Abstract
Hidradenitis suppurativa (HS) is an oftentimes debilitating condition that presents with painful nodules, abscesses, and sinus tracts. This condition is challenging to treat, in part because the pathogenesis of the condition is incompletely understood but also because there are limited therapeutic options. HS research is undergoing explosive growth with multiple new molecular pathways under study, which will hopefully lead to improved disease control for patients. Part I of this review will provide an overview of the emerging topical and systemic therapies under investigation for HS.
Keywords: Clinical trials, Hidradenitis suppurativa, Systemic, Topical, Treatment
Key Summary Points
| Hidradenitis suppurativa (HS) has a devastating impact on patients’ lives, and existing treatment options are limited. |
| The number of HS clinical trials has increased rapidly over the past few years. |
| Topical treatments under investigation include a Janus kinase (JAK) inhibitor, an aryl hydrocarbon receptor inhibitor, a lytic peptidomimetic, gentian violet, and povodine-iodine. |
| New systemic treatment pathways are also being studied such as interleukin (IL)-17 inhibition, IL-36 inhibition, Tyrosine kinase inhibition, complement blockade, leukotriene inhibition, glycan targeting, and heat shock protein inhibition. |
| These therapies herald an exciting period in HS management as options for patients continue to increase, hopefully contributing to improved symptom control and quality of life. |
Introduction
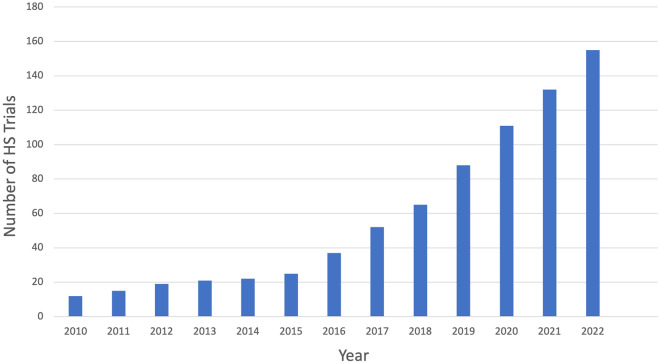
Hidradenitis suppurativa (HS) is a chronic, autoinflammatory condition that causes a severe impact on patients’ quality of life. The cause of HS is still under investigation, but it is considered a disease of follicular occlusion with various factors contributing to pathogenesis, including immune dysregulation, hormonal and metabolic factors, dysbiosis, and genetics [1]. Additionally, physical triggers for HS, including friction, deodorant use, and shaving, have been reported [2–4]. Multiple inflammatory cytokines have been found to be elevated in both lesional HS skin and in the serum of HS patients, leading to investigation of targeted immunomodulators as HS treatments. Adalimumab is currently the only drug approved by the US Food and Drug Administration and the European Medicines Agency for the treatment of HS; therefore, treatment options are limited for patients who fail to respond to the tumor necrosis factor (TNF)-alpha inhibitor. Further, delays in diagnosis, which are common in HS patients, may prevent timely initiation of adalimumab, which correlates with decreased response to treatment [5]. However, the number of clinical trials and treatments under investigation has been increasing precipitously over the past few years (Fig. 1), marking an inflection point in the management of this devastating condition that will hopefully allow clinicians to more effectively treat HS. This review will discuss emerging and investigational medical treatments for HS patients.
Fig. 1.
Number of hidradenitis suppurativa clinical trials posted on clinicaltrials.gov by year (source: clinicaltrials.gov)
Methods
For this narrative review, the term “hidradenitis suppurativa” was searched on clinicaltrials.gov on 8 January 2023. Emerging treatments with ongoing or recently completed trials were included; studies that were withdrawn or of unknown status were excluded. Other relevant articles were identified based on a search of each included drug’s name and “hidradenitis” as keywords on PubMed, and through review of reference lists of included articles. Publicly available key trial updates through March 2023 were also included. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Topical Therapies
Janus Kinases
The Janus kinase (JAK) family contains four tyrosine kinases that are involved in various signaling cascades: JAK1, JAK2, JAK3, and tyrosine kinase (TYK)2. They participate in the JAK–signal transducer and activator of transcription (STAT) pathway, which regulates multiple inflammatory signals, including interleukin (IL)-1b, IL-6, IL-17, IL-23, interferons (IFNs), and tumor necrosis factor (TNF)-α [7, 8]. JAK3 gene transcripts as well as STAT1, STAT4, and STAT5 promoter binding sites are elevated in HS lesional skin [9, 10]. IFN-induced STAT1 activation was suppressed in vitro by JAK1-selective inhibition by upadacitinib [9].
Ruxolitinib is a JAK1/2 inhibitor that decreases TNF, IL-6, IL-8, IL-1β, and C-X-C motif chemokine ligand (CXCL)3 production by HS lesional keratinocytes by a factor of 2 compared with vehicle control [11]. Two phase 2 trials are investigating the use of topical ruxolitinib 1.5% cream in patients with HS: one compared with vehicle cream in 60 participants with HS (NCT05635838), and the other as an open-label single arm study in 24 participants (NCT04414514) (Table 1).
Table 1.
Emerging topical treatments for HS
| Drug name (MOA) | Author or clinical trial # | Study type | Study goal and primary endpoint(s) | Patients enrolled | (Anticipated) completion date | Results or recruitment status |
|---|---|---|---|---|---|---|
| Ruxolitinib 1.5% cream (JAK 1/2 inhibitor) | NCT05635838 | Phase 2 randomized, double-blind vehicle-controlled |
Evaluate efficacy in patients with mild to moderate HS Endpoint: change from baseline in AN count at week 16 |
60 | March 2024 | Recruiting |
| Ruxolitinib 1.5% cream (JAK 1/2 inhibitor) | NCT04414514 | Phase 2 open label |
Evaluate efficacy and effects on skin inflammation in patients with HS Endpoint: percentage of patients who achieve HiSCR at week 16 |
24 | January 2025 | Recruiting |
| AT193 (aryl hydrocarbon agonist) | NCT04989517 | Phase 1 randomized, double-blind placebo-controlled |
Evaluate safety and tolerability in patients with HS Endpoint: incidence of TEAEs up to week 10 |
44 | February 2023 | Active, not recruiting |
| LTX-109 3% gel (lytic peptidomimetic) | NCT04756336 | Phase 2 proof of concept |
Evaluate safety and efficacy in patients with HS Endpoints: investigator and patient assessed signs and symptoms, respectively, of local reactions to the product until week 6 |
11 | July 2021 | Completed |
| Gentian violet (antiseptic) | NCT04388163 | Phase 2 open-label |
Evaluate efficacy in patients with HS Endpoint: skin redness, drainage, and pain at baseline and 1 month post-treatment |
16 | December 2023 | Not yet recruiting |
| Povidone-iodine cream (antimicrobial) | NCT01818167 | Prospective multi-center blinded, randomized, controlled clinical trial |
Compare efficacy of povidone-iodine topical cream to 10% benzoyl peroxide topical body wash for the treatment of early stage HS Endpoint: HS European Research Group scale up to 4 months |
25 | January 2015 | Results submitted |
AN abscesses and inflammatory nodules, HiSCR hidradenitis suppurativa clinical response, HS hidradenitis suppurativa, JAK Janus kinase, MOA mechanism of action, TEAEs treatment emergent adverse events
Aryl Hydrocarbon Receptor
The aryl hydrocarbon receptor (AHR) is a keratinocyte transcription factor activated by exposure to environmental toxins, including tryptophan derivatives produced by microorganisms, which mediates skin barrier function [12, 13]. It is involved in the differentiation of regulatory Treg (T) cells and Th17 cells [12]. Dysregulated tryptophan catabolism by bacterial skin flora in HS patients has been linked to decreased AHR activation [14]. Tapinarof, a topical AHR agonist, has been found to be effective in treatment of psoriasis and atopic dermatitis (AD) [13].
AT193 is a topical AHR agonist that is being studied in a phase 1b trial in 44 participants with HS (NCT04989517).
Lytic Peptidomimetic
LTX-109 is a peptide-mimic antimicrobial compound that exerts its bactericidal actions through membrane disruption and cell lysis [15]. Results are pending from a completed phase 1/2 study of LTX-109 3% gel, which enrolled 11 participants with HS (NCT04756336). LTX-109 has been studied in the treatment of nasal colonization of S. aureus, with serious adverse events (SAEs) of psychosis and post-operative bleeding [15]. Local side effects, upper respiratory infections (URIs), and headache also occurred.
Gentian Violet
Gentian violet is a dye and topical antiseptic that is effective against numerous microbes including methicillin-resistant S. aureus (MRSA) and Candida skin infections [16]. It also has anti-angiogenic properties and has been used for wound healing [16]. It will be investigated in a phase 2 study of 16 HS patients (NCT04388163).
Povidone-Iodine
A topical antimicrobial wash containing povidone-iodine acts against viral pathogens within 30 s and remains active for up to 9 h against bacterial and fungal pathogens on artificial skin [17]. It was evaluated in a prospective, blinded, randomized trial comparing it with benzoyl peroxide 10% wash in 25 participants with HS but results are not available (NCT01818167).
Systemic Therapies
TNF-α
TNF-α, a pro-inflammatory cytokine, is elevated in HS lesional skin as well as the serum of HS patients [7, 18, 19]. Inhibition of TNF-α decreases production of downstream cytokines, including interleukin (IL)-1ß, IL-6, IL-10, and IL-17A, revealing a complex interplay in the pathogenesis of HS, which remains incompletely understood [7, 20]. Several TNF-α inhibitors are effective in the treatment of HS, including adalimumab, which demonstrated efficacy in two phase 3 clinical trials [21], and infliximab, which was studied in a phase 2 trial [22].
TNF-α antagonist biosimilar medications are currently being studied. MSB11022 (an adalimumab biosimilar) has been studied in several phase 1 trials in the USA and the UK, demonstrating safety and bioequivalence compared with adalimumab [23, 24]. It has also demonstrated comparable efficacy in the treatment of psoriasis, but has not yet been studied in HS [25]. Remsima, an infliximab biosimilar, is currently in an active phase 1 trial in 16 patients with HS resistant to unspecified conventional treatments (NCT05663268) (Table 2).
Table 2.
Emerging systemic treatments for HS
| Drug name (MOA) | Author or clinical trial # | Study type | Study goal and primary endpoint(s) | Patients enrolled | (Anticipated) Completion date | Results or recruitment status |
|---|---|---|---|---|---|---|
| MSB11022 (adalimumab biosimilar/TNF-α antagonist), SQ | Sabet et al. 2022 [23] | Phase 1 randomized, open-label, parallel-group |
Evaluate PK, safety, and tolerability Endpoints: Cmax), AUC0–t, and AUC0–∞ |
216 | March 2020 | Delivery via AI or PFS was comparable in terms of PK |
| Remsima (infliximab biosimilar/TNF-α antagonist), SQ | NCT05663268 | Phase 1 open-label |
Assess efficacy and safety in patients with resistant HS Endpoints: HiSCR, HS-PGA, and DLQI after 14 weeks |
16 | September 2023 | Active, not recruiting |
| Bermekimab (MABp1, IL-1α inhibitor), SQ | Gottlieb et al. 2020 [43] | Phase 2 open-label |
Evaluate safety, tolerability, and efficacy in patients with moderate-to-severe HS who had either failed initial treatment with anti-TNF agents or never received anti-TNF treatment Endpoint: number with adverse events up to day 93 |
42 | January 2019 | 61% and 63% of anti-TNF naïve and anti-TNF failure groups, respectively, achieved HiSCR after 12 weeks. No bermekimab-related adverse events except injection site reactions |
| Bermekimab (MABp1, IL-1α inhibitor), SQ | Kanni et al. 2018 [47] | Phase 2 double-blind, randomized placebo-controlled |
Assess efficacy in patients with moderate-to-severe HS Endpoint: differences in achievement of HiSCR at week 12 between treatment and placebo group |
20 | February 2017 | 60% of patients in active arm achieved HiSCR compared with 10% in placebo arm |
| Bermekimab (MABp1, IL-1α inhibitor), SQ | NCT04988308 | Phase 2 randomized, placebo- and active comparator-controlled, double-blind |
Evaluate clinical efficacy in patients with moderate-to-severe HS Endpoint: percentage of patients achieving HiSCR |
151 | November 2022 | Terminated |
| Bermekimab (MABp1, IL-1α inhibitor), SQ | NCT04019041 | Phase 2 randomized, double-blind, placebo-controlled |
Evaluate efficacy in patients with moderate-to-severe HS Endpoint: achievement of HiSCR at week 12 |
144 | November 2020 | Completed |
| Lutikizumab/ABT-981 (ABT-981, IL-1α/β dual variable domain), SQ | NCT05139602 | Phase 2 multicenter, randomized, double-blind placebo-controlled |
Compare lutikizumab (ABT-981) versus placebo for the treatment of adults with moderate-to-severe HS who have failed anti-TNF therapy Endpoint: achievement of HiSCR at week 16 |
160 | December 2023 | Recruiting |
| PF 06650833 (IRAK4 inhibitor), oral | NCT04092452 | Phase 2 multicenter, randomized, double-blind placebo-controlled |
Compare three kinase inhibitors (PF 06650833, PF 06700841, and PF 06826647) with placebo in moderate-to-severe HS Endpoint: achievement of HiSCR at week 16 |
194a | January 2022 | Completed, results submitted |
| KT-474 (heterobifunctional small-molecule IRAK4 degrader), oral | NCT04772885 | Phase 1 randomized, placebo-controlled |
Assess safety, tolerability, and pharmacokinetics/pharmacodynamics in healthy volunteers and in patients with atopic dermatitis and HS Endpoints: incidence and severity of emergent adverse events up to 28 days, incidence and frequency of concomitant medications up to 28 days |
154 | October 2022 | Completed |
| MAS-825 (IL-1β and IL-18 inhibitor), SQ | NCT03827798 | Phase 2 randomized, double-blind, placebo-controlled |
Assess safety and efficacy in patients with moderate-to-severe HS Endpoint: achievement of HiSCR at week 16 |
200b | May 2023 | Recruiting |
| Bimekizumab (IL-17A/F inhibitor), SQ | NCT04901195 | Phase 3 open-label, parallel group extension |
Assess safety of long-term treatment in patients with moderate-to-severe HS Endpoint: percentage of patients with treatment-emergent adverse events from baseline until week 120 |
830 | April 2025 | Active, not recruiting |
| Bimekizumab (IL-17A/F inhibitor), SQ | Kimball et al. 2023 (BE HEARD II) [61] | Phase 3 randomized, double-blind, placebo-controlled |
Assess safety and efficacy in patients with moderate-to-severe HS Endpoint: achievement of HiSCR at week 16 |
509 | September 2022 | 53.8% of patients receiving monthly dosing and 52% of patients receiving twice monthly dosing achieved HiSCR at week 16, compared with 32.2% of placebo |
| Bimekizumab (IL-17A/F inhibitor), SQ | Kimball et al. 2023 (BE HEARD I) [61] | Phase 3 randomized, double-blind, placebo-controlled |
Assess safety and efficacy in patients with moderate-to-severe HS Endpoint: achievement of HiSCR at week 16 |
505 | March 2023 | 45.3% of patients receiving monthly dosing and 47.8% of patients receiving twice monthly dosing achieved HiSCR at week 16, compared with 28.7% of placebo |
| Bimekizumab (IL-17A/F inhibitor), SQ | Glatt et al. 2021 [60] | Phase 2 multicenter, investigator-blind, subject-blind, placebo-controlled |
Assess efficacy, safety, and pharmacokinetics in patients with moderate-to-severe HS compared with adalimumab and placebo Endpoint: achievement of HiSCR at week 12 |
90 | February 2019 | Efficacious compared with placebo. 57.3% of 44 patients receiving bimekizumab achieved HiSCR at week 12 compared with 26.1% receiving placebo |
| Brodalumab (IL-17 inhibitor), SQ | Frew et al. 2020 [62] | Early phase 1 |
Identify biomarkers of disease activity and clinical response in patients with moderate-to-severe HS Endpoints: biomarkers at weeks 12/24 and TEAEs until week 24 |
10 | June 2020 | 100% achieved HiSCR at weeks 4 and 24. No serious adverse events were reported |
| Brodalumab (IL-17 inhibitor), SQ | Frew et al. 2021 [63] | Early phase 1 |
Identify biomarkers of disease activity and clinical response in patients with moderate-to-severe HS Endpoints: biomarkers at weeks 12/24 and TEAEs from week 0 to week 24 |
10 | September 2020 |
100% achieved HiSCR at weeks 4 and 24 No serious adverse events or thoughts of self-harm were reported during the study |
| Brodalumab (IL-17 inhibitor), SQ | NCT04979520 | Early phase 1 open-label |
Characterize molecular response to this treatment, identify blood and tissue markers reflecting disease severity, and better understand disease mechanisms Endpoint: IL-17A receptor saturation during brodalumab administration at week 12 versus baseline |
4 | July 2022 | Completed, results not available |
| CJM112 (IL-17A inhibitor), SQ | Kimball et al. 2022 [68] | Phase 2 randomized, double-blind, placebo-controlled |
To determine efficacy and safety of multiple doses in comparison to placebo Endpoint: Decrease in HS-PGA score by at least 2 points after 16 weeks |
66 | November 2016 | Endpoint achieved in 32.3% (10/31) after 16 weeks compared with 12.5% (4/32) with placebo |
| Izokibep (IL-17A inhibitor), SQ | Acelyrin Inc. 2023 | Phase 2b randomized, double-blind |
Evaluate efficacy, safety, and immunogenicity in adults with moderate-to-severe HS Endpoint: Achievement of HiSCR after 16 weeks |
180 | February 2024 | Part A data: 71% achieved HiSCR and 33% achieved HiSCR100 at week 12 |
| Secukinumab (IL-17A inhibitor), SQ | Kimball et al. 2023 (SUNRISE) [78] | Phase 3 randomized, double-blind |
Assess efficacy, safety, and tolerability of secukinumab at week 52 in subjects with moderate-to-severe HS Endpoint: Achievement of HiSCR after 16 weeks |
544 | July 2022 | Significant HiSCR rates of 42% (every 2 weeks) and 46% (every 4 weeks) dosing compared with placebo at week 16 |
| Secukinumab (IL-17A inhibitor), SQ |
Kimball et al. 2023 (SUNSHINE) [78] |
Phase 3 randomized, double-blind |
Assess efficacy, safety, and tolerability of secukinumab at week 52 in subjects with moderate-to-severe HS Endpoint: achievement of HiSCR after 16 weeks |
545 | July 2022 | Significant HiSCR rate of 45% (every-2-week dosing) compared with placebo at week 16 |
| Secukinumab (IL-17A inhibitor), SQ | NCT04179175 | Phase 3 randomized, triple-blind | Evaluate maintenance of HiSCR response at week 104 in either continuous or interrupted therapy, comparing every-2-week dosing to every-4-week dosing in HiSCR responders after 52 weeks, or switched to placebo. Endpoint: time to loss of response during 52 week treatment duration up to week 104 | 856 | July 2026 | Recruiting |
| Sonelokimab (IL-17A/F inhibitor), SQ | NCT05322473 | Phase 2 randomized, parallel-group, double-blind, placebo-controlled |
Demonstrate clinical efficacy and safety sonelokimab compared with placebo and adalimumab in the treatment of adult participants with moderate-to-severe HS Endpoint: percentage of participants achieving HiSCR75 after 12 weeks |
210 | November 2023 | Active, not recruiting |
|
Guselkumab (IL-23 inhibitor), SQ |
NCT03628924 | Phase 2 randomized, placebo-controlled, double-blind |
Assess efficacy, safety, and tolerability of 2 doses of guselkumab compared with placebo in adults with moderate-to-severe HS Endpoint: achievement of HiSCR after 16 weeks |
184 | May 2020 | HiSCR at week 16 (not significant): 50.8% of those on 200 mg SQ every 4 weeks, 45% of those on 1200 mg IV at weeks 0, 4, and 8 followed by 200 mg SQ at week 12 and thereafter, and 38.7% in placebo group |
|
Guselkumab (IL-23 inhibitor), SQ |
NCT04061395 | Phase 2, open label |
Investigate changes in inflammatory pathways induced by IL-23p19 blockade with guselkumab, in HS lesional skin Endpoint: changes in inflammatory pathways induced by IL-23p19 blockade with guselkumab |
20 | December 2020 | Unknown |
| Risankizumab (IL-23 inhibitor), SQ | Kimball et al. 2023 [111] | Phase 2 randomized, placebo-controlled, double-blind |
Assess safety and efficacy of risankizumab 180 mg and 360 mg versus placebo for moderate-to-severe HS in adults Endpoint: achievement of HiSCR after 16 weeks |
243 | August 2022 | No significant difference in HiSCR achievement between treatment groups. Primary endpoint was not achieved |
| Spesolimab (IL-36 inhibitor), IV and SQ | Alavi et al. 2023 [122] | Phase 2 randomized, double-blind, placebo-controlled |
Evaluate efficacy in patients with moderate-to-severe HS Endpoint: change from baseline in AN count at week 12 |
52 | April 2022 | 38.8% decrease in AN count in treatment group compared with 34.7% decrease in placebo group |
| Spesolimab (IL-36 inhibitor), IV and SQ | NCT04876391 | Phase 2 open-label, long-term extension trial |
Evaluate long-term safety of spesolimab Endpoint: occurrence of TEAEs up to week 120 |
45 | April 2024 | Active, not recruiting |
| Imsidolimab/ANB019 (IL-36 inhibitor), IV and SQ | NCT04856930 | Phase 2 randomized, double-blind, placebo-controlled |
Evaluate efficacy in adults with HS and compare results with placebo Endpoint: change from baseline in AN count at week 16 |
149 | December 2022 | Completed |
| Avacopan/CCX168 (C5a receptor inhibitor), oral | ChemoCentryx 2020 [127] | Phase 2 randomized, double-blind, placebo-controlled, parallel group |
Evaluate efficacy and safety in subjects with moderate-to-severe HS Endpoint: achievement of HiSCR after 12 weeks |
435 | March 2021 | Primary endpoint not met. Subgroup analysis: 42.6% of Hurley stage III patients achieved HiSCR at higher dose compared with 22.2% of placebo (significant) |
| Vilobelimab/IFX-1 (anti-C5a antibody), IV | Giamarellos-Bourboulis et al. 2020 [128] | Phase 2 open-label trial |
Evaluate safety and tolerability in patients with moderate-to-severe HS Endpoint: number of patients with TEAEs and anti-drug antibodies up to day 134 |
12 | July 2017 | 75% achievement of HiSCR at day 50 |
| Vilobelimab/IFX-1 (anti-C5a antibody), IV | Giamarellos-Bourboulis et al. 2020 [124] | Phase 2 randomized, double-blind, placebo-controlled |
Evaluate the safety and tolerability of vilobelimab compared with placebo in patients with moderate-to-severe HS Endpoint: achievement of HiSCR after 16 weeks |
179 | January 2020 | HiSCR achievement was not superior in treatment groups compared with placebo. IHS4 scores and draining fistulae count decreased with every other week dosing |
| BDB-001 (C5a inhibitor), IV | NCT05093855 | Phase 2 open-label |
Evaluate efficacy and safety in patients with moderate-to-severe HS Endpoint: change in IHS4 score from day 0 until week 8 |
49 | December 2023 | Recruiting |
| BDB-001 (C5a inhibitor), IV | NCT05103423 | Phase 2 randomized, double-blind placebo-controlled |
Evaluate efficacy and safety in adults with moderate-to-severe HS Endpoints: TEAEs, anti-BDB-001 antibody development, PK parameters |
49 | June 2023 | Recruiting |
| Iscalimab/CFZ533 (CD40 inhibitor), SQ | NCT03827798 | Phase 2 randomized, double-blind, placebo-controlled |
Assess safety and efficacy in patients with moderate-to-severe HS Endpoint: achievement of HiSCR at week 16 |
200b | May 2023 | Recruiting |
| LYS006 (leukotriene A4 hydrolase inhibitor), oral | NCT03827798 | Phase 2 randomized, double-blind, placebo-controlled |
Assess safety and efficacy in patients with moderate-to-severe HS Endpoint: achievement of HiSCR at week 16 |
200b | May 2023 | Recruiting |
| Povorcitinib/INCB054707 (JAK-1 inhibitor), oral | NCT04476043 | Phase 2 randomized, double-blind, placebo-controlled |
Evaluate efficacy and safety of INCB054707 in participants with moderate-to-severe HS over a 16-week placebo-controlled period, followed by a 36-week extension period Endpoint: mean change from baseline in AN count at week 16 |
209 | August 2023 | Statistically significant difference in AN count across 3 doses compared with placebo at week 16 |
| Povorcitinib/INCB054707 (JAK-1 inhibitor), oral | Alavi et al. 2022 [8] | Phase 2 placebo-controlled study |
Evaluate the safety of INCB054707 over an 8-week treatment period in patients with moderate-to-severe HS Endpoint: number of TEAEs at week 12 |
35 | August 2019 | 65% achieved HiSCR in treatment group vs. 57% in placebo after 8 weeks |
| Povorcitinib/INCB054707 (JAK-1 inhibitor), oral | Alavi et al. 2022 [8] | Phase 2 open-label, single-arm study |
Evaluate the safety of INCB054707 in patients with moderate-to-severe HS Endpoint: number of TEAEs at week 12 |
10 | April 2019 | 43% achieved HiSCR at week 8; 3 participants discontinued treatment |
| Povorcitinib/INCB054707 (JAK-1 inhibitor), oral | NCT05620823 (STOP-HS1) | Phase 3 randomized, double-blind, placebo-controlled |
Evaluate the efficacy and safety of INCB054707 in participants with moderate-to-severe HS over a 12-week placebo-controlled period, followed by a 42-week extension period Endpoint: proportion of patients who achieve HiSCR at week 12 |
600 | January 2026 | Recruiting |
| Povorcitinib/INCB054707 (JAK-1 inhibitor), oral | NCT05620836 (STOP-HS2) | Phase 3 randomized, double-blind, placebo-controlled |
Evaluate efficacy and safety of INCB054707 in participants with moderate-to-severe HS over a 12-week placebo-controlled period, followed by a 42-week extension period Endpoint: proportion of patients who achieve HiSCR at week 12 |
600 | January 2026 | Recruiting |
| Upadacitinib (JAK-1 inhibitor), oral | NCT04430855 | Phase 2 randomized, double-blind, placebo-controlled |
Evaluate efficacy and safety in participants with moderate-to-severe HS Endpoint: proportion of patients who achieve HiSCR at week 12 |
68 | January 2022 | 38.3% achieved HiSCR in treatment group and 23.8% in placebo group |
| Brepocitinib/PF06700841 (TYK2/JAK1 inhibitor), oral | NCT04092452 | Phase 2 randomized, double-blind, placebo-controlled |
Compare 3 kinase inhibitors (PF 06650833, PF 06700841, and PF 06826647) with placebo in moderate-to-severe HS Endpoint: achievement of HiSCR at week 16 |
194a | January 2022 | Completed, results submitted |
| Ropsacitinib/PF06826647 (tyrosine kinase 2 inhibitor), oral | NCT04092452 | Phase 2 randomized, multicenter, double-blind, placebo-controlled |
Compare efficacy of 3 kinase inhibitors (PF 06650833, PF 06700841, and PF 06826647) with placebo in patients with moderate-to-severe HS Endpoint: achievement of HiSCR at week 16 |
194a | January 2022 | Results submitted |
| Tofacitinib (JAK inhibitor), oral | NCT04246372 | Phase 2 open-label |
Evaluate efficacy and safety of 5 mg twice daily in the treatment of multiple inflammatory conditions, including HS, in participants with Down syndrome Endpoint: number of TEAEs up to week 18 and change in interferon scores in the transcriptome of white blood cells |
47 | December 2024 | Recruiting |
| Remibrutinib/LOU064 (BTK inhibitor), oral | NCT03827798 | Phase 2 randomized, double-blind, placebo-controlled |
Assess safety and efficacy in patients with moderate-to-severe HS Endpoint: achievement of HiSCR at week 16 |
200b | May 2023 | Recruiting |
| Fostamatinib (spleen tyrosine kinase inhibitor), oral | NCT05040698 | Phase 2 exploratory, proof-of-concept |
A proof-of-concept study to evaluate efficacy Endpoint: Alterations in gene expression profiling, cell counts (CD3+, CD11c+, Neutrophil Elastase+, CD20+, CD138+) at weeks 4 and 12 compared with baseline |
20 | January 2023 | Completed |
| Apremilast (PDE-4 inhibitor), oral | Kerdel et al. 2019 [142] | Phase 2 open-label |
Evaluate efficacy and safety in patients with moderate HS Endpoint: proportion of patients who achieve HiSCR at week 16 |
20 | August 2017 | 55% HiSCR rate at week 16 |
| Apremilast (PDE-4 inhibitor), oral | Vossen et al. 2019 [143] | Phase 2 randomized, double-blind, placebo-controlled |
Evaluate efficacy and short-term safety in patients with moderate HS Endpoint: proportion of patients who achieve HiSCR at week 16 |
20 | June 2018 | 53.3% in treatment group met HiSCR and 0% in placebo met HiSCR |
| Orismilast (PDE-4 inhibitor), oral | NCT04982432 | Phase 2 open-label |
Evaluate efficacy and safety for the treatment of mild, moderate, or severe HS in adults Endpoint: change from baseline in AN count at week 16 |
24 | December 2022 | Not yet recruiting |
| PTM-001 (glycan-targeting antibody), oral | NCT05020730 | Phase 2 randomized, double-blind, placebo-controlled |
Evaluate immunomodulatory activity of PTM-001 in participants with HS Endpoint: effect of PTM-001 on IL-1β protein levels in lesional skin biopsies at week 12 |
50 | June 2024 | Recruiting |
| RIST 4721 (CXCR2 antagonist), oral | NCT05348681 | Phase 2 randomized, double-blind, placebo-controlled |
Evaluate efficacy and safety in patients with HS Endpoint: incidence of TEAEs and SAEs until week 12 |
25 | February 2023 | Terminated |
| Eltrekibart/LY3041658 (ELR and CXC chemokine neutralizer), IV | NCT04493502 | Phase 2 randomized, double-blind, placebo-controlled |
Evaluate efficacy in patients with moderate-to-severe HS Endpoint: percentage of patients who achieve HiSCR at week 16 |
67 | October 2022 | 65.6% in treatment group met HiSCR compared with 41.4% in placebo group |
| Zunsemetinib/ATI-450 (small-molecule MK2 inhibitor), oral | Aclaris Therapeutics 2023 [157] | Phase 2 randomized, double-blind placebo-controlled |
Evaluate efficacy, safety, tolerability, pharmacokinetics, and pharmacodynamics of ATI-450 versus placebo in patients with moderate-to-severe HS Endpoint: change in AN count at week 12 |
95 | January 2023 | Did not meet primary or secondary endpoints |
| CSL324 (anti-GCSF receptor), IV | NCT03972280 | Phase 1 open-label |
Evaluate safety and PK of repeat doses in subjects with HS or palmoplantar pustulosis Endpoints: incidence of TEAEs and AESIs up to 24 weeks |
39 | October 2022 | Completed |
| RGRN-305 (HSP90 inhibitor), oral | NCT05286567 | Phase 1 randomized, double-blind, placebo-controlled |
Evaluate efficacy in patients with moderate-to-severe HS Endpoint: percentage of patients who achieve HiSCR at week 16 |
15 | August 2022 | Completed |
AI autoinjector, AN abscess and inflammatory nodule, AUC0–∞ area under the concentration–time curve from time 0 extrapolated to infinity, AUC0–t area under the concentration–time curve from time 0 to the last quantifiable concentration, BTK bruton tyrosine kinase, C5a complement component 5a, Cmax maximum observed concentration, CXCR c-x-c chemokine receptor, DLQI Dermatology Life Quality Index, GCSF granulocyte colony-stimulating factor, HiSCR hidradenitis suppurativa clinical response, HS hidradenitis suppurativa, IHS4 International Hidradenitis Suppurativa Severity Score, IL interleukin, IRAK4 IL-1 receptor associated kinase 4, IV intravenous, JAK Janus kinase, MK2 mitogen-activated protein kinase (MAPK)-activated protein kinase-2, MOA mechanism of action, PDE phosphodiesterase, PFS pre-filled syringe, PGA Physician Global Assessment, PK pharmacokinetics, SQ subcutaneous, TEAEs treatment emergent adverse events, TNF tumor necrosis factor, TYK tyrosine kinase
aIncluding patients on PF 06650833, PF 06700841, and PF 06826647
bIncluding patients on MAS825, CFZ533, LYS006, and LOU064
The use of other TNF-α inhibitors, including certolizumab and golimumab, has been reported in small numbers of patients. Certolizumab is a pegylated TNF-α inhibitor that lacks a fragment crystallizable (Fc) region, preventing it from crossing the placenta during pregnancy. Therefore, it is often the first-line option when considering TNF-α inhibitors during pregnancy or in women who are trying to conceive. A total of 19 of 23 patients reported in case series demonstrated improvement, including 11 who met hidradenitis suppurativa clinical response (HiSCR) criteria [26–35]. Achievement of HiSCR requires a ≥ 50% reduction in total abscess and inflammatory nodule (AN) count, without increase in abscess or draining fistula count compared with baseline [6]. Seven patients were women of childbearing age, including two who were pregnant with no obstetric complications reported [29, 35].
Golimumab is a fully humanized monoclonal antibody [36]. Six of nine patients with relevant data available achieved HiSCR in a case series of patients who received golimumab [37]. Three out of four additional patients have improved [38–40].
The side effects of TNF-α inhibitors include injection or infusion reactions, nausea, headaches, increased risk of infections, and increased risk of malignancies, and they should be used with caution in patients with advanced heart failure, demyelinating diseases, history of malignancy, and active infections. Paradoxical HS and psoriasis have also been reported with the use of TNF inhibitors [41, 42].
IL-1
IL-1 is involved in the inflammatory cascade of HS through its interactions with TNF-α, creating a positive feedback cycle and leading to further downstream inflammation [43]. IL-1α is expressed in keratinocytes, and activation leads to recruitment of inflammatory cells and local auto-inflammation, while IL-1β is found in circulation and HS lesional skin [44]. It is also locally expressed in macrophages and dendritic cells in response to activation of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [44]. IL-1 receptor-associated kinases (IRAKs) mediate the interaction between IL-1 receptors and toll-like receptors (TLRs), resulting in downstream activation of NF-κB and MAPK [45, 46].
Bermekimab (MABp1) is a human monoclonal antibody that inhibits IL-1α. It has been evaluated in four phase 2 trials since 2017. One open label trial studied bermekimab in patients who had never tried or who had previously failed anti-TNF therapy, demonstrating 61% and 63% HiSCR achievement at week 12, respectively, [43]. A phase 2 trial evaluating efficacy of bermekimab demonstrated significantly improved HiSCR achievement of 60% compared with placebo [47]. Another trial investigating efficacy of bermekimab has been completed without available results (NCT04019041); however, an additional study was terminated prematurely after meeting prespecified futility criteria (NCT04988308).
Canakinumab is an IL-1β inhibitor that has been reported only in cases to date, comprising eight patients with mixed results [48–53]. Four patients had at least some response, including one remission, while three did not respond, and one patient’s HS worsened.
Lutikizumab (ABT-981) is an anti-IL-1 /β dual variable domain inhibitor, binding and inhibiting both IL-1α and IL-1β. A phase 2 study will assess lutikizumab in 160 patients who have failed anti-TNF therapy (NCT05139602).
Two compounds that act on IRAK4 are under investigation for their use in HS. PF06650833 is an IRAK4 inhibitor that has a completed phase 2 trial comparing multiple kinase inhibitors with placebo (NCT04092452). KT-474 is an oral heterobifunctional small molecular IRAK4 degrader; a phase 1 trial in 154 healthy volunteers, HS patients, and atopic dermatitis (AD) patients has been completed as of 2022 (NCT04772885).
A bispecific monoclonal antibody targeting both IL-1β and IL-18 is known as MAS-825. There is currently a phase 2 trial assessing efficacy and safety of MAS-825 compared with placebo (NCT03827798). This trial is also evaluating three additional compounds.
Side effects of IL-1 inhibitors include immunosuppression, increased risk of infections, diarrhea, nausea, headache, abdominal pain, and injection site reactions.
IL-4 and IL-13
HS appears to be a primarily Th1/Th17-driven condition, while IL-4 and IL-13 are Th2-associated cytokines that are elevated in AD. The level of IL-13 is inversely related to the level of Th1/Th17-associated cytokines in untreated HS lesional skin, and its role, if any, in the pathogenesis of HS is not fully understood [54]. However, patients with AD have 5.6 times the odds of being diagnosed with HS [55]. Possible links between these two conditions include dysregulated skin bacterial populations, antimicrobial peptide (AMP) production, or defects of sphingolipid metabolism [55–57].
Three cases of male patients with Hurley stage II or III HS and comorbid AD successfully treated with dupilumab have been reported [57–59].
Safety considerations with dupilumab include risk of conjunctivitis, increased reactivation of herpesvirus, increased susceptibility to parasitic infections, and injection site reactions.
IL-17
IL-17 comprises six proteins (IL-17A–F) with five receptors [IL-17 receptor (IL-17R) A–E], with IL-17A, IL-17C, and IL-17F elevated in HS lesional skin and IL-17A additionally elevated in serum of HS patients [1, 7]. The effects of activation of the IL-17 family in HS include downstream production of other inflammatory cytokines, including IL-19 and IL-36, and AMPs and recruitment of inflammatory cells [1, 7]. In terms of phase 3 trials for HS, both bimekizumab and secukinumab met their primary endpoints; however, only secukinumab is currently commercially available in the USA.
Bimekizumab is a humanized bispecific monoclonal antibody that inhibits IL-17A and IL-17F. A phase 2 trial studied bimekizumab compared with placebo and adalimumab, revealing a 57.3% HiSCR achievement rate at week 12 in the bimekizumab group [60]. A set of phase 3 trials (BE HEARD I and II) demonstrated efficacy of monthly and twice monthly dosing of bimekizumab compared with placebo, with HiSCR rates at week 16 for active arms ranging from 45.3% to 53.8%. In the observed case analysis for BE HEARD I and II, over 55% of patients achieved HiSCR 75 at week 48 [61].
Brodalumab inhibits IL-17RA, a component of the IL-17 receptor dimer, resulting in suppression of the effect of IL-17A, IL-17C, and IL-17F [1, 62]. Two early phase 1 trials of brodalumab with weekly or biweekly dosing in 20 participants (including seven who participated in both trials) demonstrated 100% achievement of HiSCR at weeks 2 or 4, maintained at week 24 [62, 63]. An additional early phase 1 open-label trial in four patients has been completed (NCT04979520). The use of brodalumab for HS has also been reported in five patients who improved on weekly (n = 1) or biweekly (n = 4) dosing [33, 64–66].
CJM112 is a fully human monoclonal antibody that inhibits IL-17A and the heterodimer IL-17A/F, targeting a different epitope than secukinumab [1, 67]. A phase 2 trial in 66 participants demonstrated that 32.3% of patients in the active group achieved the primary endpoint compared with 12.5% in the placebo group at week 16 (p = 0.03) [68].
Ixekizumab is a monoclonal antibody IL-17A inhibitor. There is no clinical trial data on ixekizumab in HS, but several cases and series have been reported, comprising nine positive results, including five patients who achieved HiSCR [28, 69–73]. Conversely, at least four patients have been reported to fail ixekizumab [28, 74, 75].
Izokibep is a selective inhibitor of IL-17A with small molecular size, which may afford it higher potency [76, 77]. In an on-going placebo-controlled phase 2b trial in 180 participants, early data showed that 71% achieved HiSCR and 33% achieved HiSCR100 at week 12 (NCT05355805) [77].
Secukinumab is a monoclonal antibody IL-17A inhibitor that has undergone two phase 3 trials (SUNSHINE and SUNRISE) that showed significantly improved HiSCR rates of 42% and 46% across two dosing regimens compared to placebo. Efficacy was shown to be durable over 52 weeks. [78] Across six studies (including retrospective series, retrospective cohort studies, and open-label trials), 83 out of 150 (55.3%) achieved HiSCR at weeks 16 or 24 [79–84]. All utilized psoriasis dosing except in two studies, one where 12 out of 47 patients were dosed bimonthly and one where 13 out of 23 patients had dose escalation to every 2 or 3 weeks depending on clinical response [82, 84]. Multiple case reports/small case series have also been published regarding secukinumab use in HS [85–96].
Sonelokimab is a trivalent camelid-derived nanobody specific to IL-17A, IL-17F, and human serum albumin administered subcutaneously [97]. Nanobodies are proprietary proteins based on antibody heavy chains. There is an ongoing phase 2 trial with sonelokimab that includes an active comparator adalimumab arm (NCT05322473).
Side effects of IL-17 inhibitors include injection site reactions, increased risk of infection, nasopharyngitis, candida infections, diarrhea, neutropenia, and nausea. IL-17 inhibition has been reported to contribute to the development or unmasking of inflammatory bowel disease, but a recent systematic review did not find a link [98]. The paradoxical development of HS has also been reported after treatment with secukinumab [34, 41]. Additionally, brodalumab carries a black box warning regarding the risk of suicidality.
IL-23
IL-23 is a member of the IL-12 family and is composed of the p40 subunit, shared with IL-12, and the p19 subunit. IL-23 induces differentiation of Th17 cells, activation of the JAK/STAT pathway, and subsequent expression of IL-17 [7, 46]. Macrophages from HS lesional skin express mRNA encoding IL-23p19 and IL-23 subunit proteins, and patients with elevated lesional levels of IL-23p19 were more likely to achieve HiSCR with IL-23 inhibition in one study of 26 patients [7, 99].
Guselkumab is a monoclonal antibody that inhibits IL-23p19. It has been studied in a phase 2b trial to evaluate the efficacy of two different dosing regimens of guselkumab compared with placebo followed by guselkumab starting at week 16 (NCT03628924). Of participants receiving guselkumab, 45% and 50.8% met HiSCR at week 16, which was not significantly higher than the placebo group (38.7%). Prior to the phase 2b trial, an open-label phase 2a trial studied guselkumab 200 mg SQ every 4 weeks in 22 patients [100]. A total of 13 out of 20 (65%) achieved HiSCR, while 7 out of 20 (35%) achieved HiSCR75 at week 16. Several cases and series have also reported the use of guselkumab for HS [49, 75, 101–110]. Across 34 patients, 25 experienced at least some improvement.
Risankizumab is a humanized monoclonal antibody that inhibits IL-23p19. A phase 2 trial in 243 participants evaluated two dosing regimens of risankizumab compared with placebo [111]. The study was terminated early, as the primary endpoint was not met: 46.8% of patients in the risankizumab 180 mg group, 43.4% of the risankizumab 360 mg group, and 41.5% of the placebo group achieved HiSCR at week 16. Prior to this trial, a prospective cohort study of 26 patients reported a HiSCR achievement rate of 69.2% (18 out of 26) at psoriasis dosing of risankizumab [99]. Additionally, there have been positive case series/reports on risankizumab use in HS. Of 11 patients treated with psoriasis dosing of risankizumab, 8 achieved HiSCR by month 6; 3 additional patients reported improvement [112–116].
Tildrakizumab is a humanized monoclonal antibody that inhibits IL-23p19. A series of nine patients treated with tildrakizumab had statistically significant decreases in mean abscess and nodule count at months 2, 5, and 15 [117]. One patient paused treatment due to pregnancy (no obstetric complications reported).
Side effects of IL-23 inhibitors include increased risk of infection, headaches, injection site reactions, rare hepatotoxicity, and arthralgias.
IL-36
The IL-36 family is a subset of the IL-1 family, which contains three agonists (IL-36α, IL-36β, and IL-36γ). These bind the receptor complex IL-36R and upregulate the NK-κB and MAPK pathways [44]. Downstream, this stimulates increased T-cell proliferation and production of inflammatory cytokines including additional interleukins, TNF-α, and members of the CXCL family [44]. The levels of all three agonist IL-36 proteins are elevated in lesional skin and serum of HS patients, while IL‐36β is elevated in perilesional skin, with higher levels of serum IL-36 proteins associated with up to 11 times increased risk of HS [44, 118–120].
Spesolimab is a humanized anti-IL-36R monoclonal antibody [121]. A phase 2a study evaluated spesolimab in 52 patients with moderate-to-severe HS [122]. At week 12, patients receiving the active arm experienced similar changes in total abscess and inflammatory nodule (AN) count compared with placebo arm (38.8% compared with 34.7%, respectively). However, a greater proportion of patients on spesolimab experienced a decrease in draining tunnel count at week 12 compared with the placebo arm (66.7% compared with 38.5%). There is an open-label extension trial (NCT04876391).
Imsidolimab (ANB019) is a humanized monoclonal antibody inhibiting IL-36 that was studied in a phase 2 trial in 149 participants with HS compared with placebo (NCT04856930) [44]. Results are pending.
Adverse events of IL-36 inhibitors observed in trials for HS and palmoplantar pustular psoriasis include headache, nausea, fatigue, injection site reactions, nasopharyngitis, and pyrexia (NCT03633396 and NCT03619902).
Complement 5a
Complement 5a (C5a) is an anaphylatoxin that is produced during complement activation, and plays an important role in immune stimulation including neutrophil chemotaxis, mast cell degranulation, and production of Th1/17 cytokines [1, 46]. Findings on complement levels in HS patients are conflicting [123–126].
Avacopan (CCX168) is an oral C5a receptor inhibitor. It underwent a phase 2 trial with 398 participants with HS (NCT03852472). A press release reported that the primary endpoint was not met in either active arm; however, more patients in the subgroup of Hurley stage III achieved HiSCR with higher dosing (42.6%) compared with placebo (22.2%) [46, 127].
Vilobelimab (IFX-1) is a monoclonal anti-C5a antibody. An open-label phase 2a trial of 12 participants who were not eligible for or had previously failed biologic therapy demonstrated 75% achievement of HiSCR at day 50 extending to 83.3% on day 134 [128]. However, a subsequent phase 2 trial comparing multiple dose regimens with placebo did not demonstrate superiority compared with placebo in HiSCR rates (NCT03487276) [124].
BDB-001 is a recombinant anti-C5a antibody developed from the same cell line as vilobelimab by Staidson (Beijing) BioPharmaceuticals (STS) [129]. It is being studied in two phase 2 trials each comprising 49 participants with HS (NCT05093855 and NCT05103423).
Serious adverse reactions reported for vilobelimab included infections and chronic obstructive pulmonary disease.
Cluster of Differentiation (CD)40
Cluster of differentiation (CD)40 is a cell surface receptor that is among the TNF receptor family [130]. It is increased in pyoderma gangrenosum (PG) lesional skin of patients with pyoderma gangrenosum, acne, and hidradenitis suppurativa (PASH) syndrome [130].
Iscalimab (CFZ533) is a human monoclonal antibody that inhibits CD40. A phase 2 trial will study iscalimab compared with placebo in 200 participants with HS, along with three other compounds (NCT03827798).
Leukotriene A4 Hydrolase
Leukotrienes are pro-inflammatory lipid mediators that are synthesized from arachidonic acid after cleavage from membrane phospholipids [131]. Leukotriene A4 hydrolase (LTA4H) catalyzes the conversion of LTA4 into LTB4, which activates neutrophil and macrophage chemotaxis [131, 132]. The level of LTB4 has been demonstrated to be significantly elevated in HS lesions, and LTA4H gene expression is increased in HS lesional macrophages [132].
LYS006 is a small-molecule inhibitor of LTA4H that is currently being studied in a phase 2 trial with three other compounds (NCT03827798).
Janus Kinases
Povorcitinib (INCB054707) is a JAK1 inhibitor that has been studied in two completed phase 2 clinical trials [8]. In the first trial, an open-label single arm study, 3 out of 7 (43%) achieved HiSCR, while in the second randomized trial, 65% of patients in the active arm achieved HiSCR compared with 57% of patients receiving placebo. An additional placebo-controlled phase 2 trial in 209 participants demonstrated a statistically significant decrease in AN count in all three dosing regimens (15 mg, 45 mg, and 75 mg daily) of povorcitinib compared with placebo at week 16 (NCT04476043). Results from the open-label extension (OLE) of this phase 2 trial with all patients on 75 mg daily found that, at 52 weeks, HiSCR 100 was achieved by 22% and 29% of patients [133]. There are two ongoing paired phase trials totaling 1200 participants (NCT05620823 and NCT05620836).
Upadacitinib is an oral JAK1 inhibitor that was evaluated in a phase 2 trial of 68 participants (NCT04430855); 38.3% of participants in the active arm achieved HiSCR compared with 23.8% in the placebo arm (p = 0.018). A retrospective cohort study was also conducted with 20 HS patients treated with upadacitinib [134]. A total of 15 out of 20 (75%) achieved HiSCR at week 4, increasing to 100% at weeks 12 and 24; those who did not achieve HiSCR at week 4 were dose increased. Of the patients, 95% also met HiSCR75 at weeks 12 and 24.
Brepocitinib (PF06700841) is a JAK1/TYK2 inhibitor that was evaluated in a phase 2a trial of three different kinases (NCT04092452). Results have been submitted but are not yet available.
Ropsacitinib (PF06826647) is a TYK2 inhibitor that was also investigated in the same phase 2a trial as above (NCT04092452).
Tofacitinib is a partial and reversible inhibitor of all four JAKs, but preferentially binds receptor dimers containing JAK1 and JAK3 over JAK2 and TYK2 [135]. A phase 2 open-label study is studying tofacitinib in the treatment of multiple inflammatory conditions, including HS, in 47 participants with Down syndrome (NCT04246372). A case series of two patients with ulcerative Hurley stage III HS reported successful treatment with tofacitinib [136].
Adverse events of JAK inhibitors include cardiac events, blood clots, immunosuppression, increased risk of infections, gastrointestinal symptoms, and acne.
Other Tyrosine Kinase Inhibitors
Tyrosine kinases are transmembrane members of signal transduction cascades that respond to extracellular stimuli [137]. Several tyrosine kinases are involved in immunity, including Bruton tyrosine kinase (BTK), which is essential for B cell development, including plasma cells, and spleen tyrosine kinase (SYK), which participates in differentiating self from non-self antigens, plasma cell expansion, and antibody responses [137, 138]. BTK and SYK signaling is up-regulated in HS lesional skin, and several inhibitors of these pathways, including fosamatinib, demonstrated decreased B cell expression of downstream inflammatory mediators in vitro [137].
Remibrutinib (LOU064) is an oral highly selective BTK inhibitor [139]. It is being investigated as part of a phase 2 trial compared with placebo (NCT03827798).
Fostamatinib is an oral SYK inhibitor. An open-label single group phase 2 trial of fostamatinib in 20 participants with HS has been completed as of January 2023, but results have not yet been posted (NCT0504069).
Adverse events reported in non-HS trials of these compounds included headache, nasopharyngitis, diarrhea, hypertension, and nausea [140, 141].
Phosphodiesterase-4 Inhibitors
Phosphodiesterase-4 (PDE-4) is an enzyme that breaks down cyclic adenosine monophosphate (cAMP), which is used as a second messenger for multiple signaling pathways, including anti-inflammatory pathways. Inhibition of PDE-4 prevents degradation of cAMP, causing reduced production of downstream pro-inflammatory compounds [46].
Apremilast is an oral small-molecule inhibitor of PDE-4. An open-label single group phase 2 trial in 20 patients reported a 55% HiSCR rate at week 16, increasing to 60% at week 24 [142]. An additional phase 2 trial studied apremilast in 20 patients with an HS-PGA score of 3, and demonstrated a HiSCR response rate of 53.3% in 15 patients at week 16 compared with 0% in the placebo group [143]. Additionally, 22 patients have been reported in case series, with 12 out of 22 reported to have some degree of improvement on apremilast [49, 74, 144–148]. Side effects of apremilast include diarrhea, nausea, headache, weight loss, and depression/suicidal ideation.
Orismilast is an oral high-potency PDE4 inhibitor with selectivity for PDE4B and PDE4D subtypes in vitro [149]. An open-label phase 2 trial will study orismilast in 24 participants with HS (NCT04982432).
Glycan-Targeting Antibody
PTM-001 is a glycan-targeting antibody that has been shown to normalize the intestinal expression of glycans in a mouse model of inflammatory bowel disease (IBD) and is undergoing development as a novel therapeutic [150, 151]. There are murine (mPTM-001) and humanized (hPTM-001) variants of this compound, both of which have demonstrated improvement of mucosal wound healing in in vitro and in vivo models [152]. PTM-001 is undergoing a phase 2 randomized controlled trial to determine its immunomodulatory activity in 50 HS patients (NCT05020730).
C-X-C Motif Chemokines
Chemokines are a class of cytokines involved in chemotaxis, tumor growth, and angiogenesis [153]. C-X-C motif chemokines (CXC) are a subtype of chemokines, which are further subdivided into the ELR+ subgroup if they express a particular motif containing glutamic acid–leucine–arginine. The CXC receptor type 2 (CXCR2) binds ELR+ CXC ligands (CXCL), which activate various inflammatory cascades including neutrophil chemotaxis and the JAK2/STAT3 and MAPK pathways [153, 154]. Notably, CXCL1-3, CXCL8/IL-8, and CXCL16 were overexpressed in PG lesional skin of patients with PASH syndrome [130].
RIST4721 is an oral small-molecule inhibitor of CXCR2. It was being investigated in a phase 2 trial in patients with HS compared with placebo; however, this study was terminated as of 22 March 2023 due to safety findings in ongoing phase 2 trials (NCT05348681).
Eltrekibart (LY3041658) is a septa-specific monoclonal antibody that neutralizes all seven ELR+ CXC chemokines (CXCL1-3 and CXCL5-8), thereby limiting signaling through CXCR1 and CXCR2 [155]. A phase 2 trial studying the efficacy of eltrekibart in 67 participants demonstrated that 65.6% in the treatment group met HiSCR compared with 41.4% receiving placebo [154]. Side effects included COVID-19 infection, nasopharyngitis, constipation, nausea, arthralgia, and fatigue.
Mitogen-Activated Protein Kinase
The mitogen-activated protein kinase (MAPK) pathway is activated in monocytes, macrophages, and dendritic cells after stimulation of toll-like receptors (TLRs) by DAMPs produced by hair follicle rupture. IL-1b, TNF-a, and IL-23 are produced downstream of the MAPK pathway, resulting in Th17 cell polarization [156]. Notch signaling, which may be dysregulated in HS, activates MAPK phosphatase-1 (MKP-1) and deactivates MAPK [156].
Zunsemetinib (ATI-450) is an oral small-molecule MAPK-activated protein kinase 2 (MK2) inhibitor being investigated in a phase 2a trial in 90 participants compared with placebo (NCT05216224). According to a press release, as of 6 March 2023, zunsemetinib did not meet primary or secondary endpoints of change from baseline in AN or percentage of patients achieving HiSCR, respectively [157]. Adverse events in a phase 1 trial in 48 healthy adults included headache, dizziness, URI, and constipation [158].
Granulocyte Colony-Stimulating Factor
Granulocyte colony-stimulating factor (GCSF) activates neutrophils and promotes their survival. It is highly elevated in HS lesional skin samples compared with perilesional skin, skin from healthy controls, and skin from patients with other inflammatory diseases [159].
CSL324 is a human recombinant GCSF receptor antagonist monoclonal antibody. Administration of systemic CSL324 decreased expression of genes associated with neutrophil migration in peripheral blood cells after stimulation in healthy participants [160]. An open-label phase 1 trial in 39 patients with HS or palmoplantar pustulosis was completed in October 2022 (NCT03972280).
Heat Shock Protein
Heat shock proteins (HSPs) are chaperones that assist protein folding to maintain cell homeostasis and ensure proper functioning. HSP90 is involved in the folding of inflammatory proteins; therefore, inhibition of HSP90 may produce anti-inflammatory effects [161]. Topical RGRN-305 has been shown to reduce expression of chemically-upregulated pro-inflammatory genes in in vitro keratinocytes, including TNF, IL-1b, IL-17A, and genes encoding the MAPK and NF-κB pathways [161].
RGRN-305 is an oral inhibitor of HSP90 that has been studied in psoriasis [162]. It has also been studied in a phase 1 trial of 15 HS patients (NCT05286567). Side effects reported in the psoriasis trial included exanthematous drug eruption, abdominal pain, headache, and blurry vision [162].
Mammalian Target of Rapamycin
Mammalian target of rapamycin (mTOR) is part of a protein kinase family that forms multiprotein complexes called mechanistic target of rapamycin complex (mTORC)1 and mTORC2 [163]. These kinases are involved in differentiation of Th17 cells, and increases in mTORC1 have been observed in psoriasis, HS, and insulin resistance [163]. Ten patients treated with sirolimus in combination with TNF inhibitors for HS have been described, with eight patients responding, including two complete responses [164, 165]. Side effects of sirolimus include immunosuppression, increased risk of malignancy, hyperlipidemia, diarrhea, headache, nausea, and leukopenia.
Semisynthetic Glycopeptide Antibacterial
Dalbavancin (BI397) is a novel semisynthetic second-generation glycopeptide antibiotic [166]. The first generation of glycopeptides includes vancomycin, though dalbavancin has increased affinity for bacterial membranes and increased in vitro bactericidal activity against gram-positive organisms [166]. It has demonstrated in vitro activity against multiple types of staphylococci, Enterococcus, and Propionibacterium species, among others, and clinical efficacy against skin and soft tissue infections including MRSA [166]. Additionally, wounds treated with dalbavancin showed decreased matrix metaloproteinase levels compared with untreated wounds in a mouse infection model [167]. It may also be effective against biofilms based on evidence in vitro and some in vivo studies, which may portend utility in HS treatment [168].
Nine patients with Hurley stage II or III HS were treated with one dose of intravenous dalbavancin, and 7 out of 9 (78%) achieved HiSCR by week 12 [169, 170]. Side effects included pyrexia, headache, oral candidiasis, diarrhea, constipation, and nausea [166].
Conclusion
Many treatments are currently under investigation for their use in HS, including multiple that target new pathways. This is especially promising given that existing therapeutic modalities are limited in their efficacy and durability. Challenges that are seen with HS trials include lack of a standardized set of trial outcomes and high placebo rates with HiSCR, the main primary endpoint for most trials at this time. Furthermore, cost and access barriers often restrict treatment options for patients outside of clinical trials. However, an expanded therapeutic armamentarium for HS is on the horizon with the tremendous increase in HS research and clinical trials. With continued study into the pathogenesis of HS, we can hope to identify new options to improve patients’ quality of life, perhaps even aiming for complete disease remission.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Medical Writing and/or Editorial Assistance
No medical writing support or editorial assistance was used for this article.
Author Contributions
Natalie M. Fragoso: Concept and design, acquisition of data, and drafting of the manuscript. Rahul Masson: Checking data and drafting of the manuscript. T. Justin Gillenwater: Critical revision of the manuscript. Vivian Y. Shi: Critical revision of the manuscript. Jennifer L. Hsiao: Concept and design, critical revision of the manuscript, and supervision.
Prior Publication
This article is based on work that has been previously published in medical journals as medical literature or in the lay press as press releases; work that has been presented at medical conferences including the American Academy of Dermatology in New Orleans, LA, in March 2023; and ongoing and completed or terminated clinical trials available on ClinicalTrials.gov.
Disclosures
Natalie M. Fragoso is an investigator for Acelyrin. T. Justin Gillenwater is on advisory boards for Avita, Exsurco, and the Musculoskeletal Transplant Foundation. Vivian Y. Shi is on the board of directors for the Hidradenitis Suppurativa Foundation (HSF), is an advisor for the National Eczema Association, is a stock shareholder of Learn Health, and has served as an advisory board member, investigator, speaker, and/or received research funding from Sanofi Genzyme, Regeneron, AbbVie, Genentech, Eli Lilly, Novartis, SUN Pharma, LEO Pharma, Pfizer, Incyte, Boehringer Ingelheim, Alumis, Aristea Therapeutics, Menlo Therapeutics, Dermira, Burt’s Bees, Galderma, Kiniksa, UCB, Target-PharmaSolutions, Altus Lab/cQuell, MYOR, Polyfins Technology, GpSkin, and Skin Actives Scientific. Jennifer L. Hsiao is on the Board of Directors for the Hidradenitis Suppurativa Foundation; has served as a consultant for Aclaris, Boehringer Ingelheim, Novartis, and UCB; and has served as a consultant and speaker for AbbVie. Rahul Masson has nothing to disclose. There was no financial transaction for the preparation of this manuscript.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Aarts P, Dudink K, Vossen ARJV, et al. Clinical implementation of biologics and small molecules in the treatment of hidradenitis suppurativa. Drugs. 2021;81(12):1397–1410. doi: 10.1007/S40265-021-01566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cutler B, Hagstrom E, Greiling TM. Deodorant/antiperspirant use and hair removal practices for hidradenitis suppurativa: recommendations from a single-center survey. Int J Women’s Dermatol. 2023;9(1):e028. doi: 10.1097/JW9.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boer J, Jemec GBE. Mechanical stress and the development of pseudo-comedones and tunnels in hidradenitis suppurativa/acne inversa. Exp Dermatol. 2016;25(5):396–397. doi: 10.1111/EXD.12926. [DOI] [PubMed] [Google Scholar]
- 4.Zouboulis CC, Benhadou F, Byrd AS, et al. What causes hidradenitis suppurativa? 15 years after. Exp Dermatol. 2020;29(12):1154–1170. doi: 10.1111/EXD.14214. [DOI] [PubMed] [Google Scholar]
- 5.Marzano AV, Genovese G, Casazza G, et al. Evidence for a ‘window of opportunity’ in hidradenitis suppurativa treated with adalimumab: a retrospective, real-life multicentre cohort study. Br J Dermatol. 2021;184(1):133–140. doi: 10.1111/BJD.18983. [DOI] [PubMed] [Google Scholar]
- 6.Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30(6):989–994. doi: 10.1111/jdv.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frew JW, Marzano AV, Wolk K, et al. A systematic review of promising therapeutic targets in hidradenitis suppurativa: a critical evaluation of mechanistic and clinical relevance. J Invest Dermatol. 2021;141(2):316–324.e2. doi: 10.1016/J.JID.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Alavi A, Hamzavi I, Brown K, et al. Janus kinase 1 inhibitor INCB054707 for patients with moderate-to-severe hidradenitis suppurativa: results from two phase II studies. Br J Dermatol. 2022;186(5):803–813. doi: 10.1111/BJD.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frings VG, Jopp L, Srivastava M, Presser D, Goebeler M, Schmidt M. Stress signalling and STAT1 activation characterize the keratinocytic gene expression pattern in Hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2022;36(12):2488–2498. doi: 10.1111/JDV.18465. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman LK, Tomalin LE, Schultz G, et al. Integrating the skin and blood transcriptomes and serum proteome in hidradenitis suppurativa reveals complement dysregulation and a plasma cell signature. PLoS One. 2018;13(9):e0203672. doi: 10.1371/JOURNAL.PONE.0203672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schell SL, Cong Z, Sennett ML, et al. Keratinocytes and immune cells in the epidermis are key drivers of inflammation in hidradenitis suppurativa providing a rationale for novel topical therapies. Br J Dermatol. 2023;188(3):407–419. doi: 10.1093/BJD/LJAC096. [DOI] [PubMed] [Google Scholar]
- 12.Yidana DB. Hidradenitis suppurativa—the role of interleukin-17, the aryl hydrocarbon receptor and the link to a possible fungal aetiology. Med Hypotheses. 2021;149:110530. doi: 10.1016/J.MEHY.2021.110530. [DOI] [PubMed] [Google Scholar]
- 13.Napolitano M, Fabbrocini G, Martora F, Picone V, Morelli P, Patruno C. Role of aryl hydrocarbon receptor activation in inflammatory chronic skin diseases. Cells. 2021 doi: 10.3390/CELLS10123559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guenin-Macé L, Morel JD, Doisne JM, et al. Dysregulation of tryptophan catabolism at the host-skin microbiota interface in hidradenitis suppurativa. JCI Insight. 2020 doi: 10.1172/JCI.INSIGHT.140598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson AC, Janson H, Wold H, et al. LTX-109 is a novel agent for nasal decolonization of methicillin-resistant and -sensitive Staphylococcus aureus. Antimicrob Agents Chemother. 2015;59(1):145–151. doi: 10.1128/AAC.03513-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pona A, Quan EY, Cline A, Feldman SR. Review of the use of gentian violet in dermatology practice. Dermatol Online J. 2020 doi: 10.5070/D3265048772. [DOI] [PubMed] [Google Scholar]
- 17.Lentini P, Retterer C, Bavari S, Cheronis J. In vivo and in vitro characterization of provodine, a long acting, alcohol-free professional antiseptic, against Ebola virus and other serious viral, bacterial and fungal pathogens. Open Forum Infect Dis. 2015 doi: 10.1093/OFID/OFV133.489. [DOI] [Google Scholar]
- 18.Matusiak Ł, Bieniek A, Szepietowski JC. Increased serum tumour necrosis factor-alpha in hidradenitis suppurativa patients: is there a basis for treatment with anti-tumour necrosis factor-alpha agents? Acta Derm Venereol. 2009;89(6):601–603. doi: 10.2340/00015555-0749. [DOI] [PubMed] [Google Scholar]
- 19.Mozeika E, Pilmane M, Nürnberg BM, Jemec GBE. Tumour necrosis factor-alpha and matrix metalloproteinase-2 are expressed strongly in hidradenitis suppurativa. Acta Derm Venereol. 2013;93(3):301–304. doi: 10.2340/00015555-1492. [DOI] [PubMed] [Google Scholar]
- 20.Jiménez-Gallo D, de la Varga-Martínez R, Ossorio-García L, Collantes-Rodríguez C, Rodríguez C, Linares-Barrios M. Effects of adalimumab on T-helper-17 lymphocyte- and neutrophil-related inflammatory serum markers in patients with moderate-to-severe hidradenitis suppurativa. Cytokine. 2018;103:20–24. doi: 10.1016/j.cyto.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375(5):422–434. doi: 10.1056/NEJMoa1504370. [DOI] [PubMed] [Google Scholar]
- 22.Grant A, Gonzalez T, Montgomery MO, Cardenas V, Kerdel FA. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62(2):205–217. doi: 10.1016/j.jaad.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 23.Sabet A, Dickerson DS, Kunina EE, Buccarello AL, Monnet J. A randomised controlled trial comparing the pharmacokinetics and tolerability of the proposed adalimumab biosimilar MSB11022 delivered via autoinjector and pre-filled syringe in healthy subjects. Rheumatol Ther. 2022;9(2):693–704. doi: 10.1007/S40744-022-00432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyland E, Mant T, Vlachos P, et al. Comparison of the pharmacokinetics, safety, and immunogenicity of MSB11022, a biosimilar of adalimumab, with Humira(®) in healthy subjects. Br J Clin Pharmacol. 2016;82(4):983–993. doi: 10.1111/BCP.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hercogová J, Papp KA, Chyrok V, Ullmann M, Vlachos P, Edwards CJ. AURIEL-PsO: a randomized, double-blind phase III equivalence trial to demonstrate the clinical similarity of the proposed biosimilar MSB11022 to reference adalimumab in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2020;182(2):316–326. doi: 10.1111/BJD.18220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esme P, Akoglu G, Caliskan E. Rapid response to certolizumab pegol in hidradenitis suppurativa: a case report. Ski Appendage Disord. 2021;7(1):58–61. doi: 10.1159/000511284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esme P, Akoglu G, Dalkıran CD, Caliskan E. Certolizumab pegol in the treatment of severe hidradenitis suppurativa after adalimumab failure: a real-life experience. Dermatol Ther. 2022 doi: 10.1111/DTH.15782. [DOI] [PubMed] [Google Scholar]
- 28.Esme P, Botsali A, Akoglu G, Caliskan E. An anti-interleukin-17A monoclonal antibody, ixekizumab, in the treatment of resistant hidradenitis suppurativa: a case series. Skin Appendage Disord. 2022;8(4):342–345. doi: 10.1159/000521860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melgosa Ramos FJ, García Ruiz R, Mateu Puchades A. Certolizumab pegol as an alternative treatment in patients with coexisting hidradenitis suppurativa and other inflammatory immune-mediated diseases: report of two cases. Dermatol Ther. 2022 doi: 10.1111/DTH.15401. [DOI] [PubMed] [Google Scholar]
- 30.Sabater Abad J, Matellanes Palacios M, Velasco Pastor M, Gimeno Carpio E. A case report of hidradenitis suppurativa treated with certolizumab. J Am Acad Dermatol. 2019 doi: 10.1016/j.jaad.2019.10.098. [DOI] [Google Scholar]
- 31.Holm JG, Jørgensen AHR, Yao Y, Thomsen SF. Certolizumab pegol for hidradenitis suppurativa: case report and literature review. Dermatol Ther. 2020 doi: 10.1111/DTH.14494. [DOI] [PubMed] [Google Scholar]
- 32.Repetto F, Burzi L, Ramondetta A, et al. Certolizumab pegol and its role in pregnancy-age hidradenitis suppurativa. Int J Dermatol. 2022;61(5):e182–e184. doi: 10.1111/IJD.15742. [DOI] [PubMed] [Google Scholar]
- 33.Tampouratzi E, Kanni T, Katsantonis J, Douvali T. Case report: treating a co-existence of hidradenitis suppurativa and psoriasis with different therapeutic approaches. F1000Research. 2020; 10.12688/f1000research.21216.2 [DOI] [PMC free article] [PubMed]
- 34.Konda S, Shetty N, Friedman B, Veenstra J. Delayed drug hypersensitivity reaction to secukinumab in a patient with hidradenitis suppurativa. BMJ Case Reports CP. 2022;15(5):e249684. doi: 10.1136/BCR-2022-249684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wohlmuth-Wieser I, Alhusayen R. Treatment of hidradenitis suppurativa with certolizumab pegol during pregnancy. Int J Dermatol. 2021;60(4):e140–e141. doi: 10.1111/IJD.15286. [DOI] [PubMed] [Google Scholar]
- 36.Chen SX, Greif C, Gibson RS, Porter ML, Kimball AB. Advances in biologic and small molecule therapies for hidradenitis suppurativa. Expert Opin Pharmacother. 2022;23(8):959–978. doi: 10.1080/14656566.2022.2070429. [DOI] [PubMed] [Google Scholar]
- 37.Melendez-Gonzalez M del M, Hamad J, Sayed C. Golimumab for the treatment of hidradenitis suppurativa in patients with previous TNF-α treatment failure. J Invest Dermatol. 2021;141(12):2975–2979. 10.1016/J.JID.2021.04.026 [DOI] [PubMed]
- 38.Tursi A. Concomitant hidradenitis suppurativa and pyostomatitis vegetans in silent ulcerative colitis successfully treated with golimumab. Dig Liver Dis. 2016;48(12):1511–1512. doi: 10.1016/j.dld.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 39.van der Zee HH, Prens EP. Failure of anti-interleukin-1 therapy in severe hidradenitis suppurativa: a case report. Dermatology. 2013;226(2):97–100. doi: 10.1159/000343221. [DOI] [PubMed] [Google Scholar]
- 40.Ramos FJM, Ruiz RG, Puchades AM. Golimumab, as an alternative treatment in patients with coexisting hidradenitis suppurativa and arthritis after adalimumab failure: report of two cases. Dermatol Ther. 2022 doi: 10.1111/DTH.15266. [DOI] [PubMed] [Google Scholar]
- 41.Ruggiero A, Martora F, Picone V, Marano L, Fabbrocini G, Marasca C. Paradoxical hidradenitis suppurativa during biologic therapy, an emerging challenge: a systematic review. Biomedicines. 2022 doi: 10.3390/BIOMEDICINES10020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen JK, Schlichte MJ, Jogi R, Alikhan M, Patel AB. A case of new-onset vitiligo in a patient on tofacitinib and brief review of paradoxical presentations with other novel targeted therapies. Dermatol Online J. 2020 doi: 10.5070/D3263047978. [DOI] [PubMed] [Google Scholar]
- 43.Gottlieb A, Natsis NE, Kerdel F, et al. A phase II open-label study of bermekimab in patients with hidradenitis suppurativa shows resolution of inflammatory lesions and pain. J Invest Dermatol. 2020;140(8):1538–1545.e2. doi: 10.1016/J.JID.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 44.Iznardo H, Puig L. IL-1 family cytokines in inflammatory dermatoses: pathogenetic role and potential therapeutic implications. Int J Mol Sci. 2022;23(16):9479. doi: 10.3390/IJMS23169479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winkler A, Sun W, De S, et al. The interleukin-1 receptor-associated kinase 4 inhibitor PF-06650833 blocks inflammation in preclinical models of rheumatic disease and in humans enrolled in a randomized clinical trial. Arthritis Rheumatol. 2021;73(12):2206–2218. doi: 10.1002/ART.41953/ABSTRACT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zouboulis CC, Frew JW, Giamarellos-Bourboulis EJ, et al. Target molecules for future hidradenitis suppurativa treatment. Exp Dermatol. 2021;30 Suppl 1(S1):8–17. 10.1111/EXD.14338 [DOI] [PubMed]
- 47.Kanni T, Argyropoulou M, Spyridopoulos T, et al. MABp1 targeting il-1α for moderate to severe hidradenitis suppurativa not eligible for adalimumab: a randomized study. J Invest Dermatol. 2018;138(4):795–801. doi: 10.1016/j.jid.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 48.Sun NZ, Ro T, Jolly P, Sayed CJ. Non-response to interleukin-1 antagonist canakinumab in two patients with refractory pyoderma gangrenosum and hidradenitis suppurativa. J Clin Aesthet Dermatol. 2017;10(9):36. /pmc/articles/PMC5749618/. Accessed March 12, 2023. [PMC free article] [PubMed]
- 49.Agud-Dios M, Arroyo-Andrés J, Rubio-Muñiz C, Postigo-Lorente C. Successful treatment of hidradenitis suppurativa and Crohn’s disease with combined guselkumab and apremilast. Dermatol Ther. 2022 doi: 10.1111/DTH.15743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tekin B, Salman A, Ergun T. Hidradenitis suppurativa unresponsive to canakinumab treatment: a case report. Indian J Dermatol Venereol Leprol. 2017;83(5):615–617. doi: 10.4103/IJDVL.IJDVL_147_16. [DOI] [PubMed] [Google Scholar]
- 51.Akdogan N, Yalici-Armagan B, Dogan S, Yılmaz R. Severe hidradenitis suppurativa (acne inversa) associated with focal segmental glomerulosclerosis and gout partially responsive to canakinumab. Dermatol Ther. 2021 doi: 10.1111/DTH.15002. [DOI] [PubMed] [Google Scholar]
- 52.Houriet C, Seyed Jafari SM, Thomi R, et al. Canakinumab for severe hidradenitis suppurativa: preliminary experience in 2 cases. JAMA Dermatol. 2017;153(11):1195–1197. doi: 10.1001/JAMADERMATOL.2017.2392. [DOI] [PubMed] [Google Scholar]
- 53.Jaeger T, Andres C, Grosber M, et al. Pyoderma gangrenosum and concomitant hidradenitis suppurativa—rapid response to canakinumab (anti-IL-1β) Eur J Dermatol. 2013;23(3):408–410. doi: 10.1684/ejd.2013.2018. [DOI] [PubMed] [Google Scholar]
- 54.Thomi R, Cazzaniga S, Morteza Seyed Jafari S, Schlapbach C, Hunger RE. Association of hidradenitis suppurativa with T helper 1/T helper 17 phenotypes: a semantic map analysis. JAMA Dermatol 2018;154(5):592–595. 10.1001/JAMADERMATOL.2018.0141 [DOI] [PMC free article] [PubMed]
- 55.Kaakati RN, Tanaka J, Liu B, et al. Atopic dermatitis is associated with hidradenitis suppurativa diagnosis: a single institution retrospective cohort study. JAAD Int. 2021;4:18. doi: 10.1016/J.JDIN.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dany M, Elston D. Gene expression of sphingolipid metabolism pathways is altered in hidradenitis suppurativa. J Am Acad Dermatol. 2017;77(2):268–273.e6. doi: 10.1016/j.jaad.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 57.Gambardella A, Calabrese G, Di Brizzi EV, Alfano R, Argenziano G. A case of atopic dermatitis and hidradenitis suppurativa successfully treated with dupilumab. J Eur Acad Dermatol Venereol. 2020;34(6):e284–e286. doi: 10.1111/JDV.16280. [DOI] [PubMed] [Google Scholar]
- 58.Cho MK, Shin JU, Kim DH, Lee HJ. Severe atopic dermatitis and concurrent severe hidradenitis suppurativa successfully treated with dupilumab. Clin Exp Dermatol. 2022 doi: 10.1111/CED.15387. [DOI] [PubMed] [Google Scholar]
- 59.Molinelli E, Sapigni C, Simonetti O, et al. Successfully and safety use of dupilumab in the management of severe atopic dermatitis and concomitant moderate-to-severe hidradenitis suppurativa. Dermatol Ther. 2022 doi: 10.1111/DTH.15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, double-blind placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157(11):1279–1288. doi: 10.1001/JAMADERMATOL.2021.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kimball AB, Zouboulis CC, Sayed C, et al. Bimekizumab in patients with moderate-to-severe hidradenitis suppurativa: 48-week efficacy and safety from BE HEARD I & II, two phase 3, randomized, double-blind, placebo-controlled, multicenter studies. In: AAD Late-Breaking Research Program; 2023.
- 62.Frew JW, Navrazhina K, Grand D, et al. The effect of subcutaneous brodalumab on clinical disease activity in hidradenitis suppurativa: an open-label cohort study. J Am Acad Dermatol. 2020;83(5):1341–1348. doi: 10.1016/J.JAAD.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frew JW, Navrazhina K, Sullivan-Whalen M, Gilleaudeau P, Garcet S, Krueger JG. Weekly administration of brodalumab in hidradenitis suppurativa: an open-label cohort study. Br J Dermatol. 2021;184(2):350–352. doi: 10.1111/BJD.19478. [DOI] [PubMed] [Google Scholar]
- 64.Yoshida Y, Oyama N, Iino S, Shimizu C, Hasegawa M. Long-standing refractory hidradenitis suppurativa responded to a brodalumab monotherapy in a patient with psoriasis: a possible involvement of Th17 across the spectrum of both diseases. J Dermatol. 2021;48(6):916–920. doi: 10.1111/1346-8138.15807. [DOI] [PubMed] [Google Scholar]
- 65.Arenbergerova M, Arenberger P, Marques E, Gkalpakiotis S. Successful treatment of recalcitrant gluteal hidradenitis suppurativa with brodalumab after anti-TNF failure. Int J Dermatol. 2020;59(6):733–735. doi: 10.1111/IJD.14792. [DOI] [PubMed] [Google Scholar]
- 66.Tee MW, Avarbock AB, Ungar J, Frew JW. Rapid resolution of pyoderma gangrenosum with brodalumab therapy. JAAD case reports. 2020;6(11):1167–1169. doi: 10.1016/J.JDCR.2020.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaul M, Jarvis P, Rozenberg I, et al. First-in-human study demonstrating the safety and clinical efficacy of novel anti-IL-17A monoclonal antibody CJM112 in moderate to severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2021;35(5):1143–1151. doi: 10.1111/JDV.17071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kimball AB, Loesche C, Prens EP, et al. IL-17A is a pertinent therapeutic target for moderate-to-severe hidradenitis suppurativa: combined results from a pre-clinical and phase II proof-of-concept study. Exp Dermatol. 2022;31(10):1522–1532. doi: 10.1111/EXD.14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Megna M, Ruggiero A, Di Guida A, Patrì A, Fabbrocini G, Marasca C. Ixekizumab: an efficacious treatment for both psoriasis and hidradenitis suppurativa. Dermatol Ther. 2020 doi: 10.1111/DTH.13756. [DOI] [PubMed] [Google Scholar]
- 70.Gul MI, Singam V, Hanson C, Neill BC, Aires DJ, Rajpara AN. Remission of refractory PASH syndrome using ixekizumab and doxycycline. J Drugs Dermatol. 2020;19(11):1123. doi: 10.36849/JDD.2020.1123. [DOI] [PubMed] [Google Scholar]
- 71.Reardon K, Levin J, Levin C. Severe hidradenitis suppurativa with herpes simplex virus 1 superinfection and clinical responsiveness to ixekizumab. JAAD Case Reports. 2021;9:7–8. doi: 10.1016/j.jdcr.2020.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iannone M, Janowska A, Tonini G, Davini G, Dini V. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine during Ixekizumab treatment for hidradenitis suppurativa. Clin Dermatol. 2021;39(4):701–702. doi: 10.1016/J.CLINDERMATOL.2021.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Odorici G, Pellacani G, Conti A. Ixekizumab in hidradenitis suppurativa in a psoriatic patient. G Ital di Dermatologia e Venereol. 2020;155(6):788–789. doi: 10.23736/S0392-0488.18.06135-7. [DOI] [PubMed] [Google Scholar]
- 74.Lanna C, Mazzilli S, Zangrilli A, Bianchi L, Campione E. One drug and two diseases: a case of multidrug-resistant hidradenitis suppurativa and psoriasis treated with apremilast. Dermatol Ther. 2019;32(6):e13089. doi: 10.1111/DTH.13089. [DOI] [PubMed] [Google Scholar]
- 75.Casseres RG, Kahn JS, Her MJ, Rosmarin D. Guselkumab in the treatment of hidradenitis suppurativa: a retrospective chart review. J Am Acad Dermatol. 2019;81(1):265–267. doi: 10.1016/j.jaad.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 76.Freeman S. Izokibep improves multiple psoriatic arthritis symptoms in phase 2 study. MDEdge Rheumatology. https://www.mdedge.com/rheumatology/article/255199/psoriatic-arthritis/izokibep-improves-multiple-psoriatic-arthritis. Published 2022. Accessed March 18, 2023.
- 77.ACELYRIN, INC. Announces Izokibep Achieves HiSCR100 Responses at 12 Weeks in Moderate-to-Severe Hidradenitis Suppurativa. https://www.prnewswire.com/news-releases/acelyrin-inc-announces-izokibep-achieves-hiscr100-responses-at-12-weeks-in-moderate-to-severe-hidradenitis-suppurativa-301775533.html. Accessed April 20, 2023.
- 78.Kimball AB, Jemec GBE, Alavi A, et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. Lancet (London, England). 2023;401(10378):747–761. doi: 10.1016/S0140-6736(23)00022-3. [DOI] [PubMed] [Google Scholar]
- 79.Ribero S, Ramondetta A, Fabbrocini G, et al. Effectiveness of secukinumab in the treatment of moderate-severe hidradenitis suppurativa: results from an Italian multicentric retrospective study in a real-life setting. J Eur Acad Dermatol Venereol. 2021;35(7):e441–e442. doi: 10.1111/JDV.17178. [DOI] [PubMed] [Google Scholar]
- 80.Prussick L, Rothstein B, Joshipura D, et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol. 2019;181(3):609–611. doi: 10.1111/BJD.17822. [DOI] [PubMed] [Google Scholar]
- 81.Casseres RG, Prussick L, Zancanaro P, et al. Secukinumab in the treatment of moderate to severe hidradenitis suppurativa: results of an open-label trial. J Am Acad Dermatol. 2020;82(6):1524–1526. doi: 10.1016/J.JAAD.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 82.Melgosa Ramos FJ, García Ruiz R, Estébanez Corrales A, Mateu Puchades A. Long-term secukinumab efficacy in patients with moderate to severe hidradenitis suppurativa: a retrospective single-centre case series (23 patients) J Eur Acad Dermatol Venereol. 2023 doi: 10.1111/JDV.18685. [DOI] [PubMed] [Google Scholar]
- 83.Reguiaï Z, Fougerousse AC, Maccari F, Bécherel PA. Effectiveness of secukinumab in hidradenitis suppurativa: an open study (20 cases) J Eur Acad Dermatol Venereol. 2020;34(11):e750–e751. doi: 10.1111/JDV.16605. [DOI] [PubMed] [Google Scholar]
- 84.Fernandez-Crehuet P, Haselgruber S, Padial-Gomez A, et al. Short-term effectiveness, safety, and potential predictors of response of secukinumab in patients with severe hidradenitis suppurativa refractory to biologic therapy: a multicenter observational retrospective study. Dermatol Ther (Heidelb) 2023 doi: 10.1007/S13555-023-00906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gutierrez E, Issa N, Resnik B. Novel regimen of IL-17A inhibitor secukinumab for the remission of severe hidradenitis suppurativa: case report. J Drugs Dermatol. 2022;21(12):1358–1360. doi: 10.36849/JDD.6752. [DOI] [PubMed] [Google Scholar]
- 86.Thorlacius L, Theut Riis P, Jemec GBE. Severe hidradenitis suppurativa responding to treatment with secukinumab: a case report. Br J Dermatol. 2018;179(1):182–185. doi: 10.1111/BJD.15769. [DOI] [PubMed] [Google Scholar]
- 87.Villegas-Romero I, Collantes-Rodríguez C, Valenzuela-Ubiña S, Jiménez-Gallo D. Moderate to severe hidradenitis suppurativa successfully treated with secukinumab. Actas Dermosifiliogr. 2020;111(8):696–698. doi: 10.1016/J.AD.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 88.Tas-Aygar G, Gonul M, Ozcan I, Ayli MD, Ertoy-Baydar D. Secukinumab may be remedy for systemic amyloidosis findings secondary to hidradenitis suppurativa. Dermatol Ther. 2020 doi: 10.1111/DTH.14205. [DOI] [PubMed] [Google Scholar]
- 89.Schuch A, Fischer T, Boehner A, Biedermann T, Volz T. Successful treatment of severe recalcitrant hidradenitis suppurativa with the interleukin-17A antibody secukinumab. Acta Derm Venereol. 2018;98(1):151–152. doi: 10.2340/00015555-2794/. [DOI] [PubMed] [Google Scholar]
- 90.Jørgensen A-HR, Yao Y, Thomsen SF. Therapeutic response to secukinumab in a 36-year-old woman with hidradenitis suppurativa. Case Rep Dermatol Med. 2018; 10.1155/2018/8685136 [DOI] [PMC free article] [PubMed]
- 91.Giuseppe P, Nicola P, Valentina C, et al. A case of moderate hidradenitis suppurativa and psoriasis treated with secukinumab. Ann Dermatol. 2018;30(4):462. doi: 10.5021/AD.2018.30.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chiricozzi A, Garcovich S, Malvaso D, Giovanardi G, Peris K. COVID-19 occurrence in one secukinumab-treated patient affected by hidradenitis suppurativa and systemic lupus erythematosus. Int J Dermatol. 2020;59(11):1423. doi: 10.1111/IJD.15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nikolakis G, Kreibich K, Vaiopoulos A, et al. Case Report: PsAPSASH syndrome: an alternative phenotype of syndromic hidradenitis suppurativa treated with the IL-17A inhibitor secukinumab. F1000Research. 2021; 10.12688/F1000RESEARCH.52100.2 [DOI] [PMC free article] [PubMed]
- 94.Enginar AU, Gundogdu M. Successful treatment of hidradenitis suppurativa with secukinumab in a patient with ankylosing spondylitis and familial mediterranean fever. Mod Rheumatol Case Rep. 2022;6(1):19–21. doi: 10.1093/MRCR/RXAB008. [DOI] [PubMed] [Google Scholar]
- 95.Li M, Xiang H, Liang Y, et al. Secukinumab for PASS syndrome: a new choice for therapeutic challenge? Dermatol Ther. 2022;35(7):e15507. doi: 10.1111/DTH.15507. [DOI] [PubMed] [Google Scholar]
- 96.Głowaczewska A, Szepietowski JC, Matusiak Ł. Severe hidradenitis suppurativa successfully treated with secukinumab. Dermatol Ther. 2020 doi: 10.1111/DTH.13845. [DOI] [PubMed] [Google Scholar]
- 97.Papp KA, Weinberg MA, Morris A, Reich K. IL17A/F nanobody sonelokimab in patients with plaque psoriasis: a multicentre, randomised, placebo-controlled, phase 2b study. Lancet. 2021;397(10284):1564–1575. doi: 10.1016/S0140-6736(21)00440-2. [DOI] [PubMed] [Google Scholar]
- 98.Truong SL, Chin J, Liew DFL, et al. Systematic review and meta-analysis of inflammatory bowel disease adverse events with anti-interleukin 17A agents and tumor necrosis factor inhibitors in rheumatic disease and skin psoriasis. Rheumatol Ther. 2021;8(4):1603. doi: 10.1007/S40744-021-00360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Flora A, Kozera EK, Jepsen R, et al. Baseline clinical, hormonal and molecular markers associated with clinical response to IL-23 antagonism in hidradenitis suppurativa: a prospective cohort study. Exp Dermatol. 2023 doi: 10.1111/EXD.14789. [DOI] [PubMed] [Google Scholar]
- 100.Dudink K, Bouwman K, Chen Y, et al. Guselkumab for hidradenitis suppurativa: a phase II, open-label, mode-of-action study. Br J Dermatol. 2023;00:1–9. doi: 10.1093/BJD/LJAD010. [DOI] [PubMed] [Google Scholar]
- 101.Burzi L, Repetto F, Ramondetta A, et al. Guselkumab in the treatment of severe hidradenitis suppurativa, a promising role? Dermatol Ther. 2021 doi: 10.1111/DTH.14930. [DOI] [PubMed] [Google Scholar]
- 102.Jørgensen AHR, Holm JG, Thomsen SF. Guselkumab for hidradenitis suppurativa in a patient with concomitant Crohn’s disease: report and systematic literature review of effectiveness and safety. Clin Case Reports. 2020;8(12):2874–2877. doi: 10.1002/CCR3.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Melgosa Ramos FJ, García Ruiz R, Mateu Puchades A, Alfageme Roldán F. Guselkumab effectiveness, and posology in patients with moderate to severe hidradenitis suppurativa: a retrospective bicentric experience. Dermatol Ther. 2022 doi: 10.1111/DTH.15558. [DOI] [PubMed] [Google Scholar]
- 104.Kovacs M, Podda M. Guselkumab in the treatment of severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2019;33(3):e140–e141. doi: 10.1111/jdv.15368. [DOI] [PubMed] [Google Scholar]
- 105.Berman HS, Villa NM, Shi VY, Hsiao JL. Guselkumab in the treatment of concomitant hidradenitis suppurativa, psoriasis, and Crohn’s disease. J Dermatolog Treat. 2021;32(2):261–263. doi: 10.1080/09546634.2019.1654067. [DOI] [PubMed] [Google Scholar]
- 106.Kearney N, Byrne N, Kirby B, Hughes R. Successful use of guselkumab in the treatment of severe hidradenitis suppurativa. Clin Exp Dermatol. 2020;45(5):618–619. doi: 10.1111/CED.14199. [DOI] [PubMed] [Google Scholar]
- 107.Montero-Vilchez T, Martinez-Lopez A, Salvador-Rodriguez L, Arias-Santiago S, Molina-Leyva A. The use of guselkumab 100 mg every 4 weeks on patients with hidradenitis suppurativa and a literature review. Dermatol Ther. 2020 doi: 10.1111/DTH.13456. [DOI] [PubMed] [Google Scholar]
- 108.Garcia-Melendo C, Vilarrasa E, Cubiró X, Bittencourt F, Puig L. Sequential paradoxical psoriasiform reaction and sacroiliitis following adalimumab treatment of hidradenitis suppurativa, successfully treated with guselkumab. Dermatol Ther. 2020;33(6):e14180. doi: 10.1111/DTH.14180. [DOI] [PubMed] [Google Scholar]
- 109.Croitoru DO, Seigel K, Nathanielsz N, et al. Treatment of severe hidradenitis suppurativa and fistulizing Crohn’s disease with guselkumab. J Eur Acad Dermatol Venereol. 2022;36(7):e563–e565. doi: 10.1111/JDV.18033. [DOI] [PubMed] [Google Scholar]
- 110.Martora F, Fabbrocini G, Marasca C, Battista T, Megna M. Paradoxical hidradenitis suppurativa induced by adalimumab biosimilar successfully treated with guselkumab in a patient with psoriasis. Comment on “Paradoxical hidradenitis suppurativa due to anti-interleukin-1 agents for mevalonate kinase deficiency successfully treated with the addition of ustekinumab.” Clin Exp Dermatol 2023; 10.1093/CED/LLAD082 [DOI] [PubMed]
- 111.Kimball AB, Prens EP, Passeron T, et al. Efficacy and safety of risankizumab for the treatment of hidradenitis suppurativa: a phase 2, randomized, placebo-controlled trial. Dermatol Ther (Heidelb). 2023 doi: 10.1007/S13555-023-00913-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Repetto F, Burzi L, Ribero S, Quaglino P, Dapavo P. Efficacy and safety of risankizumab in hidradenitis suppurativa: a case series. Acta Derm Venereol. 2022 doi: 10.2340/ACTADV.V102.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marques E, Arenberger P, Smetanová A, Gkalpakiotis S, Zimová D, Arenbergerová M. Successful treatment of recalcitrant hidradenitis suppurativa with risankizumab after failure of anti-tumour necrosis factor alpha. Br J Dermatol. 2021;184(5):966–967. doi: 10.1111/BJD.19716. [DOI] [PubMed] [Google Scholar]
- 114.Licata G, Gambardella A, Buononato D, et al. A case of moderate hidradenitis suppurativa and psoriasis successfully treated with risankizumab. Int J Dermatol. 2022;61(4):e126–e129. doi: 10.1111/IJD.15704. [DOI] [PubMed] [Google Scholar]
- 115.Flora A, Holland R, Smith A, Frew JW. Rapid and sustained remission of synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome with IL-23p19 antagonist (risankizumab) JAAD Case Reports. 2021;14:33. doi: 10.1016/J.JDCR.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Caposiena Caro RD, Pensa C, Lambiase S, Candi E, Bianchi L. Risankizumab effectiveness in a recalcitrant case of hidradenitis suppurativa after anti-TNF and anti-interleukin-17 failures. Dermatol Ther. 2021;34(6):e15116. doi: 10.1111/DTH.15116. [DOI] [PubMed] [Google Scholar]
- 117.Kok Y, Nicolopoulos J, Dolianitis C. Tildrakizumab as a potential long-term therapeutic agent for severe hidradenitis suppurativa: a 15 months experience of an Australian institution. Australas J Dermatol. 2021;62(2):e313–e316. doi: 10.1111/AJD.13559. [DOI] [PubMed] [Google Scholar]
- 118.Hayran Y, Allı N, Yücel, Akdoğan N, Turhan T. Serum IL-36α, IL-36β, and IL-36γ levels in patients with hidradenitis suppurativa: association with disease characteristics, smoking, obesity, and metabolic syndrome. Arch Dermatol Res. 2020;312(3):187–196. 10.1007/S00403-019-02012-W/FIGURES/4 [DOI] [PubMed]
- 119.Thomi R, Kakeda M, Yawalkar N, Schlapbach C, Hunger RE. Increased expression of the interleukin-36 cytokines in lesions of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2017;31(12):2091–2096. doi: 10.1111/JDV.14389. [DOI] [PubMed] [Google Scholar]
- 120.Hessam S, Sand M, Gambichler T, Skrygan M, Rüddel I, Bechara FG. Interleukin-36 in hidradenitis suppurativa: evidence for a distinctive proinflammatory role and a key factor in the development of an inflammatory loop. Br J Dermatol. 2018;178(3):761–767. doi: 10.1111/BJD.16019. [DOI] [PubMed] [Google Scholar]
- 121.Bachelez H, Choon S-E, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385(26):2431–2440. doi: 10.1056/NEJMOA2111563. [DOI] [PubMed] [Google Scholar]
- 122.Alavi A, Prens E, Kimball AB, et al. Spesolimab for hidradenitis suppurativa: a proof-of-concept study. In: American Academy of dermatology annual meeting. New Orleans, LA; 2023.
- 123.Prens LM, Ardon CB, van Straalen KR, et al. No Evident systemic terminal complement pathway activation in hidradenitis suppurativa. J Invest Dermatol. 2021;141(12):2966–2969.e1. doi: 10.1016/J.JID.2021.03.037. [DOI] [PubMed] [Google Scholar]
- 124.Giamarellos-Bourboulis EJ, Jemec GBE, Prens EP, et al. Complement split product C5a is elevated in moderate and severe hidradenitis suppurativa: clinical improvement by targeted therapy coming from the SHINE Study. In: 10th European Hidradenitis Suppurativa (EHSF) e.V. Conference. 2021.
- 125.Kanni T, Zenker O, Habel M, Riedemann N, Giamarellos-Bourboulis EJ. Complement activation in hidradenitis suppurativa: a new pathway of pathogenesis? Br J Dermatol. 2018;179(2):413–419. doi: 10.1111/BJD.16428. [DOI] [PubMed] [Google Scholar]
- 126.Hoffman LK, Tomalin LE, Schultz G, et al. Integrating the skin and blood transcriptomes and serum proteome in hidradenitis suppurativa reveals complement dysregulation and a plasma cell signature. PLoS One. 2018;13(9):e0203672. doi: 10.1371/journal.pone.0203672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.ChemoCentryx announces positive topline results of phase II AURORA clinical trial of avacopan in the treatment of hidradenitis suppurativa (HS). GlobeNewsWire. https://www.globenewswire.com/news-release/2020/10/28/2116264/19219/en/ChemoCentryx-announces-positive-topline-results-of-phase-II-AURORA-clinical-trial-of-avacopan-in-the-treatment-of-hidradenitis-suppurativa-HS.html. Published 2020. Accessed March 21, 2023.
- 128.Giamarellos-Bourboulis EJ, Argyropoulou M, Kanni T, et al. Clinical efficacy of complement C5a inhibition by IFX-1 in hidradenitis suppurativa: an open-label single-arm trial in patients not eligible for adalimumab. Br J Dermatol. 2020;183(1):176–178. doi: 10.1111/BJD.18877. [DOI] [PubMed] [Google Scholar]
- 129.InflaRx announces amendment of co-development agreement and additional equity investment by staidson in connection with regulatory filing in China for Anti-C5a-antibody for treatment of COVID-19. https://www.globenewswire.com/en/news-release/2022/12/21/2577789/0/en/InflaRx-announces-amendment-of-co-development-agreement-and-additional-equity-investment-by-staidson-in-connection-with-regulatory-filing-in-China-for-Anti-C5a-antibody-for-treatme.html. Published 2021. Accessed March 26, 2023.
- 130.Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014 doi: 10.1097/MD.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Haeggström JZ, Newcomer ME. Structures of leukotriene biosynthetic enzymes and development of new therapeutics. Annu Rev Pharmacol Toxicol. 2023;63(1):407–428. doi: 10.1146/ANNUREV-PHARMTOX-051921-085014. [DOI] [PubMed] [Google Scholar]
- 132.Penno CA, Jäger P, Laguerre C, et al. Lipidomics profiling of hidradenitis suppurativa skin lesions reveals lipoxygenase pathway dysregulation and accumulation of proinflammatory leukotriene B4. J Invest Dermatol. 2020;140(12):2421–2432.e10. doi: 10.1016/J.JID.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 133.Kirby JS, Okun MM, Alavi A, et al. Janus kinase 1 inhibition with povorcitinib (INCB054707) in hidradenitis suppurativa: efficacy and safety during the open-label extension period of a phase 2 study. In: 12th Conference of the European hidradenitis suppurativa foundation; 2023:S-0906
- 134.Kozera E, Flora A, Frew JW. Real-world safety and clinical response of Janus kinase inhibitor upadacitinib in the treatment of hidradenitis suppurativa: a retrospective cohort study. J Am Acad Dermatol. 2022 doi: 10.1016/J.JAAD.2022.07.047. [DOI] [PubMed] [Google Scholar]
- 135.Hodge JA, Kawabata TT, Krishnaswami S, et al. The mechanism of action of tofacitinib—an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34(2):318–328. https://www.clinexprheumatol.org/abstract.asp?a=9615. Accessed March 26, 2023. [PubMed]
- 136.Savage KT, Santillan MR, Flood KS, Charrow A, Porter ML, Kimball AB. Tofacitinib shows benefit in conjunction with other therapies in recalcitrant hidradenitis suppurativa patients. JAAD Case Reports. 2020;6(2):99–102. doi: 10.1016/J.JDCR.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gudjonsson JE, Tsoi LC, Ma F, et al. Contribution of plasma cells and B cells to hidradenitis suppurativa pathogenesis. JCI insight. 2020 doi: 10.1172/JCI.INSIGHT.139930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Geahlen RL. Getting Syk: spleen tyrosine kinase as a therapeutic target. Trends Pharmacol Sci. 2014;35(8):414. doi: 10.1016/J.TIPS.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mendes-Bastos P, Brasileiro A, Kolkhir P, et al. Bruton’s tyrosine kinase inhibition—an emerging therapeutic strategy in immune-mediated dermatological conditions. Allergy. 2022;77(8):2355–2366. doi: 10.1111/ALL.15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kaul M, End P, Cabanski M, et al. Remibrutinib (LOU064): A selective potent oral BTK inhibitor with promising clinical safety and pharmacodynamics in a randomized phase I trial. Clin Transl Sci. 2021;14(5):1756–1768. doi: 10.1111/CTS.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Connell NT, Berliner N. Fostamatinib for the treatment of chronic immune thrombocytopenia. Blood. 2019;133(19):2027–2030. doi: 10.1182/BLOOD-2018-11-852491. [DOI] [PubMed] [Google Scholar]
- 142.Kerdel FR, Azevedo FA, Kerdel Don C, Don FA, Fabbrocini G, Kerdel FA. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 Study. J Drugs Dermatol. 2019;18(2):170–176. [PubMed] [Google Scholar]
- 143.Vossen ARJV, van Doorn MBA, van der Zee HH, Prens EP. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80(1):80–88. doi: 10.1016/J.JAAD.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 144.Weber P, Seyed Jafari SM, Yawalkar N, Hunger RE. Apremilast in the treatment of moderate to severe hidradenitis suppurativa: a case series of 9 patients. J Am Acad Dermatol. 2017;76(6):1189–1191. doi: 10.1016/j.jaad.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 145.Garbayo-Salmons P, Expósito-Serrano V, Ribera Pibernat M, Romaní J. Hidradenitis suppurativa treated with apremilast: a case series. Actas Dermosifiliogr. 2021;112(10):936–939. doi: 10.1016/J.AD.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 146.Garcovich S, Giovanardi G, Malvaso D, De Simone C, Peris K. Apremilast for the treatment of hidradenitis suppurativa associated with psoriatic arthritis in multimorbid patients: case report and review of literature. Medicine (Baltimore). 2020;99(5):e18991. doi: 10.1097/MD.0000000000018991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Proietti I, Michelini S, Mambrin A, et al. A case of hidradenitis suppurativa successfully treated with apremilast in a patient with psoriasis and SAMPUS. Dermatol Ther. 2020;33(3):e13448. doi: 10.1111/DTH.13448. [DOI] [PubMed] [Google Scholar]
- 148.McMichael J, Stoff BK. Treatment of a perforating dermatosis with apremilast. JAAD Case Reports. 2021;16:155–157. doi: 10.1016/J.JDCR.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Warren RB, Strober B, Silverberg JI, et al. Oral orismilast: efficacy and safety in moderate-to-severe psoriasis and development of modified release tablets. J Eur Acad Dermatol Venereol. 2023 doi: 10.1111/JDV.18812. [DOI] [PubMed] [Google Scholar]
- 150.PTM-001—PTM therapeutics. https://www.ptmthera.com/ptm-001. Accessed January 8, 2023.
- 151.Walker E. New start up aims to target inflammatory bowel disease. https://www.pathology.med.umich.edu/news/924. Published 2020. Accessed March 23, 2023.
- 152.Liang TW, Presta LG, Brazil JC, Parkos CA, Cheng JC. Validation of hPTM-001, a humanized candidate therapeutic antibody for promoting mucosal wound healing in IBD. In: Crohn’s and Colitis Congress; 2021.
- 153.Xie Y, Kuang W, Wang D, Yuan K, Yang P. Expanding role of CXCR2 and therapeutic potential of CXCR2 antagonists in inflammatory diseases and cancers. Eur J Med Chem. 2023;250:115175. doi: 10.1016/J.EJMECH.2023.115175. [DOI] [PubMed] [Google Scholar]
- 154.Forman S, Patel DR, Kimball AB, et al. Safety and efficacy of LY3041658, a novel septa-specific monoclonal antibody to CXCR1 and CXCR2 ligands, in a phase 2 study in hidradenitis suppurativa. In: American Academy of Dermatology Annual Meeting 2023.
- 155.Boyles JS, Beidler CB, Strifler BA, et al. Discovery and characterization of a neutralizing pan-ELR+CXC chemokine monoclonal antibody. MAbs. 2020; 10.1080/19420862.2020.1831880 [DOI] [PMC free article] [PubMed]
- 156.Melnik BC, Plewig G. Impaired Notch-MKP-1 signalling in hidradenitis suppurativa: an approach to pathogenesis by evidence from translational biology. Exp Dermatol. 2013;22(3):172–177. doi: 10.1111/EXD.12098. [DOI] [PubMed] [Google Scholar]
- 157.Aclaris Therapeutics Announces Preliminary Topline Data from 12-Week Phase 2a Study of Oral Zunsemetinib (ATI-450) for Moderate to Severe Hidradenitis Suppurativa | BioSpace. https://www.biospace.com/article/releases/-aclaris-therapeutics-announces-preliminary-topline-data-from-12-week-phase-2a-study-of-oral-zunsemetinib-ati-450-for-moderate-to-severe-hidradenitis-suppurativa/. Accessed March 25, 2023.
- 158.Gordon D, Hellriegel ET, Hope HR, Burt D, Monahan JB. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the MK2 inhibitor ATI-450 in healthy subjects: a placebo-controlled, randomized phase 1 study. Clin Pharmacol. 2021;13:123–134. doi: 10.2147/CPAA.S305308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wolk K, Brembach TC, Šimaitė D, et al. Activity and components of the granulocyte colony-stimulating factor pathway in hidradenitis suppurativa. Br J Dermatol. 2021;185(1):164–176. doi: 10.1111/BJD.19795. [DOI] [PubMed] [Google Scholar]
- 160.Gamell C, Bankovacki A, Scalzo-Inguanti K, et al. CSL324, a granulocyte colony-stimulating factor receptor antagonist, blocks neutrophil migration markers that are upregulated in hidradenitis suppurativa. Br J Dermatol. 2023 doi: 10.1093/BJD/LJAD013. [DOI] [PubMed] [Google Scholar]
- 161.Ben Abdallah H, Seeler S, Bregnhøj A, et al. Heat shock protein 90 inhibitor RGRN-305 potently attenuates skin inflammation. Front Immunol. 2023; 10.3389/FIMMU.2023.1128897 [DOI] [PMC free article] [PubMed]
- 162.Bregnhøj A, Thuesen KKH, Emmanuel T, et al. HSP90 inhibitor RGRN-305 for oral treatment of plaque-type psoriasis: efficacy, safety and biomarker results in an open-label proof-of-concept study. Br J Dermatol. 2022;186(5):861–874. doi: 10.1111/BJD.20880. [DOI] [PubMed] [Google Scholar]
- 163.De Vita V, Melnik BC. Activated mTORC1 signaling: the common driving force of type 2 diabetes and hidradenitis suppurativa. J Am Acad Dermatol. 2018;78(5):e121. doi: 10.1016/j.jaad.2017.11.061. [DOI] [PubMed] [Google Scholar]
- 164.Bettuzzi T, Frumholtz L, Jachiet M, et al. Sirolimus as combination rescue therapy with tumor necrosis alpha inhibitors for severe, refractory hidradenitis suppurativa. J Am Acad Dermatol. 2020;83(5):1441–1444. doi: 10.1016/j.jaad.2020.06.042. [DOI] [PubMed] [Google Scholar]
- 165.Gottlieb J, Madrange M, Gardair C, et al. PAPASH, PsAPASH and PASS autoinflammatory syndromes: phenotypic heterogeneity, common biological signature and response to immunosuppressive regimens. Br J Dermatol. 2019;181(4):866–869. doi: 10.1111/BJD.18003. [DOI] [PubMed] [Google Scholar]
- 166.Chen AY, Zervos MJ, Vazquez JA. Dalbavancin: a novel antimicrobial. Int J Clin Pract. 2007;61(5):853. doi: 10.1111/J.1742-1241.2007.01318.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Simonetti O, Lucarini G, Morroni G, et al. New evidence and insights on dalbavancin and wound healing in a mouse model of skin infection. Antimicrob Agents Chemother. 2020 doi: 10.1128/AAC.02062-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Oliva A, Stefani S, Venditti M, Di Domenico EG. Biofilm-related infections in gram-positive bacteria and the potential role of the long-acting agent Dalbavancin. Front Microbiol. 2021 doi: 10.3389/FMICB.2021.749685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Molinelli E, Sapigni C, D’Agostino GM, et al. The effect of dalbavancin in moderate to severe hidradenitis suppurativa. Antibiot (Basel, Switzerland) 2022;11(11):1573. doi: 10.3390/ANTIBIOTICS11111573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Molinelli E, Sapigni C, Simonetti O, et al. Successful use of dalbavancin in the management of severe hidradenitis suppurativa. Eur J Dermatol. 2022;32(5):645–646. doi: 10.1684/EJD.2022.4329. [DOI] [PubMed] [Google Scholar]