Abstract
Chronic spontaneous urticaria (CSU) is a complex skin disease characterized by the spontaneous appearance of wheals, angioedema, or both, for more than 6 weeks. Many patients experience a relapsing–remitting disease course for years. Owing to the unpredictability of wheal recurrence and the severity of pruritis, patients suffer considerable impairment in their quality of life. Physicians face multiple challenges, not least of which is a lack of clear guidance on what constitutes “treatment success”. There is a lack of awareness of which measures should be used to best assess the various aspects of CSU, including disease activity, disease control, and quality of life—which themselves each comprise multiple components—and how to apply the results of each score to treatment decision-making. Although the overarching aim of treatment is for patients to be completely free of signs and symptoms of CSU, a more realistic definition of “treatment success” is needed to guide ongoing, long-term disease management for each individual patient. In this review, we consider what lessons can be learned from the current evidence base to provide further direction toward a universal definition of “treatment success”.
Keywords: Medical dermatology, Outcome measurement, Patients, Quality of life, Urticaria
Key Summary Points
| Many clinical and patient-reported outcomes (PROs) pertaining to disease activity, disease control, and the impact on quality of life are used during the diagnosis, management, and monitoring of patients with chronic spontaneous urticaria. |
| Physicians face multiple challenges in the management of chronic spontaneous urticaria, including inconsistent measures of treatment success in clinical practice. |
| Consensus amongst physicians is needed about what constitutes treatment success. |
| Work towards this requires universal definitions of “remission” and “recurrence”, alongside research into predictors for these disease states. Guidance on when to step down treatment is required; and PROs should be associated with treatment targets and timepoints. |
Introduction
Chronic spontaneous urticaria (CSU) is a skin disease characterized by the spontaneous appearance of wheals, angioedema, or both, for more than 6 weeks [1]. Urticaria is a common complaint within dermatology and allergy/immunology practices, with 0.6–1.0% of the population suffering from CSU [2]. Average time from symptom onset to diagnosis has been reported as 2 years [3]; the diagnostic process includes assessment of cofactors, comorbidities, predictive measures of disease activity, and treatment response [1, 4].
Although the pathogenesis of CSU is not fully understood, it is thought to be caused by autoimmune mechanisms of mast cell activation and subsequent release of immune mediators such as histamine [4]. Current evidence indicates three subtypes of CSU: type I (autoallergic), which is mediated through immunoglobulin (Ig)E; type IIb (autoimmune), which is mediated primarily through IgG autoantibodies; and CSU due to unknown causes [4]. Although the clinical profile of these endotypes remains to be fully characterized, evidence suggests that patients with type IIb CSU have higher disease activity [5, 6].
Disease duration is typically 1–5 years [7] and likely longer for patients with more severe disease, especially those with a relapsing–remitting disease course [7, 8]. Although certain clinical characteristics and biomarkers have been associated with disease activity, disease duration, and treatment response [9], none have been validated; this means considerable variability in managing patients with CSU both across and within specialties, emphasizing the need for additional biomarker research.
The most recently updated guidelines for urticaria management are the international EAACI/GA2LEN/EuroGuiDerm/APAAACI guidelines [1], which were developed in conjunction with, and are endorsed by, the American Academy of Allergy, Asthma & Immunology, the American Academy of Dermatology, and the American College of Allergy, Asthma, and Immunology, among other organizations. The treatment algorithm for CSU includes first-line, standard-dose, second-generation H1-antihistamines (H1-AH); subsequent treatments include up-dosed H1-AH, omalizumab, and cyclosporine [1]. However, it remains unclear how broadly this treatment algorithm is implemented in practice, with many physicians solely relying on their clinical experience [10]. Treating patients with additional therapies such as leukotriene antagonists (montelukast) and H2-antagonists, which have limited evidence relating to their efficacy [1, 11], delays the use of more effective treatments and prolongs patients’ suffering. This problem may be especially prevalent in patients seen by multiple physicians, with many presenting initially in the primary or urgent care setting before eventually being referred to allergists and/or dermatologists [3, 10, 12].
Physicians are currently faced with many challenges in managing patients with CSU. There is a lack of clarity concerning the clinical importance of several objective (i.e., biomarkers) and subjective (i.e., patient-reported outcome [PRO]) measures used to assess the activity, control, and impact of this multifaceted disease.
For physicians and patients, the treatment aim is to achieve and maintain a state of remission. However, with a large proportion of patients unable to achieve this [8, 13], there is a need for structured, practical, and realistic guidance of progress towards remission, i.e., “treatment success”. In this article, we evaluate the most common treatment targets in published literature and clinical trials to provide further direction toward a universal definition of “treatment success”.
Methods
This article is based on previously conducted studies and does not contain any new studies with human participants or animals that were performed by any of the authors. Initial searches were performed in PubMed using the terms “chronic spontaneous urticaria” and “treatment” in the title or abstract. Papers published within the past 5 years were included in the initial screen. Searches were extended and supplemented as needed on the basis of the initial literature review, author expertise, and relevance. The figures have been reproduced with permission from Zuberbier T, et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77:734–766. © 2022. John Wiley & Sons.
What Measures Are Used in the Literature?
An extensive range of PROs are used to determine disease activity, disease control, and the impact of CSU on a patient’s quality of life (QoL). These measures include the weekly Urticaria Activity Score (UAS7), weekly Angioedema Activity Score (AAS7), Urticaria Control Test (UCT), Angioedema Control Test (AECT), Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL), Dermatology Life Quality Index (DLQI), and Angioedema Quality of Life Questionnaire (AE-QoL) (Table 1) [2]. This section aims to evaluate the PROs recommended by the EAACI/GA2LEN/EuroGuiDerm/APAAACI guidelines, those frequently used by physicians, and the measures most prevalent in clinical trials.
Table 1.
Patient-reported outcomes
| PRO | Format (time span) | Domain | Scoring systema | Scoring range | Correlating response | MCID |
|---|---|---|---|---|---|---|
| Disease activity | ||||||
| UAS7 [23, 43] | Diary (based on the last 7 days) | Pruritus intensity and number of hives | 0–3 | 0–42b |
0 = Itch and hive free 1–6 = Well-controlled 7–15 = Mild activity 16–27 = Moderate activity 28–42 = Severe activity |
9.5–10.5 |
| AAS7 [53, 54] | Diary (based on the last 7 days) | Severity of physical discomfort, ability to perform daily activities, cosmetic impact, and global assessment of severity | 0–3 | 0–105b | – | 8 |
| Disease control | ||||||
| UCT [44, 45] | 4-item questionnaire (based on the last 4 weeks) | Physical symptoms, impact on QoL, treatment effectiveness, symptom control | 0–4 | 0–16 |
16 = Completely controlled 12–15 = Well-controlled < 12 = Uncontrolled |
3 |
| AECT [55] | 4-item questionnaire (based on the last 4 weeks) | Frequency of angioedema, angioedema-related QoL impairment, the unpredictability of angioedema attacks, and angioedema control by current treatment | 0–4 | 0–16 |
0–9 = Poorly controlled 10–16 = Controlled disease |
– |
| QoL impairments | ||||||
| CU-Q2oL [56, 57] | 23-item questionnaire (based on the last 2 weeks) | Pruritus, swelling, daily life activities, sleep, appearance, and limitations | 1–5 | 0–100 | – | 15 |
| AE-QoL [58, 59] | 17-item questionnaire (based on the last 4 weeks) | Functioning, fatigue/mood, fear/shame, and food | 1–5 | 0–100 | – | 6 |
| DLQI [60, 61] | 10-item questionnaire (based on the last 7 days) | Symptoms/feelings, daily activities, leisure, work or school, personal relationships, and treatment side effects | 0–3 | 0–30b |
0–1 = No impact 2–5 = Little impact 6–10 = Moderate impact 11–20 = Very high impact 21–30 = Extremely high impact |
4 |
Validated PROs to measure CSU symptom severity, disease control, and impact on QoL
AAS7 weekly Angioedema Activity Score, AECT Angioedema Control Test, AE-QoL Angioedema Quality of Life Questionnaire, CSU chronic spontaneous urticaria, CU-Q2oL Chronic Urticaria Quality of Life Questionnaire, DLQI Dermatology Life Quality Index, QoL health-related quality of life, HSS7 weekly Hives Severity Score, ISS7 weekly Itch Severity Score, MCID minimal clinically important difference, PRO patient-reported outcome, QoL quality of life, UAS7 weekly Urticaria Activity Score, UCT Urticaria Control Test
aEach question is scored between the range
bHigher scores for the PRO indicate a worse outcome
Treatment Targets Set by International Guidelines
Currently, there is no curative therapy for CSU; existing treatments purport to control disease activity and prevent symptom recurrence [8, 14, 15]. Guidelines provide more clarity on achieving disease control than predicting recurrence [1], which is a major clinical question.
The measures described in the EAACI/GA2LEN/EuroGuiDerm/APAAACI guidelines include UAS7 and/or AAS7 for disease activity, UCT and/or AECT for disease control, and CU-Q2oL and/or AE-QoL for the impact of CSU on a patient’s QoL (Table 1). Guidance is given on which measures should be used in particular patient populations (e.g., patients who develop wheals, with or without angioedema). The usability of measures in different settings (i.e., clinical trials, routine clinical practice) is also considered, e.g., use of the four-item UCT as a measure of disease control in routine clinical practice due to ease of administration and a clearly defined cutoff for patients with “well-controlled” versus “poorly controlled” disease [1].
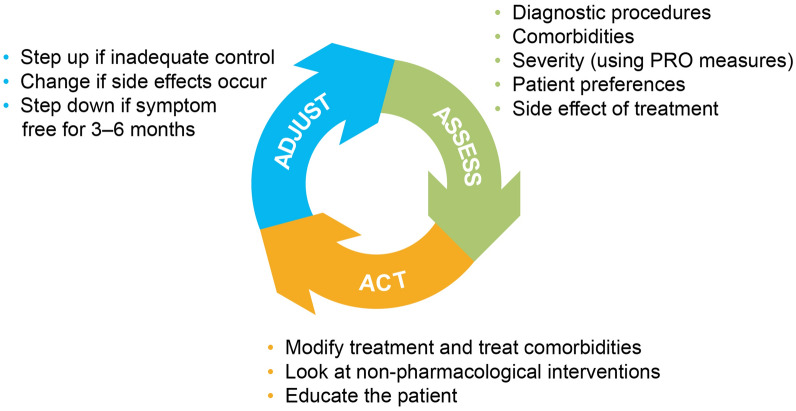
Treatment targets in CSU entail complete symptom control (UAS7 = 0) and normalizing QoL. A UAS7 = 0 score is defined as “complete control”, yet many patients do not reach this target [3, 16]; should we be asking whether this is an appropriate target for all patients with CSU? Moreover, a paradigm of “adjust, assess, and act” involves continuously assessing a patient’s disease status (using UCT) to determine whether treatment adjustments are required (Figs. 1, 2) [1], but recommendations for the continuous assessment of disease status are not well defined [1]. A comprehensive set of targets and frequency of monitoring must be defined for all relevant PROs to define treatment success.
Fig. 1.
The adjust, assess, and act paradigm [1]. A clinical decision-making aid for treatment adjustments for patients with urticaria. PRO, patient-reported outcome.
Reproduced with permission from Zuberbier T, et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77:734–766. © 2022. John Wiley & Sons
Fig. 2.
UCT score [1]. The UCT is a four-item tool with a defined cutoff for patients with “completely controlled”, “well-controlled”, and “uncontrolled” disease, with a recall period of 4 weeks.
Reproduced with permission from Zuberbier T, et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77:734–766. © 2022. John Wiley & Sons. H1-AH, second-generation H1-antihistamines; UCT, Urticaria Control Test
Treatment Targets: Physician and Patient Perspectives
A key feature of CSU is the unpredictability of recurring hives and itch intensity; this profoundly affects a patient’s QoL: their physical comfort, daily activities, and sleep [1, 3]. Consequently, reduction of itch/burning and resolution of visible hives/wheals are top treatment aims for patients [17]. The DERMLINE online survey reported that approximately half of patients were “not at all satisfied”, “not satisfied”, or “mildly satisfied” with their current medication, due to lack of response or side effects [18]. Patients have also reported that their physician did not understand the true emotional and physical burden of CSU [7]. These findings raise the question of whether physician and patient treatment aims align.
Choosing the correct treatment and when to switch treatments is a multifaceted decision. Inconsistencies in patient care have been attributed to physicians not relying on the guidelines in their decision-making [19]. Physicians have reported that guideline recommendations oversimplify the complex nature of CSU [10]. Furthermore, patients can present with comorbid disorders such as Hashimoto’s thyroiditis, type I diabetes, and rheumatoid arthritis, which share a common pathogenic mechanism based on the presence of autoantibodies and chronic inflammation [5, 20]. However, the pathophysiology of CSU is not fully understood, and its acceptance as an autoimmune disease is not universal among physicians, causing differences in treatment approaches.
A treat-to-target approach is used in several chronic diseases to improve outcomes [21], with a recently defined consensus for psoriasis [22]. Currently, there is no comprehensive treat-to-target approach for CSU that incorporates all the necessary PROs and associated targets to evaluate disease activity, disease control, and QoL. One recently proposed approach is to achieve and maintain symptom control (UAS7 ≤ 6) or symptom remission (UAS7 = 0) [21]. Although UAS7 is an effective measure of disease activity [23], PROs to determine disease control and QoL are required to encapsulate all aspects of CSU.
Clinical and Laboratory Biomarkers to Inform Treatment Decisions
Clinical characteristics and biomarkers are used increasingly within clinical practice to inform treatment decisions. Although these indicators have not been definitively established, some appear to be associated with patient outcomes. Prolonged disease duration is associated with an insufficient response to standard-dose H1-AH, comorbid chronic inducible urticaria (CIndU)—which itself is often linked to lack of response to standard-dose H1-AH—late disease onset (> 45 years), intolerance to non-steroidal anti-inflammatory drugs, presence of angioedema, and a relapsing–remitting disease course [1, 8]. With regard to laboratory biomarkers, severe disease has been associated with elevated C-reactive protein (CRP) and D-dimer [8, 24]. In a study of 549 patients with CSU, 20.2% had comorbid CIndU; this subgroup required higher doses of H1-AH and more patients experienced persistent symptoms after 5 years of follow-up than patients with isolated CSU [24]. Despite our increasing understanding of biomarkers in CSU, their clinical application remains unclear. Providers still require guidance on the appropriate timing of biomarker evaluation and how this can inform treatment decisions.
Another consideration is using biomarkers for the differential diagnosis of type IIb CSU, which has been associated with more severe disease [5, 6]. Type IIb CSU is characterized by low IgE, elevated IgG-anti-thyroid peroxidase (TPO), positive basophil histamine releasing assay (BHRA), positive autologous serum skin test, presence of IgG anti-FcεRI autoantibodies, basopenia, and eosinopenia [4, 9, 25]. Poor response to H1-AH is associated with elevated CRP and D-dimer; poor response to omalizumab is associated with a low IgE, a low peripheral blood eosinophil count, basopenia, elevated TPO, and positive BHRA; and a good response to ciclosporin is associated with positive BHRA [4, 8, 9]. The link between biomarkers and treatment response demonstrates the potential value of biomarkers in clinical practice.
Endpoints and Treatment Targets Used in Key Clinical Trials
Since the purpose of late-phase clinical trials is to inform clinical practice, the endpoints selected should measure meaningful patient outcomes [26]. We explored common measures (e.g., UAS7) and treatment targets (e.g., UAS7 ≤ 6) used in key trials of H1-AH and biologics (Table 2).
Table 2.
Key clinical trial endpoints
| Trial name | Investigated therapy | Phase | Primary endpoint | Key secondary endpoint(s) | NCT number |
|---|---|---|---|---|---|
| XTEND-CIU [39, 62] | Omalizumab | IV | Percentage of participants with CIU/CSU clinical worsening by UAS7 ≥ 12 for at least 2 consecutive weeks from W24–W48 |
Time to CIU/CSU clinical worsening by UAS7 ≥ 12 for at least 2 consecutive weeks Percentage of participants with CIU/CSU clinical worsening by UAS7 > 6 for at least 2 consecutive weeks Change from W24–W48 in UAS7 |
NCT02392624 |
| OPTIMA [38, 63] | Omalizumab | III | Number of participants with UAS7 ≤ 6 after the initial dosing period, relapsed (UAS7 ≥ 16) when treatment was discontinued and who achieved UAS7 ≤ 6 at W44 |
The difference in UAS7 between the start and end of the second dosing period Number of participants with UAS7 ≤ 6 after the second dosing period Time to relapse (UAS7 ≥ 16) after drug withdrawal in participants who responded to the initial dosing period |
NCT02161562 |
| X-ACT [64, 65] | Omalizumab | III | Change from baseline to W36 in the CU-Q2oL |
Number of angioedema-burdened days from baseline to W36 Change from baseline to W36 in the AAS7 Change from baseline to W36 in the AE-Q2oL Change from baseline to W36 in the DLQI |
NCT01723072 |
| ASTERIA I [34] | Omalizumab | III | Change from baseline to W12 in the ISS7 |
Change from baseline to W12 in the UAS7 Change from baseline to W12 in the weekly number of hives score Time to MCID response (≥ 5-point decrease) in ISS7 by W12 Percentage of participants with UAS7 ≤ 6 at W12 |
NCT01287117 |
| ASTERIA II [35] | Omalizumab | III | Change from baseline to W12 in the ISS7 |
Change from baseline to W12 in the UAS7 Change from baseline to W12 in the weekly number of hives Time to achieve MCID (≥ 5-point decrease) in the ISS7 The proportion of participants with UAS7 ≤ 6 |
NCT01292473 |
| GLACIAL [37] | Omalizumab | III | Safety |
Change from baseline to W12 in the ISS7 Change from baseline to W12 in the UAS7 Change from baseline to W12 in the weekly number of hives Time to achieve MCID (≥ 5-point decrease) in ISS7 The proportion of participants with UAS7 ≤ 6 |
NCT01264939 |
| N/A [66] | Ligelizumab | IIb | The proportion of participants with HSS7 = 0 at W12 |
The proportion of participants with HSS7 = 0 at W12 and W20 Change from baseline in HSS7, ISS7, UAS7 and AAS7 |
NCT02477332 |
| N/A [27, 67] | Benralizumab | IV | Change from baseline to W20 in the UAS7 | Safety and tolerability | NCT03183024 |
| N/A [28] | Fenebrutinib | II | Change from baseline to W8 in the UAS7 |
The proportion of participants with UAS7 ≤ 6 at W8 Change from baseline to W4 in the UAS7 |
|
| N/A [31] | Remibrutinib | IIb | Change from baseline to W4 in UAS7 |
Change from baseline to W12 in UAS7 The proportion of participants with UAS7 = 0 The proportion of participants with UAS7 ≤ 6 Safety and tolerability |
NCT03926611 |
| REMIX-1 [33] | Remibrutinib | III |
Change from baseline to W12 in UAS7 Absolute change in ISS7 at W12 Absolute change in HSS7 at W12 |
The proportion of participants with UAS7 ≤ 6 at W12 The proportion of participants with UAS7 = 0 at W12 The proportion of participants with UAS7 ≤ 6 at W2 The proportion of participants with DLQI = 0–1 at W12 The proportion of participants with AAS = 0 at W12 |
NCT05030311 |
| REMIX-2 [32] | Remibrutinib | III |
Change from baseline to W12 in UAS7 Absolute change in ISS7 at W12 Absolute change in HSS7 at W12 |
The proportion of participants with UAS7 ≤ 6 at W12 The proportion of participants with UAS7 = 0 at W12 The proportion of participants with UAS7 ≤ 6 at W2 The proportion of participants with DLQI = 0–1 at W12 The proportion of participants with AAS = 0 at W12 |
NCT05032157 |
| N/A [29] | Bilastine/levocetirizine | III | Change from baseline to W6 in the UAS7 |
Change from baseline to W6 in the DLQI Change from baseline to W6 in the VAS |
N/A |
| MUCIS [68, 69] | Methotrexate | III | Number of participants with complete urticaria remission at W18 |
Safety and tolerability Number of participants with pruritus at W18 and W26 Number of participants with complete remission at W26 |
NCT01960283 |
| N/A [30] | Levocetirizine | IV | Change from baseline to W4 in the UAS and TSS |
Change from baseline to W4 in the patient’s global assessment of disease activity Change from baseline to W4 in the physician’s global assessment of disease activity |
N/A |
| N/A [36] | Bilastine | II/III | Change from baseline to W2 in the TSS |
Change from baseline to days 1–3 in the TSS Change from baseline to W1 in the TSS |
N/A |
AAS7 weekly Angioedema Activity Score, AE-QoL Angioedema Quality of Life Questionnaire, CIU chronic idiopathic urticaria, CSU chronic spontaneous urticaria, CU-Q2oL Chronic Urticaria Quality of Life questionnaire, DLQI Dermatology Life Quality Index, HSS7 weekly Hive Severity Score, ISS7 weekly Itch Severity Score, MCID minimal clinically important difference, N/A not applicable, TSS urticaria Total Severity Score, UAS Urticaria Activity Score, UAS7 weekly Urticaria Activity Score, VAS Visual Analog Scale, W week
The most commonly used primary and secondary endpoints were change from baseline to specified timepoints in UAS7 [27–33]. Other primary endpoints included change from baseline in weekly Itch Severity Score (ISS7) [32–35] and urticaria Total Severity Score [30, 36]. UAS7 was the most commonly used PRO, particularly in recent clinical trials of biologics [27–29, 31–33]. In contrast to the primary endpoints, secondary endpoints were numerous and varied widely between trials, including change from baseline in UAS7 [28, 31, 34, 35, 37] or DLQI [35, 37]; time to a minimal clinically important difference (MCID) (≥ 5-points) reduction of ISS7 [34, 35, 37]; and proportion of patients with UAS7 ≤ 6 [28, 31–35, 37, 38]. QoL measures are commonly included in clinical trials but used inconsistently, which belies their importance to patients. Across late-stage trials, DLQI was the main indicator of QoL, generally as a secondary endpoint [32–35, 37, 39]. Although CU-Q2oL is a urticaria-specific tool recommended in the guidelines [1, 40], DLQI may be used more frequently because of its familiarity [41].
Generally, endpoints used in CSU clinical trials focus on efficacy, with minimal use of QoL and angioedema-specific measures. Unsurprisingly, UAS7 (e.g., change from baseline and UAS7 ≤ 6) is the most frequently used PRO [28, 31, 34, 35, 37, 38]. A UAS7 ≤ 6 score is defined as “well-controlled urticaria”, which indicates a good response to treatment [23]. Despite the guideline recommendation [1], UCT was not included as a primary or secondary endpoint in any key clinical trials. PRO use remains largely unchanged since the early H1-AH trials and may benefit from being made more consistent between different specialties.
Do Treatment Targets Used in Current Literature and Clinical Trials Translate into Clinical Practice?
The treatment targets described in current literature and clinical trials indicate a lack of consensus between the guidelines and clinical trial design, but what is the picture in clinical practice?
A systematic review of real-world evidence of omalizumab in CSU (N = 1507) provided valuable insights into PRO use in clinical practice [15]. Overall, treatment response was reported in 76.2% of studies. UAS was the most commonly used PRO in clinical practice, with 26.2% and 11.9% of studies using UAS7 and UAS, respectively [15]. UCT was used infrequently in only 1.2% of studies [15]. DLQI and CU-Q2oL were reported in 7.1% and 6.0% of studies, respectively [15].
AWARE and ASSURE-CSU are observational studies conducted to investigate disease burden and treatment schedules for patients with CSU [3, 19]. Both studies indicate that PROs recommended in the guidelines are increasingly common in clinical practice.
In the AWARE study, disease burden was determined by monitoring symptom control (UAS7 and Angioedema Activity Score [AAS]), disease control (UCT), QoL (DLQI, CU-Q2oL, and AE-QoL), and work productivity (Work Productivity and Activity Impairment Questionnaire [WPAI]) [19]. Of note, UAS7 and AAS scoring tools measure disease activity [1] but were described as measures of symptom control [19]. At baseline, 22.0% of patients had a score of UCT ≥ 12, compared to 71.3% after 24 months [19]. However, in a sub-analysis, less than 1 in 3 patients who should have been switched to a more effective third-line treatment were actually switched [42]. These findings indicate either guideline recommendations may not be integrated into practice or there may be a lack of concise guidance on when patients should escalate treatment.
The ASSURE-CSU study reviewed PROs, including CU-Q2oL, AE-QoL, UAS7, DLQI, European Quality of Life Five Dimensions, Urticaria Patient Daily Diary, and WPAI [3]. Overall, AWARE and ASSURE-CSU demonstrated the practical value of UAS7 and UCT, and that DLQI and CU-Q2oL were the most common PROs for QoL [3, 19].
Most measures used in clinical trials are not used in clinical practice, likely due to feasibility challenges. The lack of standardization of treatment targets in clinical trials is also reflected in clinical practice. This heterogeneity in approach highlights the need to reach a consensus in implementing a definition of “treatment success”.
Challenges in Translating Definitions of “Treatment Targets” and “Treatment Success” into Clinical Practice
With a large proportion of physicians relying on their clinical experience to inform clinical decision-making [10], the variation in patient outcomes is unsurprising. How, then, can we define “treatment success”?
Based on current guidelines, the treatment target is to achieve UAS7 = 0, complete control (UCT = 16), and normalize QoL [1]. However, the targets of UAS7 = 0 and UCT = 16 do not reflect the realities of clinical management nor the complexity of CSU. In addition, the guidelines do not specify a target to determine a “normalization of QoL”. To facilitate the long-term management of patients with CSU, PROs need to be accurately defined and implemented correctly into clinical practice: this definition would include a list of PROs that measure disease activity, disease control, QoL, and angioedema, alongside targets and any associated actions.
The PRO scores are all associated with disease status levels, which give physicians a good understanding of a patient’s disease progression over time. Using the MCID, the smallest change in score that can be considered clinically relevant, may be informative here (Table 1). For example, to determine disease activity and control, a target of UAS7 ≤ 6, defined as well-controlled urticaria, or the MCID (9.5–10.5), is a good indicator of treatment response [23, 43]. In addition, a target of UCT ≥ 12, defined as well-controlled, or a change from baseline of 3 points, could be of equal clinical value to aid a decision to step down treatment [44, 45]. A choice between the PRO score or MCID should ideally be practical, i.e., whichever is easiest to determine.
With 43–59% of patients with CSU experiencing angioedema [3, 18, 19], the lack of angioedema-specific measures is surprising. The impact of angioedema on QoL, productivity, and healthcare utilization is considerable [46]. PROs, such as AAS, AECT, and AE-QoL, are used infrequently, perhaps indicating that physicians deem other PROs adequate in measuring angioedema. More widespread use of angioedema-specific PROs is a clear area for improvement.
Clinical characteristics and laboratory measures have been associated with predicting disease duration and severity, and response to treatment. Many tests offer little or no predictive value for the individual patient during the diagnostic process [4]. Informing physicians of updates in clinically informative biomarkers should be a priority in the coming years. As the evidence base grows, predictive biomarkers may be utilized alongside specific treatment targets, which could significantly impact clinical decision-making.
Moving Toward a Universal Definition of “Treatment Success”: Remaining Questions
Current literature includes minimal guidance about what constitutes “treatment success”. In this review, we have identified several unanswered questions that should guide us toward a definition of “treatment success” and provide practical insights to support its implementation.
“Remission” remains the aim of treatment but can mean many things. Previously reported definitions of “remission” have included the absence of hives and angioedema in the last 3 months while patients were not undergoing therapy; the proportion of patients completely or fully cleared of CSU based on a self-assessment of disease symptoms, with no information provided about whether treatment is ongoing [13]; absence of urticaria for at least 4 weeks without medication [47]; and absence of urticaria treatment from any medical services for at least 1 year [48]. Many questions remain for healthcare professionals: is remission classified as permanent or temporary; for how long do patients need to be without medication; can biologic therapies lead to permanent remission; does an extended treatment course, and having neither symptoms nor active disease for a longer period, increase the chances of being in permanent remission? A recent consensus report defined remission as “the total absence of disease signs or symptoms in the absence of treatment” for 2 weeks with standard H1-AH, 4 weeks with up-dosed H1-AH, and 3–6 months with biological therapy [49]. Still, the implementation of this definition remains a challenge. For example, early evidence from omalizumab clinical trials demonstrated that patients might experience clinical worsening following treatment discontinuation after up to 6 months [50], or patients may need continuous treatment [51].
Another challenge healthcare providers face is deciding when and how to step down treatment. In the guidelines, UCT score is the only measure that informs treatment switching (Fig. 2), which poses various clinical questions: should all medications stop once disease activity has subsided; before stepping down, how long should patients be monitored if they respond; how does management change for patients predisposed to chronic spontaneous “indefinite” hives, analogous to thyroid issues, and do these patients need to be on chronic “suppression” therapy?
An understanding of recurrence is equally important. Recurrence has been defined as symptom recurrence “at least 6 months after cessation of controller therapy and resolution of prior chronic urticaria symptoms” [52]. Yet, in a recent consensus report, a definition of “recurrence” could not be agreed upon [49]. If a patient’s symptoms recur after an undefined period, do physicians treat this as a recurrence of CSU and continue treatment considering prior therapies, or consider it new acute urticaria?
Insights into improved long-term clinical management can likely be gained from comparing biomarkers and clinical features of patients with a rapid and complete response versus treatment-refractory patients; this may allow more tailored treatment approaches.
Finally, to what extent is QoL a factor in treatment success and what is the most important aspect of treatment for the patient? The reduction of itching/burning and healing of all visible skin alterations have been reported as two principal treatment aims for patients [17]; however, treatment side effects, the burden of multiple medications, and preventing recurrence may be of utmost importance to an individual patient. Accurate measurement of these factors and their incorporation into shared decision-making can help patients feel confident in their treatment plan and reach true treatment success. Although we acknowledge that completion of multiple PROs may burden the patient and clinical team, incorporating QoL and other PRO measures would help identify treatment success.
Conclusion
Prior to the development of a universal definition of “treatment success”, several questions need to be answered. A universal definition of “remission” and “recurrence” is needed, alongside research into predictors for achieving these states. Further guidance is needed on when to step down treatment. PROs should be associated with treatment targets, timepoints to determine whether current treatment is effective, and actions linked to these outcomes. Lastly, the impact CSU has on a patient’s QoL needs to be assessed, ideally over time.
Acknowledgements
Funding
Medical writing support and the journal’s Rapid Service Fee were funded by Novartis Pharmaceuticals Corporation.
Medical Writing and Editorial Assistance
Medical writing support was provided by Ella Brooks, BOLDSCIENCE®, and was funded by Novartis Pharmaceuticals Corporation. This manuscript was developed in accordance with Good Publication Practice guidelines. Authors had full control of the content and made the final decision on all aspects of this publication.
Author Contributions
April W. Armstrong, Weily Soong, and Jonathan A. Bernstein were involved in agreeing the topics to be covered; suggesting and reviewing published literature to be included; drafting, reviewing, and amending the manuscript; and approving the final manuscript for submission.
Disclosures
April W. Armstrong has served as a research investigator and/or scientific advisor to AbbVie, Almirall, Arcutis, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, EPI Health, Incyte, Leo, UCB, Janssen, Lilly, Nimbus, Novartis, Ortho Dermatologics, Sun, Dermavant, Dermira, Sanofi, Regeneron, Pfizer, and ModMed. Weily Soong has been an advisor and/or clinical investigator and/or received speaker’s honoraria and/or received a consulting fee and/or grants and/or participated as a clinical investigator for/from AbbVie, Amgen, AstraZeneca, Genentech, GlaxoSmithKline, Lilly, Incyte, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, and Teva. Jonathan A. Bernstein has served as principal investigator, advisor, and speaker for Sanofi-Regeneron, AstraZeneca, Novartis, Genentech, CSL Behring, Takeda/Shire, Biocryst and Pharming; principal investigator and advisor for Amgen, Celldex, Ionis, Biomarin, Kalvista, ONO, Escient, Cycle, TLL Pharmaceutical, and Merck; consultant for Pharvaris.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The figures have been reproduced with permission from Zuberbier T, et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77:734–766. © 2022. John Wiley & Sons.
References
- 1.Zuberbier T, Abdul Latiff AH, Abuzakouk M, et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77:734–766. doi: 10.1111/all.15090. [DOI] [PubMed] [Google Scholar]
- 2.Katelaris CH, Lima H, Marsland A, et al. How to measure disease activity, impact, and control in patients with recurrent wheals, angioedema, or both. J Allergy Clin Immunol Pract. 2021;9:2151–2157. doi: 10.1016/j.jaip.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Maurer M, Abuzakouk M, Bérard F, et al. The burden of chronic spontaneous urticaria is substantial: real-world evidence from ASSURE-CSU. Allergy. 2017;72:2005–2016. doi: 10.1111/all.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metz M, Altrichter S, Buttgereit T, et al. The diagnostic workup in chronic spontaneous urticaria - what to test and why. J Allergy Clin Immunol Pract. 2021;9:2274–2283. doi: 10.1016/j.jaip.2021.03.049. [DOI] [PubMed] [Google Scholar]
- 5.Kolkhir P, Altrichter S, Asero R, et al. Autoimmune diseases are linked to type IIb autoimmune chronic spontaneous urticaria. Allergy Asthma Immunol Res. 2021;13:545–559. doi: 10.4168/aair.2021.13.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoepke N, Asero R, Ellrich A, et al. Biomarkers and clinical characteristics of autoimmune chronic spontaneous urticaria: results of the PURIST study. Allergy. 2019;74:2427–2436. doi: 10.1111/all.13949. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein S, Eftekhari S, Mitchell L, et al. Perspectives on living with chronic spontaneous urticaria: from onset through diagnosis and disease management in the US. Acta Derm Venereol. 2019;99:1091–1098. doi: 10.2340/00015555-3282. [DOI] [PubMed] [Google Scholar]
- 8.Gonçalo M, Gimenéz-Arnau A, Al-Ahmad M, et al. The global burden of chronic urticaria for the patient and society. Br J Dermatol. 2021;184:226–236. doi: 10.1111/bjd.19561. [DOI] [PubMed] [Google Scholar]
- 9.Fok JS, Kolkhir P, Church MK, Maurer M. Predictors of treatment response in chronic spontaneous urticaria. Allergy. 2021;76:2965–2981. doi: 10.1111/all.14757. [DOI] [PubMed] [Google Scholar]
- 10.Kolkhir P, Pogorelov D, Darlenski R, et al. Management of chronic spontaneous urticaria: a worldwide perspective. World Allergy Organ J. 2018;11:14. doi: 10.1186/s40413-018-0193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 12.Macy E. Practical management of new-onset urticaria and angioedema presenting in primary care, urgent care, and the emergency department. Perm J. 2021;25:21058. doi: 10.7812/TPP/21.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balp M-M, Halliday AC, Severin T, et al. Clinical remission of chronic spontaneous urticaria (CSU): a targeted literature review. Dermatol Ther (Heidelb) 2022;12:15–27. doi: 10.1007/s13555-021-00641-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolkhir P, Altrichter S, Munoz M, et al. New treatments for chronic urticaria. Ann Allergy Asthma Immunol. 2020;124:2–12. doi: 10.1016/j.anai.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein JA, Kavati A, Tharp MD, et al. Effectiveness of omalizumab in adolescent and adult patients with chronic idiopathic/spontaneous urticaria: a systematic review of “real-world” evidence. Expert Opin Biol Ther. 2018;18:425–448. doi: 10.1080/14712598.2018.1438406. [DOI] [PubMed] [Google Scholar]
- 16.Kolkhir P, Laires PA, Salameh P, et al. The benefit of complete response to treatment in patients with chronic spontaneous urticaria-CURE results. J Allergy Clin Immunol Pract. 2023;11:610–620.e5. doi: 10.1016/j.jaip.2022.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Rönsch H, Berndt K, Bauer A. Treatment satisfaction in chronic urticaria during guideline-based therapy. J Dtsch Dermatol Ges. 2021;19:833–840. doi: 10.1111/ddg.14415. [DOI] [PubMed] [Google Scholar]
- 18.Wagner N, Zink A, Hell K, et al. Patients with chronic urticaria remain largely undertreated: results from the DERMLINE online survey. Dermatol Ther. 2021;11:1027–1039. doi: 10.1007/s13555-021-00537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurer M, Costa C, Gimenez Arnau A, et al. Antihistamine-resistant chronic spontaneous urticaria remains undertreated: 2-year data from the AWARE study. Clin Exp Allergy. 2020;50:1166–1175. doi: 10.1111/cea.13716. [DOI] [PubMed] [Google Scholar]
- 20.Bracken SJ, Abraham S, MacLeod AS. Autoimmune theories of chronic spontaneous urticaria. Front Immunol. 2019;10:627. doi: 10.3389/fimmu.2019.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lima H, Gooderham M, Dutz J, et al. Management of chronic spontaneous urticaria (CSU): a treat to target approach using a patient reported outcome. Allergy Asthma Clin Immunol. 2017;13:38. doi: 10.1186/s13223-017-0210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gisondi P, Talamonti M, Chiricozzi A, et al. Treat-to-target approach for the management of patients with moderate-to-severe plaque psoriasis: consensus recommendations. Dermatol Ther. 2021;11:235–252. doi: 10.1007/s13555-020-00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollis K, Proctor C, McBride D, et al. Comparison of urticaria activity score over 7 days (UAS7) values obtained from once-daily and twice-daily versions: results from the ASSURE-CSU study. Am J Clin Dermatol. 2018;19:267–274. doi: 10.1007/s40257-017-0331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curto-Barredo L, Archilla LR, Vives GR, et al. Clinical features of chronic spontaneous urticaria that predict disease prognosis and refractoriness to standard treatment. Acta Derm Venereol. 2018;98:641–647. doi: 10.2340/00015555-2941. [DOI] [PubMed] [Google Scholar]
- 25.Maurer M, Eyerich K, Eyerich S, et al. Urticaria: Collegium Internationale Allergologicum (CIA) update 2020. Int Arch Allergy Immunol. 2020;181:321–333. doi: 10.1159/000507218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLeod C, Norman R, Litton E, Saville BR, Webb S, Snelling TL. Choosing primary endpoints for clinical trials of health care interventions. Contemp Clin Trials Commun. 2019;16:100486. doi: 10.1016/j.conctc.2019.100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernstein JA, Singh U, Rao MB, et al. Benralizumab for chronic spontaneous urticaria. N Engl J Med. 2020;383:1389–1391. doi: 10.1056/NEJMc2016395. [DOI] [PubMed] [Google Scholar]
- 28.Metz M, Sussman G, Gagnon R, et al. Fenebrutinib in H(1) antihistamine-refractory chronic spontaneous urticaria: a randomized phase 2 trial. Nat Med. 2021;27:1961–1969. doi: 10.1038/s41591-021-01537-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Podder I, Das A, Ghosh S, Biswas D, Sengupta S, Chowdhury SN. Effectiveness, safety, and tolerability of bilastine 20 mg vs levocetirizine 5 mg for the treatment of chronic spontaneous urticaria: a double-blind, parallel group, randomized controlled trial. Dermatol Ther. 2020;33:e13946. doi: 10.1111/dth.13946. [DOI] [PubMed] [Google Scholar]
- 30.Sarkar TK, Sil A, Pal S, Ghosh C, Das NK. Effectiveness and safety of levocetirizine 10 mg versus a combination of levocetirizine 5 mg and montelukast 10 mg in chronic urticaria resistant to levocetirizine 5 mg: a double-blind, randomized, controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:561–568. doi: 10.4103/ijdvl.IJDVL_551_16. [DOI] [PubMed] [Google Scholar]
- 31.Maurer M, Berger W, Giménez-Arnau A, et al. Remibrutinib, a novel BTK inhibitor, demonstrates promising efficacy and safety in chronic spontaneous urticaria. J Allergy Clin Immunol. 2022;150:1498–1506. doi: 10.1016/j.jaci.2022.08.027. [DOI] [PubMed] [Google Scholar]
- 32.A phase 3 study of efficacy and safety of remibrutinib in the treatment of CSU in adults inadequately controlled by H1-antihistamines (REMIX-2) [Internet]. ClinicalTrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT05032157. Accessed 11 July 2022.
- 33.A phase 3 study of efficacy and safety of remibrutinib in the treatment of CSU in adults inadequately controlled by H1 antihistamines (REMIX-1) [Internet]. ClinicalTrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT05030311. Accessed 11 July 2022.
- 34.Saini SS, Bindslev-Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67–75. doi: 10.1038/jid.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maurer M, Rosén K, Hsieh H-J, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924–935. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 36.Hide M, Yagami A, Togawa M, et al. Efficacy and safety of bilastine in Japanese patients with chronic spontaneous urticaria: a multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II/III study. Allergol Int. 2017;66:317–325. doi: 10.1016/j.alit.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan A, Ledford D, Ashby M, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132:101–109. doi: 10.1016/j.jaci.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Sussman G, Hébert J, Gulliver W, et al. Omalizumab re-treatment and step-up in patients with chronic spontaneous urticaria: OPTIMA trial. J Allergy Clin Immunol Pract. 2020;8:2372–2378. doi: 10.1016/j.jaip.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 39.Casale TB, Murphy TR, Holden M, Rajput Y, Yoo B, Bernstein JA. Impact of omalizumab on patient-reported outcomes in chronic idiopathic urticaria: results from a randomized study (XTEND-CIU) J Allergy Clin Immunol Pract. 2019;7:2487–2490. doi: 10.1016/j.jaip.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 40.Moestrup K, Ghazanfar MN, Thomsen SF. Patient-reported outcomes (PROs) in chronic urticaria. Int J Dermatol. 2017;56:1342–1348. doi: 10.1111/ijd.13668. [DOI] [PubMed] [Google Scholar]
- 41.Szabó Á, Brodszky V, Rencz F. A comparative study on the measurement properties of Dermatology Life Quality Index (DLQI), DLQI-Relevant and Skindex-16. Br J Dermatol. 2022;186:485–495. doi: 10.1111/bjd.20765. [DOI] [PubMed] [Google Scholar]
- 42.Maurer M, Giménez-Arnau A, Ensina LF, Chu C-Y, Jaumont X, Tassinari P. Chronic urticaria treatment patterns and changes in quality of life: AWARE study 2-year results. World Allergy Organ J. 2020;13:100460. doi: 10.1016/j.waojou.2020.100460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathias SD, Crosby RD, Zazzali JL, et al. Evaluating the minimally important difference of the urticaria activity score and other measures of disease activity in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2012;108:20–24. doi: 10.1016/j.anai.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Weller K, Groffik A, Church MK, et al. Development and validation of the Urticaria Control Test: a patient-reported outcome instrument for assessing urticaria control. J Allergy Clin Immunol. 2014;133:1365–1372. doi: 10.1016/j.jaci.2013.12.1076. [DOI] [PubMed] [Google Scholar]
- 45.Ohanyan T, Schoepke N, Bolukbasi B, et al. Responsiveness and minimal important difference of the urticaria control test. J Allergy Clin Immunol. 2017;140:1710–1713. doi: 10.1016/j.jaci.2017.04.050. [DOI] [PubMed] [Google Scholar]
- 46.Sussman G, Abuzakouk M, Bérard F, et al. Angioedema in chronic spontaneous urticaria is underdiagnosed and has a substantial impact: analyses from ASSURE-CSU. Allergy. 2018;73:1724–1734. doi: 10.1111/all.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boonpiyathad T, Sangasapaviliya A. Autologous serum and plasma skin test to predict 2-year outcome in chronic spontaneous urticaria. Asia Pac Allergy. 2016;6:226–235. doi: 10.5415/apallergy.2016.6.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eun SJ, Lee JY, Kim D-Y, Yoon H-S. Natural course of new-onset urticaria: results of a 10-year follow-up, nationwide, population-based study. Allergol Int. 2019;68:52–58. doi: 10.1016/j.alit.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Giménez-Arnau AM, Jáuregui I, Silvestre-Salvador JF, et al. Consensus on the definition of disease control and response assessment in chronic urticaria. J Investig Allergol Clin Immunol. 2022;32:261–269. doi: 10.18176/jiaci.0820. [DOI] [PubMed] [Google Scholar]
- 50.Maurer M, Kaplan A, Rosén K, et al. The XTEND-CIU study: long-term use of omalizumab in chronic idiopathic urticaria. J Allergy Clin Immunol. 2018;141:1138–1139. doi: 10.1016/j.jaci.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Giménez-Arnau AM. Omalizumab for treating chronic spontaneous urticaria: an expert review on efficacy and safety. Expert Opin Biol Ther. 2017;17:375–385. doi: 10.1080/14712598.2017.1285903. [DOI] [PubMed] [Google Scholar]
- 52.Kim JK, Har D, Brown LS, Khan DA. Recurrence of chronic urticaria: incidence and associated factors. J Allergy Clin Immunol Pract. 2018;6:582–585. doi: 10.1016/j.jaip.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Weller K, Groffik A, Magerl M, et al. Development, validation, and initial results of the Angioedema Activity Score. Allergy. 2013;68:1185–1192. doi: 10.1111/all.12209. [DOI] [PubMed] [Google Scholar]
- 54.Can PK, Degirmentepe EN, Etikan P, et al. Assessment of disease activity and quality of life in patients with recurrent bradykinin-mediated versus mast cell-mediated angioedema. World Allergy Organ J. 2021;14:100554. [DOI] [PMC free article] [PubMed]
- 55.Weller K, Donoso T, Magerl M, et al. Validation of the Angioedema Control Test (AECT)—a patient-reported outcome instrument for assessing angioedema control. J Allergy Clin Immunol Pract. 2020;8:2050–2057. doi: 10.1016/j.jaip.2020.02.038. [DOI] [PubMed] [Google Scholar]
- 56.Baiardini I, Pasquali M, Braido F, et al. A new tool to evaluate the impact of chronic urticaria on quality of life: chronic urticaria quality of life questionnaire (CU-QoL) Allergy. 2005;60:1073–1078. doi: 10.1111/j.1398-9995.2005.00833.x. [DOI] [PubMed] [Google Scholar]
- 57.Kulthanan K, Chularojanamontri L, Tuchinda P, Rujitharanawong C, Baiardini I, Braido F. Minimal clinical important difference (MCID) of the Thai Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) Asian Pac J Allergy Immunol. 2016;34:137–145. doi: 10.12932/AP0674.34.2.2016. [DOI] [PubMed] [Google Scholar]
- 58.Weller K, Magerl M, Peveling-Oberhag A, Martus P, Staubach P, Maurer M. The Angioedema Quality of Life Questionnaire (AE-QoL)—assessment of sensitivity to change and minimal clinically important difference. Allergy. 2016;71:1203–1209. doi: 10.1111/all.12900. [DOI] [PubMed] [Google Scholar]
- 59.Kulthanan K, Chularojanamontri L, Rujitharanawong C, Weerasubpong P, Maurer M, Weller K. Angioedema quality of life questionnaire (AE-QoL)—interpretability and sensitivity to change. Health Qual Life Outcomes. 2019;17:160. doi: 10.1186/s12955-019-1229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basra MKA, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230:27–33. doi: 10.1159/000365390. [DOI] [PubMed] [Google Scholar]
- 61.Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125:659–664. doi: 10.1111/j.0022-202X.2005.23621.x. [DOI] [PubMed] [Google Scholar]
- 62.XTEND-CIU (Xolair treatment efficacy of longer duration in chronic idiopathic urticaria): a phase IV, multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of omalizumab through 48 weeks in patients with chronic idiopathic urticaria [Internet]. ClinicalTrials.gov. 2018. https://clinicaltrials.gov/ct2/show/NCT02392624. Accessed 8 Aug 2022.
- 63.OPTIMA: efficacy of optimized re-treatment and step-up therapy with omalizumab in CSU patients [Internet]. ClinicalTrials.gov. 2018. https://clinicaltrials.gov/ct2/show/NCT02161562. Accessed 8 Aug 2022.
- 64.Staubach P, Metz M, Chapman-Rothe N, et al. Omalizumab rapidly improves angioedema-related quality of life in adult patients with chronic spontaneous urticaria: X-ACT study data. Allergy. 2018;73:576–584. doi: 10.1111/all.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.A randomized, double-blind, placebo-controlled, 28-week treatment with a 8 week follow-up to investigate the impact of omalizumab on quality of life measures and the incidence and severity of angioedema despite H1-antihistamine therapy. [Internet]. ClinicalTrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT01723072. Accessed 8 Aug 2022.
- 66.Maurer M, Giménez-Arnau AM, Sussman G, et al. Ligelizumab for chronic spontaneous urticaria. N Engl J Med. 2019;381:1321–1332. doi: 10.1056/NEJMoa1900408. [DOI] [PubMed] [Google Scholar]
- 67.Treatment of chronic urticarial unresponsive to H1-antihistamines with an anti-IL5Ralpha monocloncal antibody [Internet]. ClinicalTrials.gov. 2019. https://clinicaltrials.gov/ct2/show/NCT03183024. Accessed 8 Aug 2022.
- 68.Leducq S, Samimi M, Bernier C, et al. Efficacy and safety of methotrexate versus placebo as add-on therapy to H1 antihistamines for patients with difficult-to-treat chronic spontaneous urticaria: a randomized, controlled trial. J Am Acad Dermatol. 2020;82:240–243. doi: 10.1016/j.jaad.2019.07.097. [DOI] [PubMed] [Google Scholar]
- 69.Randomized clinical trial evaluating the efficacy of methotrexate in addition to anti-H1 versus placebo and anti-H1 in the treatment of severe chronic idiopathic urticaria [Internet]. ClinicalTrials.gov. 2017. https://clinicaltrials.gov/ct2/show/NCT01960283. Accessed 8 Aug 2022.