Abstract
Background
Primary open-angle glaucoma (POAG) is the most common subtype of glaucoma. We evaluate the cost-effectiveness of polygenic risk score (PRS) profiling as a screening tool for POAG.
Methods
We used a Markov cohort model to evaluate the cost-effectiveness of implementing PRS screening in the UK and Australia, conducted from the healthcare payer’s perspective. We used published data to calculate prevalence, transition probabilities, utility, cost and other parameters in the model. Our main outcome measure was the incremental cost-effectiveness ratio (ICER) and secondary outcomes were years of blindness avoided and a ‘Blindness ICER’. We did one-way as well as two-way deterministic and probabilistic sensitivity analyses.
Results
The proposed screening programme for POAG in the UK is predicted to result in ICER of £24,783 (95% CI: £13,373–66,960) and would avoid 1 year of blindness at ICER of £10,095 (95% CI: £5513–27,656). In Australia, it is predicted to result in ICER of AU$34,252 (95% CI: AU$21,324–95,497) and would avoid 1 year of blindness at ICER of AU$13,359 (95% CI: AU$8143–37,448). Using the willingness to pay thresholds of $54,808 and £30,000, the proposed screening model is 79.2% likely to be cost-effective in Australia and is 60.2% likely to be cost-effective in the UK, respectively.
Conclusion
We describe and model the cost-efficacy of incorporating a polygenic risk score for POAG screening in Australia and the UK for the first time and results indicated this is a promising cost-effectiveness strategy.
Subject terms: Population genetics, Health care economics
Introduction
Primary open-angle glaucoma (POAG) is the leading cause of irreversible blindness globally [1], and its prevalence is estimated to range between 2–3% worldwide [1]. With an aging population, the projected number of people with POAG globally is anticipated to increase to approximately 112 million by 2040 [1].
POAG has a major disease and economic burden on both individuals and society. Vision loss from POAG is associated with higher risk of falls, depression and unemployment [2]. Direct healthcare cost estimates for glaucoma were £135 million [3] across the United Kingdom (UK) in 2002, and AU$144.2 million [4] in Australia during 2004. It is also well established that the financial burden of glaucoma increases as disease severity increases [5]. Currently, it is estimated that approximately 50% of glaucoma cases in the developed world are undiagnosed [6, 7]. Therefore, early detection of undiagnosed POAG and prevention of progression would be beneficial.
POAG is an ideal disease for screening and risk profiling given it is insidious, leads to significant morbidity if left untreated, and effective treatment is available to slow the rate of progression once diagnosed [8]. Economic evaluation of any screening programme is essential for government and decision makers due to limited resources. Prior work has shown that the costs associated with POAG are lower when the disease is in earlier stages [9, 10] and given that advanced POAG at presentation is a major risk factor for legal blindness [11], there are clear advantages to screening for POAG. However, previous economic modelling has shown that population-based clinical screening programmes are not currently cost-effective in most high-income countries such as the UK and the US due to high implementation costs and the disproportionate disease burden in the older population [12]. One potential solution to this predicament is to identify people at high-risk for POAG and then undertake targeted clinical screening and monitoring in this high-risk subgroup [12].
Given that POAG is one of the most heritable human diseases [13], genetic profiling presents as a unique means to risk stratify and prioritise clinical screening of individuals. People with a family history of POAG have significantly increased odds (between five to 10 times) for developing POAG. Recently, a polygenic risk score (PRS), which combines the weighted risk across a few thousand single-nucleotide polymorphisms (SNPs), has been shown to be a useful tool for estimating an individual’s genetic predisposition to complex diseases such as POAG [14]. This novel PRS was found to substantially outperform any previous POAG prediction tools based on common genetic variation, whilst also outperforming genetic prediction tools for any other common disease [15]. Comparing the highest and lowest deciles for the PRS, a 15-fold increased risk for developing advanced disease, as well as an earlier disease onset by up to a decade, was identified [14]. Using the PRS in combination with age, sex and family history to predict POAG diagnosis dramatically outperformed these parameters without the PRS [14]. As such, PRS profiling could have great health and economic impact on POAG prevention, early detection and intervention for high-income countries.
We sought to determine the cost-effectiveness of implementing a population-wide genetic screening programme using a PRS as an adjunct to prioritise individuals for clinical monitoring for POAG. We modelled such a genetic-risk screening programme for the general population aged between 40 to 80 years in the UK and Australia. We believe that this study will provide valuable information for the governments and policy makers of these two countries.
Methods
Study population
A cohort population was selected based on the mid 2019 population reports by the Office for National Statistics (ONS) [16] and the Australian Bureau of Statistics (ABS) [17]; we used 11,782,538 Australian and 33,618,730 UK residents aged 40 and above as our study population. Individuals with an existing diagnosis of POAG were removed from the sample, based on estimated prevalence and diagnosis rates in the published literature [6, 18].
Screening pathway and Model structure
In contrast to the conventional POAG care pathway with predominantly incidental or symptomatic detection leading straight to treatment (Fig. 1a), the proposed screening programme is to screen the age groups of interest using a genetic test to obtain the PRS, followed by comprehensive optometrist and/or ophthalmologist review for those who are identified at high-risk via the genetic test. If POAG is diagnosed in an individual, they enter the established treatment care pathway. Individuals with no POAG would be followed up with biennial optometrist check up.
Fig. 1. Overview of POAG-screening pathways and economical models.
a Current conventional and proposed PRS-based care pathway of POAG. b A schematic of Markov Model structure used for the genetic screening of primary open-angle glaucoma “pathway”. *(i) No POAG: healthy individual; (ii) Early: Abnormalities of the optic nerve without any visual field abnormalities; (iii) Mild: Damage to the optic nerve and some peripheral vision loss; (iv) Moderate: Severe optic nerve damage and advanced peripheral vision loss; (v) Severe: Visual acuity of 6/60 or worse or VF constricted to 10°.
We developed a Markov cohort model to evaluate cost-effectiveness for POAG screening in the UK and Australia (Fig. 1b) from the healthcare payor’s perspective. There were six health states in our model, including healthy individuals, death and four health states associated with POAG stages: early, mild, moderate, and severe. Our definition for the POAG severity classifications was adapted from a prior report [2] where: (1) “Early” was definite or probable POAG with no visual impairment but changes to optic nerve and/or retina; (2) “Mild” was definite POAG with mild visual impairment; (3) “Moderate” was definite POAG with moderate visual impairment; (4) “Severe” was characterised by a visual acuity of 6/60 or worse due to POAG.
Our cohort of population was divided into age groups: 40–49; 50–59; 60–69; 70–79; and 80 and above as well as into their PRS risk decile. For the base case model, three age groups: 50–59;60–69 and 70–79 were selected for screening with genetic testing and the top two PRS deciles were also selected for further screening pathway. A lifetime simulation time horizon was used, whereby individuals could begin the model as either healthy or as undiagnosed POAG at an early, mild, or moderate disease stage based on the prevalence for each disease stage. The assumption was made that patients with severe disease would have been diagnosed. Any stage can progress to the next disease stage or to death, but disease stages cannot be skipped nor can a patient remit to an earlier disease stage. The rate of progression to the next disease stage was modified by the individual’s underlying genetic profile (as per their PRS risk decile) and their age and was decreased by treatment.
TreeAge Pro Healthcare [19] was used to construct the Markov models (Fig. 1b), monitor the transition between discrete health states, and represent the cost-effectiveness of the PRS in our population cohorts. Two identical models were built, one for the UK and the other for Australia, utilising different inputs for the key parameters.
Data collection
The modelling required all parameters at specific granularity such as the prevalence of early POAG in age group 50–59 in the UK, and the transition probability from mild to moderate POAG at age group 60–69 in Australia. A thorough search was undertaken on PubMed and Google Scholar to identify these data for our modelling; limited primary source data were identified at the age/disease stage granularity required. Therefore, where available primary research data or existing systemic reviews were utilised, and other values were calculated (detailed in Appendix 1 and 2). Disease severity is based on the results of the better eye.
Modelling POAG prevalence
After excluding the population with existing diagnosis of POAG, we estimated the prevalence of early, mild, moderate and severe POAG in the screening population within the age groups of 40–49, 50–59, 60–69, 70–79, 80 and above based on published data (Appendix 1), calculated prevalences are provided in Table 1.
Table 1.
The inputs of the key parameters: the prevalence, transition probability, utility and cost.
| Country | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Source | |
|---|---|---|---|---|---|---|---|
| Prevalence at model beginning | |||||||
| No disease | Australia | 99.94% | 98.99% | 98.84% | 97.97% | 97.54% | Calculated from Keel et al. [6] |
| Early | Australia | 0.06% | 0.95% | 0.94% | 1.70% | 1.25% | Calculated from Keel et al. [6] |
| Mild | Australia | 0.00% | 0.06% | 0.03% | 0.04% | 0.15% | Calculated from Keel et al. [6] |
| Moderate | Australia | 0.00% | 0.00% | 0.20% | 0.29% | 1.05% | Calculated from Keel et al. [6] |
| Severe | Australia | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | Calculated from Keel et al. [6] |
| No disease | UK | 99.71% | 99.46% | 98.99% | 98.04% | 96.24% | Calculated from Rudnicka et al. [41] |
| Early | UK | 0.29% | 0.48% | 0.46% | 0.81% | 1.15% | Calculated from Rudnicka et al. [41] |
| Mild | UK | 0.00% | 0.07% | 0.07% | 0.14% | 0.33% | Calculated from Rudnicka et al. [41] |
| Moderate | UK | 0.00% | 0.00% | 0.49% | 1.01% | 2.28% | Calculated from Rudnicka et al. [41] |
| Severe | UK | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | Calculated from Rudnicka et al. [41] |
| Annual transition probability | |||||||
| No disease to Early | Australia | 0.02% | 0.28% | 0.28% | 0.29% | 0.29% | Calculated from Keel et al. [6] |
| Early to Mild | Australia | 0.00% | 3.80% | 9.80% | 10.00% | 17.00% | Calculated from Keel et al. [6] |
| Mild to Moderate | Australia | 85% | 85% | 85% | 85% | 85% | [2] |
| Moderate to Severe | Australia | 30% | 30% | 30% | 30% | 30% | [2] |
| No disease to Early | UK | 0.03% | 0.14% | 0.14% | 0.22% | 0.90% | Calculated from Rudnicka et al. [41] |
| Early to Mild | UK | 0.00% | 3.50% | 11.40% | 11.40% | 36.50% | Calculated from Rudnicka et al. [41] |
| Mild to Moderate | UK | 85% | 85% | 85% | 85% | 85% | [2] |
| Moderate to Severe | UK | 30% | 30% | 30% | 30% | 30% | [2] |
| Utility multipliers | Australia value | UK value | Sources |
|---|---|---|---|
| No disease | 1 | 1 | Assumption |
| Early disease stage | 1 | 1 | Assumption |
| Mild | 0.79 | 0.79 | [20] |
| Moderate | 0.64 | 0.64 | [20] |
| Severe | 0.26 | 0.26 | [21] |
| Costs (AUD and GBP respectively) | Australia value | UK value | Sources | |
|---|---|---|---|---|
| Genetic screening test per patient | 350.00 | 175.00 | [42, 43]* | |
| Annual screening costs per patient | 34.43 | 51.28 | Calculated from references [44, 45] | |
| Annual POAG treatment costs per patient | 2012.80 | 563.83 | Calculated from references [46]** | |
| Annual depression treatment per patient | 1405.89 | 2930.41 | Calculated from references [25, 27] | |
| Annual residential aged care facility costs per resident | 66,540.52 | 47,850.90 | [26, 47] | |
| Fall costs per event | 13,466.04 | 2806.00 | Calculated from references [48, 49] | |
| POAG treatment cost weighting by disease stage | ||||
| Early disease stage | 0.934 | 0.934 | [50] | |
| Mild | 1.116 | 1.116 | [50] | |
| Moderate | 1.21 | 1.21 | [50] | |
| Severe | 1.559 | 1.559 | [50] | |
*Based on commercial available tests which utilise genome-sequencing in a standard clinical setting.
**See Appendix10.
Transition probabilities
All transitions between health states take place in an annual cycle. A detailed search was conducted to identify transition probabilities by disease stage by age for POAG in Australia and the UK, using the following combined terms: “glaucoma” AND “progression” OR “transition” AND “Australia” OR “United Kingdom”. Due to lack of primary source data at the age/disease stage granularity, transition probabilities for no disease to early, and early to mild were calculated based on available prevalence data (Appendix 1, 2 and 7).
For mild to moderate and moderate to severe disease transition probabilities, we utilised the results from the CERA Tunnel Vision study [2] (Appendix 8), and then investigated in sensitivity analyses. Transition probabilities are presented in Table 1.
Utility values—QALYs and years of blindness
Glaucoma utility multipliers by disease stage were taken from the study by Burr et al. [20] and from Brown et al. [21]. No disease and early disease had a utility multiplier of 1, while death had a utility value of 0. These disease-based utility multipliers were then applied to utility-by-age data for the countries of interest [22, 23] (Appendix 9).
The model also evaluated years of blindness, with the disease stage of severe POAG contributing 1 year of blindness per year and all other disease states contributing no years of blindness.
Cost
Three distinct cost categories were identified for this model: (1) the cost of the genetic screening test and follow-up monitoring cost for high-risk individuals; (2) the cost of POAG treatment; (3) POAG-associated health-economic costs. The details of all cost categories are in Table 1 and Appendix 1 and 10.
Three major domains were included as POAG-associated health-economic costs based on previous research [24]: aged care facility admissions, falls, and depression. Costs of these events were taken from government data [25, 26] and other publications [27] and then applied to the groups based on their age [26, 28], and glaucoma relative risk [24].
Screening risk stratification
The recent study by Craig and colleagues [14] gave the odds ratio (OR) for developing POAG by PRS decile. We adjusted the ORs to be centred around 1 at the population mean risk (Appendix 3). Then, as the population prevalence for POAG is less than 10%, the rare disease assumption was used to estimate the relative risk (RR) as being the same as the OR. This relative risk was then applied to disease prevalence states at the beginning of the model and to each transition probability between POAG disease states.
Other parameters
Diagnosis rates: For the non-screening control model, and for individuals in the screening model who were not in the age groups or risk deciles identified for screening and follow-up, we assumed that the diagnosis rates were the same as those used for prevalence estimation.
Treatment impact: Patients who were diagnosed had a reduced risk of progression between disease states (excluding mortality) and incurred full treatment costs. This value was based on Garway Heath et al., which demonstrated a Hazard Ratio of 0.44 for patients with glaucoma across most disease states receiving treatment [29].
Mortality rates: mortality rates were taken by age from the Australian ABS data [30] and the UK ONS data [31] and multiplied by relative risk by POAG severity [2].
Willingness to pay (WTP): WTP for Australia was set at $54,808 based on a parliamentary review into incremental cost-effectiveness ratio (ICER) approved by PBAC for Oncology medication, as no equivalent review was found for ophthalmological treatment or screening programmes [32]. In the UK, the WTP threshold is between £20,000 to £30,000, which has been publicly identified by the National Institute for Health and Care Excellence (NICE) [33] and a value of £30,000 was utilised for this model.
Discounting
A global discount rate of 5% p.a. was used for all utility and cost variables in Australia and 3.5% for all utility and cost variables in the UK.
Outcome
The main outcome was the ICER, defined as incremental cost in the intervention group, divided by incremental QALYs. An ICER shows the additional cost per year of QALY gained by the genetic screening programme group. A secondary outcome of years of blindness avoided per person, and a “Blindness ICER”, defined as incremental cost divided by years of blindness avoided, was also calculated.
Sensitivity analysis
One-way and two-way deterministic, and probabilistic sensitivity analyses were performed to reflect the uncertainty around predicting ICERs, described in Appendix 11.
Progression rates from no POAG to early POAG, and from early to mild POAG, utilised beta distributions. The relative risk of POAG based on treatment utilised a lognormal distribution. All costs were modelled on a triangular distribution. ICER uncertainty was evaluated by recalculating the ICER over 10,000 iterations for each country, and the 95% CIs for ICERs were based on the 2.5–97.5% percentile range of results from the second order Monte Carlo analysis.
Results
The cost-effectiveness analysis for genetic screening was demonstrated as the ICER (Australian dollars and British pounds) and incremental effectiveness (QALYs) (Table 2). The results showed that the genetic screening yielded 1 QALY at the cost of AU$34,252 (95% CI: AU$21,324–95,497) and £24,783 (95% CI: £13,373–66,960) per person in our base case in Australia and the UK, respectively.
Table 2.
Base case cost-effectiveness results from genetic screening for POAG compared to current practice.
| Lifetime costs per person, Local currency | QALYs per person | Incremental costs per person, Local currency (95% CI) | Incremental QALYs per person (95% CI) | QALY ICERs, Local currency (95% CI) |
Years of blindness per person | Years of blindness avoided per person | Blindness ICERs, Local currency (95% CI) |
|
|---|---|---|---|---|---|---|---|---|
| Australia | ||||||||
| Current POAG practice | 53,943 | 12.794 | – | – | – | 0.392 | – | – |
| Genetic screening | 54,424 | 12.808 | 574 (402–741) | 0.014 (0.006–0.024) | 34,252 (21,324–95,497) | 0.356 | 0.036 (0.015–0.063) | 13,359 (8143–37,448) |
| United Kingdom | ||||||||
| Current POAG practice | 36,489 | 13.156 | – | – | – | 0.272 | – | – |
| Genetic screening | 37,127 | 13.167 | 278 (221–389) | 0.0112 (0.005–0.020) | 24,783 (13,373–66,960) | 0.244 | 0.028 (0.011–0.049) | 10,095 (5513–27,656) |
Local currency is Australian dollars for Australia and Great British Pounds for the United Kingdom. Costs, QALYs, and Years of Blindness are discounted lifetime totals per person. ICERs are calculated by comparing the Genetic Screening to the Current POAG practice. Given values are based on the expected values for the variables, whilst the 95% confidence intervals are derived from the Monte Carlo analysis.
QALY quality adjusted life year, ICER incremental cost effectiveness ratio, POAG primary open angle glaucoma
Using the WTP thresholds of $54,808 and £30,000, the proposed screening model is 79.2% likely to be cost-effective in Australia and 60.2% likely to be cost-effective in the UK (Fig. 2). Monte Carlo probabilistic sensitivity analysis showed the screening programme in Australia is likely to be cost-effective in 50, 80 and 95% of the simulated cases when the WTP threshold is AU$40,608, AU$55,467, and AU$80,170, respectively (Fig. 2a). In the UK model, the screening programme is cost-effective in 50, 80 and 95% of simulations when the WTP threshold is £27,189, £38,461 and £56,661, respectively (Fig. 2b).
Fig. 2. Cost effectiveness and sensitivity analysis for PRS-based POAG screening.
Cost-effectiveness acceptability curve for the genetic screening programme in Australia (a) and the UK (b); Tornado diagram demonstrating deterministic one-way sensitivity analysis for Australia (c) and the UK (d) models. The probability that the screening programme is cost-effective at different willingness to pay (WTP) thresholds for Australia (a) and the UK (b) models. The programme was defined as cost-effective if ICER is less than the WTP threshold, which was set at AU$54,808 in Australia and £30,000 in the UK. Costs are given in Australian dollars in c and British pounds in d.
In addition, cost-effectiveness analysis showed that the genetic screening for POAG decreased years of blindness at a reasonable cost. In Table 2, such screening in Australia would avoid 1 year of blindness at ICER of AU$13,359 (95% CI: AU$8143–37,448), which is one-sixth of the country’s GDP per capita; similarly, it would avoid 1 year of blindness at ICER of £10,095 (95% CI: £5513–27,656) which is approximately one-third of the UK GDP per capita.
Sensitivity analysis results
Sensitivity analyses were performed to determine whether the results of the Markov model would change by varying different model parameters. Running the model over 30 years returned an ICER of 34,852 compared to 34,252 for our base model and remained cost-effective (Appendix 4). This is as expected, as after 30 years the annual discounting drastically reduces the incremental impact. Similar results were obtained when comparing 30- and 80-year time frames for the UK (Appendix 4).
The tornado diagram displays the parameters with the largest influence on cost-effectiveness (Fig. 2c,d). The top variables in both countries are the annual screening costs for individuals with high PRS who have not yet developed POAG, the cost of the PRS screening test, and the probability of developing early and mild POAG. Additionally, in Australia, the annual cost of glaucoma treatment is a top parameter.
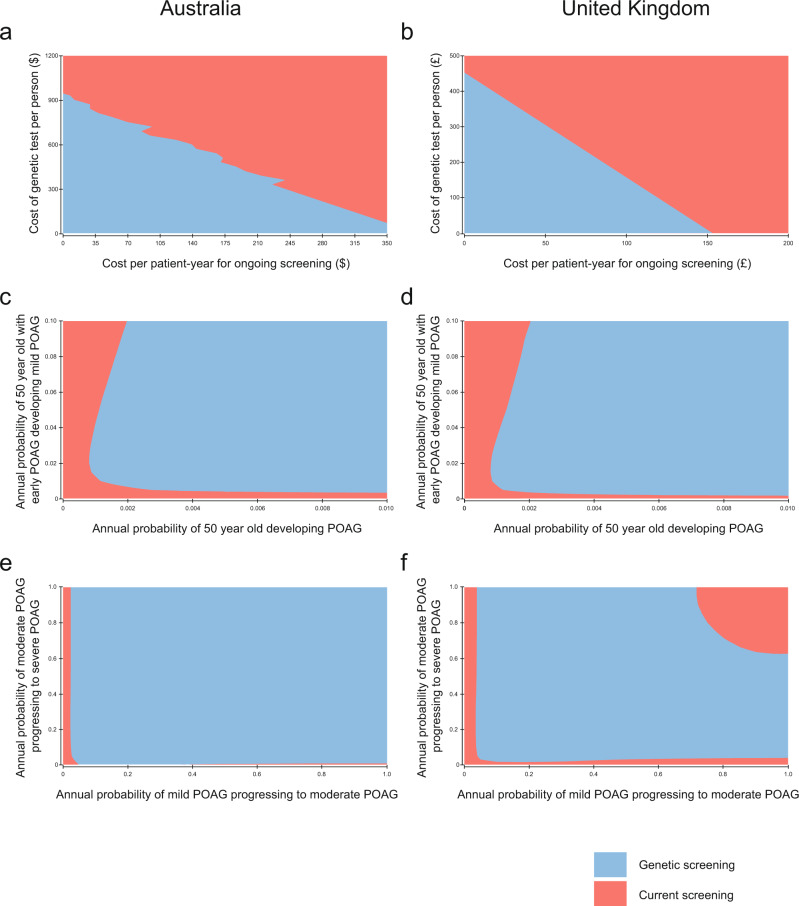
For variables with higher degrees of uncertainty surrounding the data in the literature, we completed sensitivity analyses over arbitrarily large ranges to identify areas where the programme would no longer be cost-effective. In both countries, the cut-off for either cost of genetic testing or ongoing review in terms of generating an ICER below the WTP threshold is co-dependent (Fig. 3a,d). It is obvious that the lower both costs are, the more likely the PRS will be cost-effective. We showed that the ICER is below WTP when the incidence of early and transition probability of early to mild are above 0.002 and 0.015 in Australia, and that a similar pattern emerges in the UK model when the incidence of early are above 0.002 and transition probability are above 0.005 (Fig. 3b,e). In contrast, our study demonstrated a different pattern between the countries when there is large variation in the transition probabilities of mild to moderate and moderate to severe (Fig. 3c,f). In both countries, cost-effectiveness is always below WTP if the transition probabilities of mild to moderate and moderate to severe are both above 0.05; however, in the UK there is an additional constraint where if the transition probabilities are both above 0.6, the screening is no longer cost-effective. Of interest, the region of non-cost effectiveness in the ‘top-right’ of the UK sensitivity analysis for mild to moderate and moderate to severe is cost-effective if the WTP threshold is set arbitrarily higher at £50,000 (not shown). Finally, another alternative scenario showed that the ICER would increase if the screening population includes age groups 40–49 and/or 80 and above (not shown), indicating these groups are less cost effective.
Fig. 3. Two-way sensitivity analysis of the cost-effectiveness simultaneously varying key parameters over large ranges.
These analyses included cost of screening and cost of genetic test and ongoing review (a, b), probability of developing Early and Mild POAG (c, d), and probability of developing Moderate and Severe POAG (e, f) in Australian and the UK models, respectively.
Discussion
According to the World Health Organisation (WHO) established criteria for conditions amenable to screening [8], POAG is an ideal target for screening based on benefit, risk and cost [34]. In 2007, Burr and colleagues concluded that the population-based screening programme is not cost-effective using strategies such as glaucoma-trained optometrists or dedicated techniques like IOP measurement and visual field test [12]. To date, no high-income countries have adopted a population-based screening programme for the early detection of POAG. The evidence indicates two critical factors influence the cost-effectiveness of a POAG screening programme: (1) screening cost and (2) identification of the high-risk subpopulation [12].
Our study demonstrated that using the PRS as a screening tool is likely cost-effective for the current Australian population age above 50, and is potentially cost-effective in the UK with ICERs which are lower than previous UK-based glaucoma screening models [35]. Our proposed programme incurs a one-off genetic test for each participant, and selects a high-risk subpopulation for regular surveillance. This reduces the number of people incurring screening every 3 to 5 years and reduces the cost compared to previous screening models. As a result, our proposed programme would fit the recommendation of targeting high-risk populations at a frequent interval for the POAG screening.
There is growing evidence the PRS could be incrementally valuable to traditional risk factors and implemented clinically to improve current clinical risk prediction models in many common diseases. The U.S. Food and Drug Administration (FDA) allowed marketing of the first direct-to-consumer tests that provide genetic risk information for certain conditions such as Parkinson’s and Alzheimer’s disease in 2017 [36]. Furthermore, research on PRS among the common diseases has expanded exponentially in recent years. Craig and colleagues [14] reported substantial evidence of PRS as a remarkable predictor for the risk of POAG progression to advanced POAG, and demonstrates one potential clinical use of PRS—as an adjunct for disease screening. Taking these together, we believe that POAG is a good candidate disease for the first government programme utilising PRS screening.
In most high-income countries, optometrists are well-trained to detect POAG. We believe if PRS is implemented for POAG screening, a targeted subpopulation with high-risk of developing POAG could safely undergo trained optometrist surveillance. In addition, the subpopulation of individuals with low risk could reduce or delay their monitoring, reducing the total cost of monitoring POAG.
One strength of the study is that despite the uncertainty regarding some of the input variables, our sensitivity analysis demonstrated that our results are robust with cost effectiveness remaining similar over large ranges of input variables—i.e., the results are robust to the difficulties in identifying ideal input data from primary research. For example, our sensitivity analysis also showed ICER was below WTP threshold even if transition probabilities between health states are significantly lower than the evidence suggests. For instance, the PRS programme would be cost-effective even if only 10% of individuals with mild POAG progress to moderate (Fig. 3) per year. According to published reports, the transition probability between mild and moderate POAG is 20 to 85% per year [2, 12] and moderate to severe is 10 to 30% per year [2, 12]. However, we acknowledge that our results, in particular for the UK, have a wide range. This could be minimised by the collection of more granular disease severity by age data from the populations of interest.
One limitation for this study is the difficulty in determining the appropriate WTP threshold for this programme. For example, the Parliament of Australia reported that the Pharmaceutical Benefits Advisory Committee’s acceptable ICER was in the range of $45,000–$75,000 for cancer drugs in 2015 [32]. The WTP threshold may not be comparable with other drug therapies and interventions because the PRS would be the first population-based genetic testing programme for the Australian and UK governments. Other factors that could impact the WTP include opportunities to assess genetic risk for other diseases using the same programme [37–39]. The uncertainty of an appropriate ICER for utilising a PRS to reduce vision loss remains in the political domain.
Another limitation of our analysis is the fact that the cost of computational analysis was based on an oncology genetic screening programme, and given the uncertainty of being unable to predict a cost (for a not-yet existent product) we investigated the impact of the cost of the test over a large range of values. Importantly, it is anticipated that both the consumable and computational costs of PRS profiling are likely to continue to reduce with time.
Implementing such a programme needs to consider several factors which were beyond the scope of this modelling. The data on uptake of genetic screening tests is limited in the literature but this would not impact our modelling as our cost-effective analysis is based on an individual person and does not change with population uptake rates. To be able to implement this programme, we also need to study other factors including ethics, practicalities for genetic testing, reorganising services, misdiagnosis and overtreatment (although we note the side effects from topical glaucoma treatment are usually local and well tolerated [40], minimising harm from over treatment). Therefore, a pilot study of implementing this genetic screening for POAG care in a real world setting is a reasonable next step. Such a study will gain more understanding of the true cost-effectiveness of this programme and provide data such as granular progression rates, misdiagnosis rates, and overtreatment rates in both the conventional care and proposed care pathways.
In conclusion, our study was first to report a potential cost-effective POAG screening test using PRS in Australia and the UK. Although the level of WTP for the Australian Government is uncertain, and the ICER range for the UK is broad, we showed a clear target strategy for early detection and prevention of advanced POAG in a developed nation.
Summary
What was known before
Due to the cost, to date, no high-income countries have adopted a population-based screening programme for the early detection of primary open angle glaucoma (POAG).
Recent research studies showed that polygenic risk scores (PRS) can be an important tool for disease risk profiling, including POAG.
What this study adds
We developed a health economic evaluation model to determine the cost-effectiveness of incorporating PRS for population-based POAG screening in Australia and the United Kingdom for the first time.
Our finding that POAG screening is cost-effective when incorporating PRS testing in high-income countries such as Australia and the UK is significant for both POAG and for population health in general.
Supplementary information
Acknowledgements
DAM, SM, JEC, LS and AWH are supported by the Australian National Health and Medical Research Council (NHMRC) Fellowships. XH is supported by the University of Queensland Research Training Scholarship and QIMR Berghofer Medical Research Institute PhD Top Up Scholarship. We are grateful for funding from a NHMRC Programme grant (1150144), Partnership grant (1132454) and a Centre for Research Excellence grant (1116360).
Author contributions
The contribution each author made to the manuscript is listed as following: (1) Conceptualisation: AWH, LS, JD, QL (2) Data curation: JD, QL (3) Formal Analysis: JD, LS (4) Funding acquisition: AWH, LS, DAM, SM, JEC (5) Investigation: QL, JD (6) Methodology: AWH, LS, JD, QL (7) Project administration: AWH (8) Resources: N/A. (9) Software: AWH (10) Supervision: LS, AWH (11) Validation: LS, JD (12) Visualisation: JD (13) Writing- original draft: 80% by QL, 20% by JD (14) Writing- review and editing: AWH, LS, XH, DAM, SM, JEC.
Funding
We had funding from a NHMRC Programme grant (1150144), Partnership grant (1132454) and a Centre for Research Excellence grant (1116360).
Data availability
The majority of the data generated or analysed during this study are included in this published article (and its supplementary information files) - e.g., every variable used to generate the model is available in the supplementary tables, with references to the original source. The wide range of output datasets generated during the current study are available from the corresponding author on reasonable request.
Competing interests
SM, JEC, and AWH are listed as co-inventors on a patent application (WO2019241844A1) for the use of genetic risk scores to determine risk and guide treatment for glaucoma.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lei Si, Email: l.si@westernsydney.edu.au.
Alex W. Hewitt, Email: hewitt.alex@gmail.com
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-022-02346-2.
References
- 1.Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.International Council of Ophthalmology: Resources: Tunnel Vision-The Economic Impact Of Primary Open Angle Glaucoma. [cited 2020 Oct 27]. Available from: http://www.icoph.org/resources/249/Tunnel-Vision-The-Economic-Impact-of-Primary-Open-Angle-Glaucoma.html
- 3.Rouland J-F, Berdeaux G, Lafuma A. The economic burden of glaucoma and ocular hypertension: implications for patient management: a review. Drugs Aging. 2005;22:315–21. doi: 10.2165/00002512-200522040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Taylor HR, Pezzullo ML, Keeffe JE. The economic impact and cost of visual impairment in Australia. Br J Ophthalmol. 2006;90:272. doi: 10.1136/bjo.2005.080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Real JP, Lafuente MC, Palma SD, Tártara LI. Direct costs of glaucoma: Relationship between cost and severity of the disease. Chronic Illn. 2020;16:266–74. doi: 10.1177/1742395318803660. [DOI] [PubMed] [Google Scholar]
- 6.Keel S, Xie J, Foreman J, Lee PY, Alwan M, Fahy ET, et al. Prevalence of glaucoma in the Australian National Eye Health Survey. Br J Ophthalmol. 2019;103:191–5. doi: 10.1136/bjophthalmol-2017-311786. [DOI] [PubMed] [Google Scholar]
- 7.Gupta P, Zhao D, Guallar E, Ko F, Boland MV, Friedman DS. Prevalence of glaucoma in the United States: the 2005–2008 national health and nutrition examination survey. Invest Ophthalmol Vis Sci. 2016;57:2905–13. doi: 10.1167/iovs.15-18469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson JMG, Jungner G. Principles and practice of screening for disease. Geneva: World Health Organization; 1968:163.
- 9.Traverso CE, Walt JG, Kelly SP, Hommer AH, Bron AM, Denis P, et al. Direct costs of glaucoma and severity of the disease: a multinational long term study of resource utilisation in Europe. Br J Ophthalmol. 2005;89:1245–9. doi: 10.1136/bjo.2005.067355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee PP, Walt JG, Doyle JJ, Kotak SV, Evans SJ, Budenz DL, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol. 2006;124:12–9. doi: 10.1001/archopht.124.1.12. [DOI] [PubMed] [Google Scholar]
- 11.Gessesse GW, Damji KF. Advanced glaucoma: management pearls. Middle East Afr J Ophthalmol. 2013;20:131–41. doi: 10.4103/0974-9233.110610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–190. doi: 10.3310/hta11410. [DOI] [PubMed] [Google Scholar]
- 13.Fingert JH. Primary open-angle glaucoma genes. Eye. 2011;25:587–95. doi: 10.1038/eye.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig JE, Han X, Qassim A, Hassall M, Cooke Bailey JN, Kinzy TG, et al. Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat Genet. 2020;52:160–6. doi: 10.1038/s41588-019-0556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50:1219–24. doi: 10.1038/s41588-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park N. Analysis of population estimates tool. Office for National Statistics; 2020 [cited 2022 Feb 7]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/analysisofpopulationestimatestool
- 17.Commonwealth of Australia, Estimated resident population. 31010 / Jun 2019 / Australian Demographic Statistics / Estimated resident population / Details [Internet]. Australian Bureau of Statistics. 2019 [cited 2020 Oct 16]; Available from: https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3101.0Jun%202019?OpenDocument
- 18.National Institute for Health and Care Excellence. The Way Forward: Glaucoma - options to help meet demand for the current and future care of patients with eye disease [Internet]. [cited 2020 Oct 8]. Available from: https://www.evidence.nhs.uk/document?id=1618377&returnUrl=Search%3Fps%3D40%26q%3Deye%2Bcare&q=eye+care
- 19.TreeAge Pro Healthcare [Internet]. [cited 2020 Oct 1]. Available from: https://www.treeage.com/product/treeage-pro-healthcare/
- 20.Burr JM, Kilonzo M, Vale L, Ryan M. Developing a preference-based Glaucoma Utility Index using a discrete choice experiment. Optom Vis Sci. 2007;84. [cited 2020 Sep 29]; Available from: https://pubmed.ncbi.nlm.nih.gov/17700343/ [DOI] [PubMed]
- 21.Brown MM, Brown GC, Sharma S, Kistler J, Brown H. Utility values associated with blindness in an adult population. Br J Ophthalmol. 2001;85:327–31. doi: 10.1136/bjo.85.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCaffrey N, Kaambwa B, Currow DC, Ratcliffe J. Health-related quality of life measured using the EQ-5D–5L: South Australian population norms. Health Qual Life Outcomes. 2016;14:1–12. doi: 10.1186/s12955-016-0537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York Centre for health economics. University of York; 1999:8.
- 24.Dirani M, Crowston JG, Taylor PS, Moore PT, Rogers S, Pezzullo ML, et al. Economic impact of primary open-angle glaucoma in Australia. Clin Experiment Ophthalmol. 2011;39.[cited 2020 Oct 2]; Available from: https://pubmed.ncbi.nlm.nih.gov/21631669/ [DOI] [PubMed]
- 25.Australian Institute of Health and Walfare (AIHW). Disease expenditure in Australia [Internet]. [cited 2020 Oct 2]. Available from: https://www.aihw.gov.au/reports/health-welfare-expenditure/disease-expenditure-australia/contents/summary
- 26.Australian Institute of Health and Welfare (AIHW). Aged care service list: 30 June 2019 [Internet]. [cited 2020 Oct 2]. Available from: https://www.gen-agedcaredata.gov.au/Resources/Access-data/2019/September/Aged-care-service-list-30-June-2019
- 27.McCrone P, Dhanasiri S, Patel A, Knapp M, Lawton-Smith S. Paying the Price: the cost of mental health care in England to 2026. King’s Fund 2008. Website [Internet]. [cited 2020 Oct 15]. Available from: https://www.kingsfund.org.uk/sites/default/files/Paying-the-Price-the-cost-of-mental-health-care-England-2026-McCrone-Dhanasiri-Patel-Knapp-Lawton-Smith-Kings-Fund-May-2008_0.pdf
- 28.Australian Institute of Health and Welfare (AIHW). Trends in hospitalised injury due to falls in older people 2007–08 to 2016–17 [Internet]. [cited 2020 Oct 2]. Available from: https://www.aihw.gov.au/reports/injury/trends-in-hospitalised-injury-due-to-falls/contents/table-of-contents
- 29.Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385:1295–304. doi: 10.1016/S0140-6736(14)62111-5. [DOI] [PubMed] [Google Scholar]
- 30.Australian Bureau of Statistics. Deaths, Year of occurrence, Age at death, Age-specific death rates, Sex, States, Territories and Australia [Internet]. [cited 2020 Oct 15]. Available from: http://stat.data.abs.gov.au/Index.aspx?DataSetCode=DEATHS_AGESPECIFIC_OCCURENCEYEAR
- 31.Morgan E. Mortality rates (qx), principal projection, England and Wales [Internet]. Office for National Statistics; 2019 [cited 2020 Oct 16]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/mortalityratesqxprincipalprojectionenglandandwales
- 32.Parliament of Australia. Report – Availability of new, innovative and specialist cancer drugs in Australia [Internet]. [cited 2020 Sep 30]. Available from: https://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Community_Affairs/Cancer_Drugs/Report
- 33.McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold. PharmacoEconomics. 2008;26:733–44. doi: 10.2165/00019053-200826090-00004. [DOI] [PubMed] [Google Scholar]
- 34.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 35.Burr J, Hernández R, Ramsay C, Prior M, Campbell S, Azuara-Blanco A, et al. Is it worthwhile to conduct a randomized controlled trial of glaucoma screening in the United Kingdom? J Health Serv Res Policy. 2014;19:42–51. doi: 10.1177/1355819613499748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Office of the Commissioner. FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions [Internet]. 2017 [cited 2020 Sep 28]. Available from: https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-tests-provide-genetic-risk-information-certain-conditions
- 37.Callender T, Emberton M, Morris S, Eeles R, Kote-Jarai Z, Pharoah PDP, et al. Polygenic risk-tailored screening for prostate cancer: A benefit–harm and cost-effectiveness modelling study. PLOS Med. 2019;16:e1002998. doi: 10.1371/journal.pmed.1002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naber SK, Kundu S, Kuntz KM, Dotson WD, Williams MS, Zauber AG, et al. Cost-effectiveness of risk-stratified colorectal cancer screening based on polygenic risk: current status and future potential. JNCI Cancer Spectr. 2020;4:kz086. doi: 10.1093/jncics/pkz086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuchenbaecker KB, McGuffog L, Barrowdale D, Lee A, Soucy P, Dennis J, et al. Evaluation of polygenic risk scores for breast and ovarian cancer risk prediction in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2017;109. Available from: 10.1093/jnci/djw302 [DOI] [PMC free article] [PubMed]
- 40.Beckers HJM, Schouten JSAG, Webers CAB, van der Valk R, Hendrikse F. Side effects of commonly used glaucoma medications: comparison of tolerability, chance of discontinuation, and patient satisfaction. Graefes Arch Clin Exp Ophthalmol. 2008;246:1485–90. doi: 10.1007/s00417-008-0875-7. [DOI] [PubMed] [Google Scholar]
- 41.Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, Ashby D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Investigative Opthalmology Vis Sci. 2006;47:4254. doi: 10.1167/iovs.06-0299. [DOI] [PubMed] [Google Scholar]
- 42.Manchanda R, Patel S, Gordeev VS, Antoniou AC, Smith S, Hopper JL, et al. Cost-effectiveness of population-based BRCA1, BRCA2, RAD51C, RAD51D, BRIP1, PALB2 mutation testing in unselected general population women. JNCI: J Natl Cancer Inst. 2018;110:714–25. doi: 10.1093/jnci/djx265. [DOI] [PubMed] [Google Scholar]
- 43.Sonic Genetics. Solid tumour panel (Find It) [Internet]. [cited 2022 July 17]. Available from: https://www.sonicgenetics.com.au/our-tests/all-tests/solid-tumour-panel-find-it/
- 44.Department of Health, Australian Government. Medicare Benefits Schedule -Item 10910 [Internet]. [cited 2020 Nov 15]. Available from: http://www9.health.gov.au/mbs/fullDisplay.cfm?type=item&qt=ItemID&q=10910
- 45.Violato M, Dakin H, Chakravarthy U, Reeves BC, Peto T, Hogg RE, et al. Cost-effectiveness of community versus hospital eye service follow-up for patients with quiescent treated age-related macular degeneration alongside the ECHoES randomised trial. BMJ Open. 2016;6:e011121. doi: 10.1136/bmjopen-2016-011121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahman MQ, Beard SM, Discombe R, Sharma R, Montgomery DM. Direct healthcare costs of glaucoma treatment. Br J Ophthalmol. 2013;97. [cited 2020 Oct 15]; Available from: https://pubmed.ncbi.nlm.nih.gov/23590855/ [DOI] [PubMed]
- 47.Care Markets and LaingBuisson. Annual Survey of UK Local Authority Usual Costs 2017/2018 [Internet]. [cited 2020 Nov 5]. Available from: https://www.laingbuisson.com/wp-content/uploads/sites/3/2017/07/CareMarkets_UsualCosts_20172018.pdf
- 48.Australian Institute of Health and Welfare (AIHW). Admitted patient care 2017-18 [Internet]. [cited 2020 Nov 5]. Available from: https://www.aihw.gov.au/getmedia/df0abd15-5dd8-4a56-94fa-c9ab68690e18/aihw-hse-225.pdf.aspx?inline=true
- 49.McGinley P, Ansari E, Sandhu H, Dixon T. The cost burden of falls in people with glaucoma in National Health Service Hospital Trusts in the UK. [cited 2020 Nov 5]. Available from: 10.1080/13696998.2019.1646262 [DOI] [PubMed]
- 50.Varma R, Lee PP, Goldberg I, Kotak S. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol. 2011;152:515. doi: 10.1016/j.ajo.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The majority of the data generated or analysed during this study are included in this published article (and its supplementary information files) - e.g., every variable used to generate the model is available in the supplementary tables, with references to the original source. The wide range of output datasets generated during the current study are available from the corresponding author on reasonable request.