Abstract
The borylation of unreactive carbon-hydrogen bonds is a valuable method for transforming feedstock chemicals into versatile building blocks. Here, we describe a transition metal-free method for the photoredox-catalyzed borylation of unactivated C(sp3)−H bond, initiated by 1,5-hydrogen atom transfer (HAT). The remote borylation was directed by 1,5-HAT of the amidyl radical, which was generated by photocatalytic reduction of hydroxamic acid derivatives. The method accommodates substrates with primary, secondary and tertiary C(sp3)−H bonds, yielding moderate to good product yields (up to 92%) with tolerance for various functional groups. Mechanistic studies, including radical clock experiments and DFT calculations, provided detailed insight into the 1,5-HAT borylation process.
Subject terms: Homogeneous catalysis, Photocatalysis, Synthetic chemistry methodology
Alkyl boronic esters are commonly used building blocks in organic synthesis, causing efficient C-H borylation methods to be in high demand. Here, the authors develop a method for the photoredox-catalyzed borylation of unreactive C(sp3)-H bonds, mediated by amidyl radicals to achieve C-H borylation at the λ position of carbonyl compounds.
Introduction
Alkyl boronic esters are highly versatile building blocks in organic synthesis because C−B bonds can be easily transformed into various useful functional groups1. C−H borylation is desirable and powerful because it directly transforms common C−H bonds into synthetically valuable C−B bonds. Borylation of nonactivated C(sp3)−H is one of the most challenging subjects in the area. In the past decades, a series of methods have been established2–4. and most of them require transition-metals as catalysts, including W5, Rh6, Pd7–10, Ir11–24 (Fig. 1a), and the reaction conditions could be very harsh (temperature 100–150 °C). In 2020, the Aggarwal group reported the metal-free photoinduced borylation of alkanes (Fig. 1b)25. However, due to the low reactivity of alkanes, the reaction requires high equivalency of alkane substrates (10 equiv.), and the yields based on diboron are relatively low (lower than 50% in most cases). Given that boronic esters are important in organic synthesis and medicinal chemistry, it is of great significance to develop substrate equivalent and efficient C−H borylation methods.
Fig. 1. Unactivated C(sp3)−H bonds borylation.

Previously reported: a Transition metal catalyzed C(sp3)−H borylation. b The metal-free photoinduced borylation of alkanes by Aggarwal group.
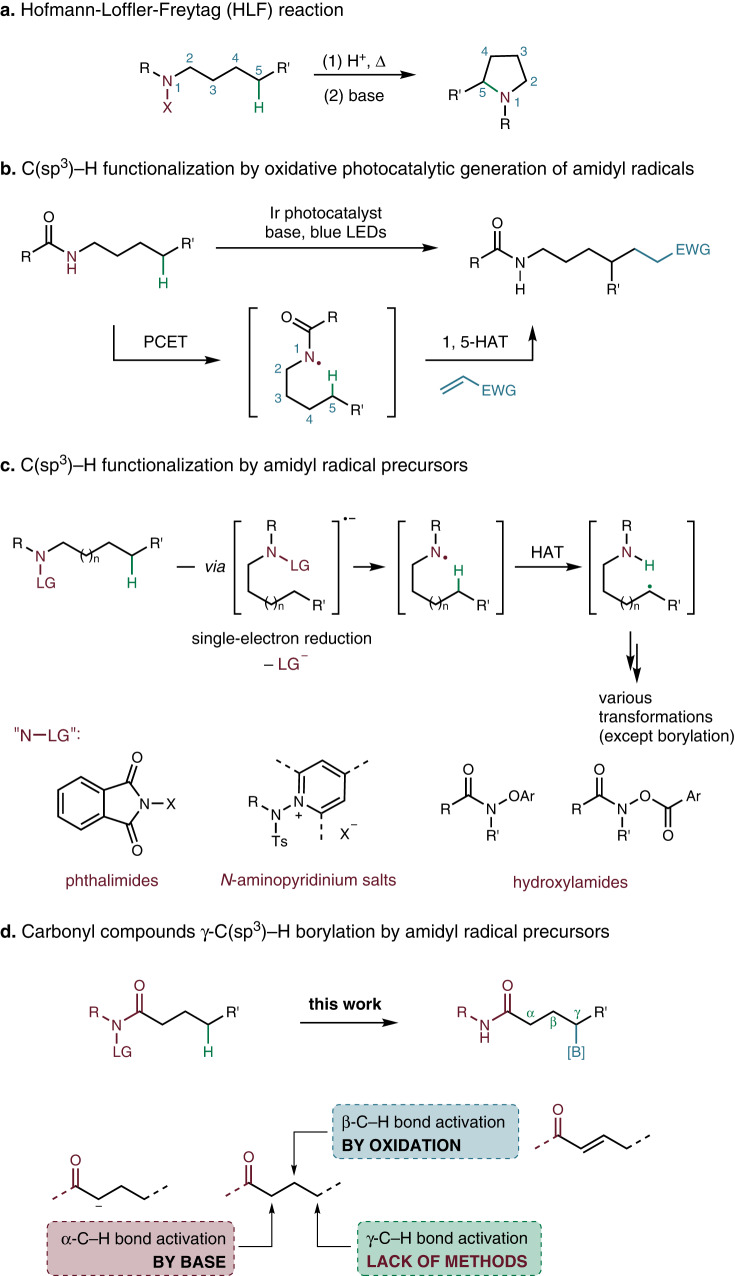
Hydrogen atom transfer (HAT) is a general strategy in organic synthesis, and it represents an attractive approach toward remote C−H functionalization26–28. The earliest example of remote C−H functionalization by means of HAT was Hofmann–Löffler–Freytag (HLF) reaction (Fig. 2a)29,30, the key step is the 1,5-hydrogen atom abstraction via nitrogen-centered radical, where the bond-dissociation energy (BDE) of N−H bond is higher than the C−H bond. In past decades, the nitrogen-centered radical-based HLF type reaction has shown its advances in regioselective functionalization of inert C−H bonds31,32, by trapping the carbon-centered radicals towards the formations of C−C33,34, C−O35,36, C−N37–40, and C−X (X=halogen)41–44 bonds. Particularly, amidyl radical is widely explored nitrogen-centered radical in above mentioned processes. In 2016, Knowles45 and Rovis46 independently developed oxidative photocatalytic generation of amidyl radicals by direct N−H bond cleavage, after 1,5-HAT process, followed by the Michael addition of the translocated carbon-centered radical, affording the remote functionalized amides (Fig. 2b). Another way to generate amidyl radical is using activated precursors47, for example, single-electron photoreduction of N-acyloxy(halo)phthalimides48,49, N-aminopyridinium salts50–53, and hydroxylamides40,47,54–58 give the corresponding amidyl radicals upon heterolytic cleavage of the respective leaving groups (Fig. 2c). Despite these advances, trapping the carbon-centered radical with diboron compounds to build C−B bonds, is still challenging and remains unexplored.
Fig. 2. HLF type remote C−H functionalization reactions.
a HLF reaction. b C(sp3)−H functionalization via amidyl radical by Knowles and Rovis group independently. c C(sp3)−H functionalization by amidyl radical precursors. “N-LG" showed some of the precursors. d This work: carbonyl compounds γ-C(sp3)−H borylation.
In our previous studies, we focused on developing transition metal-free radical borylation reactions59–63. We therefore turned our interest towards the use of amidyl radicals for remote C−H bond (γ-position of a carbonyl compound) borylation reaction (Fig. 2d). With a properly designed amidyl radical precursor that facilitates an intramolecular 1,5-HAT process, site-selective borylation of γ-C−H bond could be achieved. It is known that α-C−H bond and β-C−H bond could be simply activated64. For the direct γ-C−H functionalization, only a few of examples have been reported to date65–68. Thus, our research offers another significant aspect worthy of consideration. Here we present a method that does not require transition metals for the photoredox-catalyzed borylation of unreactive C(sp3)−H bonds initiated through 1,5-HAT.
Results and discussion
Reaction optimization
Recently, visible-light photoredox catalysis has been the leading efficient access to N-radicals under mild conditions. With their weak N−O bond, hydroxylamine derivatives have recently been exploited as nitrogen-centered radical precursors in visible-light photocatalysis, given their higher oxidation state, it would be necessary to apply reductive conditions54,55,57. Thus, 4-trifluoromethylbenzoyl hydroxamic acid derivative (1a) was used as the model substrate to establish optimal reaction parameters, including the equivalency of bis(catecholato)diboron (B2cat2), the choice of photocatalysts (Ir and Ru complexes, or organic photocatalysts), light sources, and additives (Table 1 and Supplementary Table 1 and Fig. 1). We investigated that with Ir(p-CF3ppy)3 (2%) as photocatalyst, 40 W 456 nm light source, Et3N as additive, the desired product was obtained in 45% GC yield. Other amines, such as DABCO, DIPEA or no additive (entry 2–4), were tested, and DIPEA gave improved yield (47%), almost same yield was obtained with a longer reaction time (30 h) (entry 5). Replacing photocatalyst to Ir(ppy)3, [Ru(bpy)3]Cl2, or 4CzIPN could not give the improved results (entry 6–8). Gratifyingly, the reaction gave 47% yield by using Eosin Y, which is a non transition metal photocatalyst, and importantly, much cheaper than Ir(p-CF3ppy)3 (entry 9). Furthermore, We examined the influence of the light source. 467 nm wavelength LED turned out to be better than 456 nm LED, leading a jump increasing yield (from 47% to 73%, entry 10). Higher equivalent photocatalyst (5%) resulted in 77% yield (entry 11). Reducing the B2cat2 loading from 3.0 to 2.5 equiv. led to a slightly improved yield (81% GC yield, isolated in 73%), and 4.0 equiv. B2cat2 gave significantly lower yield (entry 13), which showed that the product yield is not always proportional to the equivalent of borylating reagent. Decreasing the loading of additive DIPEA, the yield dropped to 54% (entry 14). Control experiments revealed that no borylation product formed in the absence of photocatalyst, light or additive (entry 15–17). To rule out the potential for substrate thermal decomposition, we conducted a control experiment by replacing the light with heating at 50 °C for 12 h in the absence of light. However, no borylation product was detected under these conditions (entry 18). Continuous irradiation is necessary, as turning off the light after 30 min resulted in no product formation (entry 19). The standard reaction in air gave a 76% yield, indicating that the reaction is not sensitive to oxygen (entry 20).
Table 1.
Optimization of reaction conditionsa.
| Entry | B2cat2 | PC (mol%) | Light | Additive | Yielda,b(%) |
|---|---|---|---|---|---|
| 1 | 3.0 | Ir(p-CF3ppy)3 (2 mol%) | 456 nm | Et3N (2.0) | 45 |
| 2 | 3.0 | Ir(p-CF3ppy)3 (2 mol%) | 456 nm | DABCO (2.0) | 15 |
| 3 | 3.0 | Ir(p-CF3ppy)3 (2 mol%) | 456 nm | DIPEA (2.0) | 47 |
| 4 | 3.0 | Ir(p-CF3ppy)3 (2 mol%) | 456 nm | none | trace |
| 5c | 3.0 | Ir(p-CF3ppy)3 (2 mol%) | 456 nm | DIPEA (2.0) | 49 |
| 6 | 3.0 | Ir(ppy)3 (2 mol%) | 456 nm | DIPEA (2.0) | 30 |
| 7 | 3.0 | [Ru(bpy)3]Cl2 (2 mol%) | 456 nm | DIPEA (2.0) | trace |
| 8 | 3.0 | 4CzIPN (2 mol%) | 456 nm | DIPEA (2.0) | trace |
| 9 | 3.0 | Eosin Y (2 mol%) | 456 nm | DIPEA (2.0) | 47 |
| 10 | 3.0 | Eosin Y (2 mol%) | 467 nm | DIPEA (2.0) | 73 |
| 11 | 3.0 | Eosin Y (5 mol%) | 467 nm | DIPEA (2.0) | 77 |
| 12 | 2.5 | Eosin Y (5 mol%) | 467 nm | DIPEA (2.0) | 81 (73) |
| 13 | 4.0 | Eosin Y (5 mol%) | 467 nm | DIPEA (2.0) | 40 |
| 14 | 2.5 | Eosin Y (5 mol%) | 467 nm | DIPEA (1.5) | 54 |
| 15 | 2.5 | None | 467 nm | DIPEA (2.0) | trace |
| 16 | 2.5 | Eosin Y (5 mol%) | none | DIPEA (2.0) | trace |
| 17 | 2.5 | Eosin Y (5 mol%) | 467 nm | None | trace |
| 18d | 2.5 | Eosin Y (5 mol%) | none | DIPEA (2.0) | trace |
| 19e | 2.5 | Eosin Y (5 mol%) | 467 nm | DIPEA (2.0) | trace |
| 20f | 2.5 | Eosin Y (5 mol%) | 467 nm | DIPEA (2.0) | 76 |
aReactions run on 0.2 mmol scale, using Kessil 40 W blue LED as light source.
bGC-fid yields are measured by using decane as an internal standard. The yield in bracket is isolated yield.
cThe reaction time is 30 h.
d50 °C for 12 h, in the dark.
eTurned off the light after 30 min.
fThe reaction was set up in air.
Subtrate scope
With the optimal reaction conditions, we next sought to explore the substrate scope of this visible light photoredox-catalyzed remote C(sp3)-H borylation. As summarized in Fig. 3, a variety of hydroxamic acid derivatives were synthesized and tested. The reactions of the substrates with tertiary C−H bonds in the γ-position all gave borylation products in good to excellent yields (2a–2d), while the reactions of the substrate possessing secondary C−H bonds could also afford moderate yield (2e, 2h-2j). Substrates containing tertiary C−H bond in cyclic structure were also tested, and cyclopentyl (2f) and cyclohexyl (2g) products were formed in 83% and 58%, respectively. Specially, we found that this method can activate secondary C(sp3)-H bonds in cyclic substrates, producing the corresponding syn product 2h with excellent diastereoselectivity. Moreover, for bicyclic substrate which have more than one γ-positions, such as norbornyl derivative, high site-selectivity was obtained (2i, 65%; 2j, 64%) (as indicated by NMR NOE test, see Supplementary Data 2 Figs. 1–3 for details). With the replacement of tertbutyl group to isopropyl group or cyclohexyl group, the substrates can undergo the desired transformation to form borylation products in moderate to good yields (2k-2o), albeit the undesired hydrogenation by-products accompanied. It may cause by replacing the N-connected alkyl group from tertiary one to secondary one, which weakens the N−H bond, and the 1,5-HAT process was affected thermodynamically (vide infra, a DFT calculation was performed). Interestingly, we found that a benzene ring can be involved in this six-membered ring 1,5-HAT process, as exemplified in the cases of (2p-2u). For isolation of these products, we found the partial instability of the benzylic pinacol boronic esters in silica gel, and that in situ oxidation with H2O2 allowed isolation of the corresponding alcohols in high yields. Various functional groups such as methyl(2q), methoxy (2r), chlorine (2s) and bromine (2t) were well tolerated, and the yields were up to 92%. For secondary benzylic substrates(2u) or benzylic position on the carbon chain substrates(2v-2w), the products were obtained in good yields. α-hydroxy hydroxamic acids derivatives were also tested, including methoxy (2x), acetoxy (2y). Amino acid leucine-derived hydroxamic acid also underwent this remote borylation, giving the desired product in 45% (2z). Our method can also work on complex molecules, such as lithocholic acid and dehydroabietylamine derivatives (in the cases of 2aa and 2ab). It is worth noting that the last example (2ab) in the substrate scope is a different type other than the rest ones. The borylation of C−H bond took place on the alkyl chain of the amine site.
Fig. 3. Substrate scope.
a Reaction conditions: substrate (0.3 mmol), B2cat2 (0.75 mmol), Eosin Y (5%), DMA (3 mL), DIPEA (0.6 mmol), irradiated by Kessil 40 W blue LED for 12 h, then with pinacol (4.0 equiv.) and Et3N (1 mL); b Products were formed as mixtures of borylation product and hydrogenation side product; c Yields were of isolated products after in-situ oxidation with hydrogen peroxide (4.0 equiv.); d1H NMR yield was reported. e Reaction time was 24 h.
Mechanistic studies
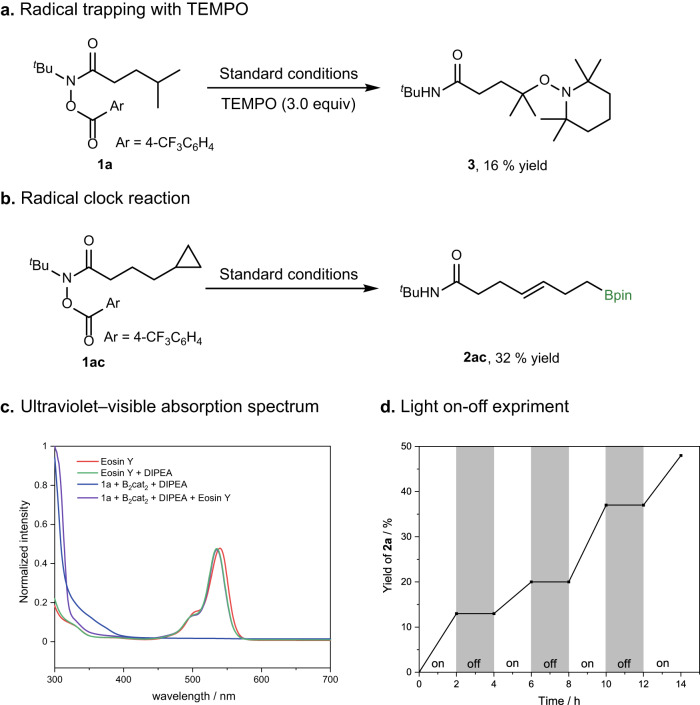
To gain further insight into the mechanism of this 1,5-HAT borylation reaction, we performed a series of mechanistic investigations. First, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) was added to standard conditions, and the reaction yielded 16% of the corresponding TEMPO-trapped product 3 as a white solid, instead of the desired borylation product (Fig. 4a). Second, we designed and managed to synthesize the remote cyclopropyl substrate 1ac, and the reaction of 1ac in standard conditions gave the ring-opened terminal borylation product in 32% yield (Fig. 4b). These two experiments indicated the involvement of carbon radical intermediates in this borylation reaction. The photocatalyst Eosin Y was found to be the only absorbing species in the reaction near the excitation wavelength (λmax = 467 nm) from ultraviolet-visible absorption spectroscopy (Fig. 4c and Supplementary Fig. 2). To determine whether the reaction involves an efficient radical chain mechanism, we performed a light on- off experiment showing no product formation in the dark phases (Fig. 4d and Supplementary Table 2 and Fig. 3).
Fig. 4. Mechanistic studies.
a The standard reaction with TEMPO (3.0 equiv.) as a radical scavenger. b Radical clock ring-opening reaction of 1ac. c In the ultraviolet-visible absorption spectrum, the photocatalyst Eosin Y was found to be the only absorbing species in the reaction near the excitation wavelength (λmax = 467 nm). d The light on-off expriment showed no product formation in dark.
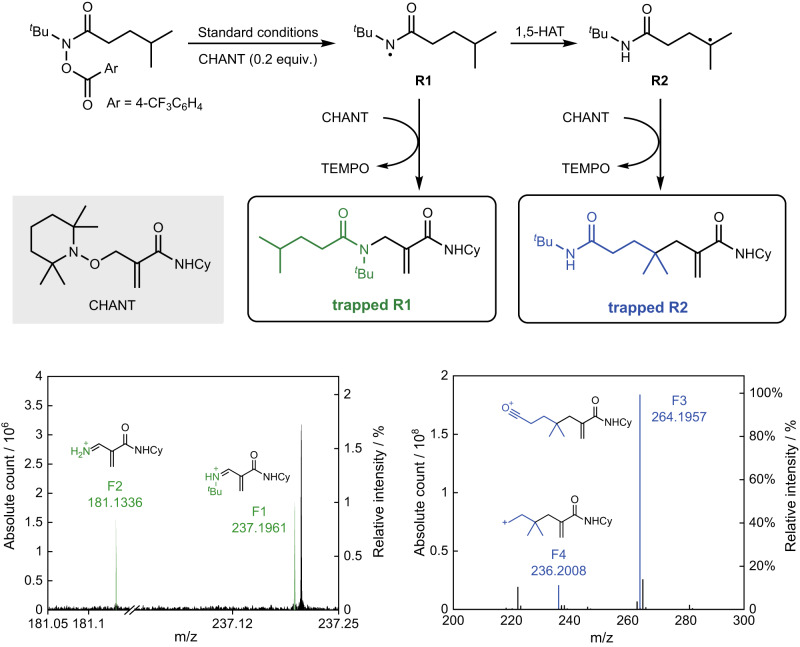
In recent days, the Chechik group reported a new type of radical scavenger called CHANT to detect short-lived radical intermediates (Supplementary Fig. 4)69. In this method, the radical trapping product is a bench-stable terminal alkene that can be analyzed by mass spectrometry (MS). To further testify the 1,5-HAT process, we chose CHANT as radical trapping reagent, and carried out the reaction in standard conditions (Fig. 5). The trapped radicals were then analyzed by electrospray ionization HRMS (Supplementary Tables 3, 4 and Figs. 5–7). The MS peak at m/z 337.2849 indicates the trapped radical intermediates, whereas it cannot distinguish the nitrogen-centered radical R1 or the carbon-centered radical R2, since they are isomers. In order to distinguish them, the tandem mass spectrometry was analyzed at m/z 337.2849, the two low-intensity peaks F1 (m/z 237.1961) and F2 (m/z 181.1336) which contain two nitrogen atoms, could only be attributed to the trapped R1, the fragments showed that the acyl group RCO was dissociated. The most strong-intensity peaks F3 (m/z 264.1957) is a stable acylium ions, and F4 (m/z 236.2008) is F3 losing CO. They could only be attributed to the trapped R2, and many other strong peaks could also be attributed to it (see Supplementary Table 4 for details). This experiment proved that the existence of R1 and R2. The intensity of the trapped R2 is 100 times greater than the trapped R1. Assuming a similar ionization efficiency of the trapped R1 and R2 species, this suggests that the 1,5-HAT process is very fast and that the carbon-centered radical R2 is the resting state for this radical process.
Fig. 5. The radical trapping experiment with CHANT and MS analysis.
R1: carbon-centered radical. R2: nitrogen-centered radical. Trapped R1: R1 trapped by CHANT. Trapped R2: R2 trapped by CHANT. CHANT: N-cyclohexyl-2-[(2,2,6,6-tetramethylpiperidin-1-yl)oxy]methylacrylamide. Cy = cyclohexyl. The fragments F1 and F2 were attributed to trapped R1; The fragments F3 and F4 were attributed to trapped R2.
To better understand the photoredox mechanism of this 1,5-HAT process, we carried out density functional theory (DFT) calculations (see Supplementary Information section 6 for details). The computed free energy profile of the key steps in the photo irradiation process is shown in Fig. 6. Eosin Y transfers from ground state EY2− to excited singlet state (S1) upon being irradiated with the vertical excitation energy of +65.7 kcal mol−1 (equals to 2.8 eV, corresponds to the energy of 442 nm wavelength light)70–72. Then this singlet state transforms to its more stable triplet counterpart (T1)73. Next, this triplet state species is oxidatively quenched by 1a to give EY− and 1a−, which further undergoes N−O bond cleavage to form a nitrogen-centered radical, accompanied with the leaving of group. The N_radical overcomes a relatively low barrier (7.7 kcal mol−1) to the six-member ring transition state HAT_TS, which allows the hydrogen atom to transfer from C atom to N atom to give the C_radical, this step is quite favorable thermodynamically. Subsequently, the C_radical is borylated by diboron to give the desired product59.
Fig. 6. DFT-computed pathways for the generation of the N-centered radical and 1,5-HAT process.
1a is reducted by EY2−(T1)* to give 1a−, followed the leaving of group, which is thermodynamically favorable. The N_radical overcomes a relatively low barrier (7.7 kcal ⋅ mol−1) to give C_radical. The Gaussian 09 level of M06-2X/6-311++G(d,p)-SMD(DMAc)//B3LYP/6-31+G(d) was used.
As shown in substrate scope studies, it is noted that the borylation reactivity is in a order of tertiary C−H bond substrates > secondary ones > primary ones. For tertiary C−H bond substrates, the reactivity of the N-tert-butyl substituted ones are better than that of the N-isopropyl ones. To further elucidate these reactivity patterns, we calculated the standard Gibbs free energy and the activation Gibbs free energy in the process of 1,5-HAT of radicals I to IV (Table 2 and Supplementary Tables 5–7). Our results indicate that the tertiary C−H bonds are the most thermodynamically and dynamically favorable for 1,5-HAT process, due to the more stability effect of σ-p hyperconjugation. When replacing tertbutyl with isopropyl in tertiary C−H bond substrates, the activation energy raises from 7.7 to 10.8 kcal mol−1 (IV), leading to the formation of a certain amount of hydrogenation product.Similar behavior was observed for secondary C−H bond substrates, where the activation energy increases from 7.7 to 10.4 kcal mol−1 (II). For the primary C−H bond substrates, the barrier is 13.1 kcal mol−1 (I), the reaction all gave hydrogenation products and no borylation products were obtained.
Table 2.
The correlation between the type of remote C−H bonds and reaction parameters.
| kcal mol−1 | I | II | III | IV |
|---|---|---|---|---|
| ΔGΘ | −8.3 | −12.2 | −14.4 | −14.7 |
| ΔG‡ | 13.1 | 10.4 | 7.7 | 10.8 |
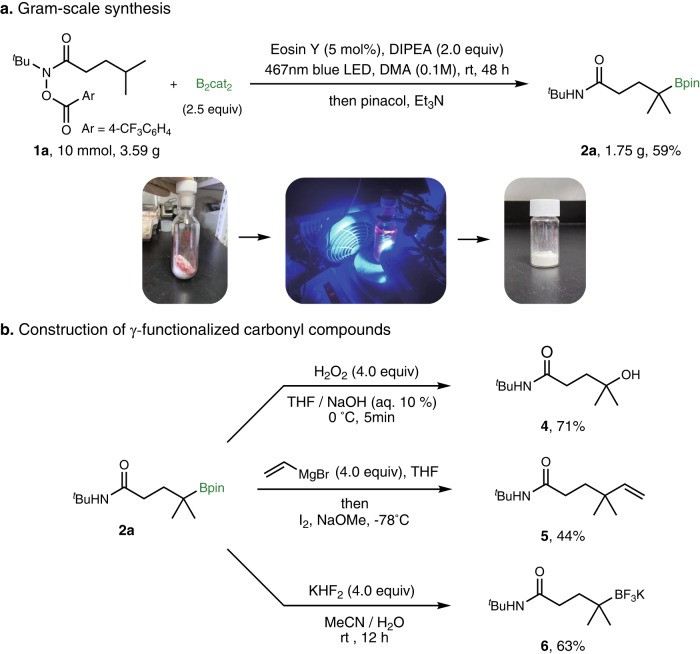
To further demonstrate the utility of this borylation method, a gram-scale reaction of 1a was carried out under air (Fig. 7a and Supplementary Fig. 8), furnishing 2a in 59% isolated yield. The resulting borylation products could be further transformed to a series of functional groups (Fig. 7b). For example, the tertiary alcohol in γ-position of carbonyl compounds could be obtained by oxidation of the boron compound 2a. In addition, tertiary boronic esters underwent C−C bond formation with vinyl Grignard reagent to afford vinylation products 574. Treatment of 1a with KHF2 yields the potassium trifluoroborate salt 6 in 63% yield.
Fig. 7. The gram-scale synthesis and further transformations of the borylation products.
a Gram-scale synthesis set up in air, resulting 1.59 g 2a as a white solid, the following pictures depict the step-by-step procedure involved in the synthesis. b Conversion of 2a into γ-functionalized carbonyl compounds: oxidation to alcohol; vinylation of boronic ester; transformation to potassium trifluoroborate salts.
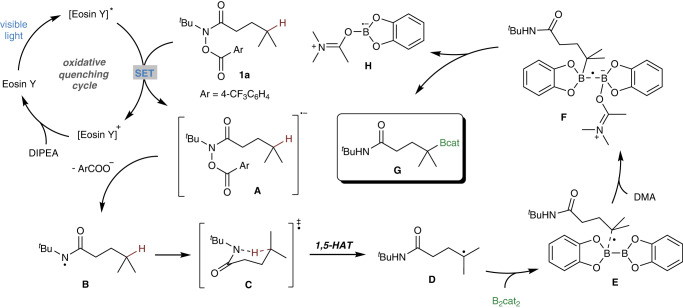
Based on the experimental and computational results as well as studies on previously developed radical borylation reactions59,62,75–79, a proposed mechanism for the photoredox-catalyzed 1,5-HAT borylation process is presented in Fig. 8. First, substrate 1a is reducted by exicited photocatalyst Eosin Y to give radical A, followed by N−O bond cleavage to give nitrogen-center radical B, due to the higer BDE of N−H bond, radical B forms the six-membered ring transition state C to allow intramolecular 1,5-HAT process to give carbon-centered radical D, which adds to diboron B2cat2 to deliver the adduct E, with the help of DMA, the adduct F’s B−B bond is dissociated to form C−B bond, affords the borylated product G, along with the DMA-Bcat radical H.
Fig. 8. Proposed mechanism for the remote C(sp3)−H borylation reaction enabled by 1,5-HAT process.
The carbon radical D is then borylated by B2cat2/DMA to give G.
Conclusion
In conclusion, we have developed a transition-metal free visible light photoredox-catalyzed remote C(sp3)-H borylation reaction, which enabled by 1,5-HAT process based on hydroxamic acid derivatives. A series of mechanism experiments, as well as DFT calculations resolved the radical mechanism in detail. This investigation afforded remote C−H bond functionalization of γ position of carbonyl compounds, thus provided scarce synthetic method of such type. We anticipate that this approach will find broad applications in synthetic community.
Methods
General procedure of C(sp3)−H borylation enabled by 1,5-HAT
In a glovebox under a nitrogen atmosphere, sequentially added B2cat2 (0.75 mmol, 1.5 equiv., 178 mg), the substrate (0.3 mmol, 1.0 equiv.), Eosin Y (5%, 0.015 mmol, 10.5 mg) and 3 mL DMA to a 10 mL Schlenk tube with a stir bar, followed by DIPEA (0.6 mmol, 2.0 equiv., 105 μL). The capped Schlenk tube was removed from the glovebox, and the reaction mixture was irradiated by 467 nm Kessil 40 W LED at a 3 cm distance for 12 h, with a fan for cooling. As the catechol boronate esters are sensitive to hydrolysis, RBcat was transformed to RBpin for isolation. After cooling to room temperature, pinacol (142 mg, 4.0 equiv.) and 1.0 mL triethylamine were added, and the mixture was stirred at room temperature for 1 h. Water was added to the reaction mixture, the aqueous layer was extracted with EtOAc (20 mL × 2). If phase separation was slow, brine was added. The organic phases were combined and washed with 50 mL of saturated brine, the organic phase was dried over anhydrous sodium sulfate and filtered, then concentrated under reduced pressure, purified by column chromatography to give the product. The product was monitored by thin-layer chromatography with phosphomolybdic acid stain.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work is supported by the Natural Science Foundation of China (Grant Nos. 22071004, 21933001, 22150013).
Author contributions
B.S. discovered the method and optimized the reaction condition, performed the substrate synthesis, substrate scope and characterization of the resulting compounds, conducted mechanism studies except DFT calculations, and prepared the manuscript. W.L. contributed to the synthesis of part of the substrates and performed characterizations. Q.L. conducted DFT calculations as part of the mechanism studies. G.Z. helped with reaction condition optimization. F.M. directed the study and prepared the manuscript.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
The authors declare that the data supporting the study are available within the article and Supplementary Information. For experimental details and compounds characterization, see Supplementary Information. For Cartesian coordinates of the structures, see Supplementary Data 1. For NMR spectra, see Supplementary Data 2.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-023-00960-z.
References
- 1.Hall, D. G. Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine (John Wiley & Sons, Weinheim, Germany, 2006).
- 2.Mkhalid IA, Barnard JH, Marder TB, Murphy JM, Hartwig JF. C−H activation for the construction of C−B bonds. Chem. Rev. 2010;110:890–931. doi: 10.1021/cr900206p. [DOI] [PubMed] [Google Scholar]
- 3.Xu L, et al. Recent advances in catalytic C−H borylation reactions. Tetrahedron. 2017;73:7123–7157. doi: 10.1016/j.tet.2017.11.005. [DOI] [Google Scholar]
- 4.Tian Y-M, Guo X-N, Braunschweig H, Radius U, Marder TB. Photoinduced borylation for the synthesis of organoboron compounds: focus review. Chem. Rev. 2021;121:3561–3597. doi: 10.1021/acs.chemrev.0c01236. [DOI] [PubMed] [Google Scholar]
- 5.Waltz KM, Hartwig JF. Selective functionalization of alkanes by transition-metal boryl complexes. Science. 1997;277:211–213. doi: 10.1126/science.277.5323.211. [DOI] [Google Scholar]
- 6.Chen H, Schlecht S, Semple TC, Hartwig JF. Thermal, catalytic, regiospecific functionalization of alkanes. Science. 2000;287:1995–1997. doi: 10.1126/science.287.5460.1995. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L-S, et al. Direct borylation of primary C−H bonds in functionalized molecules by palladium catalysis. Angew. Chem. Int. Ed. 2014;53:3899–3903. doi: 10.1002/anie.201310000. [DOI] [PubMed] [Google Scholar]
- 8.He J, et al. Ligand-promoted borylation of C(sp3)−H bonds with palladium(ii) catalysts. Angew. Chem. Int. Ed. 2016;55:785–789. doi: 10.1002/anie.201509996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J, Shao Q, Wu Q, Yu J-Q. Pd (ii)-catalyzed enantioselective C(sp3)−H borylation. J. Am. Chem. Soc. 2017;139:3344–3347. doi: 10.1021/jacs.6b13389. [DOI] [PubMed] [Google Scholar]
- 10.Chandrashekar HB, et al. Ligand-enabled δ-C(sp3)−H borylation of aliphatic amines. Angew. Chem. Int. Ed. 2021;60:18194–18200. doi: 10.1002/anie.202105204. [DOI] [PubMed] [Google Scholar]
- 11.Ohmura T, Torigoe T, Suginome M. Catalytic functionalization of methyl group on silicon: iridium-catalyzed C(sp3)−H borylation of methylchlorosilanes. J. Am. Chem. Soc. 2012;134:17416–17419. doi: 10.1021/ja307956w. [DOI] [PubMed] [Google Scholar]
- 12.Liskey CW, Hartwig JF. Iridium-catalyzed C−H borylation of cyclopropanes. J. Am. Chem. Soc. 2013;135:3375–3378. doi: 10.1021/ja400103p. [DOI] [PubMed] [Google Scholar]
- 13.Ohmura T, Torigoe T, Suginome M. Functionalization of tetraorganosilanes and permethyloligosilanes at a methyl group on silicon via iridium-catalyzed C(sp3)−H borylation. Organometallics. 2013;32:6170–6173. doi: 10.1021/om400900z. [DOI] [Google Scholar]
- 14.Li Q, Liskey CW, Hartwig JF. Regioselective borylation of the C−H bonds in alkylamines and alkyl ethers. observation and origin of high reactivity of primary C−H bonds beta to nitrogen and oxygen. J. Am. Chem. Soc. 2014;136:8755–8765. doi: 10.1021/ja503676d. [DOI] [PubMed] [Google Scholar]
- 15.Murakami R, Tsunoda K, Iwai T, Sawamura M. Stereoselective C−H borylations of cyclopropanes and cyclobutanes with silica-supported monophosphane-ir catalysts. Chem.–A Eur. J. 2014;20:13127–13131. doi: 10.1002/chem.201404362. [DOI] [PubMed] [Google Scholar]
- 16.Ohmura T, Torigoe T, Suginome M. Iridium-catalysed borylation of sterically hindered C(sp3)−H bonds: remarkable rate acceleration by a catalytic amount of potassium tert-butoxide. Chem. Commun. 2014;50:6333–6336. doi: 10.1039/C4CC01262C. [DOI] [PubMed] [Google Scholar]
- 17.Huang G, Kalek M, Liao R-Z, Himo F. Mechanism, reactivity, and selectivity of the iridium-catalyzed C(sp3)−H borylation of chlorosilanes. Chem. Sci. 2015;6:1735–1746. doi: 10.1039/C4SC01592D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamura S, Araki M, Suzuki T, Yamaguchi J, Itami K. Stereodivergent synthesis of arylcyclopropylamines by sequential C−H borylation and suzuki-miyaura coupling. Angew. Chem. Int. Ed. 2015;3:846–851. doi: 10.1002/anie.201409186. [DOI] [PubMed] [Google Scholar]
- 19.Cook AK, Schimler SD, Matzger AJ, Sanford MS. Catalyst-controlled selectivity in the C−H borylation of methane and ethane. Science. 2016;351:1421–1424. doi: 10.1126/science.aad9289. [DOI] [PubMed] [Google Scholar]
- 20.Larsen MA, Cho SH, Hartwig J. Iridium-catalyzed, hydrosilyl-directed borylation of unactivated alkyl C−H bonds. J. Am. Chem. Soc. 2016;138:762–765. doi: 10.1021/jacs.5b12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith KT, et al. Catalytic borylation of methane. Science. 2016;351:1424–1427. doi: 10.1126/science.aad9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Yang Y, Liu L, Gao Q, Xu S. Iridium-catalyzed enantioselective α-C(sp3)−H borylation of azacycles. J. Am. Chem. Soc. 2020;142:12062–12068. doi: 10.1021/jacs.0c06756. [DOI] [PubMed] [Google Scholar]
- 23.Dannatt JE, Yadav A, Smith III MR, Maleczka Jr RE. Amide directed iridium C(sp3)−H borylation catalysis with high n-methyl selectivity. Tetrahedron. 2022;109:132578. doi: 10.1016/j.tet.2021.132578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y, Yang Y, Xu S. Iridium-catalyzed enantioselective C(sp3)−H borylation of aminocyclopropanes. Angew. Chem. Int. Ed. 2022;61:e202201463. doi: 10.1002/anie.202201463. [DOI] [PubMed] [Google Scholar]
- 25.Shu C, Noble A, Aggarwal VK. Metal-free photoinduced C(sp3)−H borylation of alkanes. Nature. 2020;586:714–719. doi: 10.1038/s41586-020-2831-6. [DOI] [PubMed] [Google Scholar]
- 26.Stateman LM, Nakafuku KM, Nagib DA. Remote C−H functionalization via selective hydrogen atom transfer. Synthesis. 2018;50:1569–1586. doi: 10.1055/s-0036-1591930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarkar S, Cheung KPS, Gevorgyan V. C−H functionalization reactions enabled by hydrogen atom transfer to carbon-centered radicals. Chem. Sci. 2020;11:12974–12993. doi: 10.1039/D0SC04881J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capaldo L, Ravelli D, Fagnoni M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C−H bonds elaboration. Chem. Rev. 2021;122:1875–1924. doi: 10.1021/acs.chemrev.1c00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofmann A. Ueber die einwirkung des broms in alkalischer lösung auf die amine. Ber. Dtsch. Chem. Ges. 1883;16:558–560. doi: 10.1002/cber.188301601120. [DOI] [Google Scholar]
- 30.Löffer K, Kaim H. Synthese des inaktiven δ-coniceins. Ber. Dtsch. Chem. Ges. 1909;42:94–107. doi: 10.1002/cber.19090420112. [DOI] [Google Scholar]
- 31.Guo W, Wang Q, Zhu J. Visible light photoredox-catalysed remote C−H functionalisation enabled by 1,5-hydrogen atom transfer (1, 5-HAT) Chem. Soc. Rev. 2021;50:7359–7377. doi: 10.1039/D0CS00774A. [DOI] [PubMed] [Google Scholar]
- 32.Wu X, Zhu C. Radical functionalization of remote C(sp3)−H bonds mediated by unprotected alcohols and amides. CCS Chem. 2020;2:813–828. doi: 10.31635/ccschem.020.202000234. [DOI] [Google Scholar]
- 33.Chen H, Yu S. Remote C−C bond formation via visible light photoredox-catalyzed intramolecular hydrogen atom transfer. Org. Biomol. Chem. 2020;18:4519–4532. doi: 10.1039/D0OB00854K. [DOI] [PubMed] [Google Scholar]
- 34.Guo Q, et al. Visible-light promoted regioselective amination and alkylation of remote C(sp3)−H bonds. Nat. Commun. 2020;11:1463. doi: 10.1038/s41467-020-15167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivero AR, Fodran P, Ondrejková A, Wallentin C-J. Alcohol etherification via alkoxy radicals generated by visible-light photoredox catalysis. Org. Lett. 2020;22:8436–8440. doi: 10.1021/acs.orglett.0c03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim K, Kim N, Hong S. Visible light-induced intramolecular C−O bond formation via 1, 5-hydrogen atom transfer strategy. Bull. Korean Chem. Soc. 2021;42:548–552. doi: 10.1002/bkcs.12234. [DOI] [Google Scholar]
- 37.Zhao Y, Xia W. Recent advances in radical-based C−N bond formation via photo-/electrochemistry. Chem. Soc. Rev. 2018;47:2591–2608. doi: 10.1039/C7CS00572E. [DOI] [PubMed] [Google Scholar]
- 38.Min Q-Q, Yang J-W, Pang M-J, Ao G-Z, Liu F. Copper-catalyzed remote C(sp3)−H amination of carboxamides. Org. Lett. 2020;22:2828–2832. doi: 10.1021/acs.orglett.0c00829. [DOI] [PubMed] [Google Scholar]
- 39.Wappes EA, Fosu SC, Chopko TC, Nagib DA. Triiodide-mediated δ-amination of secondary C−H bonds. Angew. Chem. 2016;128:10128–10132. doi: 10.1002/ange.201604704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies J, Svejstrup TD, Fernandez Reina D, Sheikh NS, Leonori D. Visible-light-mediated synthesis of amidyl radicals: transition-metal-free hydroamination and n-arylation reactions. J. Am. Chem. Soc. 2016;138:8092–8095. doi: 10.1021/jacs.6b04920. [DOI] [PubMed] [Google Scholar]
- 41.Morcillo SP, et al. Photoinduced remote functionalization of amides and amines using electrophilic nitrogen radicals. Angew. Chem. Int. Ed. 2018;57:12945–12949. doi: 10.1002/anie.201807941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Armas P, et al. Synthesis of 1, 4-epimine compounds. iodosobenzene diacetate, an efficient reagent for neutral nitrogen radical generation. Tetrahedron Lett. 1985;26:2493–2496. doi: 10.1016/S0040-4039(00)94862-7. [DOI] [Google Scholar]
- 43.Martínez C, Muniz K. An iodine-catalyzed hofmann–löffler reaction. Angew. Chem. Int. Ed. 2015;54:8287–8291. doi: 10.1002/anie.201501122. [DOI] [PubMed] [Google Scholar]
- 44.Qin Q, Yu S. Visible-light-promoted remote C(sp3)−H amidation and chlorination. Org. Lett. 2015;17:1894–1897. doi: 10.1021/acs.orglett.5b00582. [DOI] [PubMed] [Google Scholar]
- 45.Choi GJ, Zhu Q, Miller DC, Gu CJ, Knowles RR. Catalytic alkylation of remote C−H bonds enabled by proton-coupled electron transfer. Nature. 2016;539:268–271. doi: 10.1038/nature19811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu JC, Rovis T. Amide-directed photoredox-catalysed C−C bond formation at unactivated sp3 C−H bonds. Nature. 2016;539:272–275. doi: 10.1038/nature19810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung H, Keum H, Kweon J, Chang S. Tuning triplet energy transfer of hydroxamates as the nitrene precursor for intramolecular C(sp3)−H amidation. J. Am. Chem. Soc. 2020;142:5811–5818. doi: 10.1021/jacs.0c00868. [DOI] [PubMed] [Google Scholar]
- 48.Kim H, Kim T, Lee DG, Roh SW, Lee C. Nitrogen-centered radical-mediated C−H imidation of arenes and heteroarenes via visible light induced photocatalysis. Chem. Commun. 2014;50:9273–9276. doi: 10.1039/C4CC03905J. [DOI] [PubMed] [Google Scholar]
- 49.Allen LJ, Cabrera PJ, Lee M, Sanford MS. N-acyloxyphthalimides as nitrogen radical precursors in the visible light photocatalyzed room temperature C−H amination of arenes and heteroarenes. J. Am. Chem. Soc. 2014;136:5607–5610. doi: 10.1021/ja501906x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim N, Lee C, Kim T, Hong S. Visible-light-induced remote C(sp3)−H pyridylation of sulfonamides and carboxamides. Org. Lett. 2019;21:9719–9723. doi: 10.1021/acs.orglett.9b03879. [DOI] [PubMed] [Google Scholar]
- 51.Moon Y, et al. Visible light induced alkene aminopyridylation using N-aminopyridinium salts as bifunctional reagents. Nat. Commun. 2019;10:4117. doi: 10.1038/s41467-019-12216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang H, Studer A. Amidyl radicals by oxidation of α-amido-oxy acids: transition-metal-free amidofluorination of unactivated alkenes. Angew. Chem. Int. Ed. 2018;57:10707–10711. doi: 10.1002/anie.201804966. [DOI] [PubMed] [Google Scholar]
- 53.Greulich TW, Daniliuc CG, Studer A. N-aminopyridinium salts as precursors for N-centered radicals−direct amidation of arenes and heteroarenes. Org. Lett. 2015;17:254–257. doi: 10.1021/ol503338b. [DOI] [PubMed] [Google Scholar]
- 54.Chen H, Guo L, Yu S. Primary, secondary, and tertiary γ-C(sp3)−H vinylation of amides via organic photoredox-catalyzed hydrogen atom transfer. Org. Lett. 2018;20:6255–6259. doi: 10.1021/acs.orglett.8b02737. [DOI] [PubMed] [Google Scholar]
- 55.Chen H, Fan W, Yuan X-A, Yu S. Site-selective remote C(sp3)−H heteroarylation of amides via organic photoredox catalysis. Nat. Commun. 2019;10:4743. doi: 10.1038/s41467-019-12722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu K, Wang L, Colón-Rodríguez S, Flechsig G-U, Wang T. Amidyl radical directed remote allylation of unactivated sp3 C−H bonds by organic photoredox catalysis. Angew. Chem. Int. Ed. 2019;58:1774–1778. doi: 10.1002/anie.201811004. [DOI] [PubMed] [Google Scholar]
- 57.Chen H, Jin W, Yu S. Enantioselective remote C(sp3)−H cyanation via dual photoredox and copper catalysis. Org. Lett. 2020;22:5910–5914. doi: 10.1021/acs.orglett.0c02008. [DOI] [PubMed] [Google Scholar]
- 58.Jin W, Yu S. Photoinduced and palladium-catalyzed remote desaturation of amide derivatives. Org. Lett. 2021;23:6931–6935. doi: 10.1021/acs.orglett.1c02509. [DOI] [PubMed] [Google Scholar]
- 59.Liu Q, et al. Transition-metal-free borylation of alkyl iodides via a radical mechanism. Org. Lett. 2019;21:6597–6602. doi: 10.1021/acs.orglett.9b01951. [DOI] [PubMed] [Google Scholar]
- 60.Liu Q, Zhang L, Mo F. Organic borylation reactions via radical mechanism. Acta Chim. Sin. 2020;78:1297–1308. doi: 10.6023/A20070294. [DOI] [Google Scholar]
- 61.Mo F, Qiu D, Wang J. Synthesis of arylboronic pinacol esters from corresponding arylamines. Org. Synth. 2020;97:1–11. doi: 10.15227/orgsyn.097.0001. [DOI] [Google Scholar]
- 62.Sun B, Zheng S, Mo F. Transition metal-and light-free radical borylation of alkyl bromides and iodides using silane. Chem. Commun. 2021;57:5674–5677. doi: 10.1039/D1CC02134F. [DOI] [PubMed] [Google Scholar]
- 63.Liu Q, et al. Synthesis of alkylboronic esters from alkyl iodides. Org. Synth. 2022;99:15–28. doi: 10.15227/orgsyn.099.0015. [DOI] [Google Scholar]
- 64.Wang C, Dong G. Catalytic β-functionalization of carbonyl compounds enabled by α,β-desaturation. ACS Catal. 2020;10:6058–6070. doi: 10.1021/acscatal.0c01519. [DOI] [Google Scholar]
- 65.Zhang F-L, Hong K, Li T-J, Park H, Yu J-Q. Functionalization of C(sp3)−H bonds using a transient directing group. Science. 2016;351:252–256. doi: 10.1126/science.aad7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu R-Y, Li Z-Q, Park HS, Senanayake CH, Yu J-Q. Ligand-enabled γ-C(sp3)−H activation of ketones. J. Am. Chem. Soc. 2018;140:3564–3568. doi: 10.1021/jacs.8b01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang H, Studer A. α-aminoxy-acid-auxiliary-enabled intermolecular radical γ-C(sp3)−H functionalization of ketones. Angew. Chem. 2018;130:1708–1712. doi: 10.1002/ange.201712066. [DOI] [PubMed] [Google Scholar]
- 68.Hu R, Chen F-J, Zhang X, Zhang M, Su W. Copper-catalyzed dehydrogenative γ-C(sp3)−H amination of saturated ketones for synthesis of polysubstituted anilines. Nat. Commun. 2019;10:1–10. doi: 10.1038/s41467-019-11624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams PJ, et al. New approach to the detection of short-lived radical intermediates. J. Am. Chem. Soc. 2022;144:15969–15976. doi: 10.1021/jacs.2c03618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daly S, et al. The gas-phase photophysics of Eosin Y and its maleimide conjugate. J. Phys. Chem. A. 2016;120:3484–3490. doi: 10.1021/acs.jpca.6b01075. [DOI] [PubMed] [Google Scholar]
- 71.Fan X-Z, et al. Eosin Y as a direct hydrogen-atom transfer photocatalyst for the functionalization of C−H bonds. Angew. Chem. Int. Ed. 2018;57:8514–8518. doi: 10.1002/anie.201803220. [DOI] [PubMed] [Google Scholar]
- 72.Yan D-M, Zhao Q-Q, Rao L, Chen J-R, Xiao W-J. Eosin Y as a redox catalyst and photosensitizer for sequential benzylic C−H amination and oxidation. Chem.−A Eur. J. 2018;24:16895–16901. doi: 10.1002/chem.201804229. [DOI] [PubMed] [Google Scholar]
- 73.Mau AW-H, Johansen O, Sasse WHF. Xanthene dyes as sensitizers for the photoreduction of water. Photochem. Photobiol. 1985;41:503–509. doi: 10.1111/j.1751-1097.1985.tb03519.x. [DOI] [Google Scholar]
- 74.Sonawane RP, et al. Enantioselective construction of quaternary stereogenic centers from tertiary boronic esters: methodology and applications. Angew. Chem. Int. Ed. 2011;50:3760–3763. doi: 10.1002/anie.201008067. [DOI] [PubMed] [Google Scholar]
- 75.Fawcett A, et al. Photoinduced decarboxylative borylation of carboxylic acids. Science. 2017;357:283–286. doi: 10.1126/science.aan3679. [DOI] [PubMed] [Google Scholar]
- 76.Hu D, Wang L, Li P. Decarboxylative borylation of aliphatic esters under visible-light photoredox conditions. Org. Lett. 2017;19:2770–2773. doi: 10.1021/acs.orglett.7b01181. [DOI] [PubMed] [Google Scholar]
- 77.Hu J, Wang G, Li S, Shi Z. Selective C−N borylation of alkyl amines promoted by lewis base. Angew. Chem. Int. Ed. 2018;57:15227–15231. doi: 10.1002/anie.201809608. [DOI] [PubMed] [Google Scholar]
- 78.Wu J, He L, Noble A, Aggarwal VK. Photoinduced deaminative borylation of alkylamines. J. Am. Chem. Soc. 2018;140:10700–10704. doi: 10.1021/jacs.8b07103. [DOI] [PubMed] [Google Scholar]
- 79.Friese FW, Studer A. Deoxygenative borylation of secondary and tertiary alcohols. Angew. Chem. Int. Ed. 2019;58:9561–9564. doi: 10.1002/anie.201904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the data supporting the study are available within the article and Supplementary Information. For experimental details and compounds characterization, see Supplementary Information. For Cartesian coordinates of the structures, see Supplementary Data 1. For NMR spectra, see Supplementary Data 2.