Abstract
Catalytic dynamic kinetic asymmetric transformation (DyKAT) provides a powerful tool to access chiral stereoisomers from racemic substrates. Such transformation has been widely employed on the construction of central chirality, however, the application in axial chirality remains underexplored because its equilibrium of substrate enantiomers is limited to five-membered metalacyclic intermediate. Here we report a tetracoordinate boron-directed dynamic kinetic asymmetric cross-coupling of racemic, configurationally stable 3-bromo-2,1-azaborines with boronic acid derivatives. A series of challenging C-B axially chiral compounds were prepared with generally good to excellent enantioselectivities. Moreover, this transformation can also be extended to prepare atropisomers bearing adjacent C-B and C-C diaxes with excellent diastereo- and enantio-control. The key to the success relies on the rational design of a reversible tetracoordinate boron intermediate, which is supported by theoretical calculations that dramatically reduces the rotational barrier of the original C-B axis and achieves the goal of DyKAT.
Subject terms: Synthetic chemistry methodology, Synthetic chemistry methodology, Reaction mechanisms
The application of catalytic dynamic kinetic asymmetric transformation (DyKAT) in axial chirality is limited to fivemembered metalacyclic intermediate-directed equilibrium of substrate enantiomers. Here, the authors report a tetracoordinate boron-directed DyKAT of racemic, configurationally stable 3-bromo- 2,1-azaborines for the construction of C-B axial chirality.
Introduction
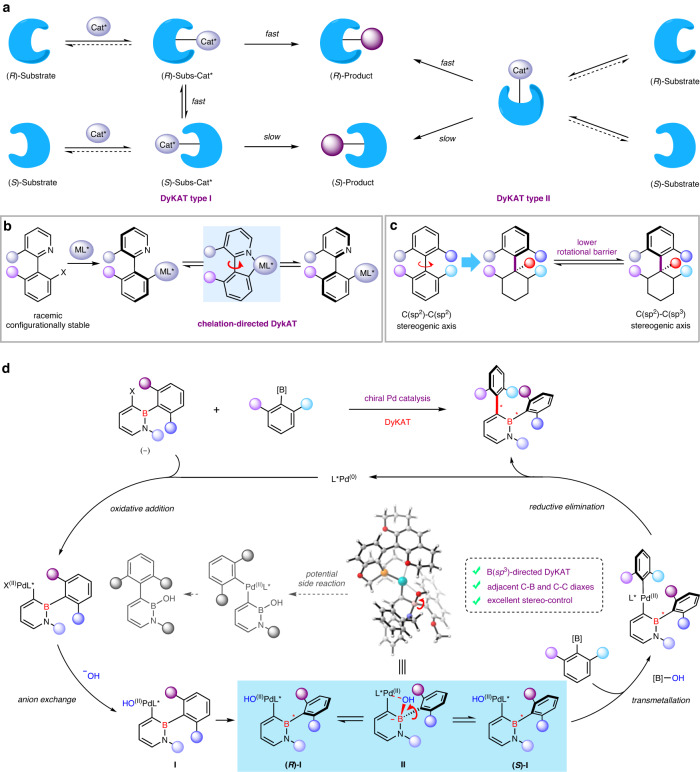
Catalytic dynamic kinetic asymmetric transformation (DyKAT) has emerged as a powerful platform for 100% theoretical conversion of racemic, configurationally stable substrates into high-value optically pure compounds1,2 like numerous pharmaceuticals and natural products3–6. Mechanistically, these reactions normally entail a chiral catalyst-mediated equilibration of substrate enantiomers, involving formation of diastereomeric substrate−catalyst intermediates with unstable configuration (DyKAT type I, Fig. 1a) or a common chiral intermediate that has lost the substrate’s chiral center (DyKAT type II, Fig. 1a). DyKAT strategy has been widely employed on central chirality2, in recent years, it has also demonstrated important applications on the construction of heterobiaryl atropisomers7, which are prevalent in natural products, medicines, ligands, catalysts and materials8–17. The transition-metal-catalyzed DyKAT of racemic, configurationally stable heterobiaryl substrates have been applied in the synthesis of axially chiral heterobiaryl compounds18–25 since the pioneering works by Lassaletta & Fernandez18 and Virgil & Stoltz19. The key of these elegant works all depends on the fast interconversion of diastereomeric substrate−catalyst intermediates promoted by five-membered metalacyclic intermediates18–25 (Fig. 1b). However, the monotonous reaction mode significantly restricts the wide application of DyKAT on axial chirality, and the challenge is to explore and find more modes to promote equilibrium of substrate enantiomers.
Fig. 1. Previous transition-metal-catalyzed atroposelective cross-coupling reactions and our reaction design.
a Dynamic kinetic asymmetric transformation (DyKAT). b Chelation-directed DyKAT of racemic heterobiaryls. c C(sp2)−C(sp3) axial chirality. d A tetracoordinate boron-directed DyKAT to access the challenging atropisomers bearing C-B stereogenic axis, or adjacent C-B and C-C diaxes (this work).
Compared with the common C(sp2)−C(sp2) atropisomers, the C(sp2)−C(sp3) atropisomers have a lower rotational barrier because the conical space of sp3 carbon is more conducive to rotation26–33 (Fig. 1c). It can be imagined that (1) if an atom of the stereogenic axis of the diastereomeric intermediates in DyKAT changes from sp2 to sp3 under the action of additional reagents, the group on the stereogenic axis may be easier to rotate; (2) if the conversion from sp2 to sp3 is reversible, it might be a new model for the interconversion of diastereomeric intermediates in DyKAT. As a result of our continuous interest in tetracoordinate boron chemistry34, we envisioned that the reversibility between B(sp2) and B(sp3)35–40 could support B(sp3)-directed DyKAT and fabricate optically pure C-B axially chiral molecules41,42 (Fig. 1d), which as elusive atropisomers and this type of chiral organoborons are underdeveloped and represent a big hurdle and challenge in boron chemistry as well as in axial chirality compared to their congeners with C-C or C-N axis, owing to the lower rotational barrier which is caused by longer C-B bond43–47. If successful, this reaction would develop an interesting diastereomeric intermediate equilibrium process that differs from previous chelation-directed DyKAT of racemic heterobiaryls18–25. Herein, we present a palladium-catalyzed dynamic kinetic asymmetric cross-coupling of racemic, configurationally stable 3-bromo-2,1-azaborines48–53 with boronic acid derivatives via an equilibrium mode of DyKAT mediated by tetracoordinate boron intermediates. By doing so, the DyKAT strategy could be employed to the assembly of challenging atropisomers with C-B axis or adjacent C-B and C-C diaxes.
On the basis of the previous reports of DyKAT2,7 and asymmetric Suzuki−Miyaura coupling reactions54–67, our proposed catalytic DyKAT version is shown in Fig. 1d. Oxidative addition of racemic 3-bromo-2,1-azaborines to chiral Pd(0) species and subsequent anion exchange afford diastereomeric intermediate I. The fast equilibration of intermediate (R)-I and (S)-I could occur through the tetracoordinate boron intermediate II formed by the transfer of the hydroxy group from Pd68. Then, the transmetalation between intermediate I and boronic acid derivatives and final reductive elimination generates C-B axial chirality and regenerates the chiral Pd(0) catalysis. It is important that one of the diastereomeric intermediate I ((R)-I or (S)-I) undergoes the transmetalation step faster than the other, so as to achieve the goal of DyKAT. Although mechanistically appealing, there are several considerable challenges: (1) the sterically hindered environment around B atom may inhibit the formation of tetracoordinate boron intermediates; (2) it is still uncertain whether the tetracoordinate boron intermediate could really reduce the rotation barrier and facilitate rotation of the aryl group on B atom around the C-B stereogenic axis; (3) competitive intramolecular self-coupling side reactions might occur69; (4) the simultaneous diastereoselective and enantioselective synthesis of axially chiral molecules with multiple axes by one-step reactions is still in its infancy70–74.
Results
To validate our hypothesis, we first designed and synthesized racemic 3-bromo-2,1-borazaronaphthalene 1a. Preliminary density functional theory (DFT) calculations were performed to evaluate the feasibilities of the racemization processes of three different species. As depicted in Fig. 2a, substrate 1a with a C–B axis has a rotation barrier of 31.8 kcal/mol to 1a’ since the congested steric environment of the planar geometry in transition states induces large distortions of aromatic rings. Compared with 1a, substrate 1a-C for the traditional asymmetric Suzuki-Miyaura coupling possesses not only a shorter C–C axis but also stronger aromaticity, which renders a much higher rotational barrier of 47.8 kcal/mol to 1a-C’, making direct dynamic kinetic asymmetric transformation from the substrate (DyKAT) even more unattainable (Fig. 2b). However, the Lewis acidic boronic complex 1a-Pd, the intermediate after oxidative addition of 1a to Pd followed by ligand exchange, allows the coordination of the hydroxide ligand to form a chiral tetracoordinate boron species. In TS-Pd, the tetracoordinate boron species own elongated C–B axis. The corresponding rotational barrier from 1a-Pd to 1a-Pd’ is significantly reduced to 16.7 kcal/mol (Fig. 2c), which makes the free rotation of the aryl group around C–B stereogenic axis feasible and fully supports our conjecture.
Fig. 2. Preliminary density functional theory (DFT) calculations on the racemization processes and condition optimization.
a Activation energy barrier for axial rotation of 3-bromo-2,1-borazaronaphthalene 1a. b Activation energy barrier for axial rotation of 3-bromo-naphthalene 1a-C. c Activation energy barrier for axial rotation of 3-Pd-2,1-borazaronaphthalene via a TS featuring tetracoordinate boron geometry, TS-Pd. d Condition optimization for palladium-catalyzed dynamic kinetic asymmetric cross-coupling.
Encouraged by the results from theoretical calculations, we then investigated this envisioned dynamic kinetic cross-coupling using racemic 3-bromo-2,1-borazaronaphthalene 1a and trifluoroborate 2a as model substrates (Fig. 2d). Delightfully, this reaction with Pd2(dba)3 as catalyst, the P-chiral monophosphorus ligand L1 as ligand and Cs2CO3 as base in toluene/H2O furnished the desired C-B axially chiral product (R)-3a in 81% NMR yield with 25% enantioselectivity excess (ee) at 40 °C (Fig. 2d, entry 1). This result proved the feasibility of our hypothesis and encouraged us to further evaluate other ligands. The P-chiral monophosphorus ligands with small steric hindrance led to higher ee values (Fig. 2d, entries 1–3). The substituents on the aryl units of the ligands have an effect on this reaction (Fig. 2d, entries 4-5), and a better result (Fig. 2d, entry 5, 92% yield and 76% ee) was obtained when ligand L5 with tetrahydrobenzofuran group was used. Subsequently, we investigated the effect of bases and found that these bases all promoted this reaction well, but the enantioselectivities of this reaction were sensitive to bases (Fig. 2d, entries 6–10). In general, weak bases were more favorable for enantioselectivities than strong bases. Overall, the optimized reaction conditions for this DyKAT are shown below: 1a (1 equiv), 2a (1.3 equiv), Pd2(dba)3 (2 mol%), L5 (6 mol%), NaHCO3 (2 equiv) in toluene/H2O at 40 °C for 34 h (Fig. 2d, entry 11). In addition, the same yield and enantioselectivity were obtained by reducing the proportion of water when 3-methoxyphenylboronic acid (2a’) was used as the substrate (Fig. 2d, entry 12).
To better understand the racemization process of the DyKAT, the following experiments were performed. As illustrated in Fig. 3a, the profile of the ee values or yields of the recovered 1a and the product 3a versus time indicated that two enantiomers of 1a were consumed together and one of the enantiomers was decreased more rapidly, suggesting a kinetic resolution (KR) process. In addition, the reactions of enantioenriched 1a (37% ee) with two ligands with different configurations were carried out, and the profile of the ee values of the recovered 1a versus time was shown in Fig. 3b. The results also supported a KR process. Finally, no obvious racemization of enantioenriched 3-bromo-2,1-azaborine 1a under standard conditions without aryl trifluoroborates, excluding a dynamic kinetic resolution (DKR) pathway. To demonstrate that the process is indeed a DyKAT, DFT calculations were performed to probe the mechanism of the racemization process. After oxidative addition and anion ligand exchange, benefiting from the boron Lewis acidity, IM0 first underwent an intramolecular hydroxide migration to form a tetracoordinate boron species IM1 via TS1. The C-B bond in IM1 is free to rotate with a small barrier of 5.2 kcal/mol. The analysis of the geometry of TS2 indicates that owing to the formation of tetracoordinate structure, the naphthalene moiety undergoing rotation is placed on the axial position to avoid repulsions with the benzylic group sprouted on the equatorial position. Meanwhile, the C–B bond is elongated by ~0.1 Å, which also provides more space to relax the strain in TS2. Interestingly, IM2 is more stable than its diastereomer IM1 due to the formation of an intramolecular hydrogen bond. The overall energy barrier for the racemization process is 13.8 kcal/mol, endorsing our strategy that the rotation around C–B axis could be realized even with very bulky ligands.
Fig. 3. Mechanistic studies.
a Yields and ee values of product 3a (recovered 1a) at different reaction times. b Ee values of recovered 1a at different reaction times by using ligands L5 and (-)-L5. c Racemization experiment under standard conditions without aryl trifluoroborates. d Free energy profile calculated for the racemization process with L5 as ligand.
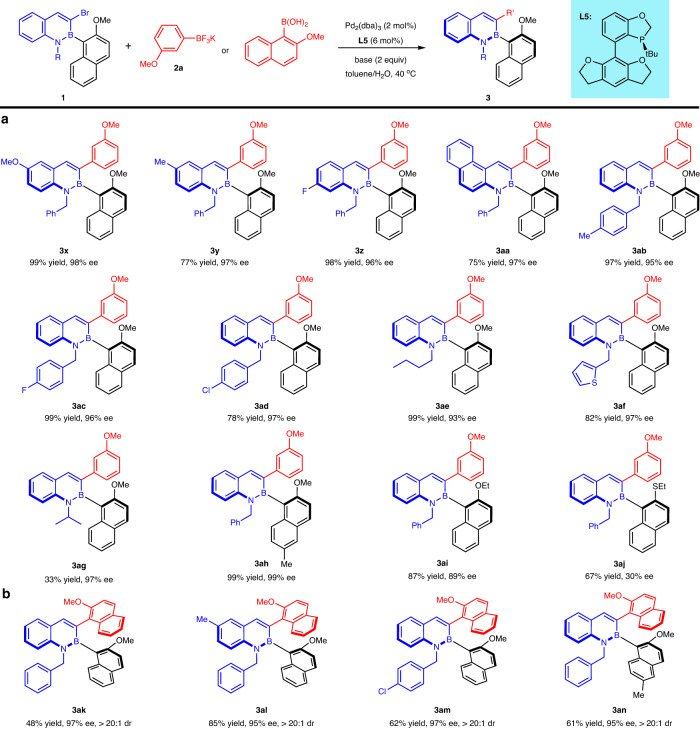
Applying the optimized reaction conditions to a range of substrates demonstrates the generality of this DyKAT (Fig. 4). This approach was compatible with aryl trifluoroborate bearing electron-rich groups, including alkoxy (3a, 3b, and 3e–3j), methylthio (3c), and N,N-diphenyl (3d), delivering the corresponding C-B axially chiral products in high yields with good to excellent enantioselectivities (80–96% ee). The absolute configuration of (Ra)-3a was determined by X-ray crystallographic analysis (CCDC 2245394, the CIF file is provided in Supplementary Data 1). Aryl trifluoroborate with an electron-withdrawing group was tolerated well under the standard conditions (3k, 70% yield and 93% ee). The tetrastyryl group could also be introduced into the desired product 3l by this method, which provides the possibility for a chiral AIE molecule. Polycyclic aryl trifluoroborates (3m and 3n) and unsubstituted phenyl trifluoroborate (3q) were successfully coupled with excellent enantioselectivities to desired products. Moreover, aryl trifluoroborates bearing heteroaromatic components, including carbazoles (3o and 3p), furan (3r), thiophene (3s), and benzothiophene (3t), could be smoothly converted into the target products with good to excellent enantioselectivities (82–96% ee). Alkenyl trifluoroborates underwent this reaction well, and the better enantioselectivities of 1-substituted alkenyl trifluoroborates (3v and 3w) than (E)-styryl trifluoroborate (3u) may be due to steric hindrance.
Fig. 4. Substrate scope for trifluoroborates.
Reaction conditions: 1 (0.1 mmol), 2 (0.13 mmol), Pd2(dba)3 (2 mol%), L5 (6 mol%), NaHCO3 (2 equiv) in toluene (1.5 mL)/H2O (0.3 mL) at 40 °C; isolated yields are provided.
Next, a wide range of racemic 3-bromo-2,1-borazaronaphthalenes could all undergo this DyKAT to render the corresponding enantiomerically enriched C-B axially chiral molecules (Fig. 5a). Methoxy (3x), methyl (3y and 3ah), and fluoro (3z)-substituted racemic 3-bromo-2,1-borazaronaphthalenes could successfully deliver the desired products in excellent efficiency (77–98% yields and 96–98% ee). Notably, BN-phenanthrene (3aa) was a viable framework for this transformation, providing the corresponding product with excellent enantioselectivity. Moreover, substituents on the N atom of the 2,1-borazaronaphthalene including benzyls (3ab-3ad), n-butyl (3ae), and thiophen-2-ylmethyl (3af) were readily tolerated well. Despite lower yield, the transformation also tolerated bulky (isopropyl) moiety on the N atom of the 2,1-borazaronaphthalene with excellent enantioselectivity (3ag, 33% yield and 97% ee). Low enantioselectivities were obtained when the OMe group was changed to the OEt (3ai) or SEt (3aj) groups with larger steric hindrance.
Fig. 5. Substrate scope for racemic 3-bromo-2,1-borazaronaphthalenes and diaxially chiral compounds.
a Scope for racemic 3-bromo-2,1-borazaronaphthalenes. Reaction conditions: 1 (0.1 mmol), 2a (0.13 mmol), Pd2(dba)3 (2 mol%), L5 (6 mol%), NaHCO3 (2 equiv) in toluene (1.5 mL)/H2O (0.3 mL) at 40 °C; isolated yields are provided. b Scope of adjacent C-B and C-C diaxially chiral compounds. Reaction conditions: 1 (0.1 mmol), 2-methoxy-1-naphthyl)boronic acid (1.3–4.0 equiv), Pd2(dba)3 (2 mol%), L5 (6 mol%), Li2CO3 (2.0–4.0 equiv) in toluene (1.5 mL)/H2O (0.15 mL) at 40 °C; isolated yields are provided.
In view of the successful application of the DyKAT strategy to prepare the C-B axially chiral compounds, we turned our attention to the synthesis of atropisomers with C-B adjacent diaxes of C-B and C-C bonds (Fig. 5b). (2-Methoxy-1-naphthyl)boronic acid was tested in the reaction, and to our delight, the desired axially chiral products 3ak-3an were obtained with excellent diastereoselectivities and enantioselectivities (>20:1 dr, 95–97% ee). The absolute configuration of 3ak was determined by ECD and two-dimensional NMR experiments (for details, see Supplementary Figs. 1–4 and 6–10)75–77.
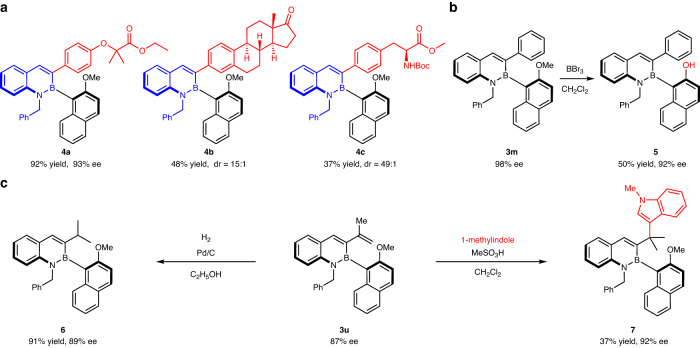
This transformation is also applicable to the synthesis of C-B axially chiral compounds bearing complex fragments derived from natural products or therapeutic agents, whose high functional-group compatibility is fully linchpinned. Aryl trifluoroborates derived from clofibrate (4a), estrone (4b), and tyrosine (4c) were transformed into the corresponding C-B axially chiral compounds with ease (Fig. 6a). In addition, C-B axially chiral compounds could be further modified. Firstly, demethylation of product 3m could generate a C-B axially chiral molecule 5 with free naphthol, which has the potential for further transformations (Fig. 6b). Meanwhile, product 3u could be converted to isopropyl-substituted C-B axially chiral molecule 6 via hydrogenation, and could also react with indole under acid catalysis to afford compound 7 with high retention of the enantiopurity (Fig. 6c).
Fig. 6. Functionalization of complex molecules and synthetic transformations.
a Functionalization of complex molecules. b Demethylation of 3m. c Palladium-catalyzed hydrogenation of 3u and Brønsted acid-catalyzed alkylation of indole with 3u as alkylation reagent. Boc = t-butoxycarbonyl.
In conclusion, we developed a palladium-catalyzed DyKAT process of racemic, configurationally stable 3-bromo-2,1-azaborines for the construction of C-B axial chirality. The experiments and calculations demonstrated that the reaction is a DyKAT process and that the reversible tetracoordinate boron intermediate is the key to its success. This chemistry offers practical access to chiral organoborons bearing C-B axis or adjacent C-B and C-C diaxes in generally high yields with excellent diastereoselevtivities and enantioselctivities.
Methods
General procedure for the synthesis of atropisomers with a single C-B stereogenic axis
In air, a 25 mL Schlenk tube was charged with 1 (0.1 mmol, 1 equiv), 2 (0.13 mmol, 1.3 equiv), Pd2(dba)3 (2 mol%), L5 (6 mol%), and NaHCO3 (0.2 mmol, 2.0 equiv). The tube was evacuated and filled with argon for three cycles. Then, 1.5 mL of toluene and 0.3 ml of water was added under argon. The reaction was allowed to stir at 40 °C for 34 h. Upon completion, a proper amount of silica gel was added to the reaction mixture. After the removal of the solvent, the crude reaction mixture was purified on silica gel (petroleum ether and ethyl acetate) to afford the desired products.
General procedure for the synthesis of atropisomers with adjacent diaxes of C-B bond and C-C bond
In air, a 25 mL Schlenk tube was charged with 1 (0.1 mmol, 1 equiv), 2-methoxy-1-naphthyl)boronic acid (1.3–4.0 equiv), Pd2(dba)3 (2 mol%), L5 (6 mol%), and Li2CO3 (2.0–4.0 equiv). The tube was evacuated and filled with argon for three cycles. Then, 1.5 mL of toluene and 0.15 ml of water was added under argon. The reaction was allowed to stir at 40 °C for 46–76 h. Upon completion, a proper amount of silica gel was added to the reaction mixture. After the removal of the solvent, the crude reaction mixture was purified on silica gel (petroleum ether and ethyl acetate) to afford the desired products.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
Financial support from National Natural Science Foundation of China (21931013, 22001038, 22271105, and 22271048), the Natural Science Foundation of Fujian Province (2022J02009 and 2022J05016), Fuzhou University (510578), Open Research Fund of School of Chemistry and Chemical Engineering (Henan Normal University), Guangdong Provincial Key Laboratory of Catalysis (2020B121201002), and Shenzhen Higher Education Institution Stable Support Plan (20200925152921001) is gratefully acknowledged. Computational work was supported by the Center for Computational Science and Engineering at the Southern University of Science and Technology and the CHEM high-performance supercomputer cluster (CHEM-HPC) located at the Department of Chemistry, Southern University of Science and Technology.
Source data
Author contributions
Q.S. conceived and directed the project. K.Y. and Y.M. performed experiments and prepared supplementary information. J.X., H.W., and Y.H. helped collect some new compounds and analyze the data. P.Y. and Z.Z. performed the DFT calculations and drafted the DFT parts. Q.S., P.Y., K.Y., and Z.Z. wrote the paper. All authors discussed the results and commented on the manuscript.
Peer review
Peer review information
Nature Communications thanks Laurent Chabaud, Donghui Wei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data that support the findings of this study are available within the article and its Supplementary Information files. All other data are available from the corresponding author upon request. Supplementary Tables 1 and 2 for mechanism experiment results, Supplementary Table 3 for rotational barrier of 3a, Supplementary Figs. 1–4 and 10 for additional computational results, Supplementary Fig. 5 for the plot of ln(ee0/eet) vs time of 3a, Supplementary Figs. 6–9 for two-dimensional NMR analysis of 3ai, Supplementary Figs. 11–237 for NMR spectra, Supplementary Figs. 238–283 for HPLC spectra. The X-ray crystallographic coordinates for the structure reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 2245394 (3a). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The cartesian coordinates of the optimized structures are provided in a source data file. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kai Yang, Yanfei Mao, Zhihan Zhang.
Contributor Information
Peiyuan Yu, Email: yupy@sustech.edu.cn.
Qiuling Song, Email: qsong@fzu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-40164-6.
References
- 1.Bhat V, Welin ER, Guo X, Stoltz BM. Advances in stereoconvergent catalysis from 2005 to 2015: Transition-metal-mediated stereoablative reactions, dynamic kinetic resolutions, and dynamic kinetic asymmetric transformations. Chem. Rev. 2017;117:4528–4561. doi: 10.1021/acs.chemrev.6b00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinreiber J, Faber K, Griengl H. De-racemization of enantiomers versus de-epimerization of diastereomers—classification of dynamic kinetic asymmetric transformations (DYKAT) Chem. Eur. J. 2008;14:8060–8072. doi: 10.1002/chem.200701643. [DOI] [PubMed] [Google Scholar]
- 3.Leijondahl K, Borén L, Braun R, Bäckvall J-E. Enzyme- and ruthenium-catalyzed dynamic kinetic asymmetric transformation of 1,5-diols. Application to the synthesis of (+)-solenopsin A. J. Org. Chem. 2009;74:1988–1993. doi: 10.1021/jo8025109. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz A, et al. Scalable synthesis of the potent HIV inhibitor BMS-986001 by non-enzymatic dynamic kinetic asymmetric transformation (DYKAT) Angew. Chem. Int. Ed. 2015;54:7185–7188. doi: 10.1002/anie.201502290. [DOI] [PubMed] [Google Scholar]
- 5.Trost BM, Bunt RC, Lemoine RC, Calkins TL. Dynamic kinetic asymmetric transformation of diene monoepoxides: a practical asymmetric synthesis of vinylglycinol, vigabatrin, and ethambutol. J. Am. Chem. Soc. 2000;122:5968–5976. doi: 10.1021/ja000547d. [DOI] [Google Scholar]
- 6.Trost BM, Toste FD. Palladium catalyzed kinetic and dynamic kinetic asymmetric transformations of γ-acyloxybutenolides. Enantioselective total synthesis of (+)-aflatoxin B1 and B2a. J. Am. Chem. Soc. 2003;125:3090–3100. doi: 10.1021/ja020988s. [DOI] [PubMed] [Google Scholar]
- 7.Carmona JA, Rodríguez-Franco C, Fernández R, Hornillos V, Lassaletta JM. Atroposelective transformation of axially chiral (hetero)biaryls. From desymmetrization to modern resolution strategies. Chem. Soc. Rev. 2021;50:2968–2983. doi: 10.1039/D0CS00870B. [DOI] [PubMed] [Google Scholar]
- 8.Bao X, Rodriguez J, Bonne D. Enantioselective synthesis of atropisomers with multiple stereogenic axes. Angew. Chem. Int. Ed. 2020;59:12623–12634. doi: 10.1002/anie.202002518. [DOI] [PubMed] [Google Scholar]
- 9.Cheng JK, Xiang S-H, Li S, Ye L, Tan B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 2021;121:4805–4902. doi: 10.1021/acs.chemrev.0c01306. [DOI] [PubMed] [Google Scholar]
- 10.Cheng JK, Xiang S-H, Tan B. Organocatalytic enantioselective synthesis of axially chiral molecules: development of strategies and skeletons. Acc. Chem. Res. 2022;55:2920–2937. doi: 10.1021/acs.accounts.2c00509. [DOI] [PubMed] [Google Scholar]
- 11.Liu C-X, Zhang W-W, Yin S-Y, Gu Q, You S-L. Synthesis of atropisomers by transition-metal-catalyzed asymmetric C–H functionalization reactions. J. Am. Chem. Soc. 2021;143:14025–14040. doi: 10.1021/jacs.1c07635. [DOI] [PubMed] [Google Scholar]
- 12.Mei G-J, Koay WL, Guan C-Y, Lu Y. Atropisomers beyond the C–C axial chirality: advances in catalytic asymmetric synthesis. Chem. 2022;8:1855–1893. doi: 10.1016/j.chempr.2022.04.011. [DOI] [Google Scholar]
- 13.Zhang D, Wang Q. Palladium catalyzed asymmetric Suzuki–Miyaura coupling reactions to axially chiral biaryl compounds: chiral ligands and recent advances. Coord. Chem. Rev. 2015;286:1–16. doi: 10.1016/j.ccr.2014.11.011. [DOI] [Google Scholar]
- 14.Rokade BV, Guiry PJ. Axially chiral P,N-Ligands: some recent twists and turns. ACS Catal. 2018;8:624–643. doi: 10.1021/acscatal.7b03759. [DOI] [Google Scholar]
- 15.Pu L. Enantioselective fluorescent sensors: a tale of BINOL. Acc. Chem. Res. 2012;45:150–163. doi: 10.1021/ar200048d. [DOI] [PubMed] [Google Scholar]
- 16.Toenjes ST, Gustafson JL. Atropisomerism in medicinal chemistry: challenges and opportunities. Future Med. Chem. 2018;10:409–422. doi: 10.4155/fmc-2017-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bringmann G, Gulder T, Gulder TAM, Breuning M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 2011;111:563–639. doi: 10.1021/cr100155e. [DOI] [PubMed] [Google Scholar]
- 18.Ros A, et al. Dynamic kinetic cross-coupling strategy for the asymmetric synthesis of axially chiral heterobiaryls. J. Am. Chem. Soc. 2013;135:15730–15733. doi: 10.1021/ja4087819. [DOI] [PubMed] [Google Scholar]
- 19.Bhat V, Wang S, Stoltz BM, Virgil SC. Asymmetric synthesis of QUINAP via dynamic kinetic resolution. J. Am. Chem. Soc. 2013;135:16829–16832. doi: 10.1021/ja409383f. [DOI] [PubMed] [Google Scholar]
- 20.Hornillos V, et al. Synthesis of axially chiral heterobiaryl alkynes via dynamic kinetic asymmetric alkynylation. Chem. Commun. 2016;52:14121–14124. doi: 10.1039/C6CC08997F. [DOI] [PubMed] [Google Scholar]
- 21.Ramírez-López P, et al. A dynamic kinetic C–P cross–coupling for the asymmetric synthesis of axially chiral P,N ligands. ACS Catal. 2016;6:3955–3964. doi: 10.1021/acscatal.6b00784. [DOI] [Google Scholar]
- 22.Ramírez-López P, et al. Synthesis of IAN-type N,N-ligands via dynamic kinetic asymmetric Buchwald–Hartwig amination. J. Am. Chem. Soc. 2016;138:12053–12056. doi: 10.1021/jacs.6b07972. [DOI] [PubMed] [Google Scholar]
- 23.Carmona JA, et al. Dynamic kinetic asymmetric Heck reaction for the simultaneous generation of central and axial chirality. J. Am. Chem. Soc. 2018;140:11067–11075. doi: 10.1021/jacs.8b05819. [DOI] [PubMed] [Google Scholar]
- 24.Han S-J, Bhat V, Stoltz BM, Virgil SC. Atroposelective synthesis of PINAP via dynamic kinetic asymmetric transformation. Adv. Synth. Catal. 2019;361:441–444. doi: 10.1002/adsc.201801248. [DOI] [Google Scholar]
- 25.Jiang X, et al. Construction of axial chirality via asymmetric radical trapping by cobalt under visible light. Nat. Catal. 2022;5:788–797. doi: 10.1038/s41929-022-00831-1. [DOI] [Google Scholar]
- 26.Bertuzzi G, et al. Organocatalytic enantioselective construction of conformationally stable C(sp2)–C(sp3) atropisomers. J. Am. Chem. Soc. 2022;144:1056–1065. doi: 10.1021/jacs.1c12619. [DOI] [PubMed] [Google Scholar]
- 27.Ford WT, Thompson TB, Snoble KAJ, Timko JM. Hindered rotation in 9-arylfluorenes. Resolutions of the mechanistic question. J. Am. Chem. Soc. 1975;97:95–101. doi: 10.1021/ja00834a017. [DOI] [Google Scholar]
- 28.Wu X, et al. Catalyst control over sixfold stereogenicity. Nat. Catal. 2021;4:457–462. doi: 10.1038/s41929-021-00615-z. [DOI] [Google Scholar]
- 29.Di Iorio N, Filippini G, Mazzanti A, Righi P, Bencivenni G. Controlling the C(sp3)–C(sp2) axial conformation in the enantioselective Friedel–Crafts-type alkylation of β-naphthols with inden-1-ones. Org. Lett. 2017;19:6692–6695. doi: 10.1021/acs.orglett.7b03415. [DOI] [PubMed] [Google Scholar]
- 30.He X-L, et al. Asymmetric Barton–Zard reaction to access 3-pyrrole-containing axially chiral skeletons. ACS Catal. 2019;9:4374–4381. doi: 10.1021/acscatal.9b00767. [DOI] [Google Scholar]
- 31.Nguyen TT. Traceless point-to-axial chirality exchange in the atropselective synthesis of biaryls/heterobiaryls. Org. Biomol. Chem. 2019;17:6952–6963. doi: 10.1039/C9OB01304K. [DOI] [PubMed] [Google Scholar]
- 32.Quinonero O, et al. Combining organocatalysis with central-to-axial chirality conversion: atroposelective hantzsch-type synthesis of 4-arylpyridines. Angew. Chem. Int. Ed. 2016;55:1401–1405. doi: 10.1002/anie.201509967. [DOI] [PubMed] [Google Scholar]
- 33.Raut VS, et al. Enantioselective syntheses of furan atropisomers by an oxidative central-to-axial chirality conversion strategy. J. Am. Chem. Soc. 2017;139:2140–2143. doi: 10.1021/jacs.6b11079. [DOI] [PubMed] [Google Scholar]
- 34.Yang K, Song Q. Tetracoordinate boron intermediates enable unconventional transformations. Acc. Chem. Res. 2021;54:2298–2312. doi: 10.1021/acs.accounts.1c00132. [DOI] [PubMed] [Google Scholar]
- 35.Frath D, Massue J, Ulrich G, Ziessel R. Luminescent materials: locking π-conjugated and heterocyclic ligands with boron(III) Angew. Chem. Int. Ed. 2014;53:2290–2310. doi: 10.1002/anie.201305554. [DOI] [PubMed] [Google Scholar]
- 36.Li D, Zhang H, Wang Y. Four-coordinate organoboron compounds for organic light-emitting diodes (OLEDs) Chem. Soc. Rev. 2013;42:8416–8433. doi: 10.1039/c3cs60170f. [DOI] [PubMed] [Google Scholar]
- 37.Mellerup SK, Wang S. Boron-doped molecules for optoelectronics. Trends Chem. 2019;1:77–89. doi: 10.1016/j.trechm.2019.01.003. [DOI] [Google Scholar]
- 38.Matteson DS, Ray R. Directed chiral synthesis with pinanediol boronic esters. J. Am. Chem. Soc. 1980;102:7590–7591. doi: 10.1021/ja00545a046. [DOI] [Google Scholar]
- 39.Miyaura, N. & Suzuki, A. Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. J. Chem. Soc., Chem. Commun. 866–867 10.1039/C39790000866 (1979).
- 40.Petasis NA, Akritopoulou I. The boronic acid mannich reaction: a new method for the synthesis of geometrically pure allylamines. Tetrahedron Lett. 1993;34:583–586. doi: 10.1016/S0040-4039(00)61625-8. [DOI] [Google Scholar]
- 41.Yang K, et al. Construction of axially chiral arylborons via atroposelective miyaura borylation. J. Am. Chem. Soc. 2021;143:10048–10053. doi: 10.1021/jacs.1c04345. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, et al. Chiral phosphoric acid-catalyzed remote control of axial chirality at boron–carbon bond. J. Am. Chem. Soc. 2021;143:12924–12929. doi: 10.1021/jacs.1c05079. [DOI] [PubMed] [Google Scholar]
- 43.Birepinte M, Robert F, Pinet S, Chabaud L, Pucheault M. Non-biaryl atropisomerism at the C–B bond in sterically hindered aminoarylboranes. Org. Biomol. Chem. 2020;18:3007–3011. doi: 10.1039/D0OB00421A. [DOI] [PubMed] [Google Scholar]
- 44.Mancinelli M, Bencivenni G, Pecorari D, Mazzanti A. Stereochemistry and recent applications of axially chiral organic molecules. Eur. J. Org. Chem. 2020;2020:4070–4086. doi: 10.1002/ejoc.201901918. [DOI] [Google Scholar]
- 45.Mazzanti A, Boffa M, Marotta E, Mancinelli M. Axial chirality at the boron–carbon bond: synthesis, stereodynamic analysis, and atropisomeric resolution of 6-aryl-5,6-dihydrodibenzo[c,e][1,2]azaborinines. J. Org. Chem. 2019;84:12253–12258. doi: 10.1021/acs.joc.9b01550. [DOI] [PubMed] [Google Scholar]
- 46.Mazzanti A, Mercanti E, Mancinelli M. Axial chirality about boron–carbon bond: atropisomeric azaborines. Org. Lett. 2016;18:2692–2695. doi: 10.1021/acs.orglett.6b01159. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X-L, et al. Stepwise asymmetric allylic substitution-isomerization enabled mimetic synthesis of axially chiral B,N-heterocycles. Angew. Chem. Int. Ed. 2022;61:e202210456. doi: 10.1002/anie.202210456. [DOI] [PubMed] [Google Scholar]
- 48.Bélanger-Chabot G, Braunschweig H, Roy DK. Recent developments in azaborinine chemistry. Eur. J. Inorg. Chem. 2017;2017:4353–4368. doi: 10.1002/ejic.201700562. [DOI] [Google Scholar]
- 49.Bhattacharjee A, Davies GHM, Saeednia B, Wisniewski SR, Molander GA. Selectivity in the elaboration of bicyclic borazarenes. Adv. Synth. Catal. 2021;363:2256–2273. doi: 10.1002/adsc.202001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell PG, Marwitz AJV, Liu S-Y. Recent advances in azaborine chemistry. Angew. Chem. Int. Ed. 2012;51:6074–6092. doi: 10.1002/anie.201200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giustra ZX, Liu S-Y. The state of the art in azaborine chemistry: new synthetic methods and applications. J. Am. Chem. Soc. 2018;140:1184–1194. doi: 10.1021/jacs.7b09446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McConnell CR, Liu S-Y. Late-stage functionalization of BN-heterocycles. Chem. Soc. Rev. 2019;48:3436–3453. doi: 10.1039/C9CS00218A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhuang F-D, Wang J-Y, Pei J. Recent progress and challenges in the synthesis of boron-nitrogen fused polycyclic aromatic hydrocarbons. Sci. Sin. Chim. 2020;50:1205. doi: 10.1360/SSC-2020-0113. [DOI] [Google Scholar]
- 54.Yin J, Buchwald SL. A catalytic asymmetric suzuki coupling for the synthesis of axially chiral biaryl compounds. J. Am. Chem. Soc. 2000;122:12051–12052. doi: 10.1021/ja005622z. [DOI] [Google Scholar]
- 55.Cammidge, A. N. & Crépy, K. V. L. The first asymmetric Suzuki cross-coupling reaction. Chem. Commun. 1723–1724 10.1039/B004513F (2000).
- 56.Cammidge AN, Crépy KVL. Synthesis of chiral binaphthalenes using the asymmetric Suzuki reaction. Tetrahedron. 2004;60:4377–4386. doi: 10.1016/j.tet.2003.11.095. [DOI] [Google Scholar]
- 57.Bermejo A, Ros A, Fernández R, Lassaletta JM. C2-symmetric bis-hydrazones as ligands in the asymmetric suzuki−miyaura cross-coupling. J. Am. Chem. Soc. 2008;130:15798–15799. doi: 10.1021/ja8074693. [DOI] [PubMed] [Google Scholar]
- 58.Ding L, Sui X, Gu Z. Enantioselective synthesis of biaryl atropisomers via Pd/norbornene-catalyzed three-component cross-couplings. ACS Catal. 2018;8:5630–5635. doi: 10.1021/acscatal.8b01037. [DOI] [Google Scholar]
- 59.Ji W, Wu H-H, Zhang J. Axially chiral biaryl monophosphine oxides enabled by palladium/wj-phos-catalyzed asymmetric suzuki–miyaura cross-coupling. ACS Catal. 2020;10:1548–1554. doi: 10.1021/acscatal.9b04354. [DOI] [Google Scholar]
- 60.Patel ND, et al. Computationally assisted mechanistic investigation and development of pd-catalyzed asymmetric suzuki–miyaura and negishi cross-coupling reactions for tetra-ortho-substituted biaryl synthesis. ACS Catal. 2018;8:10190–10209. doi: 10.1021/acscatal.8b02509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qiu S-Q, et al. Asymmetric construction of an aryl-alkene axis by palladium-catalyzed suzuki–miyaura coupling reaction. Angew. Chem. Int. Ed. 2022;61:e202211211. doi: 10.1002/anie.202211211. [DOI] [PubMed] [Google Scholar]
- 62.Shen D, Xu Y, Shi S-L. A bulky chiral n-heterocyclic carbene palladium catalyst enables highly enantioselective suzuki–miyaura cross-coupling reactions for the synthesis of biaryl atropisomers. J. Am. Chem. Soc. 2019;141:14938–14945. doi: 10.1021/jacs.9b08578. [DOI] [PubMed] [Google Scholar]
- 63.Shen X, Jones GO, Watson DA, Bhayana B, Buchwald SL. Enantioselective synthesis of axially chiral biaryls by the pd-catalyzed suzuki−miyaura reaction: substrate scope and quantum mechanical investigations. J. Am. Chem. Soc. 2010;132:11278–11287. doi: 10.1021/ja104297g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uozumi Y, Matsuura Y, Arakawa T, Yamada YMA. Asymmetric suzuki–miyaura coupling in water with a chiral palladium catalyst supported on an amphiphilic resin. Angew. Chem. Int. Ed. 2009;48:2708–2710. doi: 10.1002/anie.200900469. [DOI] [PubMed] [Google Scholar]
- 65.Xu G, Fu W, Liu G, Senanayake CH, Tang W. Efficient syntheses of korupensamines A, B and michellamine B by asymmetric suzuki-miyaura coupling reactions. J. Am. Chem. Soc. 2014;136:570–573. doi: 10.1021/ja409669r. [DOI] [PubMed] [Google Scholar]
- 66.Yang H, Sun J, Gu W, Tang W. Enantioselective cross-coupling for axially chiral tetra-ortho-substituted biaryls and asymmetric synthesis of gossypol. J. Am. Chem. Soc. 2020;142:8036–8043. doi: 10.1021/jacs.0c02686. [DOI] [PubMed] [Google Scholar]
- 67.Yang H, Tang W. Enantioselective construction of ortho-sulfur- or nitrogen-substituted axially chiral biaryls and asymmetric synthesis of isoplagiochin D. Nat. Commun. 2022;13:4577. doi: 10.1038/s41467-022-32360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Emslie DJH, Harrington LE, Jenkins HA, Robertson CM, Britten JF. Group 10 Transition-metal complexes of an ambiphilic psb-ligand: investigations into η3(bcc)-triarylborane coordination. Organometallics. 2008;27:5317–5325. doi: 10.1021/om800670e. [DOI] [Google Scholar]
- 69.Molander GA, Wisniewski SR. Accessing 2,1-borazaronaphthols: self-arylation of 1-alkyl-2-aryl-3-bromo-2,1-borazaronaphthalenes. J. Org. Chem. 2014;79:8339–8347. doi: 10.1021/jo501638q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bao X, Rodriguez J, Bonne D. Enantioselective synthesis of atropisomers with multiple stereogenic axes. Angew. Chem. Int. Ed. 2020;59:12623–12634. doi: 10.1002/anie.202002518. [DOI] [PubMed] [Google Scholar]
- 71.Beleh OM, Miller E, Toste FD, Miller SJ. Catalytic dynamic kinetic resolutions in tandem to construct two-axis terphenyl atropisomers. J. Am. Chem. Soc. 2020;142:16461–16470. doi: 10.1021/jacs.0c08057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao Q, et al. Catalytic synthesis of atropisomeric o-terphenyls with 1,2-diaxes via axial-to-axial diastereoinduction. J. Am. Chem. Soc. 2021;143:7253–7260. doi: 10.1021/jacs.1c02405. [DOI] [PubMed] [Google Scholar]
- 73.Huang S, et al. Organocatalytic enantioselective construction of chiral azepine skeleton bearing multiple-stereogenic elements. Angew. Chem. Int. Ed. 2021;60:21486–21493. doi: 10.1002/anie.202108040. [DOI] [PubMed] [Google Scholar]
- 74.Teng F, et al. Palladium-catalyzed atroposelective coupling–cyclization of 2-isocyanobenzamides to construct axially chiral 2-aryl- and 2,3-diarylquinazolinones. J. Am. Chem. Soc. 2021;143:2722–2728. doi: 10.1021/jacs.1c00640. [DOI] [PubMed] [Google Scholar]
- 75.Ambrogi M, Ciogli A, Mancinelli M, Ranieri S, Mazzanti A. Atropisomers of arylmaleimides: stereodynamics and absolute configuration. J. Org. Chem. 2013;78:3709–3719. doi: 10.1021/jo400200v. [DOI] [PubMed] [Google Scholar]
- 76.Mancinelli M, Perticarari S, Prati L, Mazzanti A. Conformational analysis and absolute configuration of axially chiral 1-aryl and 1,3-bisaryl-xanthines. J. Org. Chem. 2017;82:6874–6885. doi: 10.1021/acs.joc.7b01010. [DOI] [PubMed] [Google Scholar]
- 77.Pagano A, et al. Stereodynamic analysis of new atropisomeric 4,7-di(naphthalen-1-yl)-5,6-dinitro-1H-indoles. Synlett. 2018;29:2161–2166. doi: 10.1055/s-0037-1609908. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data that support the findings of this study are available within the article and its Supplementary Information files. All other data are available from the corresponding author upon request. Supplementary Tables 1 and 2 for mechanism experiment results, Supplementary Table 3 for rotational barrier of 3a, Supplementary Figs. 1–4 and 10 for additional computational results, Supplementary Fig. 5 for the plot of ln(ee0/eet) vs time of 3a, Supplementary Figs. 6–9 for two-dimensional NMR analysis of 3ai, Supplementary Figs. 11–237 for NMR spectra, Supplementary Figs. 238–283 for HPLC spectra. The X-ray crystallographic coordinates for the structure reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 2245394 (3a). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The cartesian coordinates of the optimized structures are provided in a source data file. Source data are provided with this paper.