Abstract
Background
We aim to characterise the ophthalmic findings and retinal vasculature changes in patients with WS, and to analyse the correlation between ophthalmic manifestations and the associated systemic diseases.
Methods
This retrospective case-control study included 27 WS patients and 28 age-matched healthy participants. Stellate pattern of iris, central macular thickness (CMT), foveal width, retinal vessel diameter, superficial vascular density (SVD) of macula and foveal avascular zone (FAZ) were compared between WS patients and healthy participants.
Results
Twenty-five patients (93%) had the classic stellate iris presentation. Compared with healthy controls, WS patients had decreased CMT, increased foveal width and a lower SVD of macula (all P < 0.001). Significantly decreased mean retinal arterial (117.9 ± 9.9 µm vs. 133.0 ± 6.7 µm in WS and controls, respectively; p < 0.001) and venous (158.9 ± 11.2 µm vs. 174.0 ± 8.0 µm in WS and controls, respectively; p < 0.001) outer diameters, as well as mean arterial wall thickness (11.2 ± 1.3 µm vs. 12.2 ± 0.8 µm in WS and controls, respectively; p < 0.01) were found in WS. Stellate iris grading was significantly associated with CMT, foveal width, retinal vessel diameter (all p < 0.05), and a significant increase in the odds of having hypertension (Odds ratio (OR), 5.63; P < 0.05). The severity of stellate iris in WS seemed to have the trend of increasing risk of having pulmonary stenosis, tricuspid regurgitation and mitral regurgitation.
Conclusions
This study provides the first in vivo evidence reflecting current knowledge on vessel morphology in WS patients that deficient circumferential growth is the predominant pathophysiologic changes resulting from elastin deficiency. The ophthalmic characteristics may serve as a complementary tool to diagnose and follow-up patients suffering from WS.
Subject terms: Predictive markers, Eye manifestations
Introduction
Williams-Beuren syndrome, also known as Williams syndrome (WS), is a multisystem disorder that affects ~1 in every 10,000 births and caused by a genetic deletion of chromosome 7q11.23 [1, 2]. It typically presents in childhood with a variety of phenotypic abnormalities, including distinct dysmorphic facies, developmental delay, cardiovascular (CV) diseases and hypercalcemia [3, 4]. Cardiovascular abnormalities, largely comprising stenotic lesions, occur in about 80% of cases, and is the leading cause of morbidity and mortality in patients with WS [3–5]. Deletion of the ELN gene, which encodes protein elastin that comprises ~50% of the dry weight of the aorta, results in widespread vascular abnormalities including decreased elasticity, thickening of the tunica media and smooth muscle cell hypertrophy seen in WS [6–8].
Previously reported ocular anomalies in WS include a typical stellate iris pattern, astigmatism, strabismus, retinal vascular tortuosity and wider foveal pit [9–12]. Although ocular changes were described in a number of WS patients, none of the previous studies had reported in-depth ophthalmologic examinations about these features, including optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA).
Recent advances in ophthalmic imaging technology have revolutionised fundus examination and offered a unique yet noninvasive “window” to visualise retinal vessels. Furthermore, existing evidence leans toward the possibility that impaired human circulatory system may result in suboptimal peripheral circulation in the eye and elsewhere, and microvascular morphology in the retina has been shown to reflect in vivo changes in systemic microcirculation [13–15]. Previously published guidelines for WS patients recommend routine CV evaluation and screening [16]; However, advanced CV imaging may not be applicable in all circumstances. Thus, it is worth exploring the changes in retinal structures and vascular networks in relation to systemic vascular alteration, which might serve as an easily measured surrogate for the systemic circulation.
To date, there have been no studies identifying relationships between ocular anomalies and systemic diseases in WS. The aim of this study is to determine the full spectrum of ocular manifestations in patients with WS and to investigate the association between fundamental CV abnormalities and ocular changes. We believe the understanding of these correlations may also provide further insight into pathogenetic mechanisms of WS.
Methods
Study population
In this cross-sectional study, 27 patients with WS and 28 age-matched healthy control subjects were enrolled. The study adhered to the tenets of the Declaration of Helsinki and was approved by the internal review board (IRB) at the Taipei Veterans General Hospital. Written informed consent was waived by the IRB because of the retrospective design. Diagnosis of WS was made by array comparative genomic hybridisation (array-CGH) accompanied by clinical evaluation including typical morphology for WS. All subjects received two-dimensional echocardiography and renal doppler ultrasound according to a standard protocol. Serum calcium levels, free thyroxin, triiodothyronine and thyroid-stimulating hormone levels and average blood pressure were also measured.
Ophthalmic examinations
All participants received a comprehensive ophthalmologic examination included a detailed ocular history and measurements in five ophthalmic categories: (1) Best corrected visual acuity (BCVA); (2) Orthoptic testing including ocular position; (3) Intraocular pressure (IOP) with non-contact tonometry; (4) Anterior segment evaluation with slit-lamp biomicroscopy, including grading of stellate iris severity. (5) Fundus examinations, including colour fundus photography, spectral-domain optical coherence tomography (SD-OCT) and OCTA.
A characteristic “stellate” pattern on the iris can be seen in WS patients, which is believed to be caused by stromal hypoplasia of the iris. A stellate pattern of the iris is defined as raised trabeculae extending from the usual stroma of the iris accompanied by absence or displacement of the iris collarette. Its involvement typically extends from pupillary margin towards peripheral iris root [9]. The severity of stellate iris was scored from 0 to 3 visually with slit-lamp (Fig. 1).
Fig. 1. Examples of slit-lamp photography for stellate iris grading.

Stellate pattern of the iris is defined as “raised trabeculae extending from the usual stroma of the iris accompanied by absence or displacement of the iris collarette”. A Grade 0, indicating normal iris without stellate pattern. B Grade I stellate iris, (0 < stellate pattern diameter <½ total iris diameter), defined as stellate pattern diameter less than half of the iris diameter. C Grade II stellate iris, (½ total iris diameter ≤ stellate pattern diameter < total iris diameter), defined as the diameter of stellate pattern more than half of the iris diameter but less than the total iris diameter. D Grade III stellate iris, (stellate pattern diameter = total iris diameter), defined as the extent of stellate pattern involving the total of iris.
Optical coherence tomography (OCT) and OCT angiography (OCTA) imaging
The Optovue RTVue (Optovue Inc., Fremont, CA, USA) SD-OCT was used in this study. For each participant, fovea-centred cross-line macular scans were acquired, which consists of 2 orthogonally oriented 6 mm lines made up by of 1024 A-scans, and the central macular thickness (CMT) with a thickness map were generated with the built-in analysis software.
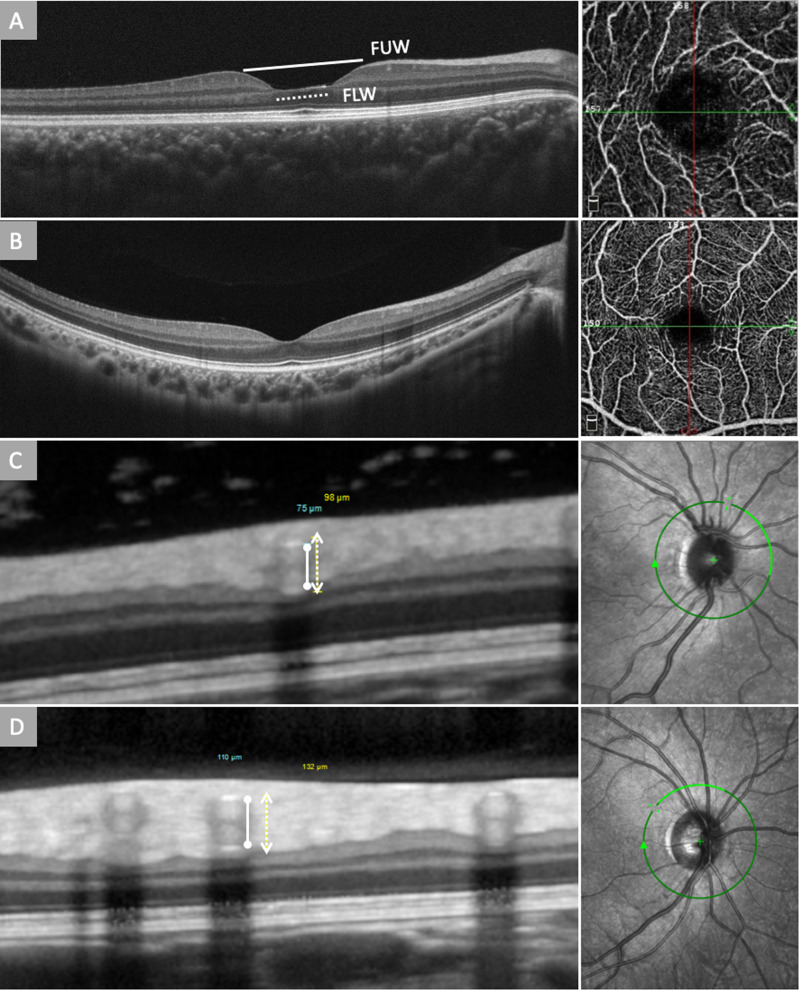
Using the inbuilt manual calibres, foveal upper width (FUW) was measured as the horizontal distance between foveal crests, identified as the maximal retinal thickness nearest to the foveal centre on the nasal and temporal side (Fig. 2A, B). Similarly, fovea lower width (FLW) was measured as the horizontal distance between the lowest point of the temporal and nasal slope, identified as the minimum retinal thickness nearest to the foveal centre on the nasal and temporal side (Fig. 2A, B). This approach has been validated previously in several studies [17, 18].
Fig. 2. Optical coherence tomography (OCT) and OCT angiography of Williams syndrome (WS) and healthy control.
A A macular OCT scan of a WS patient showing deepening and widening of the fovea. Foveal upper width (FUW, solid line) was measured as the horizontal distance between foveal crests on the nasal and temporal side, and fovea lower width (FLW, dotted line) was measured as the horizontal distance between the lowest point of the temporal and nasal slope; A macular OCTA of a WS patient showing reduced vascular density of the fovea and enlarged foveal avascular zone (FAZ). B Macular OCT and OCTA scan of a healthy subject showing normal foveal contour and vascular density of the fovea. C The green circle indicates the position of corresponding SD-OCT scan. Retinal vessel caliber measurements used in our study were based on 4 major arteries and veins arising from the edge of the optic disc. Sections of major retinal vessels appearing as a group of heterogeneous reflectivities in a hourglass configuration. The top and bottom of the vessel walls correlate to the innermost and outermost hyper-reflectivities. The solid line marks the inner reflections of the arterial vessel wall, and the dotted line marks the outer reflections of the arterial vessel wall of a William syndrome patient and (D) a healthy control. The arterial walls generally have higher reflectivity compared to the venous walls.
The Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany) was used in measuring retinal vessel diameter and vessel wall thickness. Outer and inner diameters of the 4 largest retinal arteries and veins were measured using OCT vascular wall reflections, and vessel wall thickness was calculated accordingly. A circular OCT scan, centred on the optic disc, provided cross-sectional images of all major retinal arteries and veins (Fig. 2C, D). Major retinal vessels radiate from the optic disc through the peripapillary area, running parallel to the retinal surface. This allows scanning beam to be vertically projected onto the vessel walls and the lumen, resulting in distinct heterogeneous hyper-reflectivities in a linear configuration [19]. The top and bottom of the vessel walls correlate to the innermost and outermost hyper-reflectivities, while the arterial walls generally have higher reflectivity compared with the venous walls. Vessel wall thickness was then calculated according to the following formula: wall thickness = (outer vessel diameter − inner vessel diameter)/2. The data were shown to be highly reproducible and valid as compared to previous studies [20].
OCTA measurement was performed with Optovue RTVue (Optovue, Fremont, CA, USA) with a scan rate of 70,000 A-scans/s, scan beam wavelength of 840 ± 10 nm, consisting 304 × 304 A-scans. The superficial vascular density (SVD) and the foveal avascular zone (FAZ) were automatically calculated by the built-in software. In addition, the vascular density was further divided into quadrants with two diagonal lines including the central foveal area (<1 mm), superior, inferior, nasal, and temporal region.
Statistical analysis
Statistics were calculated using SPSS software (IBM SPSS, Version 26.0, IBM Corporation, Armonk, NY). Continuous variables are presented as mean ± SD. Multivariable linear regression analysis was used to test the association between iris grading and each parameter on OCT/OCTA. Logistic regression analysis was used to see if iris grading or retinal vascular caliber is associated with increase of risk of medical/CV comorbidity. All p values were two-tailed, and p < 0.05 was considered statistically significant.
Results
A total of 27 patients with WS and 28 control subjects were enrolled in this study, with the mean age of 13.81 years in WS group and 13.64 years in control group. Of the 27 patients, 13 were male and 14 were female. The mean logMAR BCVA was 0.77 ± 0.11 in the WS and 0.54 ± 0.11 in the control group, respectively. There were no statistically significant differences of age, sex, BCVA and refraction between the two groups (all p > 0.05). Of the 27 WS patients, 13 (48.1%) had strabismus, and the predominant form of strabismus was exotropia (8 of 13 strabismus patients). Twenty-five (92.6%) WS patients had a classic stellate pattern of the iris. This iris presentation was exclusively seen in WS cases as compared to controls. Among 27 WS patients, 7 (25.9%) had hypertension and 10 (37.0%) had hypothyroidism. Hypercalcemia was noted in 11 (40.7%) patients. As for CV diseases, the most common CV manifestation in WS patients was supravalvular aortic stenosis (11 patients, 40.7%), followed by tricuspid regurgitation (TR) (22 patients, 81.5%), mitral regurgitation (MR) (13 patients, 48.1%), pulmonary stenosis (PS) (3 patients, 53.8%), and pulmonary regurgitation (PR) (4 patients, 14.8%). Among 24 patients with CV abnormalities, 21 (87.5%) had more than one CV abnormalities. In addition, renal artery stenosis occurs in 3 (11.1%) WS patients (Table 1).
Table 1.
Baseline demographics of participants.
| Characteristic | Mean (SD) | P Value | |
|---|---|---|---|
| Williams syndrome (n = 27) | Healthy control (n = 28) | ||
| Age, y | 13.81 (8.41) | 13.64 (11.00) | 0.948 |
| Sex, No. (%) | |||
| Male | 13 (48.1) | 12 (42.9) | 0.694 |
| Female | 14 (51.9) | 16 (57.1) | |
| BCVA (logMAR) | 0.774 (0.113) | 0.540 (0.108) | 0.445 |
| IOP (mmHg) | 14.72 (2.23) | 17.10 (3.08) | 0.003 |
| Refraction (SE) | −0.083 (1.225) | −1.257 (3.062) | 0.069 |
| Strabismus, No. (%) | 13 (48.1) | 0 (0) | <0.001 |
| Iris Grading, No. (%) | |||
| Grade 0 | 2 (7.4) | 28 (100) | <0.001 |
| Grade 1 | 9 (33.3) | 0 (0) | |
| Grade 2 | 9 (33.3) | 0 (0) | |
| Grade 3 | 7 (25.9) | 0 (0) | |
| Hypertension, No. (%) | 7 (25.9) | 0 (0) | <0.001 |
| Hyperthyroidism, No. (%) | 10 (37.0) | — | — |
| Hypercalcemia, No. (%) | 11 (40.7) | — | — |
| Cardiovascular Disease, No. (%) | |||
| Supravalvular Aortic Stenosis | 11 (40.7) | — | — |
| Pulmonary Artery Stenosis | 3 (11.1) | — | — |
| Tricuspid Regurgitation | |||
| Grade 1 | 17 (63.0) | — | — |
| Grade 2 | 4 (14.8) | — | — |
| Grade 3 | 1 (3.7) | — | — |
| Mitral Regurgitation | |||
| Grade 1 | 4 (14.8) | — | — |
| Grade 2 | 6 (22.2) | — | — |
| Grade 3 | 3 (11.1) | — | — |
| Pulmonary Regurgitation | |||
| Grade 1 | 2 (7.4) | — | — |
| Grade 2 | 2 (7.4) | — | — |
| Renal Artery Stenosis, No. (%) | 3 (11.1) | — | — |
BCVA best corrected visual acuity, IOP intraocular pressure, logMAR logarithm of the Minimum Angle of Resolution, SD standard deviation, SE spherical equivalent.
Quantitative measurements in SD-OCT and OCTA
The mean CMT of 219.1 μm in WS group was significantly thinner than that of 242.7 μm in control group (P < 0.001). Patients with WS had significant wider FUW (mean, 621.3 μm vs. 533.9 μm in WS and control group separately, p = 0.001) and FLW (mean, 151.7 μm vs. 31.6 μm in WS and control group respectively, p < 0.001) compared to controls (Table S1).
WS patients had significant smaller arterial outer (mean, 117.9 μm vs. 133.0 μm in WS and controls respectively, p < 0.001) and inner diameter (mean, 95.1 μm vs. 108.6 μm in WS and controls respectively, p < 0.001), and thinner arterial vessel wall thickness (mean, 11.2 μm vs. 12.2 μm in WS and controls respectively, p = 0.01) compared to controls. Both of venular outer (mean, 158.9 μm vs. 174.0 μm in WS and controls respectively) and inner diameter (mean, 133.1 μm vs. 149.4 μm in WS and controls respectively) were thinner in WS patients (all p < 0.001). However, the venular vessel wall thickness did not differ significantly between the two groups (Table 2).
Table 2.
The results of multivariable linear regression analysis in patients with williams syndrome and control subjectsa.
| Iris grading | ||||||
|---|---|---|---|---|---|---|
| Grade I | Grade II | Grade III | ||||
| β Coefficient (95% CI) | P Value | β Coefficient (95% CI) | P Value | β Coefficient (95% CI) | P Value | |
| LogMAR BCVA | 0.03 [−0.06–0.11] | 0.543 | 0.03 [−0.06–0.12] | 0.472 | 0.02 [−0.07–0.11] | 0.693 |
| Vessel Density | −2.67 [−4.97–−0.38] | 0.024 | −3.52 [−6.57–−0.47] | 0.025 | 0.17 [−2.63–2.96] | 0.905 |
| Vessel Density-Fovea | −7.20 [−12.41–−2.00] | 0.008 | −10.20 [−17.12–−3.27] | 0.005 | −2.15 [−8.48–4.19] | 0.496 |
| Foveal Avascular Zone | 0.11 [0.01–0.21] | 0.033 | 0.20 [0.07–0.34] | 0.005 | 0.03 [−0.10–0.16] | 0.586 |
| Central Macular Thickness | −17.29 [−31.48–−3.11] | 0.018 | −29.46 [−44.26–−14.66] | <0.001 | −21.68 [−37.08–−6.28] | 0.007 |
| Fovea Upper Width | 98.35 [34.58–162.11] | 0.003 | 127.08 [57.07–197.10] | 0.001 | 36.96 [−32.22–106.15] | 0.288 |
| Fovea Lower Width | 128.69 [80.60–176.77] | <0.001 | 132.91 [80.12–185.71] | <0.001 | 84.30 [32.13–136.47] | 0.002 |
| Arterial Outer Diameter | −17.04 [−24.26–−9.82] | <0.001 | −7.48 [−15.31–0.36] | 0.061 | −20.04 [−27.72–−12.36] | <0.001 |
| Arterial Inner Diameter | −15.55 [−22.09–−9.00] | <0.001 | −6.26 [−13.37–0.84] | 0.082 | −18.16 [−25.13–−11.19] | <0.001 |
| Arterial Vessel Wall | −0.81 [−1.75–0.14] | 0.091 | −0.85 [−1.87–0.18] | 0.102 | −0.93 [−1.93–0.08] | 0.069 |
| Venous Outer Diameter | −11.00 [−18.73–−3.26] | 0.007 | −9.39 [−18.60–−0.17] | 0.046 | −21.84 [−30.89–−12.80] | <0.001 |
| Venous Inner Diameter | −12.77 [−21.21–−4.33] | 0.004 | −7.47 [−17.53–2.59] | 0.141 | −26.05 [−35.93–−16.18] | <0.001 |
| Venous Vessel Wall | 1.01 [−0.37–2.39] | 0.147 | −0.73 [−2.37–0.91] | 0.373 | 0.66 [−0.96–2.27] | 0.413 |
logMAR logarithm of the Minimum Angle of Resolution, BCVA best corrected visual acuity, CI confidence interval.
aAdjusting for Age and Gender, subjects with iris grade 0 were used as controls.
Patients with WS had significantly lower SVD in the whole retina (mean, 42.0 vs. 44.0 in WS and controls respectively, p = 0.04, in %) and fovea (mean, 14.4 vs. 20.2 in WS and controls respectively, p = 0.007) on OCTA, compared to controls. WS patients also had larger FAZ compared to controls (mean, 0.38 mm2 vs. 0.27 mm2 in WS and controls respectively, p = 0.006). However, the SVD of the other quadrants of macular area did not show significant differences between the two groups (Table 2).
Ophthalmic parameters associated with severity of stellate iris
In multivariate linear regression models adjusting for age and gender, the relationship between stellate iris severity and various ophthalmic parameters was found (Table 2). There were significant associations between stellate iris severity and several quantitative markers on OCT, including CMT (Grade I: β = −17.29; Grade II: β = −29.46; Grade III: β = −21.68, all P < 0.05), and FLW (Grade I: β = 128.69; Grade II: β = 132.91; Grade III: β = 84.30, all P < 0.01). Regarding OCTA findings, the associations with stellate iris severity were observed in SVD in the whole retina and fovea, as well as the FAZ area. Similarly, associations were also observed between the arterial and venous diameters, and the stellate iris severity (Table 2). If we re-grouped the iris grading into grade I + II and grade III, the association between arterial, venous diameter and each group would be more significant, which shown that higher iris grading had narrower arterial and venous diameter compared to controls (grade 0) (arterial outer diameter: grade I + II, β = −12.75 vs. grade III, β = −19.68, all p < 0.001; arterial inner diameter: grade I + II, β = −11.38 vs. grade III, β = −17.81, all p < 0.001; venous outer diameter: grade I + II, β = −10.37 vs. grade III, β = −21.79 all p < 0.01; venous inner diameter: grade I + II, β = −10.72 vs. grade III, β = −25.87, all p < 0.01). Noteworthy, the vessel wall thickness in both arteries and veins did not correlate with the stellate iris severity (Table 2).
Systemic diseases associated with severity of stellate iris
In multivariable logistic regression model, we found that the severity of stellate iris in WS patients was associated with a significant increase in the odds of having hypertension (Odds ratio (OR), 5.63; 95% confidence interval (CI), 1.48–21.48, P < 0.05). In addition, the severity of stellate iris in WS seemed to have the trend of increasing risk of having PS, TR and MR, which did not attain statistically significance (OR, 11.13 in PS; OR, 7.31 in TR; OR, 1.52 in MR; all p > 0.05) (Table 3).
Table 3.
The results of logistic regression analysis in patients with Williams syndrome, focusing on medical comorbidities and iris grading.
| Iris grading | ||||||
|---|---|---|---|---|---|---|
| Crude | Adjusteda | |||||
| Odds ratio | 95% CI | P Value | Odds ratio | 95% CI | P Value | |
| Medical Comorbidities | ||||||
| Hypertension | 3.225 | [1.398–7.441] | 0.006 | 5.632 | [1.477–21.48] | 0.011 |
| Hyperthyroidism | 0.712 | [0.298–1.698] | 0.443 | 0.711 | [0.246–2.057] | 0.529 |
| Hypercalcemia | 0.903 | [0.39–2.09] | 0.812 | 0.95 | [0.362–2.496] | 0.917 |
| Cardiovascular Comorbidities | ||||||
| Aortic Stenosis | 0.321 | [0.097–1.06] | 0.062 | 0.128 | [0.013–1.302] | 0.082 |
| Pulmonary Stenosis | 9.767 | [0.858–111.213] | 0.066 | 11.132 | [0.699–177.353] | 0.088 |
| Tricuspid Regurgitation | 2.91 | [0.743–11.397] | 0.125 | 7.312 | [0.748–71.441] | 0.087 |
| Mitral Regurgitation | 1.193 | [0.452–3.15] | 0.721 | 1.524 | [0.486–4.778] | 0.470 |
CI confidence interval.
aAdjusting for age and gender.
Discussion
In this study, we observed a variety of ocular manifestations in WS patients and quantitatively analysed these parameters using OCT and OCTA. We found significantly enlarged FAZ, FUW, FLW, and decreased CMT, as well as decreased retinal arterial and venous calibre in patients with WS as compared to age-matched controls. Noteworthy, we demonstrated that there were robust associations between stellate iris severity and OCT measurements at the macula. Our findings also suggest that hypertension and a series of changes in cardiac structure were associated with stellate iris severity, implicating that hypoplasia of the stroma of the iris might be informative for predicting systemic CV abnormalities.
Our study echoes the ocular findings with earlier cohort studies of WS [9–12]. In our patients, strabismus was a common finding, with an incidence of up to 78% described in the literature [11, 21]. We found stellate iris pattern to be a common abnormality as seen in previous studies [9–11, 21], observed in ~93% of our patients with variable severity.
Wider and deeper foveal pit has been described in literatures [22], yet none of them provided quantitative assessment. Our study is the first in the literature to evaluate the CMT and foveal width in WS. CMT was significantly decreased in WS than in the control group, accompanied with deepening and widening of the fovea. Tick et al. showed that a greater pit diameter and deeper foveal pit were correlated with a thinner central fovea in normal eyes [18]. Using immunohistochemistry and ELISA, Chen et al. demonstrated the presence of elastin in the human retina, showing that 3.73 ± 0.55% of the normal retinal tissues were elastin. Elastin was found to be intervened with the collagen fibres at the outer basement membranes of retinal vessels [23]. We suggest that the decreased macular thickness and enlarged foveal width could be associated with decreased or disorganised elastin fibres in central macula due to deletion in the critical region at 7q11.23 which includes the elastin gene (ELN).
We found that the SVD on OCTA was significantly lower in WS than controls. Although we found a lower SVD in the central foveal zone, the difference was not statistically significant in other portions of the macular area (superior, nasal, inferior, temporal). In the previous studies, SVD and retinal thickness appear to be significantly correlated in myopic eyes [24]. The lowered SVD among patients with WS in our study may be associated with enlarged FAZ and the CMT thinning, resulted from elastin deficiency.
Prior studies have suggested that the lack of elastin in blood vessel walls results in vascular smooth muscle hyperplasia which causes narrowing of the vessel lumen and arterial stenosis [25, 26]. However, recent work by Jiao et al. showed that the lack of elastin results in deficient circumferential growth of arteries rather than vascular smooth muscle cell hyperplasia, is the primary determinant of aortic luminal narrowing [27]. This represents a significant shift in the understanding of the pathophysiology of arterial stenosis in WS, which may revolutionise future medical approach. Decreased retinal vessel diameter and arterial wall thickness in WS patients certainly is an interesting finding in our study, and it might be interpreted as a result of elastin haploinsufficiency. This in vivo evidence from OCT is consistent with previous studies, which may provide new insights into its pathogenesis [5, 27].
In this study, we detailed the characteristic ocular manifestations in WS patients and developed a grading system to assess the extent and severity of stellate iris more objectively. This grading system was designed to be easy and convenient to use in clinical practice. As shown in Table 2, severity of stellate iris can represent a potential indicator of a significant number of ocular abnormalities, such as foveal width and retinal vessel diameter. We found that increased severity of stellate iris independently related to enlarged foveal width, especially in FLW, and decreased retinal arterial and venous diameter. The stellate iris grading system introduced here can be used in the initial evaluation and the follow-up of ocular abnormalities in WS patients.
Currently, there is little information on the possible correlation of ocular manifestations with systemic diseases such as CV abnormalities, hypertension, hypercalcemia and hypothyroidism in WS. However, there have been a number of studies supporting close relations between ophthalmic findings and systemic conditions. Lee et al. reported that hypertension was strongly correlated with retinal microcirculatory changes [28]. In addition, a range of ocular manifestations including retinal vascular changes have been proposed as biomarkers of Alzheimer’s disease [29]. Our study provides a potential link between stellate iris grading and systemic hypertension. We found that increased severity of stellate iris was associated with increased risks of developing hypertension (Table 3). According to the literatures, hypertension occasionally beginning in childhood, ultimately develops in ~ 50% of WS patients [3, 30]. Animal model suggest that the increased blood pressures may reflect a physiological change to abnormal vasculature [31]. In fact, CV complications are the major cause of death in patients with WS, and patients often need lifelong medical attention [5]. In this study, we also found that the severity of stellate iris in WS seemed to have the trend of increasing risk of having PS, TR and MR, though not attaining statistically significance. A plausible explanation is that our analyses may have had insufficient sample sizes that limited the power to demonstrate statistical significance. Nonetheless, these findings still indicated that ocular features were associated with systemic comorbidities, and perhaps a powerful noninvasive tool to help further identify patients with cardiac structural changes in addition to conventional tools that are reflective of CV disease risk.
To our knowledge, this is the first comprehensive study regarding ocular manifestations and systemic diseases in patients with WS. However, we acknowledge there are some limitations. First, WS is a rare genetic disorder and the limited sample size may have hampered the statistical power to detect all possible associations. Second, the normal systolic and diastolic cardiac cycle may inject random variation into the measured retinal vessel calibre. However, fluctuations were found to be small and random and should not cause major miscalculations [32]. Finally, the retrospective nature of our study design precludes inferences regarding the nature of the observed associations. Future longitudinal follow-up studies may provide insights into the pathogenesis between ocular and systemic findings.
In conclusion, our results advance current knowledge on pathophysiologic changes of CV abnormality in WS patients. This is the first study to report quantitative retinal morphological characteristics and in vivo retinal vessel measurements in WS patients. It confirms the bench finding of elastin-deficient mice that deficient circumferential growth resulting from elastin deficiency is the predominant pathophysiologic changes for clinical vasculopathies in WS patients. In addition, elastin deficiency may also result in other ophthalmic abnormalities, such as stellate iris, decreased CMT, enlarged FAZ, and widening foveal width. We demonstrated that the stellate iris grading might represent as a novel marker of risk for developing systemic hypertension, and possibly correlate to the cardiac abnormalities. In the future, non-invasive ophthalmic imaging such as slit-lamp biomicroscopy and OCT may serve as a complementary tool to diagnose and follow-up patients suffering from WS, and ultimately provide important insights into the causation and progression of disease.
Supplemental information is available at nature.com/eye
Summary
What was known before
Williams Syndrome is an inherited disorder caused by microdeletions of chromosome 7q11.23. Ocular findings such as a stellate iris pattern, retinal vascular tortuosity, and wider foveal pit can be seen in patients with Williams Syndrome.
What this study adds
This study highlights novel evidence of obstructive vasculopathy in Williams Syndrome, showing decreased external retinal vessel diameter attributable to elastin deficiency.
Compared with healthy controls, Williams Syndrome patients had decreased CMT, increased foveal width and a lower SVD of macula.
The severity of stellate iris was found to be correlated with CMT, foveal width and retinal vessel diameter.
The severity of stellate iris in Williams Syndrome patients was associated with a significant increase in the odds of having hypertension.
Supplementary information
Author contributions
(I) Conception and design: T-CY, H-CC, D-MN, A-GW (II) Administrative support: H-YL, SCC, H-YY, J-YY. (III) Provision of study materials or patients: H-CC, A-GW. (IV) Collection and assembly of data: T-CY. (V) Data analysis and interpretation: T-CY, H-CC, A-GW. (VI) Paper writing: T-CY, H-CC. (VII) Final approval of paper: All authors.
Funding
This study is supported by Taipei Veterans General Hospital (grant no. VGH V110C-052, V111C-099) and Ministry of Science and Technology of Taiwan (grant no. MOST 110-2314-B-075-054-MY3, MOST 111-2324-B-075-42).
Data availability
The data that support the findings of this study are available upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tsai-Chu Yeh, Hui-Chen Cheng, Dau-Ming Niu, An-Guor Wang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-022-02328-4.
References
- 1.Strømme P, Bjørnstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J Child Neurol. 2002;17:269–71. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- 2.Korenberg JR, Chen XN, Hirota H, Lai Z, Bellugi U, Burian D, et al. VI. Genome structure and cognitive map of Williams syndrome. J Cogn Neurosci. 2000;12:89–107. doi: 10.1162/089892900562002. [DOI] [PubMed] [Google Scholar]
- 3.Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362:239–52. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- 4.Collins RT, Kaplan P, Somes GW, Rome JJ. Long-Term Outcomes of Patients With Cardiovascular Abnormalities and Williams Syndrome. Am J Cardiol. 2010;105:874–8. doi: 10.1016/j.amjcard.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 5.Collins RTI. Cardiovascular disease in Williams syndrome. Curr Opin Pediatrics. 2018;30:609–15. doi: 10.1097/MOP.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 6.Leung DY, Glagov S, Mathews MB. Elastin and collagen accumulation in rabbit ascending aorta and pulmonary trunk during postnatal growth. Correlation of cellular synthetic response with medial tension. Circ Res. 1977;41:316–23. doi: 10.1161/01.RES.41.3.316. [DOI] [PubMed] [Google Scholar]
- 7.Keating MT. Genetic Approaches to Cardiovascular Disease. Circulation. 1995;92:142–7. doi: 10.1161/01.CIR.92.1.142. [DOI] [PubMed] [Google Scholar]
- 8.Parks WC, Pierce RA, Lee KA, Mecham RP. Elastin. In: Bittar EE, editor. Advances in Molecular and Cell Biology. Vol 6. Extracellular Matrix. Elsevier; Volume 6; 1993. p. 133–181.
- 9.Holmström G, Almond G, Temple K, Taylor D, Baraitser M. The iris in Williams syndrome. Arch Dis Child. 1990;65:987–9. doi: 10.1136/adc.65.9.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winter M, Pankau R, Amm M, Gosch A, Wessel A. The spectrum of ocular features in the Williams-Beuren syndrome. Clin Genet. 1996;49:28–31. doi: 10.1111/j.1399-0004.1996.tb04320.x. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg F, Lewis RA. The Williams syndrome. Spectrum and significance of ocular features. Ophthalmology. 1988;95:1608–12. doi: 10.1016/S0161-6420(88)32959-3. [DOI] [PubMed] [Google Scholar]
- 12.Weber Sarah LP, Souza Rodrigo B, Ribeiro Lorena G, Tavares Marcela F, Goldchmit M. Williams Syndrome: Ophthalmological Examination and Review of Systemic Manifestations. J Pediatr Ophthalmol Strabismus. 2014;51:209–13. doi: 10.3928/01913913-20140423-01. [DOI] [PubMed] [Google Scholar]
- 13.Popovic N, Radunovic M, Badnjar J, Popovic T. Fractal dimension and lacunarity analysis of retinal microvascular morphology in hypertension and diabetes. Microvascular Res. 2018;118:36–43. doi: 10.1016/j.mvr.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Cabrera DeBuc D, Somfai GM, Koller A. Retinal microvascular network alterations: potential biomarkers of cerebrovascular and neural diseases. Am J Physiol-Heart Circulatory Physiol. 2016;312:H201–H212.. doi: 10.1152/ajpheart.00201.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung CY, Thomas GN, Tay W, Ikram MK, Hsu W, Lee ML, et al. Retinal Vascular Fractal Dimension and Its Relationship With Cardiovascular and Ocular Risk Factors. Am J Ophthalmol. 2012;154:663–674.e1. doi: 10.1016/j.ajo.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Genetics C on. Health Care Supervision for Children With Williams Syndrome. Pediatrics. 2001;107:1192–204. doi: 10.1542/peds.107.5.1192. [DOI] [PubMed] [Google Scholar]
- 17.Ctori I, Huntjens B. Repeatability of Foveal Measurements Using Spectralis Optical Coherence Tomography Segmentation Software. PLoS One. 2015;10:e0129005. [DOI] [PMC free article] [PubMed]
- 18.Tick S, Rossant F, Ghorbel I, Gaudric A, Sahel J-A, Chaumet-Riffaud P, et al. Foveal shape and structure in a normal population. Investig Ophthalmol Vis Sci. 2011;52:5105–10. doi: 10.1167/iovs.10-7005. [DOI] [PubMed] [Google Scholar]
- 19.Muraoka Y, Tsujikawa A, Kumagai K, Akiba M, Ogino K, Murakami T, et al. Age- and hypertension-dependent changes in retinal vessel diameter and wall thickness: an optical coherence tomography study. Am J Ophthalmol. 2013;156:706–14. doi: 10.1016/j.ajo.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Alten F, Motte J, Ewering C, Osada N, Clemens CR, Kadas EM, et al. Multimodal Retinal Vessel Analysis in CADASIL Patients. PLOS ONE. 2014;9:e112311. doi: 10.1371/journal.pone.0112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams JCP, Barratt-Boyes BG, Lowe JB. Supravalvular Aortic Stenosis. Circulation. 1961;24:1311–8. doi: 10.1161/01.CIR.24.6.1311. [DOI] [PubMed] [Google Scholar]
- 22.Castelo-Branco M, Mendes M, Sebastião AR, Reis A, Soares M, Saraiva J, et al. Visual phenotype in Williams-Beuren syndrome challenges magnocellular theories explaining human neurodevelopmental visual cortical disorders. J Clin Investig. 2007;117:3720–9. doi: 10.1172/JCI32556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen K, Weiland JD. Discovery of retinal elastin and its possible role in age-related macular degeneration. Ann Biomed Eng. 2014;42:678–84. doi: 10.1007/s10439-013-0936-x. [DOI] [PubMed] [Google Scholar]
- 24.Milani P, Montesano G, Rossetti L, Bergamini F, Pece A. Vessel density, retinal thickness, and choriocapillaris vascular flow in myopic eyes on OCT angiography. Graefes Arch Clin Exp Ophthalmol. 2018;256:1419–27. doi: 10.1007/s00417-018-4012-y. [DOI] [PubMed] [Google Scholar]
- 25.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, et al. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–23. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 26.Urbán Z, Riazi S, Seidl TL, Katahira J, Smoot LB, Chitayat D, et al. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am J Hum Genet. 2002;71:30–44. doi: 10.1086/341035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao Y, Li G, Korneva A, Caulk AW, Qin L, Bersi MR, et al. Deficient Circumferential Growth Is the Primary Determinant of Aortic Obstruction Attributable to Partial Elastin Deficiency. Arteriosclerosis Thrombosis Vasc Biol. 2017;37:930–41. doi: 10.1161/ATVBAHA.117.309079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee WH, Park J-H, Won Y, Lee M-W, Shin Y-I, Jo Y-J, et al. Retinal Microvascular Change in Hypertension as measured by Optical Coherence Tomography Angiography. Sci Rep. 2019;9:156. doi: 10.1038/s41598-018-36474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majeed A, Marwick B, Yu H, Fadavi H, Tavakoli M. Ophthalmic Biomarkers for Alzheimer’s Disease: A Review. Front Aging Neurosci. 2021;13:720167. doi: 10.3389/fnagi.2021.720167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wessel A, Motz R, Pankau R, Bürsch JH. Arterial hypertension and blood pressure profile in patients with Williams-Beuren syndrome. Z Kardiol. 1997;86:251–7. doi: 10.1007/s003920050056. [DOI] [PubMed] [Google Scholar]
- 31.Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, et al. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Investig. 2003;112:1419–28. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54:74–95. doi: 10.1016/j.survophthal.2008.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.