Abstract
Introduction
Ixekizumab, a highly selective interleukin-17A monoclonal antibody, was approved for the treatment of moderate-to-severe psoriasis (PsO) in 2016. Limited real-world data are available on its effectiveness from a patient’s perspective shortly (2 to 4 weeks) after initiation and upon continuation for 24 weeks.
Objective
To describe patient-reported clinical and quality-of-life outcomes after initiating ixekizumab using data collected from the United States Taltz® Customer Support Program.
Methods
This was a 24-week prospective, observational study of commercially insured diagnosis-confirmed adults with PsO. Surveys were completed at weeks 0 (baseline), 2, 4, 8, 12, and 24 and included the Patient Report of Extent of Psoriasis Involvement questionnaire to assess the extent of body surface area (BSA) affected by PsO, itch and pain numeric rating scales, Patient Global Assessment of Disease Severity (PatGA), and Dermatology Life Quality Index (DLQI).
Results
523 patients were included in the analysis. Proportions of patients with ≤ 2% BSA involvement were 34.5%, 40.1%, 50.9%, and 79.9% at weeks 0, 2, 4, and 24, respectively; 54.8% and 75.1% achieved National Psoriasis Foundation preferred (BSA ≤ 1%) and acceptable (BSA ≤ 3% or ≥ 75% improvement) responses at week 12, respectively. Improvements of ≥ 4 points in itch and pain were seen by week 2 in 21.1% and 28.0% of patients, respectively, which increased to 63.1% and 64.8% at week 24. Proportions of patients with PatGA scores of 0 (clear) or 1 were 13.4%, 24.1%, 34.0%, and 69.6% at weeks 0, 2, 4, and 24, respectively; and proportions with DLQI total scores of 0 or 1 [no or minimal impact] were 8.4%, 17.6%, 27.3%, and 53.8% at weeks 0, 2, 4, and 24, respectively.
Conclusion
Patient-reported improvements in BSA, itch, skin pain, dermatology-specific quality of life, and overall PsO severity were seen as early as 2 weeks after initiation and continued through week 24.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-023-00969-1.
Keywords: Customer support program, Ixekizumab, Patient-reported outcomes, Psoriasis
Key Summary Points
| Why carry out this study? |
| • Although ixekizumab has demonstrated significant benefits in clinical and real-world studies for psoriasis (PsO), limited real-world data are available on the effectiveness of ixekizumab from a patient’s perspective shortly (2 to 4 weeks) after the initiation of treatment and upon continuation for 24 weeks. |
| • This study evaluated the clinical effectiveness, quality of life, sleep, and treatment satisfaction outcomes after initiating ixekizumab based on data collected from the United States (US) Taltz® Customer Support Program (CSP), a comprehensive, no-cost service that offers reimbursement support, injection training, and symptom tracking to assist patients throughout their treatment journey. |
| What was learned from this study? |
| • Patient-reported improvements in BSA involvement, itch, skin pain, overall PsO severity, dermatology-specific health-related quality of life, and treatment satisfaction were seen as early as 2 weeks after initiation and continued through week 24. Patient-reported BSA involvement findings from this study were consistent with those reported by physicians in other real-world studies. |
| • Together these findings demonstrate the benefit of ixekizumab and the benefit of using a CSP for evaluating treatment benefit for patients with a confirmed diagnosis of PsO at initiation and over time. |
Introduction
Psoriasis (PsO) is a chronic, immune-mediated, inflammatory skin disorder characterized by scaly erythematous plaques frequently occurring on the extensor surfaces of the extremities and scalp; lesions may be painful or itchy, leading to a significant impact on patients’ health-related quality of life (HRQL) [1, 2]. Although the skin is the most prominent manifestation of PsO, it may be associated with other inflammatory disorders, such as psoriatic arthritis, inflammatory bowel disease, and coronary artery disease [2].
Treatment goals for PsO include alleviating the signs and symptoms of skin disease, improving function, and managing related comorbidities [2], with treat-to-target guidelines recommending that body surface area (BSA) involvement 3 months after biologic treatment initiation should be reduced to ≤ 1% (preferred) or ≤ 3% (acceptable) [3]. Ixekizumab, a highly selective interleukin-17A monoclonal antibody, was approved for the treatment of adults with moderate-to-severe PsO in 2016 and subsequently approved for adults with active psoriatic arthritis, active ankylosing spondylitis, and non-radiographic axial spondyloarthritis [4, 5]; it has been shown to improve clinical and patient-reported outcomes (PROs) such as the Psoriasis Area and Severity Index (PASI) and Itch Numeric Rating Scale (NRS) in phase III clinical trials [6–9].
Ixekizumab has demonstrated significant benefits in clinical and real-world studies, but limited real-world data are available on its clinical effectiveness and PROs from a patient’s perspective shortly (2 to 4 weeks) after initiation and upon continuation for 24 weeks. Recruitment through a customer support program (CSP) can provide patients the chance to participate in research and scientists the opportunity to collect real-world data from large samples of patients initiating treatment.
No formal hypotheses were tested; however, the study objective was to describe the clinical effectiveness, quality of life, sleep, and treatment satisfaction outcomes after initiating ixekizumab based on data collected from the United States (US) Taltz® CSP, a comprehensive, no-cost service that offers reimbursement support, injection training, and symptom tracking to assist patients throughout their treatment journey.
Methods
Study Design and Setting
This was a 24-week prospective, observational, web-based survey study of commercially insured, diagnosis-confirmed patients with PsO enrolling in the US Taltz CSP; and was conducted between November 2020 and September 2022.
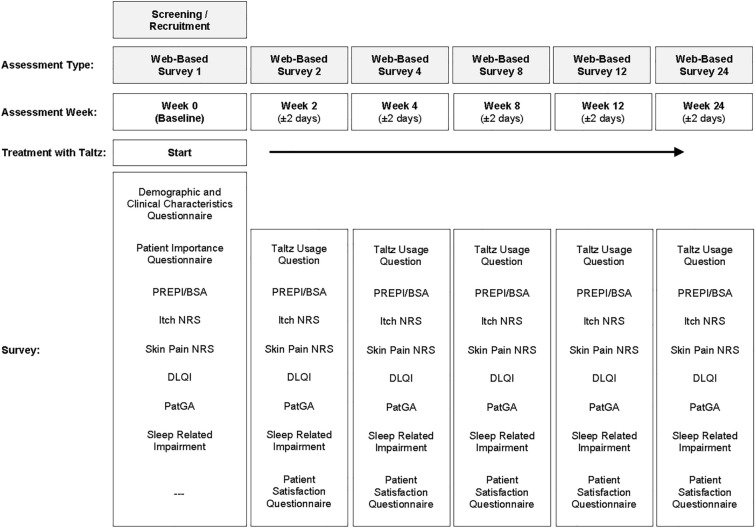
CSP enrollees were invited to participate by a third-party recruitment and web-survey hosting vendor, Global Perspectives, which specializes in health outcomes market research. Potentially eligible participants were e-mailed an invitation to consider the study. Interested participants opened the survey link and completed the screening questions, consent form, and baseline survey. Subsequent surveys were emailed at weeks 2, 4, 8, 12, and 24, which reflect the study visits from the Taltz phase III trials (Fig. 1) [7]. Participants were allowed 7 days to complete each survey, and reminder e-mails were sent after 3 days if survey links were not opened or completed. Participants were remunerated for their time.
Fig. 1.
Overall study design. BSA body surface area, DLQI Dermatology Life Quality Index, NRS numeric rating scale, PatGA Patient Global Assessment of Disease Severity, PREPI Patient Report of Extent of Psoriasis Involvement
Compliance with Ethics Guidelines
The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and was approved by an institutional review board (IRB), Advarra CIRBI™ (Pro00046597; 30 September 2020). Informed consent was provided before the commencement of survey procedures.
Study Sample
Participants were eligible for the study if they were at least 18 years of age, were diagnosed with PsO, were enrolled in the Taltz US CSP, had commercial insurance, had agreed to be contacted for future research studies, had access to a smartphone/iPhone or computer with internet access, and had not started Taltz® treatment or had been on Taltz® treatment for ≤ 7 days.
Survey
Participants completed the web-based survey after providing electronic informed consent; the survey took approximately 25 min to complete and captured the following areas of interest: clinical outcomes, disease and treatment impact, the importance of treatment attributes, treatment satisfaction, and demographic and clinical characteristics.
For clinical outcomes, participants completed the Patient Report of Extent of Psoriasis Involvement (PREPI) questionnaire, a single-item, self-administered, validated questionnaire used to assess the extent of BSA affected by PsO [10]. Patients are asked to cover their PsO patches with the palms of their hands, where one palm equals 1% BSA, with higher scores indicating more extensive PsO. Scores are interpreted as follows: little to no psoriasis visible (< 1 palm or < 1% BSA), only a few patches (1–2 palms or 1–2% BSA), scattered patches (3–10 palms or 3–10% BSA), and extensive psoriasis covering large areas of the body (> 10 palms or > 10% BSA).
Itch and skin pain in the past 24 h were each rated on single-item, 11-point NRS ranging from 0 (no itch/pain) to 10 (worst itch/pain imaginable). Scores of 0 or 1 reflect no or minimal itch or pain. A single-item, 6-point scale, the Patient Global Assessment of Disease Severity (PatGA), was used to assess overall PsO severity “today;” scores range from 0 (clear) to 5 (severe).
For functional and HRQL outcomes, participants completed the Patient-Reported Outcomes Measurement Information System Sleep-Related Impairment (PROMIS SI) Short Form 4a, a four-item measure assessing perceptions during usual waking hours (i.e., sleepiness) and perceived functional impairments due to poor sleep over the past 7 days [11]. Higher scores indicate greater sleep impairment. The 10-item Dermatology Life Quality Index (DLQI) was used to assess HRQL over the past week. DLQI total scores range from 0 to 30, with higher scores indicating greater HRQL impairment; a score > 10 indicates that the patient’s life is being severely affected by their skin condition. Scores of 0 or 1 (i.e., DLQI 0,1) reflect no impact or minimal impact [12].
Treatment attribute importance and treatment satisfaction were each evaluated using bespoke measures that assessed eight PsO treatment attributes: effectiveness (skin being clear, clearing quickly, and staying clear), itch, skin pain, unwanted effects, ease of use, and convenience. For each questionnaire, items were scored individually on a 5-point scale: from 0 (not at all important) to 4 (extremely important) on the Patient Importance Questionnaire and from 1 (strongly dissatisfied) to 5 (strongly satisfied) on the Patient Satisfaction Questionnaire. Demographic and clinical characteristics also were evaluated using a bespoke measure.
The PREPI, DLQI, and itch NRS were validated in PsO populations [10, 13, 14] and the PROMIS SI measure and pain NRS were validated in other chronic conditions [11, 15].
Statistical Analysis
Descriptive statistics were used to summarize the demographics, clinical characteristics, and PROs for all enrolled patients who had a baseline value. Continuous variables were summarized using means and standard deviations or medians and ranges; categorical variables were summarized using frequencies and percentages. PROs were evaluated at each administration timepoint, and changes in scores were evaluated from baseline across all timepoints (i.e., 24 weeks). For the National Psoriasis Foundation (NPF) preferred (BSA ≤ 1%) and acceptable (BSA ≤ 3% or ≥ 75% improvement in BSA) treatment goals, responder analyses were performed on those who had a baseline BSA > 1% and BSA > 3%, respectively. Responder analysis on a 4-point improvement in the itch NRS, which is based on the minimum clinically important difference (MCID) of 4 points [16], was performed for those who had a baseline itch NRS score of 4 or higher. All statistical analyses were conducted using the observed data.
Results
Patient Baseline Demographic and Clinical Characteristics
A total of 523 patients with PsO completed the baseline survey and were included in the analysis (Table 1). The average age of the sample was 47.5 (± 12.0) and most patients were white (86.2%), female (63.5%), and non-Hispanic (89.5% data not shown). Most participants had completed at least some college (89.9%; data not shown) and were employed full-time (74.4%).
Table 1.
Demographic and clinical characteristics of survey participants
| Demographic parameter | Baseline (N = 523) | |
|---|---|---|
| Agea | Mean (SD) | 47.5 (12.0) |
| Min–max | 18–82 | |
| Median (Q1, Q3) | 49 (38, 56) | |
| 95% CI | (46.5, 48.6) | |
| Sex | Female | 332 (63.5%) |
| Male | 191 (36.5%) | |
| Race | White | 451 (86.2%) |
| Multi-racial (no primary race) | 31 (5.9%) | |
| Asian | 15 (2.9%) | |
| Black or African American | 14 (2.7%) | |
| American Indian or Alaska Native | 2 (0.4%) | |
| Native Hawaiian or other Pacific Islander | 1 (0.2%) | |
| Other | 9 (1.7%) | |
| Employment status | Employed, full-time | 389 (74.4%) |
| Retired | 36 (6.9%) | |
| Employed, part-time | 34 (6.5%) | |
| Unemployed | 24 (4.6%) | |
| Homemaker | 17 (3.3%) | |
| Disabled | 15 (2.9%) | |
| Student | 3 (0.6%) | |
| Other | 5 (1.0%) | |
| Psoriasis duration (months) | Mean (SD) | 197.1 (167.4) |
| Min–max | 0–720 | |
| Median (Q1, Q3) | 144 (54, 301) | |
| 95% CI | (182.7, 211.5) | |
| PREPI/BSA | BSA 0% | 17 (3.3%) |
| BSA 1% to 2% | 163 (31.2%) | |
| BSA 3% to 4% | 90 (17.2%) | |
| BSA 5% to 10% | 133 (25.4%) | |
| BSA 11% to 20% | 71 (13.6%) | |
| BSA > 20% | 49 (9.4%) | |
| Psoriasis location | Upper extremities | 372 (71.1%) |
| Lower extremities | 369 (70.6%) | |
| Head and neck | 357 (68.3%) | |
| Trunk | 254 (48.6%) | |
| Nails (fingernails or toenails) | 140 (26.8%) | |
| Biologics used in the past 2 years | Yes | 254 (48.6%) |
| No | 269 (51.4%) | |
| Comorbid psoriatic arthritis | Yes | 287 (54.9%) |
| No | 236 (45.1%) | |
BSA body surface area, CI confidence interval, PREPI Patient Report of Extent of Psoriasis Involvement, Q quartile, SD standard deviation
aParticipants were at least 18 years of age or the age of majority (19 or 21 years) in Nebraska, Alabama, and Mississippi
Participants experienced PsO for 197.1 ± 167.4 months (16.4 ± 13.9 years), with it most often appearing on their upper extremities (71.1%), lower extremities (70.6%), head and neck (68.3%), and trunk (48.6%) (Table 1). Participants reported experiencing PsO in difficult-to-treat areas such as the scalp (62.1%), fingernails and toenails (26.8%), face (24.9%), genital area (22.0%), and palms (14.0%) (Supplemental Table 1).
In the 2 years prior to using ixekizumab, participants were treated most often with topical treatments (83.6%), biologics (48.6%), and non-biologics (43.8%) (Supplemental Table 1). Of the biologics, adalimumab (29.6%), secukinumab (21.8%), and etanercept (12.6%) were used most often. Approximately one-third of patients most recently used a different biologic prior to ixekizumab initiation (35.0%)—most frequently using secukinumab (14.5%) and adalimumab (13.2%). The most frequent comorbid conditions were psoriatic arthritis (54.9%) (Table 1), obesity (34.8%; data not shown), and anxiety (33.1%; data not shown).
Patient-Reported Clinical, Quality of Life, and Sleep Outcomes
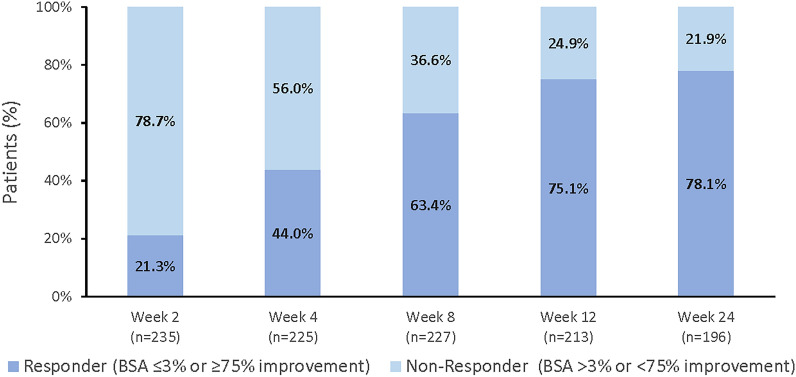
At baseline, 34.5% of patients had PsO involvement of ≤ 2% BSA. The proportion of patients with ≤ 2% BSA involvement increased to 40.1%, 50.9%, and 79.9% by weeks 2, 4 and 24, respectively (Fig. 2). Twenty-three percent (23.0%) of patients had extensive PsO plaques (BSA > 10%) at baseline that decreased to 18.0%, 13.5%, and 3.8% by weeks 2, 4, and 24, respectively. By week 12, 54.8% and 75.1% of patients that had BSA involvement > 1% and > 3% at baseline, respectively, and achieved the NPF preferred and acceptable target responses of BSA ≤ 1% and BSA ≤ 3% or ≥ 75% improvement, respectively [3] (Figs. 3 and 4). The preferred and acceptable responses increased to 64.8% and 78.1% by week 24. Mean BSA scores over time are provided in Supplemental Table 2.
Fig. 2.
BSA percent involvement: baseline to week 24.a BSA body surface area. aBSA based on the PREPI
Fig. 3.
Percent preferred treat-to-target responders (BSA ≤ 1%): weeks 2 to 24.a,b BSA body surface area. aResponder definition based on National Psoriasis Foundation guidelines. bFor patients with BSA > 1% at baseline
Fig. 4.
Percent acceptable treat-to-target responders (BSA ≤ 3% or ≥ 75% improvement): weeks 2 to 24.a,b BSA body surface area. aResponder definition based on National Psoriasis Foundation guidelines. bFor patients with BSA > 3% at baseline
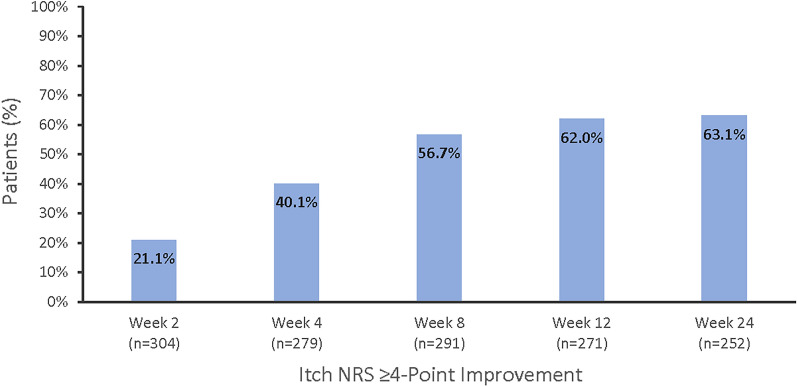
At baseline, 11.5% of patients reported itch NRS scores of 0 or 1 on an 11-point scale from 0 (no itch) to 10 (worst itch imaginable), which improved to 17.9%, 30.0%, and 53.4% at weeks 2, 4, and 24, respectively (Fig. 5). Similarly for the skin pain NRS, 20.6% of patients reported scores of 0 or 1 at baseline, which improved to 31.3%, 44.9%, and 65.8% at weeks 2, 4, and 24, respectively (Fig. 6). A minimal clinically important improvement in itch (≥ 4 points) was seen as early as week 2 in 21.1% of patients, which increased to 63.1% at week 24 (Fig. 7). Similar findings were seen for skin pain, where 28.0% of patients achieved improvement at week 2 and 64.8% at week 24 (Supplemental Fig. 1).
Fig. 5.
Percent of patients with an itch NRS of 0 or 1: baseline to week 24.a NRS numeric rating scale. a0 (no itch) to 10 (worst itch imaginable)
Fig. 6.
Percent of patients with a pain NRS of 0 or 1: baseline to week 24.a NRS numeric rating scale. a0 (no pain) to 10 (worst pain imaginable)
Fig. 7.
Proportion of patients with a ≥ 4-point improvement in itch NRS.a,b NRS numeric rating scale. aFor patients with itch scores greater than or equal to 4 at baseline. b0 (no itch) to 10 (worst itch imaginable)
At baseline, 13.4% of patients rated their PsO overall as 0 (clear on PatGA) or 1, which improved to 24.1%, 34.0%, and 69.6% at weeks 2, 4, and 24, respectively. PatGA scores of 5 (severe) decreased from 21.6% of patients at baseline to 8.7% by week 2, which remained low through week 24 (2.3%) (Fig. 8).
Fig. 8.
Patient Global Assessment of Severity scores: baseline to week 24.a a0 (clear, no psoriasis) to 5 (severe)
Only 8.4% of patients reported a DLQI 0,1 at baseline, and the proportion with DLQI 0,1 increased to 17.6%, 27.3%, and 53.8% at weeks 2, 4, and 24, respectively (Fig. 9).
Fig. 9.
Percent of patients with a DLQI total score of 0 or 1: baseline to week 24.a DLQI Dermatology Life Quality Index. a0 or 1 (no effect at all on patient’s life)
Approximately one-half of patients reported “not at all” or “a little bit” of sleep-related impairment due to PsO at baseline (range PROMIS SI Q1 to Q4: 45.5% to 55.5%), which increased to one-half to slightly less than two-thirds of patients at week 2 (range Q1 to Q4: 52.0% to 62.1%), approximately two-thirds of patients at week 4 (range Q1 to Q4: 59.4% to 69.9%), and approximately three-fourths of patients at week 24 (range Q1 to Q4: 70.5% to 79.5%) (Supplemental Table 3). Across all timepoints, the percent of patients reporting “not at all” or “a little bit” was lowest for Q4 (I was sleepy during the daytime) and highest for Q3 (I had a hard time concentrating because of poor sleep).
Treatment Attribute Importance and Treatment Satisfaction Outcomes
At least three-fourths of patients rated the following PsO medication attributes as “moderately” or “extremely” important: PsO staying clear or almost clear (94.4%), PsO being clear or almost clear (91.8%), skin not being itchy (89.5%), skin not being painful (89.5%), not having unwanted effects (84.1%), and PsO clearing quickly (74.9%) (Supplemental Table 4).
At least one-third of patients were satisfied (satisfied or strongly satisfied) with their ixekizumab treatment based on these six attributes by week 2 (range 30.6% [PsO being clear or almost clear] to 53.0% [not having unwanted effects]), which increased to at least one-half of patients at week 4 (range 46.6% [skin not being itchy] to 60.6% [PsO clearing quickly]) and to at least two-thirds at week 24 (range 64.6% [skin not being itchy] to 76.4% [PsO being clear or almost clear]).
Discussion
In this study of 523 adults with moderate-to-severe PsO, we evaluated the real-world, self-reported effectiveness of ixekizumab over 24 weeks. Findings indicate an early treatment benefit and continued improvement over time. Patient-reported improvements in BSA, itch, skin pain, dermatology-specific HRQL, sleep, and overall PsO severity were seen as early as 2 weeks after treatment initiation and continued through week 24.
These findings are consistent with those reported in other real-world studies for ixekizumab, which showed improvements in BSA involvement, dermatology-specific HRQL, and disease severity over time with ixekizumab treatment [17–19].
Patient characteristics for our sample were comparable to those for other ixekizumab real-world studies in terms of age (mean 47 to 51 years) [17–21] and average duration of PsO (15 to 17 years) [18–22]. Forty-nine percent of patients in our study had prior experience with biologic therapy, compared with 68% to 83% in the other real-world studies [17–21]. This may be due to differences in data collected by chart review versus that collected by survey, where patients were asked to report biologic treatments taken in the past 2 years.
Also, our registry is only commercially insured and has a high percentage of highly educated patients (college graduates). These are limitations of the study.
Nineteen percent (19.3%) and 70.3% of patients in this study reported clear to minimal PsO involvement (BSA ≤ 1%) at baseline and week 24, respectively. A similar trend was seen in the study by Leonardi and colleagues [18], where the percent of patients with clear or minimal involvement improved from 19.6% at baseline to 84% (from figure) at week 24. Patients in this study and a study by Shahriari and colleagues [19] demonstrated similar magnitudes of improvement in the proportion of patients with BSA involvement of ≤ 3%, from 43.8% and 18.4% at baseline, respectively, to 84.6% and 72.1% at week 24; and mean BSA scores improved 74.8% and 77.5% in these studies, respectively. The consistency in findings across real-world studies is particularly impressive given that BSA involvement in our study was measured by patients via the PREPI, rather than by physicians as in the other studies [18, 19].
Similar findings for overall disease severity also were noted; the percent of patients with scores of 0 (clear) or 1 on global assessment scales was 13.3%, 34.0%, and 69.6% at baseline and weeks 4 and 24 for our study (patient-rated; 6-point scale) and 7.2%, 58.8%, and 76% (from figure) at the same timepoints in Leonardi and colleagues’ work [18] (physician-rated; 7-point scale). In another study, the percent of patients with scores of 0 (clear) or 1 on a 5-point, physician-rated global assessment scale was 7.4% and 50.7% at baseline and week 24, respectively [19]. Similar magnitudes of improvement from baseline to week 24 also were seen for itch and pain using an 11-point NRS in our study and a 0 to 100 visual analog scale in another study [19]. Though the findings are generally consistent, comparisons may be impacted by differences in the scale structures.
Improvements in dermatology-specific HRQL also were similar to findings from other real-world studies. The percent of patients in this study reporting DLQI total scores of 0 or 1 (no impact) improved by 18.9% and 45.3% at weeks 4 and 24, respectively, which is comparable to 35.0% and 48.5% for the same timepoints in the study by Leonardi and colleagues [18]. Improvements also were seen in mean DLQI total scores, with reductions of 6.7 points from baseline to week 24 in our study and reductions of 4.2 points [19] and 17.5 points (approximate—based on figure) [17] over the same timeframe in other studies.
Findings from this study also are consistent with those from the ixekizumab phase III trials (UNCOVER 1, UNCOVER 2, and UNCOVER 3), which showed early improvements in psoriasis plaques, itch and skin pain, HRQL, and bothersomeness of skin disease [7, 8].
Patient characteristics for our sample were comparable to those for the ixekizumab phase III trials in terms of age (average 45.5 years vs. 47.5 years) and duration of PsO (18.8 years vs. 16.4 years) [8]. Patient baseline characteristics differed, however, between this real-world study and the phase III trials in terms of sex distribution and itch severity. Males comprised most of the sample in the phase III trials but a minority of the sample in our study (68.2% vs. 36.5%, respectively). Itch severity was higher on an 11-point scale (0 to 10) in the phase III trials (range 6.2 to 7.2 across trials) compared with our study (average 5.2). The findings regarding itch severity are not unexpected, as entry criteria for clinical trials aim to recruit participants with more severe disease and “washout” periods prior to the baseline visit, which may limit the benefit from treatments received prior to trial start.
Real-world benefit based on BSA involvement was relatively similar to that seen in the UNCOVER 1 trial, although PsO was measured differently in different study settings. Among the patients in UNCOVER 1, approximately 50% and 80% of patients who received ixekizumab had minimal PsO involvement based on physician ratings at baseline and week 12, respectively, based on a static physical global rating scale (0 [clear] or 1 [minimal PsO]; data from figures) [7]. In this study, 34.5% and 75.3% of patients had clear to minimal PsO involvement at baseline and week 12, respectively, based on patient-reported BSA involvement (PREPI BSA < 3%). Also at week 12, similar proportions of patients in this study and the UNCOVER trials achieved the NFP preferred (54.8% and 51.8%) and acceptable (75.1% and 73.9%) responses [23].
Skin pain also was measured differently in the clinical trials (pain visual analog scale) and this real-world study (pain NRS), but was demonstrated to be a consistent benefit of ixekizumab (data from figures) [8]. In the three phase III trials, skin pain scores for patients who received ixekizumab were approximately 45 at baseline and 8 at week 12 on a 0 (no skin pain) to 100 (severe skin pain) visual analog scale [8]. In this study, skin pain scores were 4.3 at baseline and 1.6 at week 12 on a 0 (no skin pain) to 10 (worst skin pain imaginable) NRS.
Among patients in the three phase III trials with a baseline itch NRS score of 4 or higher, clinically meaningful improvements of 4 or more points were achieved by 76.8% to 85.9% of patients who received ixekizumab at week 12 [8]. In this real-world study, 62.0% of patients with a baseline NRS score of 4 or higher achieved clinically meaningful improvements at week 12 and 63.1% at week 24. The difference in the percentage achieving a clinically meaningful improvement between these populations may reflect differences in itch severity at entry, where baseline scores for those in the phase III trials were 2 points higher on average compared to our study. However, most participants in this real-world study and the clinical trials demonstrated clinically meaningful improvements, confirming the benefit of ixekizumab outside the trial setting [14].
In addition to being generally consistent with clinical outcome findings from the ixekizumab clinical trial program, this study demonstrated the benefit of ixekizumab for dermatology-specific HRQL and sleep-related impairment, where one-half and three-fourths of participants had no or minimal impact of PsO on their HRQL and sleep-related impairment at week 24. Findings from Kimball and colleagues [8] show a strong correlation between improvement in itch severity and HRQL based on the DLQI, which appear to be reflected in this study.
This study showed that most participants were satisfied with ixekizumab treatment based on attributes they considered most relevant; that is, having and maintaining skin that is clear or almost clear and having skin that is not itchy or painful. As early as week 2, approximately one-third of participants reported being satisfied based on these attributes, which increased to three-fourths by week 24. Most participants also were satisfied with not experiencing any unwanted effects. Research evaluating the primary reasons for discontinuing biologics in PsO were related to patient perceptions of poor efficacy (did not work or stopped working) and having adverse events [24]. In this context, satisfaction with ixekizumab is suggestive of continued use and benefit. Affordability or a lack of insurance coverage was another reason cited for treatment discontinuation. Being enrolled in a CSP designed to mitigate financial barriers to treatment also supports continued ixekizumab use and benefit.
Findings from this study should be viewed with caution due to limitations associated with a survey design study and enrollment through a CSP. As patients included in the study were required to enroll in a CSP and consent to future research, unknown characteristics of this sample may be different from those of a general PsO population. The CSP was suitable for a single-arm, prospective design for ixekizumab, but did not allow for comparisons to control groups. In addition, patients who were insured by public payers (Medicaid and Medicare) were not included in the sample, which may limit generalizability to minority and elderly populations. Attrition over the 6-month study period (which occurred during the COVID-19 pandemic) also may have impacted our findings, as participants dropped out over time and 35% of the sample at baseline did not complete the week 24 assessment.
This study also demonstrates the potential benefits, to researchers and patients, of conducting CSP-based survey studies. The CSP can provide a large, real-world sample of patients with an established diagnosis for prospective, observational research. Data can be collected early after treatment initiation and over time to better understand the onset and duration of treatment effectiveness. Key endpoints that may not be available from other sources, such as claims databases, can be collected directly from patients in a manner that is virtual (i.e., not requiring an office visit) and non-intrusive. In addition to providing financial and educational support, the CSP can provide opportunities for interested patients to participate in research and help researchers better understand the outcomes that are most relevant to their disease and treatment experience.
Conclusion
This study gives context to the real-world benefit of ixekizumab from a patient’s perspective and demonstrates early and sustained clinical and functional improvements similar to those observed in the UNCOVER phase III trials and other real-world studies. The study also supports the use of a CSP for evaluating treatment benefit for patients with a confirmed diagnosis of PsO at initiation and over time.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Scott A. Lawrence and Jiaying Guo for their contributions to this research. The authors thank the patients who participated in this survey study.
Funding
This work and the Rapid Service Fee were funded by Eli Lilly and Company. Eli Lilly and Company provided data analysis services.
Medical Writing and Editorial Assistance
The authors did not use any medical writing or editorial assistance for this article.
Author Contributions
Alice B. Gottlieb, Russel Burge, William N. Malatestinic, Baojin Zhu, Julie McCormack, Miriam Kimel, and Joseph F. Merola contributed to the study conception and design. Material preparation, data collection, and/or analysis were performed by Baojin Zhu, Yunyang Zhoa, Julie McCormack, and Miriam Kimel. All authors contributed to the drafting of the manuscript and approved the final manuscript.
Prior Presentation
This manuscript is based on work that has been previously presented as follows: Gottlieb AB, et al. Patient Demographics and Disease Characteristics of Psoriasis Patients from the U.S. Ixekizumab Customer Support Program. Presented at: Innovations in Dermatology Meeting; April 27–30, 2022; Scottsdale, AZ, USA. Gottlieb AB, et al. Real-World Effectiveness of Ixekizumab in Patients with Psoriasis from the US Ixekizumab Customer Support Program. Presented at: European Academy of Dermatology and Venereology (EADV) Meeting; September 7–10, 2022; Milan, Italy. Gottlieb AB, et al. Ixekizumab Real-World Effectiveness at 24 Weeks in Patients with Psoriasis: Data from the United States Taltz Customer Support Program. American Academy of Dermatology (AAD) Meeting; March 17–21, 2023; Virtual/New Orleans, LA, USA. Gottlieb AB, et al. Achievement of the National Psoriasis Foundation Treatment Treat-to-Target Goals in the US Ixekizumab Customer Support Program. Winter Clinical Dermatology Conference (WCDC); January 13–18, 2023; Kohala Coast, HI, USA.
Disclosures
Alice B. Gottlieb: Amgen, AnaptysBio, Avotres Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Dice Therapeutics, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi, Sun Pharma, UCB Pharma, and Xbiotech (stock options for an RA project)—honoraria as an advisory board member, non-promotional speaker or consultant. AnaptysBio, Janssen, Novartis, Ortho Dermatologics, Sun Pharma, BMS, and UCB Pharma—research/educational grants; all funds go to the Icahn School of Medicine at Mount Sinai. Russel Burge, William N. Malatestinic, Baojin Zhu: Eli Lilly—employee and stockholder. Yunyang Zhao: Eli Lilly—employee. Julie McCormack, Miriam Kimel: Eli Lilly—consultant. Joseph F. Merola: AbbVie, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi-Regeneron, Sun Pharmaceuticals, and UCB—consultant and/or investigator.
Compliance with Ethics Guidelines
The study was conducted in accordance with the protocol, applicable regulations, and the ethical principles that have their origin in the Declaration of Helsinki. Advarra CIRBI™, a central IRB, approved the study protocol and related documents (Pro00046597). Patients provided electronic informed consent for participation and publication prior to the commencement of the study procedures.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the language in the patient informed consent, which does not permit the dataset to be made public.
References
- 1.Kimel M, Bell JA, Sexton C, Revicki DA. HRQL outcomes for assessing effectiveness in clinical trials of biological agents for psoriasis: a systematic review. Adv Psor Inflam Skin Dis. 2009;1(1):7–14. [Google Scholar]
- 2.Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi: 10.1016/j.jaad.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong AW, Siegel MP, Bagel J, Boh EE, Buell M, Cooper KD, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76(2):290–298. doi: 10.1016/j.jaad.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Lu J, Allan BW, Tang Y, Tetreault J, Chow CK, et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J Inflamm Res. 2016;9:39–50. doi: 10.2147/JIR.S100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Drug Administration. Ixekizumab prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125521s024lbl.pdf.
- 6.Gooderham MJ, Elewski B, Augustin M, Iversen L, Torii H, Burge R, et al. Effect of Ixekizumab on patient reported outcomes and quality of life in patients with moderate-to-severe plaque psoriasis: 5-year results from the UNCOVER-1 and -2 studies. J Drugs Dermatol. 2021;20(4):394–401. [DOI] [PubMed]
- 7.Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 trials of Ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–56. [DOI] [PubMed]
- 8.Kimball AB, Luger T, Gottlieb A, Puig L, Kaufmann R, Nikai E, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75(6):1156–1161. doi: 10.1016/j.jaad.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 9.Yosipovitch G, Reich A, Steinhoff M, Beselin A, Kent T, Dossenbach M, et al. Impact of ixekizumab treatment on itch and psoriasis area and severity index in patients with moderate-to-severe plaque psoriasis: an integrated analysis of two phase III randomized studies. Dermatol Ther (Heidelb). 2018;8(4):621–37. [DOI] [PMC free article] [PubMed]
- 10.Dommasch ED, Shin DB, Troxel AB, Margolis DJ, Gelfand JM. Reliability, validity and responsiveness to change of the patient report of extent of psoriasis involvement (PREPI) for measuring body surface area affected by psoriasis. Br J Dermatol. 2010;162(4):835–842. doi: 10.1111/j.1365-2133.2009.09589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, et al. Development of short forms from the PROMIS sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2011;10(1):6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finlay AY, Khan GK. Dermatology life quality index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6. [DOI] [PubMed]
- 13.Shikiar R, Willian MK, Okun MM, Thompson CS, Revicki DA. The validity and responsiveness of three quality of life measures in the assessment of psoriasis patients: results of a phase II study. Health Qual Life Outcomes. 2006;4:71. doi: 10.1186/1477-7525-4-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimball AB, Naegeli AN, Edson-Heredia E, Lin CY, Gaich C, Nikai E, et al. Psychometric properties of the itch numeric rating scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(1):157–162. doi: 10.1111/bjd.14464. [DOI] [PubMed] [Google Scholar]
- 15.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS Pain), numeric rating scale for pain (NRS Pain), McGill pain questionnaire (MPQ), short-form mcgill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 16.Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the dermatology life quality index (DLQI): further data. Dermatology. 2015;230(1):27–33. doi: 10.1159/000365390. [DOI] [PubMed] [Google Scholar]
- 17.Chiricozzi A, Burlando M, Caldarola G, Conti A, Damiani G, De Simone C, et al. Ixekizumab effectiveness and safety in the treatment of moderate-to-severe plaque psoriasis: a multicenter, retrospective observational study. Am J Clin Dermatol. 2020;21(3):441–447. doi: 10.1007/s40257-019-00490-2. [DOI] [PubMed] [Google Scholar]
- 18.Leonardi C, Tao R, Setayeshgar S, Wang S, McMullen S, Barge R, et al. Clinical and quality of life outcomes among Ixekizumab treated psoriasis patients in a real-world setting. J Clin Dermatol Ther. 2021;7:087. [Google Scholar]
- 19.Shahriari M, Harrison RW, Burge R, Lin CY, Malatestinic WN, Goldblum OM, et al. Disease response and patient-reported outcomes among initiators of ixekizumab. J Dermatolog Treat. 2022;33(3):1538–1546. doi: 10.1080/09546634.2020.1853023. [DOI] [PubMed] [Google Scholar]
- 20.Lockshin B, Cronin A, Harrison RW, McLean RR, Anatale-Tardiff L, Burge R, et al. Drug survival of ixekizumab, TNF inhibitors, and other IL-17 inhibitors in real-world patients with psoriasis: the Corrona Psoriasis Registry. Dermatol Ther. 2021;34(2):e14808. doi: 10.1111/dth.14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu JJ, Harrison RW, Zhu B, Goldblum OM, Malatestinic WN, Burge R, et al. Understanding characteristics of patients newly initiating ixekizumab: findings from the Corrona Psoriasis Registry. J Comp Eff Res. 2021;10(2):157–167. doi: 10.2217/cer-2020-0113. [DOI] [PubMed] [Google Scholar]
- 22.Gonulal M, Altunay IK, Dogan S, Turkmen M, Balci DD, Ozturkcan S. Ixekizumab for the treatment of the patients with moderate to severe plaque psoriasis: clinical data from a real-world experience. Dermatol Ther. 2022;35(12):e15955. doi: 10.1111/dth.15955. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong A, Amato D, Huster W, Ojeh C, Van Voorhees AS. Achievement of the National Psoriasis Foundation treatment targets with ixekizumab: pooled analyses from 4 clinical studies. J Am Acad Dermatol. 2021;85(2):330–336. doi: 10.1016/j.jaad.2019.09.030. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013;149(10):1180–1185. doi: 10.1001/jamadermatol.2013.5264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the language in the patient informed consent, which does not permit the dataset to be made public.