grain length QTLs, qGL1.2 and qGL10.1. qGL10.1 was identified by the rapid Pseudo-NIF2 strategy. To isolate qGL1.2, the Pseudo-NIF2 method should be preferentially used. We believe that applying Pseudo-NIF2 in gene cloning will express its high efficiency.
Conclusion
The rapidness and efficiency of the Pseudo-NIF2 approach for QTL cloning are validated by cloning qGL10.1. A large Pseudo-NIF2 population generated at F3 in rice would accelerate the speed of QTL isolation.
Supplementary Information
Acknowledgements
The authors thank Mr. JB. Wang for his outstanding fieldwork in managing the field experiments.
Author contributions
Sherif A wrote the paper. Sherif A, Zhang B, Wu B, Hu Y, Li S, and Zhou X conducted all experiments and analyzed the data. Ayaad M and Hassan IO developed the Egyptian mutant (Egy316). Ayaad M, El-Badri AM, El-Badawy MEM, Sedhom SA, and Abo-Yousef M developed the F2 population. Xing YZ designed, guided this study, and revised the paper.
Funding
This work is partially supported by the National Natural Science Foundation of China (32061143042), and a bilateral project entitled “mapping of early heading and yield-related trait genes in Chinese and Egyptian rice resources” between Benha University and Huazhong Agricultural University. The Talented Young Scientists Program China 2019 (TYSP).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Soybean Functional Genomics
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alonso-Blanco C, Bentsink L, Hanhart CJ, Vries HB-D, Koornneef M. Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana. Genetics. 2003;164(2):711–729. doi: 10.1093/genetics/164.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Huang Y, Hu Y, Liu H, Zhang B, Smaczniak C, Hu G, Han Z, Xing Y. Duplication of an upstream silencer of FZP increases grain yield in rice. Nat Plants. 2017;3(11):885–893. doi: 10.1038/s41477-017-0042-4. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Pirrung M, McCue LA. FQC Dashboard: integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics. 2017;33(19):3137–3139. doi: 10.1093/bioinformatics/btx373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Li G, Yin M, Adegoke TV, Wang Y, Tong X, Zhang J, Ying J. Verification and dissection of one quantitative trait locus for grain size and weight on chromosome 1 in rice. Sci Rep. 2021;11(1):18252–18265. doi: 10.1038/s41598-021-97622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed Y, Abu-Abied M, Saranga Y, Zamir D. Lycopersicon esculentum lines containing small overlapping introgressions from L. pennellii. Theor Appl Genet. 1992;83(8):1027–1034. doi: 10.1007/bf00232968. [DOI] [PubMed] [Google Scholar]
- Fan C, Xing Y, Mao H, Lu T, Han B, Xu C, Li X, Zhang Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor Appl Genet. 2006;112(6):1164–1171. doi: 10.1007/s00122-006-0218-1. [DOI] [PubMed] [Google Scholar]
- Feng L, Ma A, Song B, Yu S, Qi X. Mapping causal genes and genetic interactions for agronomic traits using a large F2 population in rice. G3. 2021;11(11):jkab318. doi: 10.1093/g3journal/jkab318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff SA, Ricke D, Lan T-H, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica) Science. 2002;296(5565):92–100. doi: 10.1126/science.1068275. [DOI] [PubMed] [Google Scholar]
- Hu J, Wang Y, Fang Y, Zeng L, Xu J, Yu H, Shi Z, Pan J, Zhang D, Kang S. A rare allele of GS2 enhances grain size and grain yield in rice. Mol Plant. 2015;8(10):1455–1465. doi: 10.1016/j.molp.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Hu W, Zhou T, Wang P, Wang B, Song J, Han Z, Chen L, Liu K, Xing Y. Development of whole-genome agarose-resolvable LInDel markers in rice. Rice. 2020;13(1):1–11. doi: 10.1186/s12284-019-0361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Bai X, Cheng N, Xiao J, Li X, Xing Y. Wide Grain 7 increases grain width by enhancing H3K4me3 enrichment in the OsMADS1 promoter in rice (Oryza sativa L.) The Plant J. 2020;102(3):517–528. doi: 10.1111/tpj.14646. [DOI] [PubMed] [Google Scholar]
- Ishimaru K, Hirotsu N, Madoka Y, Murakami N, Hara N, Onodera H, Kashiwagi T, Ujiie K, Shimizu B-i, Onishi A, Miyagawa H, Katoh E. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat Gen. 2013;45(6):707–711. doi: 10.1038/ng.2612. [DOI] [PubMed] [Google Scholar]
- Klee HJ. Genetic control of floral architecture: insights into improving crop yield. Cell. 2017;169(6):983–984. doi: 10.1016/j.cell.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Subgroup GPDP. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fan C, Xing Y, Jiang Y, Luo L, Sun L, Shao D, Xu C, Li X, Xiao J, He Y, Zhang Q. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat Genet. 2011;43(12):1266–1269. doi: 10.1038/ng.977. [DOI] [PubMed] [Google Scholar]
- Li Q, Lu L, Liu H, Bai X, Zhou X, Wu B, Yuan M, Yang L, Xing Y. A minor QTL, SG3, encoding an R2R3-MYB protein, negatively controls grain length in rice. Theor Appl Genet. 2020;133(8):2387–2399. doi: 10.1007/s00122-020-03606-z. [DOI] [PubMed] [Google Scholar]
- Li S, Zou J, Fan J, Guo D, Tan L. Identification of quantitative trait loci for important agronomic traits using chromosome segment substitution lines from a japonica × indica cross in rice. Mol Breed. 2022;42(12):73–85. doi: 10.1007/s11032-022-01343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Zhan W, Hu G, Liu H, Xing Y, Li Z, Han Z. Five plants per RIL for phenotyping traits of high or moderate heritability ensure the power of QTL mapping in a rice MAGIC population. Mol Breed. 2022;42(5):28–41. doi: 10.1007/s11032-022-01299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Liu T, Xu C, Li X, Xing Y. Epistasis and complementary gene action adequately account for the genetic bases of transgressive segregation of kilo-grain weight in rice. Euphytica. 2011;180(2):261–271. doi: 10.1007/s10681-011-0395-0. [DOI] [Google Scholar]
- Meng L, Li H, Zhang L, Wang J. QTL IciMapping: integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. The Crop J. 2015;3(3):269–283. doi: 10.1016/j.cj.2015.01.001. [DOI] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi P, Lin Y-S, Song X-J, Shen J-B, Huang W, Shan J-X, Zhu M-Z, Jiang L, Gao J-P, Lin H-X. The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin-T1;3. Cell Res. 2012;22(12):1666–1680. doi: 10.1038/cr.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao W, Qi L, Cheng Z, Su L, Li J, Sun Y, Ren J, Zheng X, Yang Q. Development and characterization of chromosome segment substitution lines derived from Oryza rufipogon in the genetic background of O. sativa spp. indica cultivar 9311. BMC Genom. 2016;17(1):580–591. doi: 10.1186/s12864-016-2987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Yun P, Zhu Y, Wang L, Li P, Lou G, Xia D, Zhang Q, Xiao J, Li X, He Y, Gao G. Fine mapping of qTGW2b and qGL9, two minor QTL conferring grain size and weight in rice. Mol Breed. 2022;42(11):68–81. doi: 10.1007/s11032-022-01328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomura A, Izawa T, Ebana K, Ebitani T, Kanegae H, Konishi S, Yano M. Deletion in a gene associated with grain size increased yields during rice domestication. Nat Genet. 2008;40(8):1023–1028. doi: 10.1038/ng.169. [DOI] [PubMed] [Google Scholar]
- Si L, Chen J, Huang X, Gong H, Luo J, Hou Q, Zhou T, Lu T, Zhu J, Shangguan Y, Chen E, Gong C, Zhao Q, Jing Y, Zhao Y, Li Y, Cui L, Fan D, Lu Y, Weng Q, Wang Y, Zhan Q, Liu K, Wei X, An K, An G, Han B. OsSPL13 controls grain size in cultivated rice. Nat Genet. 2016;48(4):447–456. doi: 10.1038/ng.3518. [DOI] [PubMed] [Google Scholar]
- Song X-J, Huang W, Shi M, Zhu M-Z, Lin H-X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet. 2007;39(5):623–630. doi: 10.1038/ng2014. [DOI] [PubMed] [Google Scholar]
- Song XJ, Kuroha T, Ayano M, Furuta T, Nagai K, Komeda N, Segami S, Miura K, Ogawa D, Kamura T. Rare allele of a previously unidentified histone H4 acetyltransferase enhances grain weight, yield, and plant biomass in rice. Proc Natl Acad Sci U S A. 2015;112(1):76–81. doi: 10.1073/pnas.1421127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Li X, Fu Y, Zhu Z, Tan L, Liu F, Sun X, Sun X, Sun C. GS6, a member of the GRAS gene family, negatively regulates grain size in rice. J Integr Plant Biol. 2013;55(10):938–949. doi: 10.1111/jipb.12062. [DOI] [PubMed] [Google Scholar]
- Tang Z, Gao X, Zhan X, Fang N, Wang R, Zhan C, Zhang J, Cai G, Cheng J, Bao Y, Zhang H, Huang J. Natural variation in OsGASR7 regulates grain length in rice. Plant Biotechnol J. 2021;19(1):14–16. doi: 10.1111/pbi.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinstra MR, Ejeta G, Goldsbrough PB. Heterogeneous inbred family (HIF) analysis: a method for developing near-isogenic lines that differ at quantitative trait loci. Theor Appl Genet. 1997;95(5):1005–1011. doi: 10.1007/s001220050654. [DOI] [Google Scholar]
- Van der Auwera G, O’Connor B. Genomics in the cloud: Using Docker, GATK, and WDL in Terra. 1. O’Reilly Media; 2020. [Google Scholar]
- Wang S, Wu K, Yuan Q, Liu X, Liu Z, Lin X, Zeng R, Zhu H, Dong G, Qian Q, Zhang G, Fu X. Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet. 2012;44(8):950–954. doi: 10.1038/ng.2327. [DOI] [PubMed] [Google Scholar]
- Wang S, Li S, Liu Q, Wu K, Zhang J, Wang S, Wang Y, Chen X, Zhang Y, Gao C, Wang F, Huang H, Fu X. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat Genet. 2015;47(8):949–954. doi: 10.1038/ng.3352. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xiong G, Hu J, Jiang L, Yu H, Xu J, Fang Y, Zeng L, Xu E, Xu J, Ye W, Meng X, Liu R, Chen H, Jing Y, Wang Y, Zhu X, Li J, Qian Q. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat Genet. 2015;47(8):944–948. doi: 10.1038/ng.3346. [DOI] [PubMed] [Google Scholar]
- Wang P, Qi F, Yao H, Xu X, Li W, Meng J, Zhang Q, Xie W, Xing Y. Fixation of hybrid sterility genes and favorable alleles of key yield-related genes with dominance contribute to the high yield of the Yongyou series of intersubspecific hybrid rice. J Genet Genomics. 2022;49(5):448–457. doi: 10.1016/j.jgg.2022.02.027. [DOI] [PubMed] [Google Scholar]
- Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, Zhang Q. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet. 2008;40(6):761–767. doi: 10.1038/ng.143. [DOI] [PubMed] [Google Scholar]
- Yan W, Wang P, Chen H, Zhou H, Li Q, Wang C, Ding Z, Zhang Y, Yu S, Xing Y, Zhang Q. A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol Plant. 2011;4(2):319–330. doi: 10.1093/mp/ssq070. [DOI] [PubMed] [Google Scholar]
- Yang W, Guo Z, Huang C, Duan L, Chen G, Jiang N, Fang W, Feng H, Xie W, Lian X, Wang G, Luo Q, Zhang Q, Liu Q, Xiong L. Combining high-throughput phenotyping and genome-wide association studies to reveal natural genetic variation in rice. Nat Comm. 2014;5(1):5087–5095. doi: 10.1038/ncomms6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan P, Ma S, Xiao Z, Li F, Wei X, Lin S, Wang X, Ji Z, Fu Y, Pan J, Zhou M, Liu Y, Chang Z, Li L, Bu S, Liu Z, Zhu H, Liu G, Zhang G, Wang S. Natural variations in grain length 10 (GL10) regulate rice grain size. J Genet Genomics. 2022;49(5):405–413. doi: 10.1016/j.jgg.2022.01.008. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma B, Bian Z, Li X, Zhang C, Liu J, Li Q, Liu Q, He Z. Grain size selection using novel functional markers targeting 14 genes in rice. Rice. 2020;13(1):63–78. doi: 10.1186/s12284-020-00427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang D, Fan Y, Li C, Xu P, Li W, Sun Q, Huang X, Zhang C, Wu L, Yang H, Wang S, Su X, Li X, Song Y, Wu M-e, Lian X, Li Y. The identification of grain size genes by RapMap reveals directional selection during rice domestication. Nat Comm. 2021;12(1):5673–5690. doi: 10.1038/s41467-021-25961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H (2013) Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv: Genomics:1303-3997
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.