Abstract
The purpose of this study was to perform a systematic review of existing literature on OCT screening before cataract surgery. Available literature was evaluated and projections on how it could be applied to enhance postoperative outcomes of cataract surgery were summarised. The PubMed, Embase and Cochrane databases were searched for articles pertaining to preoperative OCT screening. Selected articles were qualitatively and quantitatively analysed. Across 9 studies, the addition of OCT macular screening resulted in preoperative detection of macular pathology in 13.7% of eyes that were determined to be normal on fundoscopic examination alone. The types of macular pathology most frequently detected through preoperative OCT screening were interface abnormalities followed by macular degeneration. Comparative analysis of SS-OCT biometer and SD-OCT found that SS-OCT had a sensitivity of 0.48–0.81 in the detection of macular pathology in eyes with pathology diagnosed by SD-OCT. OCT screening prior to cataract surgery results in the detection of occult macular pathology that may influence postoperative visual outcomes in approximately 1 in 10 eyes (13.7%). As a result, OCT screening should be considered in the routine preoperative workup for cataract surgery.
Subject terms: Outcomes research, Refractive errors
Abstract
本文旨在对白内障术前的OCT对眼底筛查的现有文献进行系统综述。对现有文献进行了评估, 并总结了将其应用在白内障术后的预测的方法。
我们检索了PubMed, Embase和Cochrane数据库中与白内障术前OCT对眼底病变筛查相关的文献。对选定的文章进行了定性和定量分析。
在九项研究中, OCT可筛查出13.7%的黄斑病变患者, 而这些患者仅通过眼底镜检查时诊断为正常。通过术前OCT筛查到最多的黄斑病变类型是玻璃体视网膜交界面异常, 其次是黄斑病变。通过比较SS-OCT和SD-OCT可以发现, SS-OCT对SD-OCT诊断的黄斑病变的检测敏感性为0.48-0.81。白内障术前的OCT筛查可发现隐匿性黄斑病变, 这可能会影响术后约1/10 (13.7%) 患者的视力预后。因此, 本文的结果强烈推荐OCT作为白内障术前的常规术前检查。虽然SS-OCT确实有所帮助, 但SD-OCT仍然是白内障患眼术前诊断的黄金标准。
Introduction
The development of cataracts is among the most common causes of age-related decreased vision [1]. Phacoemulsification with intraocular lens implantation (IOL) is the standard of care for visually significant cataracts and remains the most frequently performed surgical procedure in developed countries [2]. With recent advances in surgical techniques and IOL implants, the postoperative visual outcomes of cataract surgery are steadily improving [3]. Accompanying these advances are the higher patient expectations about postoperative visual function. Cataract surgery is considered a procedure to improve vision rather than simply to restore to a point before cataract formation [3]. Suboptimal visual outcomes following cataract surgery cause substantial patient dissatisfaction [3]. Therefore, all potential causes of decreased postoperative vision must be addressed adequately before cataract surgery.
A considerable portion of elderly patients being evaluated for cataract surgery present with co-existing macular pathologies [3]. Based on the characteristics of the cataract at presentation, routine fundus examination may miss subtle macular pathologies [4]. This may be due to poor fundus view due to a dense cataract, poor pupillary dilation or photophobia [5]. As a result, important posterior segment pathology may go undetected when determining the patient’s final postoperative visual outcome [4]. This may cause patient dissatisfaction, particularly among patients who opt for premium IOLs [6]. Furthermore, this may also lead to litigation due to pre-operative pathology not being discussed during the consent process [7].
Current techniques to prevent these adverse outcomes have been aimed at integrating the detection of occult macular pathology as part of the routine preoperative workup [5, 8–17]. The most widely used strategy has been to use spectral-domain optical coherence tomography (SD-OCT). Recent studies have also shown swept source optical coherence tomography (SS-OCT) to be a feasible alternative [10, 16, 17]. Both of these imaging techniques allow for non-invasive cross-sectional evaluation of the macula in the presence of media opacities [17].
The purpose of this review is to summarise the literature on the use of SD-OCT and SS-OCT in detecting macular pathology during cataract surgery diagnostics and to appraise the efficacy of this intervention. Furthermore, an appraisal of the advantages and disadvantages of the SD-OCT and SS-OCT is made.
Methodology
The PubMed, Embase and Cochrane Library were searched using the following keywords: ‘spectral domain optical coherence tomography’, ‘swept source optical coherence tomography’, ‘cataract surgery’, ‘macular pathology’ and ‘screening’. The searches were conducted on 16 February 2021. References of included studies were evaluated to find additional potential manuscripts. The search was restricted to literature published in the English language and published literature only. Duplicate records were identified via Zotero and then manually reviewed and deduplicated on a case-by-case basis. There was no prior registered protocol, and the study was conducted independent from PRISMA reporting guidelines. The study selection process is illustrated in Fig. 1.
Fig. 1. A flowchart of the study selection process.
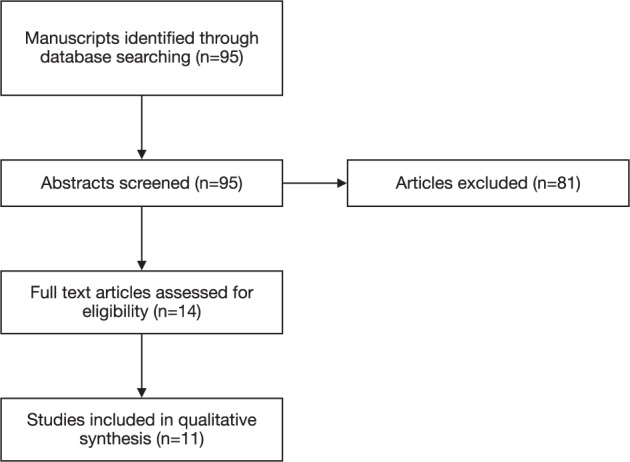
Arrows represent the order in which included studies were filtered out.
Data extraction & analysis
Data from the selected articles were extracted on a spreadsheet. This data included the design of the study, the number of participants and the outcomes. The primary outcome recorded was the rate of macular pathology detected using SD-OCT or SS-OCT. The type of macular pathology and its reportable frequency and the type of OCT technique (SS, SD) used by each study was also documented. Other outcomes measured were the study sample size, country, year of publication and fundoscopic findings. Data was secondarily analysed both qualitatively and quantitively. Quantitative analysis included calculating the mean increase in detection of macular pathology with OCT-augmented preoperative screening. This was conducted by individually aggregating the total number of patients and pathology detected across all included studies rather than aggregating the reported percentage increase across all studies which would not have accounted for study size. Most studies excluded eyes based on abnormal fundoscopic findings to gauge the incremental benefit of OCT screening in the absence of clinically detectable pathology. The fundoscopy-based inclusion criteria of all studies were therefore also documented.
Results
The findings of all included studies have been individually summarised in Table 1. The outcomes of the studies encompassed by this review fell into one of three assigned categories:
Studies that gauged the incremental benefit of pre-cataract surgery macular screening augmented with OCT.
Studies that attempted to quantify the prevalence of underlying macular pathology amongst individuals undergoing cataract surgery.
Studies that compared pre-cataract macular screening with SS-OCT biometer vs SD-OCT.
Table 1.
Overview of individual study attributes.
| Article | Country | Journal | Year | Study Type | Intervention Used | Participants | Fundoscopic Exclusion | Rate of Macular Pathology Detection | Type of Pathology detected |
|---|---|---|---|---|---|---|---|---|---|
| Sudhalkar et al. [8] | India | AJO | 2019 | Case series | SD-OCT | 1444 eyes of 1444 patients |
- Fundoscopy done - Eyes appearing abnormal on fundoscopic exam were excluded |
SD-OCT: 133 eyes (9.21%) |
SD-OCT: Interface abnormalities = 94 Macular degeneration = 26 Foveal attenuation = 8 Macular oedema = 5 Miscellaneous = 3 |
| Tognetto et al. [17] | Italy | Scientific Reports | 2019 | Cross-sectional study | SD-OCT & SS-OCT (Biometer) | 1089 eyes of 732 patients | - Fundoscopy not done |
SD-OCT 449 eyes (41.2%) SS-OCT 364 eyes (33.4%) |
SD-OCT Full-thickness macular holes = 16 (1.5%), Interface abnormalities = 33 (3%) Macular degeneration = 210 (19.4%) Macular oedema = 169 (15.6%) |
| Pinto et al. [15] | Brazil | JCRS | 2019 | Case series | SD-OCT | 952 eyes of 614 patients |
- Fundoscopy done - Eyes appearing abnormal on fundoscopic exam were excluded |
SD-OCT: 47 eyes (4.9%) |
SD-OCT Interface abnormalities = 35 (3.7%) Macular degeneration = 7 (0.4%) Macular oedema = 4 (0.4%) FTMH = 1 (0.1%) |
| Abdelmassih et al. [14] | France | JCRS | 2018 | Case series | SD-OCT | 401 eyes of 245 patients |
- Fundoscopy done - Eyes with abnormal fundoscopy findings were included in OCT intervention group |
SD-OCT: 107 (26.7%) Fundoscopy: 37 (9.23%) |
SD-OCT Macular degeneration = 66 (61.7%) Interface abnormalities = 32 (30%) Miscellaneous = 5 (4.6%) Macular oedema = 4 (3.7%) |
| Kowallic et al. [11] | Germany | PLoS One | 2018 | Case series | SD-OCT | 162 eyes of 123 patients |
- Fundoscopy done - Eyes appearing abnormal on fundoscopic exam were excluded |
SD-OCT: 20 eyes (12.35%) |
Interface abnormalities = 10 (6.17%) Macular degeneration = 6 (3.71%) Miscellaneous = 4 (2.47%) |
| Huang et al. [9] | China | Scientific Reports | 2018 | Case series | SD-OCT | 992 eyes of 992 patients |
- Fundoscopy done - Eyes appearing abnormal on fundoscopic exam were excluded |
SD-OCT: 294 eyes (25%) |
SD-OCT: Interface abnormalities = 135 Macular degeneration = 160 Miscellaneous = 46 Macular oedema = 22 FTMH = 14 |
| Zafar et al. [16] | Pakistan | JCRS | 2017 | Case series | SS-OCT | 155 eyes of 155 patients |
- Fundoscopy done - Eyes appearing abnormal on fundoscopic exam were excluded |
SS-OCT: 17 eyes (10.9%) |
SS-OCT: Macular degeneration = 5 Interface abnormality = 6 Macular oedema = 2 |
| Hirnschall et al. [10] | Austria | JCRS | 2016 | Case series | SD-OCT & SS-OCT (Biometer) | 125 eyes of 125 patients | - Fundoscopy not done |
SD-OCT: 65 eyes (54.2%) SS-OCT: 38 eyes (28%) |
SD-OCT: Interface abnormalities = 30 (25%) FTMH = 5 (4.2%) Macular degeneration = 16 (13.4%) Macular oedema = 16 (13.3%) |
| Klein et al. [13] | USA | JCRS | 2016 | Case series | SD-OCT | 265 eyes of 149 patients |
- Fundoscopy done - Eyes appearing abnormal on fundoscopic exam were excluded |
SD-OCT: 35 eyes (13.2%) |
SD-OCT: Macular degeneration = 20 (7.55%) Interface abnormalities = 11 (4.15%) Macular oedema = 3 (1.13%) |
| Neto et al. [12] | Germany | Arq Bras Oftalmol | 2015 | Case series | SD-OCT | 98 eyes of 98 patients |
- Fundoscopy done - Eyes with abnormal fundoscopy findings were included in OCT intervention group |
SD-OCT: 21 eyes (21.4%) Fundoscopy: 11 eyes (11.2%) |
Fundoscopy: Macular degeneration = 11 SD-OCT: Macular degeneration = 7 Interface abnormalities = 3 |
| Creese et al. [5] | Austria | Clinical and Experimental Ophthalmology | 2011 | Case series | SD-OCT | 218 eyes of 149 patients |
- Fundoscopy done - Eyes appearing abnormal on fundoscopic exam were excluded |
SD-OCT: 10 eyes (4.6%) |
SD-OCT Interface abnormalities = 5 Macular degeneration = 3 FTMH = 1 Macular oedema = 1 |
Incremental benefit studies
Of the 11 included studies, the primary aim of 9 of these studies was to quantify the incremental benefit that macular screening with OCT may impart over standard biomicroscopic fundal exam [5, 8, 9, 11–16]. All these studies except for Zafar et al. who used SS-OCT, used SD-OCT for this purpose [16]. These studies attempted to gauge outcomes by randomly selecting patients undergoing cataract surgery. Upon enrolment, the patients would undergo biomicroscopic fundal examination. This examination, in most studies, would then be repeated by another ophthalmologist to check for any discrepancies between the findings and to minimise human error. If any abnormalities were detected on any of these examinations, the patient would be excluded from subsequent OCT screening. This was to ensure that all patients undergoing OCT assessment who were subsequently discovered to have macular pathology could confidently be labelled as having clinically undetectable pathology that was only detectable through OCT augmented screening [16]. To this end, all included studies reported a statistically significant figure in the rates of macular pathology detection with OCT [5, 8, 9, 11–16]. The range of OCT- detected macular pathology varied from 4.6% in a study by Creese et al. up to as high 26.4% as reported by Hirnschall et al. [5, 10]. The largest study of this type was Sudhalkar et al. in which 1444 eyes of 1444 patients were recruited [8]. Sudhalkar et al. reported a macular pathology detection rate of 9.21% after excluding eyes appearing abnormal on fundoscopic examination [8].
Notably, of these 9 studies, 2 kept patients with abnormal fundoscopic findings in the OCT intervention group instead of excluding them [12, 14]. These studies reported the rate of pathology detected on biomicroscopic examination as well as the rate detected by subsequent OCT screening. Given this, determination of the incremental benefit of OCT screening was done by looking at how much pathology was detected through OCT screening compared to biomicroscopy. Through this, the rate of macular pathology detection in these 2 studies by Neto et al. and Abdelmassih et al. was deduced to be 10.2% and 17.5%, respectively [12, 14]. Statistical meta-analysis shows the mean incremental detection of macular pathology across all studies was calculated at 13.7% per eye screened. This yields a number needed to screen to detect one additional pathology at 7.3 eyes screened. This is in line with the conclusion made by Sudhalkar et al. that OCT screening will detect occult pathology that may affect vision post cataract surgery in roughly 1 in every 10 eyes [8].
SD-OCT vs SS-OCT
SS-OCT has also shown to be a capable imaging modality that uses longer wavelength and has a higher scanning speed as compared to SD-OCT [18]. This, theoretically, allows for acquisition of higher resolution images that may detect subtle macular pathologies missed on SD-OCT [4]. Only a single study (Zafar et al.) used a standard SS-OCT. There was no comparison with SD-OCT. Two studies compared SD-OCT with SS-OCT biometery devices. The drawback of SS-OCT biometer scan is that the fovea appears flatter than in conventional SD-OCT scans due to the smaller scan zone of 1.0 mm [17]. As a result, when screening the macula it may have a lower sensitivity than SD-OCT devices [4]. To this end, there were 2 studies that directly evaluated this. The first study by Hirnschall et al. found that SS-OCT biometer had a moderate sensitivity of 0.48–0.62 compared to SD-OCT and detected macular pathology in 38 of the 65 eyes confirmed to have macular pathology on SD-OCT [10]. The second study by Tognetto et al. found that SS-OCT biometer had a mean sensitivity of 0.81 compared to SD-OCT and that it detected pathology in 364 of 449 eyes detected as abnormal on SD-OCT [17].
Hirnschall et al. also found that SS-OCT biometer scan had difficulty in detecting geographic atrophy and epiretinal membranes (ERM) [10]. However, it was shown that SS-OCT biometer was equally effective as SD-OCT in detecting macular holes and intraretinal fluid [10]. Hirnschall et al. also suggested that enlarging the SS-OCT biometer scan area of 1.0 mm could bring about significant improvement in its sensitivity to near SD-OCT levels [10].
Characteristics of underlying occult pathologies
Studies that documented the rate of macular pathology detected also described the type of pathology detected [5, 8–17]. These pathologies were broadly categorised into five groups: interface abnormalities, macular degeneration, oedema, full-thickness macular hole (FTMH) and miscellaneous. The results have been summarised in Table 2.
Table 2.
Group attributes of included studies.
| Study attributes | Study outcome |
|---|---|
| All studies | 11 |
| Type of pathology detected | |
| Interface abnormalities | 394 (32.2%) |
| Macular degeneration | 526 (42.9%) |
| Oedema | 226 (18.4%) |
| Full-thickness macular hole | 21 (1.7%) |
| Miscellaneous | 58 (4.73%) |
| Years of publication | |
| 2011–2015 | 2 |
| 2016–2020 | 9 |
| OCT Technique | |
| SD-OCT | 10 |
| SS-OCT | 3 |
The pathology most frequently detected on OCT screening was macular degeneration. This is because of the ability of the OCT to analyse the macula in detail and identify pathologies not visible on fundoscopic exam [18]. The effect macular pathologies may have on postoperative visual acuity ranges from being clinically negligible to very significant [4]. Given the frequency of macular pathology detection, an understanding of its nature is necessary to guide the decision to pursue cataract surgery.
Notably, the majority of the interface abnormalities were attributed to ERMs. Fine ERMs are particularly challenging to detect on slit-lamp biomicroscopy and are therefore unlikely to be detected without OCT screening [9]. Eyes with ERMs have also been shown to have a greater tendency to develop cystoid macular oedema (CMO) postoperatively [19]. The development of CMO after cataract surgery can alter the postoperative course by lengthening the time on topical medications and even necessitating more invasive adjunctive therapy [20]. Given the established risk between ERM and CMO, preoperative detection of ERM through OCT screening can allow for informed consent to be obtained from the patient and for discussion of a contingency plan preoperatively as opposed to a difficult unexpected discussion upon the development of complications postoperatively.
Discussion
Optical coherence tomography is rapidly gaining recognition as part of preoperative cataract workup, particularly when premium IOLs are considered [4]. As expectations of patients following cataract surgery continue to rise, steps should be taken to match them. Undiagnosed ocular diseases are currently the main cause for discrepancies between preoperative expectations and postoperative outcomes. The value of preoperative OCT screening in detecting such comorbidities is significant and recommendations on its use as part of routine preoperative diagnostics warrant consideration.
In favour of routine use, we must first consider the large body of evidence suggesting that preoperative OCT can consistently pick up clinically undetectable pathology [5, 8, 9, 11–16]. Our review reported that when OCT was employed preoperatively, it detected clinically undetectable pathology in 13.7% of eyes undergoing cataract surgery. Prior to the routine recommendation of this additional screening test, it is important to consider if this increase in detection of occult pathology has any effect on management and outcomes. There are three benefits of early detection in cases where pathology is detected:
It allows for a more well-informed discussion of postoperative expectations with the patient [4]. This is particularly important in low-income countries where IOL costs primarily determine the type of IOLs patients opt for.
Certain retinal pathology, such as macular oedema, requires treatment before cataract surgery and early detection can ensure these cases do not slip through the cracks of a rushed diagnostic process [21].
Certain retinal pathology such as advanced macular degeneration may preclude cataract surgery [22].
Our review found that the majority of the undetectable pathologies consisted of varying forms of macular degeneration and interface abnormalities. Both of these groups of pathologies are refractory to refractive correction and are difficult to symptomatically differentiate from decreased visual acuity secondary to cataracts [22].
Early detection in these cases should avoid unnecessary procedures and their associated procedural risks and allow for timely referral to retinal specialists. Given these advantages, it is hard to argue that there is no benefit to the patient when OCT screening is employed.
However, before routine recommendation, the benefits must also be balanced with the cost of the intervention. Leung et al. conducted a cost-effectiveness analysis on the use of OCT in the preoperative evaluation of cataracts in which they measured the incremental cost- effectiveness ratio and incremental cost-utility ratio measured in quality-adjusted life years (QALYs) [23]. Based on this, Leung et al. concluded that while OCT screening increased diagnostic costs, it also improved QALYs proportionally to a point where it was cost-effective from a third-party payer and societal perspective [23].
One potential drawback to the routine use of OCT has been its association with a potential delay in cataract surgery and an increase in risk-of-falls (OR 1.1) [23]. However, the research suggesting this is currently limited by its assumption that the control group of patients not undergoing OCT testing were operated on immediately after biometry, which is rarely the case. In routine use, we would suggest stratifying patients based on preoperative visual acuity and risk of falls. Scheduling their cataract surgery earlier would be prudent. Barring high risk-of- fall-patients who should be managed on a case-to-case basis, we believe the adjunctive benefit of routine OCT screening on a populational level outweighs the risks associated with potential surgical delays.
Knowing the optimal time for routine OCT screening in cataract workup is necessary. To this end, we suggest an algorithmic approach on when to employ OCT in the workup of patients presenting for cataract evaluation based on cost in a particular setup (Fig. 2).
Fig. 2. Options for potential application of OCT screening among patients undergoing cataract surgery.
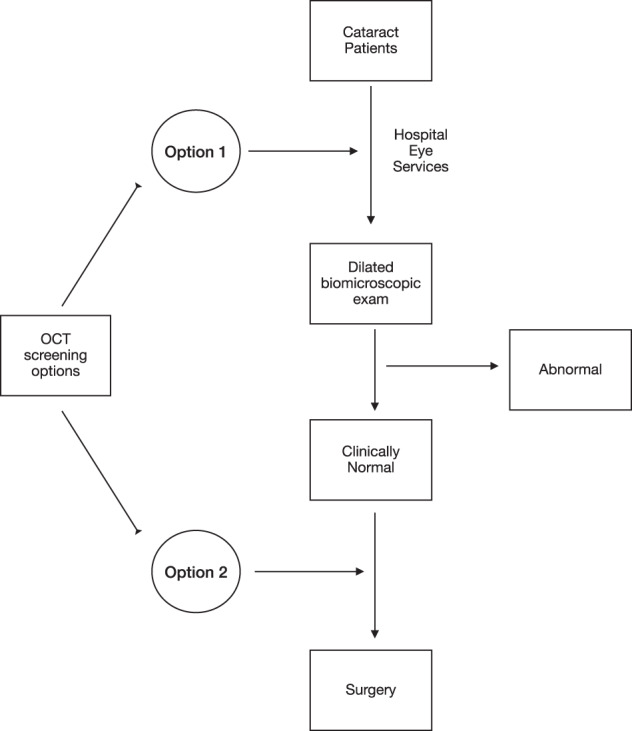
In option 1, all patients undergo OCT screening, whereas in option 2, only patients deemed ‘normal’ on clinical exam undergo OCT screening.
Given the different types of OCT machines currently in the market, it is necessary to discuss how recommendations on routine use vary between them. Zafar et al. demonstrated that SS-OCT shows promising results [16]. There is a slightly lower sensitivity of SS-OCT biometry devices when compared to SD-OCT, likely stemming from a smaller scan area. In so far as OCT is being employed solely as a screening test, a lower sensitivity value is suggestive of an objectively less effective screening tool. Therefore, SD-OCT should remain the current standard for screening purposes. There were no direct head to head comparison studies of standard SS-OCT with SD-OCT.
Conclusion
There is a growing body of evidence recognising the value of preoperative OCT screening in cataract surgery. Our review found that screening before cataract surgery results in detection of occult macular pathology that may influence postoperative visual outcomes in approximately 1 in 10 eyes (13.7%). Patient expectations following cataract surgery are at an all-time high and steps need to be taken to ensure corresponding satisfaction. Given the excellent safety profile, cost-effectiveness and value-added to the diagnostic process we recommend that OCT screening becomes part of a routine preoperative workup for patients being considered for cataract surgery. While SS-OCT may have better utility, more studies with direct head-to-head comparison are needed.
Summary
What is known about this topic
A considerable portion of elderly patients being evaluated for cataract surgery present with co-existing macular pathologies.
Routine fundus examination may miss subtle macular pathologies.
What this study adds
OCT screening prior to cataract surgery detects occult macular pathology in 1 in 10 eyes.
OCT is a cost-effective diagnostic intervention that should be considered in the routine preoperative workup of cataract surgery.
Author contributions
TMA was responsible for conducting the search, screening potentially eligible studies, extracting. and analysing data, writing the report, interpreting results, updating reference lists and creating ‘Summary of findings’ tables. MARS and BH were responsible for designing the review protocol, screening potentially eligible studies and providing feedback on the manuscript. All the authors reviewed and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28:98–103. doi: 10.1097/ICU.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 2.Allen D, Vasavada A. Cataract and surgery for cataract. BMJ. 2006;333:128–32. doi: 10.1136/bmj.333.7559.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panagiotopoulou EK, Ntonti P, Vlachou E, Georgantzoglou K, Labiris G. Patients’ expectations in lens extraction surgery: a systematic review. Acta Med (Hradec Kralove) 2018;61:115–24. doi: 10.14712/18059694.2018.129. [DOI] [PubMed] [Google Scholar]
- 4.Goldhardt R, Rosen BS. Optical coherence tomography: critical tool to manage expectations after cataract extraction. Curr Ophthalmol Rep. 2020;8:129–35. doi: 10.1007/s40135-020-00243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creese K, Ong D, Zamir E. Should macular optical coherence tomography be part of routine preoperative cataract assessment? Clin Exp Ophthalmol. 2012;40:e118–9. doi: 10.1111/j.1442-9071.2011.02623.x. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons A, Ali TK, Waren DP, Donaldson KE. Causes and correction of dissatisfaction after implantation of presbyopia-correcting intraocular lenses. Clin Ophthalmol. 2016;10:1965–70. [DOI] [PMC free article] [PubMed]
- 7.Ali N, Little BC. Causes of cataract surgery malpractice claims in England 1995–2008. Br J Ophthalmol. 2011;95:490–2. doi: 10.1136/bjo.2010.182774. [DOI] [PubMed] [Google Scholar]
- 8.Sudhalkar A, Vasavada V, Bhojwani D, Raju CVG, Vasudev P, Jain S, et al. Incorporating optical coherence tomography in the cataract preoperative armamentarium: additional need or additional burden? Am J Ophthalmol. 2019;198:209–14. doi: 10.1016/j.ajo.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Zhang Z, Wang J, Meng X, Chen T, Wu Z. Macular assessment of preoperative optical coherence tomography in ageing Chinese undergoing routine cataract surgery. Sci Rep. 2018;8:5103. doi: 10.1038/s41598-018-22807-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirnschall N, Leisser C, Radda S, Maedel S, Findl O. Macular disease detection with a swept-source optical coherence tomography-based biometry device in patients scheduled for cataract surgery. J Cataract Refract Surg. 2016;42:530–6. doi: 10.1016/j.jcrs.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Kowallick A, Fischer CV, Hoerauf H. Optical coherence tomography findings in patients prior to cataract surgery regarded as unremarkable with ophthalmoscopy. PLoS One. 2018;13:e0208980. doi: 10.1371/journal.pone.0208980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreira Neto CA, Moreira Júnior CA, Moreira ATR. Optical coherence tomography in patients undergoing cataract surgery. Arq Bras Oftalmol. 2015;78:241–5. doi: 10.5935/0004-2749.20150062. [DOI] [PubMed] [Google Scholar]
- 13.Klein BR, Brown EN, Casden RS. Preoperative macular spectral-domain optical coherence tomography in patients considering advanced-technology intraocular lenses for cataract surgery. J Cataract Refract Surg. 2016;42:537–41. doi: 10.1016/j.jcrs.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 14.Abdelmassih Y, El-Khoury S, Georges S, Guindolet D, Gabison E, Cochereau I. Preoperative spectral-domain optical coherence tomography in patients having cataract surgery. J Cataract Refract Surg. 2018;44:610–4. doi: 10.1016/j.jcrs.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Pinto WP, Rabello LP, Ventura MC, Rocha CS, Ventura BV. Prevalence of macular abnormalities identified only by optical coherence tomography in Brazilian patients with cataract. J Cataract Refract Surg. 2019;45:915–8. doi: 10.1016/j.jcrs.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Zafar S, Siddiqui MAR, Shahzad R, Shahzad MH. Swept-source optical coherence tomography to screen for macular pathology in eyes having routine cataract surgery. J Cataract Refract Surg. 2017;43:324–7. doi: 10.1016/j.jcrs.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Tognetto D, Pastore MR, De Giacinto C, Merli R, Franzon M, D’Aloisio R, et al. Swept- source optical coherence tomography biometer as screening strategy for macular disease in patients scheduled for cataract surgery. Sci Rep. 2019;9:9912. doi: 10.1038/s41598-019-46243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adhi M, Duker JS. Optical coherence tomography—current and future applications. Curr Opin Ophthalmol. 2013;24:213–21. doi: 10.1097/ICU.0b013e32835f8bf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardin JS, Gauldin DW, Soliman MK, Chu CJ, Yang YC, Sallam AB. Cataract surgery outcomes in eyes with primary epiretinal membrane. JAMA Ophthalmol. 2018;136:148–54. doi: 10.1001/jamaophthalmol.2017.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han JV, Patel DV, Squirrell D, McGhee CN. Cystoid macular oedema following cataract surgery: A review. Clin Exp Ophthalmol. 2019;47:346–56. doi: 10.1111/ceo.13513. [DOI] [PubMed] [Google Scholar]
- 21.Johnson MW. Etiology and treatment of macular edema. Am J Ophthalmol. 2009;147:11–21.e1. doi: 10.1016/j.ajo.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Tennant MTS, Connolly BP. Cataract surgery in patients with retinal disease. Curr Opin Ophthalmol. 2002;13:19–23. doi: 10.1097/00055735-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Leung EH, Gibbons A, Koch DD. Cost-effectiveness of preoperative OCT in cataract evaluation for multifocal intraocular lens. Ophthalmology. 2020;127:859–65. doi: 10.1016/j.ophtha.2020.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]


