Abstract
Background
Numerous studies have indicated that alopecia areata is associated with a chronic systemic inflammation, which is considered as a risk factor for venous thromboembolism. The aim of the study was to evaluate the following markers of venous thromboembolism risk: soluble fibrin monomer complex (SFMC), thrombin–antithrombin complex (TATC), and prothrombin fragment 1 + 2 (F1 + 2) in patients with alopecia areata and compare them with healthy controls.
Methods
In total, 51 patients with alopecia areata [35 women and 16 men; mean age: 38 (19–54) years] and 26 controls [18 women and 8 men; mean age: 37 (29–51) years] were enrolled in the study. The serum concentrations of thromboembolism markers were measured using an enzyme-linked immunosorbent assay (ELISA) kit.
Results
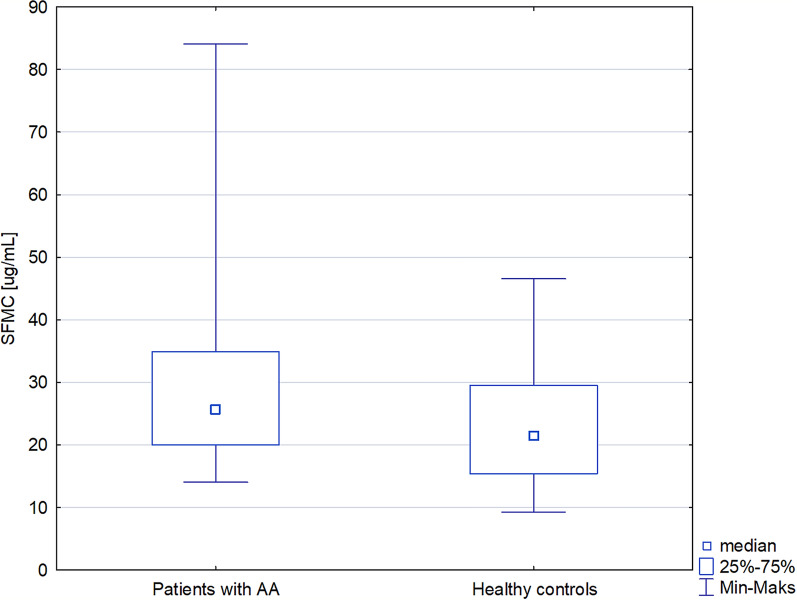
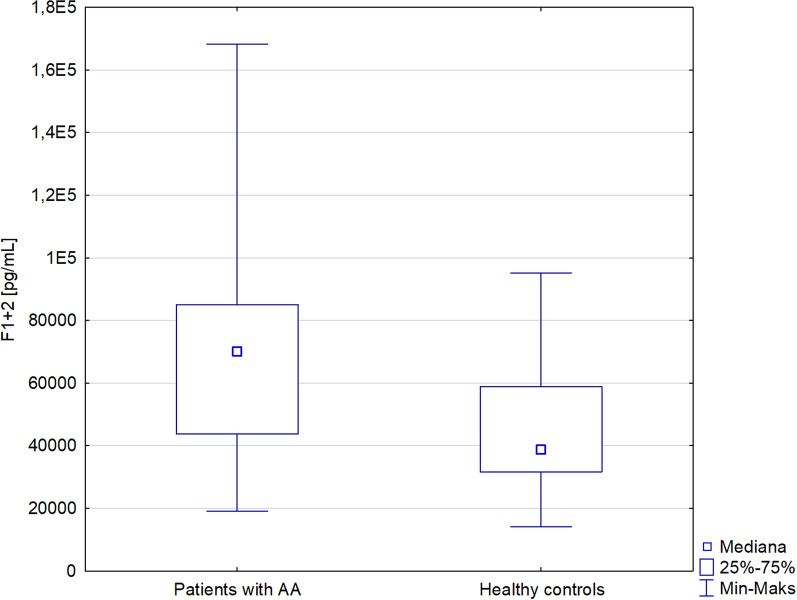
An increased level of SFMC was detected in patients with alopecia areata compared with the controls [25.66 (20–34.86) versus 21.46 (15.38–29.48) µg/ml; p < 0.05)]. In addition, a higher level of F1 + 2 was observed in patients with alopecia areata in comparison with the control group [70150 (43720–86070) versus 38620 (31550–58840) pg/ml; p < 0.001]. No significant correlation was detected among SFMC or F1 + 2 and the Severity of Alopecia Tool (SALT) score, disease duration, or the number of the hair loss episodes.
Conclusion
Alopecia areata may be associated with an increased risk of venous thromboembolism. Regular screening and preventive management of venous thromboembolism may be beneficial in patients with alopecia areata, especially before and during systemic Janus kinase (JAK) inhibitors or glucocorticoid therapy.
Keywords: Autoimmune disease, Blood clot, Embolism, Hair loss, Thrombotic risk, Thrombosis
Key Summary Points
| There are numerous risk factors for venous thromboembolism, including systemic inflammation. Markers of venous thromboembolism risk include soluble fibrin monomer complex, thrombin–antithrombin complex, and prothrombin fragment 1 + 2. |
| Numerous studies have indicated that alopecia areata is associated with chronic systemic inflammation, with an increased level of proinflammatory cytokines. |
| In the present study, an increased level of soluble fibrin monomer complex and prothrombin fragment 1 + 2 in patients with alopecia areata compared with the control group was observed. |
| Alopecia areata may be associated with an increased risk of venous thromboembolism. Regular screening and preventive management of venous thromboembolism may be beneficial in patients with alopecia areata. |
Introduction
Alopecia areata is an autoimmune condition characterized by nonscarring hair loss. The pathogenesis of the disease has not been fully elucidated. However, the role of the collapse of the hair follicle immune privilege has been well described [1]. Although alopecia areata was considered as an organ-specific disorder limited to the hair follicles, numerous studies have indicated that the disease is associated with a systemic inflammation [2]. In patients with alopecia areata, an increased level of proinflammatory cytokines such as interleukin 1 beta (IL-1β), IL-6, tumor necrosis factor (TNF), and interferon gamma (IFN-γ) has been observed. Moreover, a higher prevalence of numerous diseases associated with systemic inflammation such as coronary artery disease, stroke, hyperlipidemia, diabetes mellitus, and metabolic syndrome has been reported in patients with alopecia areata compared with control patients.
Numerous therapeutic agents, both topical (glucocorticoids) and systemic (glucocorticoids, cyclosporine, methotrexate) [3] have been used off label for alopecia areata. Janus kinase (JAK) inhibitors are small molecules that are used in the treatment of numerous autoimmune conditions. Baricitinib, a JAK1/2 inhibitor, is the first drug, approved in 2022, by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of alopecia areata.
The treatment of autoimmune diseases such as alopecia areata is usually long lasting, so the safety profile of the therapy is important. It is well known that systemic glucocorticoids are associated with an increased risk of venous thromboembolism [4]. Indeed, pulmonary thromboembolism was described in a patient with alopecia areata treated with systemic steroids for 6 months [5]. Moreover, the FDA has warned about an increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors. It has been shown in patients with rheumatoid arthritis that the use of tofacitinib, a JAK 1/2 inhibitor, was associated with an increased risk of major adverse cardiovascular events (including venous thromboembolism) when compared with TNF inhibitors [6]. An increased risk of deep venous thromboembolism was also observed in patients with rheumatoid arthritis treated with baricitinib [7] and upadacitinib (a JAK1 inhibitor) [8]. To date, pulmonary embolism has been only reported in one patient with alopecia areata treated with baricitinib.
Objectives
The aim of the study was to evaluate the following markers of venous thromboembolism risk: soluble fibrin monomer complex (SFMC), thrombin–antithrombin complex (TATC), and prothrombin fragment 1 + 2 (F1 + 2) in patients with alopecia areata and compare them with healthy controls.
Methods
Patients
All patients with alopecia areata at the age of 18 years or older consulted in our department between March 2021 and September 2021 were screened for inclusion into this study. The exclusion criteria were as follows: autoimmune diseases other than alopecia areata, malignancy, pregnancy and puerperium, breastfeeding, a history of venous thromboembolism, hypercoagulable states or renal failure, the use of hormonal contraceptives, hormone-replacement therapy, systemic glucocorticoids, JAK inhibitors or anticoagulants, immobilization, smoking and a history of trauma, and surgery or hospitalization in the last 3 months. The control group comprised healthy persons matched for age, gender, and body mass index (BMI). Control group subjects followed the same exclusion criteria.
Demographic data and clinical variables (age, gender, weight, and height) were recorded for all individuals. BMI was calculated as: weight (kg)/height2 (m). Data considering the number and duration of the present episode of hair loss were collected in patients with alopecia areata. The severity of alopecia tool (SALT) was used to assess the severity of hair loss [9]. The activity of hair loss was evaluated and defined as follows: (1) progressive alopecia areata with an increase in total hair loss of more than 5%, (2) remitting alopecia areata with a decrease in total hair loss of more than 5%, or (3) stable with a change in total hair loss of less than 5% over the month prior to the examination.
Assessment of Thrombotic Markers
Venous blood samples were collected after an overnight 12 hour fast. The serum concentrations of SFMC, TATC, and F1 + 2 were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (EIAab, Wuhan, China) according to the manufacturer’s instructions.
Statistical Analysis
All statistical analyses were carried out using STATISTICA 13.1 (StatSoft, Cracow, Poland). The Shapiro–Wilk test was used to evaluate the normality of data distribution. Nonnormally distributed variables were presented as a median and interquartile range (IQR), while categorical data as counts and percentages. Nonparametric continuous variables were compared using the Mann–Whitney U test, while the chi-squared test was used to analyze categorical data. The correlation coefficient Spearman’s rank test was used to assess possible linear associations between two continuous variables. The values of p < 0.05 were considered statistically significant.
Compliance with Ethics Guidelines
The study protocol conformed to the principles of the World Medical Association’s Declaration of Helsinki and was approved by the Medical University of Warsaw Review Board for Ethics in Human Research (KB/142/2020). Written informed consent was obtained from all the participants of the study.
Results
In total, 51 patients with alopecia areata [35 women and 16 men; mean age: 38 (19–54) years] and 26 controls [18 women and 8 men; mean age: 37 (29–51) years] were enrolled in the study. With regards to the study design, the patient groups did not differ with respect to age, sex distribution, or BMI. The demographic and clinical characteristics of both groups are presented in Table 1.
Table 1.
Markers of venous thromboembolism and characteristics of patients with alopecia areata and healthy controls
| Parameter | Patients with alopecia areata (n = 51) | Healthy controls (n = 26) | Statistical significance |
|---|---|---|---|
| Thromboembolism markers | |||
| Soluble fibrin monomer complex (µg/ml), median (IQR) | 25.66 (20–34.86) | 21.46 (15.38–29.48) | < 0.05 |
| Thrombin–antithrombin complex (pg/ml), median (IQR) | 5690 (4177–9617) | 7417.50 (4453–12,530) | 0.42 |
| Prothrombin fragment 1 + 2 (pg/ml), median (IQR) | 70150 (43720–86,070) | 38620 (31550–58840) | < 0.001 |
| Demographic and clinical data | |||
| Age (years), median (IQR) | 38 (29–54) | 37 (29–51) | 0.8 |
| Sex (men), n (%) | 16 (31%) | 8 (31%) | 0.95 |
| BMI (kg/m2), median (IQR) | 24.38 (21.51–28.09) | 22.7 (20.43–26.12) | 0.15 |
| Age at the first episode of alopecia (years), mean (range) | 32 (3–71) | NA | |
| Number of episodes of hair loss, n (range) | 4 (1–30) | NA | |
| Duration of the present episode of alopecia (months), mean (range) | 34 (1–300) | NA | |
| SALT, mean (range) | 42 (1–100) | NA | |
| Activity of the disease, n (%) | |||
| Active | 21 (41%) | NA | |
| Stable | 22 (43%) | ||
| Remitting | 8 (16%) | ||
An increased level of SFMC was detected in patients with alopecia areata compared with the controls [25.66 (20–34.86) versus 21.46 (15.38–29.48) µg/ml; p < 0.05)] (Table 1 and Fig. 1). Moreover, a higher level of F1 + 2 was observed in patients with alopecia areata in comparison with the control group [70150 (43720–86070) versus 38620 (31550–58840) pg/ml; p < 0.001] (Table 1 and Fig. 2). No significant difference was observed in the TATC level between patients with alopecia areata and the control group.
Fig. 1.
Soluble fibrin monomer complex in patients with alopecia areata and healthy controls
Fig. 2.
Prothrombin fragment 1 + 2 in patients with alopecia areata and healthy controls
No significant correlation was detected among SFMC or F1 + 2 and SALT score, disease duration, or the number of hair loss episodes (Table 2). However, the F1 + 2 level positively correlated with BMI (r = 0.357, p < 0.05) in patients with alopecia areata.
Table 2.
Spearman’s correlation coefficients between soluble fibrin monomer complex/prothrombin fragment 1 + 2 and selected clinical parameters in patients with alopecia areata
| Parameter | Age (years) | BMI (kg/m2) | SALT score (%) | Disease duration (months) | Number of hair loss episodes (n) |
|---|---|---|---|---|---|
| Soluble fibrin monomer complex (pg/ml) | 0.045 | 0.250 | 0.021 | 0.124 | 0.847 |
| Prothrombin fragment 1 + 2 (pg/ml) | 0.186 | 0.357* | 0.004 | 0.120 | −0.057 |
*p < 0.05
Discussion
Alopecia areata has been considered as a disorder limited to the hair follicles. However, recent studies have indicated that the disease is associated with the dysregulation of numerous systemic proinflammatory cytokines [2].
It is well known that chronic systemic inflammation is associated with an increased risk of developing metabolic syndrome, cardiovascular disorders, depression, neurodegenerative and autoimmune diseases [10]. Indeed, a higher frequency of numerous autoimmune conditions, such as autoimmune thyroiditis, vitiligo, and lupus erythematosus has been reported in patients with alopecia areata compared with controls [11]. Moreover, depression and anxiety were more commonly diagnosed in patients with alopecia areata compared with the control group [12]. Finally, alopecia areata is associated with an increased frequency of metabolic syndrome, insulin resistance, diabetes mellitus and dyslipidemia [13]. Cardiovascular disorders, such as coronary artery disease, and stroke, were also more commonly reported in patients with alopecia areata in comparison with the control group [14].
There are numerous risk factors of venous thromboembolism including age over 40 years, surgery, trauma, immobilization, cancer, obesity, the use of oral contraceptives or hormone-replacement therapy, pregnancy and puerperium, a history of venous thromboembolism, and inherited and acquired disorders of hypercoagulation [15–17]. Recently, chronic inflammation has also been described as being associated with an increased risk of venous thromboembolism. Numerous effects of inflammation on the hemostatic balance have been described [18]. An increased level of fibrinogen and a decreased concentration of antithrombin were observed in inflammation. Moreover, inflammatory mediators, such as IL-6, were found to increase platelet formation and reactivity. It was confirmed that endotoxin, TNF, or CD40 ligand induce the expression of a tissue factor known as the primary cellular initiator of blood coagulation, while C-reactive protein (CRP) promotes both plasminogen activator inhibitor-1 and tissue factor [18].
Thromboembolic conditions are the leading cause of mortality, but they may be prevented [19]. A d-dimer is a protein fragment considered as a marker of venous thromboembolism. However, it has a low positive predictive value and low specificity. More specific markers of venous thromboembolism risk may be listed, i.e., SFMC, TATC, F1 + 2, P- and E-selectin [20, 21].
To date, data considering venous thromboembolism risk in patients with alopecia areata have been limited. A study performed by Schneeweiss et al. [22], which included 17,889 patients with alopecia areata and 1,570,387 patients without chronic inflammatory skin disease revealed no significant difference in the risk of venous thromboembolism between the groups. A study conducted by Shakoei et al. [23] showed an elevated level of d-dimers in patients with alopecia areata compared with the control group. However, no differences were detected in the fibrinogen and CRP levels between groups. Sudnik et al. [24] observed a higher level of P- and E-selectin in patients with alopecia areata than in healthy controls. Serum concentrations of E- and L-selectins correlated with the severity of the disease, while E-selectin correlated with the activity of hair loss. Finally, the presented study revealed a higher level of SFMC and F1 + 2 in patients with alopecia areata compared with the controls. Our study revealed no correlation between venous thromboembolism markers and the severity of alopecia areata.
The limitation of the present study is a relatively small number of patients included in the analysis. Further studies are needed to assess thromboembolism markers in patients with alopecia areata, especially before and during treatment with JAK inhibitors.
Conclusion
Alopecia areata may be associated with an increased risk of venous thromboembolism. Regular screening and the preventive management of venous thromboembolism may be beneficial in patients with alopecia areata, especially before and during JAK or systemic glucocorticoid therapy. Patients with alopecia areata should be encouraged to avoid risk factors of systemic chronic inflammation that may lead to thromboembolism, such as a diet rich in fats and sugar, smoking, stress, and sleep deprivation.
Acknowledgements
We thank the participants of the study.
Funding
Anna Waśkiel-Burnat received a grant (1M4/1/M/MBS/N/21) for this project from the Medical University of Warsaw. No funding or sponsorship was received for this study or publication of this article.
Medical Writing and Editorial Assistance
The authors did not receive medical writing or editorial assistance for this article.
Author Contributions
Conceptualization: Lidia Rudnicka, Małgorzata Olszewska, Anna Waśkiel-Burnat. Methodology: Anna Waśkiel-Burnat, Adriana Rakowska, Bianca Maria Piraccini. Formal analysis and investigation: Anna Waśkiel-Burnat, Leszek Blicharz, Magdalena Maciejewska, Michał Zaremba. Writing - original draft preparation: Anna Waśkiel-Burnat, Matilde Iorizzo, Michela Starace. Writing - review and editing: Adriana Rakowska, Leszek Blicharz, Magdalena Maciejewska, Michał Zaremba, Bianca Maria Piraccini, Małgorzata Olszewska, Lidia Rudnicka. Funding acquisition: Anna Waśkiel-Burnat. Supervision: Lidia Rudnicka, Anna Waśkiel-Burnat.
Disclosures
Anna Waskiel-Burnat, Adriana Rakowska, Michal Zaremba, Magdalena Maciejewska, Leszek Blicharz, Michela Starace, Matilde Iorizzo, Bianca Maria Piraccini, Malgorzata Olszewska and Lidia Rudnicka have nothing to disclose.
Compliance with Ethics Guidelines
The study protocol conformed to the principles of the World Medical Association’s Declaration of Helsinki and was approved by the Medical University of Warsaw Review Board for Ethics in Human Research (KB/142/2020). Written informed consent was obtained from all the participants of the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Dai Z, Chen J, Chang Y, Christiano AM. Selective inhibition of JAK3 signaling is sufficient to reverse alopecia areata. JCI Insight. 2021 doi: 10.1172/jci.insight.142205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waśkiel-Burnat A, Osińska M, Salińska A, Blicharz L, Goldust M, Olszewska M, et al. The role of serum Th1, Th2, and Th17 cytokines in patients with alopecia areata: clinical implications. Cells. 2021 doi: 10.3390/cells10123397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waśkiel-Burnat A, Kołodziejak M, Sikora M, Stochmal A, Rakowska A, Olszewska M, et al. Therapeutic management in paediatric alopecia areata: a systematic review. J Eur Acad Dermatol Venereol. 2021;35(6):1299–1308. doi: 10.1111/jdv.17187. [DOI] [PubMed] [Google Scholar]
- 4.Ayodele OA, Cabral HJ, McManus DD, Jick SS. Glucocorticoids and risk of venous thromboembolism in asthma patients aged 20–59 years in the United Kingdom’s CPRD 1995–2015. Clin Epidemiol. 2022;14:83–93. doi: 10.2147/clep.S341048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rakshith M. Pulmonary thromboembolism in a case of alopecia areata. Dig J Clin Med. 2019;1(1):62–69. [Google Scholar]
- 6.Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 7.Taylor PC, Weinblatt ME, Burmester GR, Rooney TP, Witt S, Walls CD, et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol. 2019;71(7):1042–1055. doi: 10.1002/art.40841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genovese MC, Fleischmann R, Combe B, Hall S, Rubbert-Roth A, Zhang Y, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139):2513–2524. doi: 10.1016/s0140-6736(18)31116-4. [DOI] [PubMed] [Google Scholar]
- 9.Olsen EA, Roberts J, Sperling L, Tosti A, Shapiro J, McMichael A, et al. Objective outcome measures: collecting meaningful data on alopecia areata. J Am Acad Dermatol. 2018;79(3):470–8.e3. doi: 10.1016/j.jaad.2017.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Lee H, Lee CH, Lee WS. Comorbidities in alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80(2):466–77.e16. doi: 10.1016/j.jaad.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Sellami R, Masmoudi J, Ouali U, Mnif L, Amouri M, Turki H, et al. The relationship between alopecia areata and alexithymia, anxiety and depression: a case-control study. Indian J Dermatol. 2014;59(4):421. doi: 10.4103/0019-5154.135525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waśkiel-Burnat A, Niemczyk A, Chmielińska P, Muszel M, Zaremba M, Rakowska A, et al. Lipocalin-2 and insulin as new biomarkers of alopecia areata. PLoS ONE. 2022;17(5):e0268086. doi: 10.1371/journal.pone.0268086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conic RRZ, Chu S, Tamashunas NL, Damiani G, Bergfeld W. Prevalence of cardiac and metabolic diseases among patients with alopecia areata. J Eur Acad Dermatol Venereol. 2021;35(2):e128–e129. doi: 10.1111/jdv.16864. [DOI] [PubMed] [Google Scholar]
- 15.Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007;44(2):62–69. doi: 10.1053/j.seminhematol.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barczyk A, Pierzchała W. Risk factors of venous thromboembolism. Wiad Lek. 2001;54(5–6):311–324. [PubMed] [Google Scholar]
- 17.Aksu K, Donmez A, Keser G. Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des. 2012;18(11):1478–1493. doi: 10.2174/138161212799504731. [DOI] [PubMed] [Google Scholar]
- 18.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131(4):417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 19.Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118(9):1340–1347. doi: 10.1161/circresaha.115.306841. [DOI] [PubMed] [Google Scholar]
- 20.Lin Z, Sun H, Li D, Cai Z, Chen M, Zhang W, et al. Thrombin antithrombin complex concentration as an early predictor of deep vein thrombosis after total hip arthroplasty and total knee arthroplasty. BMC Musculoskelet Disord. 2022;23(1):574. doi: 10.1186/s12891-022-05532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Feng G, Yan J, Wu L, Wang F, Ding D, et al. Plasminogen activator inhibitor-1, thrombin-antithrombin, and prothrombin fragment F1+2 have higher diagnostic values than d-dimer for venous thromboembolism after TKA. Clin Appl Thromb Hemost. 2022;28:10760296221097383. doi: 10.1177/10760296221097383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneeweiss MC, Kim SC, Wyss R, Jin Y, Chin K, Merola JF, et al. Incidence of venous thromboembolism in patients with dermatologist-diagnosed chronic inflammatory skin diseases. JAMA Dermatol. 2021;157(7):805–816. doi: 10.1001/jamadermatol.2021.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shakoei S, Ghiasi M, Ziaee K. Coagulation status in patients with alopecia areata: a cross-sectional study. Ital J Dermatol Venerol. 2021;156(5):588–592. doi: 10.23736/s2784-8671.20.06628-6. [DOI] [PubMed] [Google Scholar]
- 24.Sudnik W, Dańczak-Pazdrowska A, Silny W, Osmola-Mańkowska A, Pazdrowski J, Polańska A. The role of selectins in alopecia areata. Postepy Dermatol Alergol. 2015;32(1):27–32. doi: 10.5114/pdia.2014.40946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.