Abstract
Introduction:
Physical activity (PA) is prospectively inversely associated with dementia risk, but few studies examined accelerometer measures of PA and sitting with rigorously-adjudicated MCI and dementia risk.
Methods:
We examined the associations of accelerometer measures (PA and sitting) with incident MCI/probable dementia in the Women’s Health Initiative (n=1,334; mean age=82±6 years)
Results:
Over a median follow-up of 4.2 years, 267 MCI/probable dementia cases were identified. Adjusted Cox regression HRs (95% CI) across moderate-to-vigorous PA (MVPA) minutes/day quartiles were 1.00 (reference), 1.28 (0.90–1.81), 0.79 (0.53–1.17), and 0.69 (0.45–1.06); P-trend=0.01. Adjusted HRs (95% CI) across steps/day quartiles were 1.00 (reference), 0.73 (0.51–1.03), 0.64 (0.43–0.94), and 0.35 (0.22–0.58); P-trend<0.001. The HR (95% CI) for each 1-SD increment in MVPA (31 minutes/day) and steps/day (1,865) were 0.79 (0.67–0.94) and 0.67 (0.54–0.82), respectively. Sitting was not associated with MCI/probable dementia.
Discussion:
Findings suggest ≥moderate intensity PA, particularly stepping, associates with lower MCI and dementia risk.
Keywords: Physical activity, sedentary behavior, accelerometer-measured physical activity, accelerometer-measured sedentary behavior, sitting, aging, epidemiology, public health, mild cognitive impairment, dementia
INTRODUCTION
The number of Americans with dementia is estimated to approximately double from ~5.3 million in 2019 to 10.5 million in 2050. [1] Data from the Framingham Heart Study suggests that the lifetime risk of dementia among older women is 24.6%, compared to 15.5% for older men. [2,3] Given that dementia neuropathology begins 20 or more years before symptom onset, early invention for delaying or preventing cognitive decline and dementia for older adults is essential. [3,4]
The National Academy of Sciences, Engineering, and Medicine identified physical activity (PA) as one of three promising intervention targets for cognitive decline and dementia including Alzheimer’s disease (AD) and related dementias (ADRD). [5] Sedentary behavior (SB), defined as waking behavior involving sitting or reclining with low energy expenditure (<1.5 metabolic equivalents), is associated with cardiovascular disease (CVD) and mortality independent of PA and therefore could be an additional target for delaying or preventing cognitive decline and dementia. [6–9] However, much of the currently published literature on the associations of PA and SB with cognitive decline and dementia is based on self-reported measures. [10,11] Accelerometry more accurately and more completely captures ambulatory movement, particularly light intensity PA (LPA) and SB patterns among older adults. [12] In the UK Biobank population-based cohort study, higher device-measures of moderate-to-vigorous (MV) PA (MVPA) and LPA were inversely prospectively associated with lower risk of incident ICD-classified dementia risk. [13] Those with ≥ ~6,000 metabolic equivalent (MET) minutes/week of LPA had a 40% lower risk of dementia compared to those with ~4,424 MET minutes/week of LPA. [13] These findings warrant replication in cohorts followed carefully for incident mild cognitive impairment (MCI) and dementia among women for whom dementia burden is high. Additionally, few studies have examined the associations of alternative PA metrics (e.g., steps/day) with MCI and dementia.
In the present study among older ambulatory community-living women, we hypothesized that: (1) higher amounts of accelerometer-measured PA and lower amounts of accelerometer-measured sitting would be associated with lower risk of MCI and probable dementia; (2) the shapes of these associations would be linear, and (3) these associations would be consistent in magnitude across age, BMI, physical functioning, APOE ε4 carrier status (0 or ≥1 ε4 allele), and CVD risk factor profile.
METHODS
Study Population
The Women’s Health Initiative (WHI) is a prospective study of morbidity and mortality among 161,808 US postmenopausal women aged 50–79 years enrolled in the WHI Clinical Trial Studies or Observational Study from 1993–1998 across 40 sites. [14] The WHI Memory Study (WHIMS), ancillary to the WHI Hormone Trial among 27,347 women, was designed to investigate the effect of estrogen therapy on incident dementia risk starting in June 1995. Details about WHIMS design and data collection procedures are published. [15,16] The Objective Physical Activity and Cardiovascular Health (OPACH) Study, also ancillary to WHI, collected accelerometry data from 6,489 ambulatory community-living women aged 63 years and older at baseline in May 2012-April 2014. Details about OPACH design and data collection procedures are published. [17]
The study sample consisted of 1,346 women enrolled in both WHIMS and OPACH with adherent accelerometer wear (≥1 day with ≥10 hours of wear). [18] At OPACH baseline, 59 women had WHIMS-ascertained MCI, 12 had probable dementia, and 69 had MCI or probable dementia. The final analytic samples consisted of 1,287 women free of MCI, 1,334 women free of probable dementia, and 1,277 women free of MCI or probable dementia. Of these women, 96.9% had ≥4 adherent accelerometer wear days.
Consent statement
The Fred Hutchinson Cancer Research Center approved the present study protocols and all women provided informed consent in writing or by phone.
MCI and probable dementia ascertainment
The outcomes examined were incident MCI, probable dementia, and combined MCI/probable dementia from May 2012 through May 2020. We additionally examined combined MCI/probable dementia as an endpoint as both are stages on the continuum of cognitive decline. Prior to the start of OPACH, WHIMS administered an annual multi-stage clinical evaluation of participants’ cognitive functioning followed by independent review and adjudication by a panel of experienced clinicians to identify MCI and probable dementia cases. [15] Beginning in 2008, annual cognitive assessments were conducted by telephone using a validated cognitive battery. [19] For women who scored below age and education adjusted cutpoints on the Telephone Interview for Cognitive Status-modified, a telephone interview with a pre-identified proxy respondent was conducted using the Dementia Questionnaire. [20] All participant data were reviewed by a centralized adjudication panel of experts who assigned participants to outcome classifications of no impairment, MCI based on Petersen’s criteria, or probable dementia based on the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria. [21,22]
Accelerometer measures of PA and SB
In OPACH, the ActiGraph GT3X+ was worn over the right hip secured with a belt for 24 hours/day, except when bathing or swimming, for up to 7 consecutive days. The 30 Hz data were aggregated into 15-second epochs using ActiLife version 6 software and periods of accelerometer non-wear were identified for removal using the Choi algorithm. [23] Sleep time was removed using participants’ self-reported in-bed and out-of-bed times from sleep diaries concurrent with accelerometer wear.
We applied OPACH Calibration Study vector magnitude-based cutpoints to define LPA (19–518 counts/15 seconds) and MVPA (≥519 counts/15 seconds). [24] We used the normal frequency (i.e., default) filter to process GT3X+ data to classify steps. Steps/day were determined by calculating steps for each 15-second epoch using ActiLife’s proprietary algorithm and dividing the total number of steps by the number of adherent wear days. [17,25] A systematic review suggested that the GT3X+ has good criterion validity. [26] Steps/day are a straightforward metric of movement that can be clearly translated into public health recommendations. [27] Light intensity steps/day were those taken during 15-second epochs assigned to LPA while MV steps were those taken during 15-second epochs assigned to MVPA.
SB, measured as sitting time (ST; minutes/day) and mean sitting bout duration (MSBD; minutes), were classified using the Convolutional Neural Network Hip Accelerometer Pattern (CHAP) algorithm. [28] Briefly, the CHAP algorithm was developed on 709 older adults in the Adult Changes in Thought study who concurrently wore a GT3X+ secured over the hip with an elastic belt and an activPAL micro3 inclinometer on the thigh which served as the criterion sitting measure. [28] A machine-learning model architecture extracted GT3X+ features for identifying sitting, refined these features by considering neighboring time-points and likely sequence of events, and converted the extracted features to a final classification of sitting or non-sitting. [28] The CHAP algorithm has high agreement with the activPAL micro3 for classifying minute-level sitting (sensitivity=97.1%, specificity=88.6%, balanced accuracy=92.9%) and sit-to-stand transitions (sensitivity=83.2%, positive predictive value=82.9%), outperforming the commonly used ActiGraph cutpoint of <100 counts/minute on the vertical axis for SB. [28]
Covariates
All covariates were measured at OPACH baseline. Questionnaires ascertained age, self-identified race and/or ethnicity (Black, Hispanic/Latina, or White), education (≤high school equivalent, some college, or college graduate), alcohol consumption in the past 3 months (non-drinker, <1 drink/week, ≥1 drink/week, or unknown), current smoking status, and history of vision or hearing impairment. Physical functioning was measured using the RAND-36 questionnaire, which ranged from 0–100 with higher scores indicating higher physical functioning. [29] History of diabetes and hypertension were determined by self-report of physician diagnosis with medication use reported by the participant at OPACH baseline. [14] Trained study staff measured weight with a bathroom scale, height with a tape measure, and collected fasting (12 hour) blood samples. BMI was calculated as kg/m2. Systolic blood pressure (SBP) was recorded as the average of 2 measures using an aneroid sphygmomanometer. CRP and total and HDL cholesterol were measured using standardized Clinical Laboratory Act-approved methods. [30] The Reynolds Risk Score (RRS), a summary measure of CVD risk, was calculated using age, smoking, diabetes, SBP, CRP, total and HDL cholesterol, and family history of myocardial infarction. [31] Apolipoprotein E (APOE) ε4 carrier status (0 or ≥1 ε4 allele) was based on two single nucleotide polymorphisms, rs429358 and rs7412, which were imputed and harmonized across WHI genome-wide association studies using the 1000 Genomes Project reference panel and MaCH algorithms. [32]
Statistical analysis
All statistical analyses used R 4.1.3 in RStudio 1.3.1093 (https://rstudio.com/). Means and standard deviations (SD) or counts and proportions were calculated and compared across quartiles of steps/day using F-tests for continuous variables or chi-square tests for categorical variables. Pearson correlations between accelerometer measures were examined.
Adjusted hazard ratios (HR) and corresponding 95% confidence intervals (95% CI) for MCI and dementia were calculated using multivariable Cox proportional hazards regression models across quartiles and in 1-standard deviation increments of PA and sitting measures. Follow-up time was the number of days from OPACH baseline to the first occurrence of MCI or probable dementia, or the date of the last obtained annual medical update for which the participant was free of MCI and probable dementia. Trend tests across PA and SB exposure categories were calculated by replacing the exposure quartile variable in the model with the continuous variable. We tested the proportional hazards assumption by inspecting Schoenfeld residuals. Models were progressively adjusted for confounders and mediators selected from prior studies of PA and dementia. [13,33,34] Model 1 adjusted for age, race and ethnicity, and education. Model 2 additionally contained alcohol use, smoking status, diabetes, hypertension, RAND-36 physical functioning score, APOE ε4 carrier status, and BMI. We calculated linear trend tests in Cox models by modeling accelerometer measures as continuous variables. Kaplan-Meier survival curves were estimated for each cognitive outcome across quartiles of PA and SB measures. The linearity of associations for PA and SB measures was evaluated using restricted cubic splines with knots at the 10th, 50th, and 90th percentiles using the rms package in R and chi-squared tests for nonlinearity were performed. To evaluate consistency of associations, we carried out stratified analysis across age (<83 years, ≥83 years; median split), BMI (<30 kg/m2, ≥30 kg/m2), RAND-36 physical functioning (<75, ≥75), RRS (<12.2, ≥12.2; median split), and APOE ε4 carrier status and evaluated effect modification using cross-product terms between continuous PA or SB measures and stratification variables. Nominal p-values were presented for all tests. A total of 75 tests were performed; thus, 4 tests would be expected to have a p-value <0.05 based on chance. We imputed missing covariate data for women with accelerometry and APOE ε4 data using multiple imputation by chained equations (MICE) using the mice package for 100 imputations and 5 iterations, specifying all covariates.
In sensitivity analyses, we additionally adjusted for self-reported vision and hearing impairment. We repeated the quartile models excluding data from women without ≥4 days of adherent accelerometer wear. To evaluate potential reverse causation, we repeated the quartile models excluding outcome events that occurred during the first two years of follow-up. To account for the competing risk of death, as individuals who died were assumed to be at risk of MCI or probable dementia, we repeated the quartile models using the Fine and Gray method for competing risks. [35]
RESULTS
Study population characteristics
On average, women had approximately 3,216 steps/day, 276 minutes/day in LPA, 45.5 minutes/day in MVPA, 633 minutes/day in ST, and an MSBD of approximately 14 minutes (Table 1). Across incremental quartiles of steps/day, women were younger, more likely to have a higher education, less likely to smoke, more likely to consume alcohol, had a lower BMI, had higher RAND-36 physical functioning scores, were less likely to have diabetes and hypertension, had a more favorable Reynolds CVD risk score, and had higher LPA and MVPA and lower ST and MSBD (Table 1). The Pearson correlations between steps/day and MVPA, LPA, ST, and MSBD were 0.78, 0.42, −0.53, and −0.44, respectively (Figure S1).
Table 1.
Mean (SD) or count (%) of Objective Physical Activity and Cardiovascular Health (OPACH; n=1277) study baseline (2012–2014) sociodemographic and health-related characteristics across quartiles of steps/day.
| Steps/day quartiles | |||||
|---|---|---|---|---|---|
| Total | Q1 (≤ 1867) |
Q2 (1868–2809) |
Q3 (2810–4049) |
Q4 (≥ 4050) |
|
| Age, years, mean (SD) | 81.8 (6.2) | 84.2 (5.0) | 83.1 (5.3) | 81.7 (5.9) | 78.7 (6.9) |
| Race and ethnicity, n (%) | |||||
| White | 1133 (88.7) | 258 (88.7) | 292 (89.6) | 292 (89.6) | 291 (87.1) |
| Black | 100 (7.8) | 26 (8.9) | 28 (8.6) | 18 (5.5) | 28 (8.4) |
| Hispanic/Latina | 44 (3.4) | 7 (2.4) | 6 (1.8) | 16 (4.9) | 15 (4.5) |
| Highest education level, n (%) | |||||
| High school or less | 268 (21) | 75 (25.9) | 64 (19.6) | 70 (21.6) | 59 (17.7) |
| Some college | 474 (37.2) | 107 (36.9) | 138 (42.3) | 118 (36.4) | 111 (33.2) |
| College graduate | 532 (41.8) | 108 (37.2) | 124 (38) | 136 (42) | 164 (49.1) |
| Health behavior/status | |||||
| Current smoker, n (%) | 27 (2.1) | 13 (4.5) | 7 (2.1) | 3 (0.9) | 4 (1.2) |
| Alcohol Intake in past 3 months, n (%) | |||||
| Non-drinker | 398 (31.2) | 122 (41.9) | 114 (35) | 81 (24.8) | 81 (24.3) |
| Less than 1 drink per week | 410 (32.1) | 89 (30.6) | 101 (31) | 121 (37.1) | 99 (29.6) |
| 1 or more drinks per week | 378 (29.6) | 48 (16.5) | 89 (27.3) | 103 (31.6) | 138 (41.3) |
| Unknown | 91 (7.1) | 32 (11) | 22 (6.7) | 21 (6.4) | 16 (4.8) |
| Body Mass Index, kg/m2, mean (SD) | 27.6 (5.6) | 28.5 (6.13) | 28.7 (5.92) | 27.4 (5.19) | 25.7 (4.7) |
| Self-rated health | |||||
| Excellent or very Good | 704 (55.2) | 98 (33.8) | 158 (48.5) | 203 (62.3) | 245 (73.4) |
| Good | 484 (37.9) | 156 (53.8) | 147 (45.1) | 102 (31.3) | 79 (23.7) |
| Fair or poor | 88 (6.9) | 36 (12.4) | 21 (6.4) | 21 (6.4) | 10 (3) |
| RAND-36 physical functioning score, mean (SD) | 66.5 (25.1) | 47.7, (25.6) | 60.0 (22.8) | 73.3 (20.2) | 82.4 (17.0) |
| Diabetes, n (%) | 230 (18) | 70 (24.1) | 72 (22.1) | 55 (16.9) | 33 (9.9) |
| Hypertension, n (%) | 906 (70.9) | 238 (81.8) | 250 (76.7) | 227 (69.6) | 191 (57.2) |
| Vision impairment | 82 (7.1) | 21 (8.4) | 17 (5.9) | 23 (7.8) | 21 (6.5) |
| Hearing impairment | 269 (23.2) | 63 (25.2) | 76 (26.3) | 70 (23.6) | 60 (18.7) |
| CVD biomarkers | |||||
| Reynolds risk score, mean (SD) | 14.6 (11.6) | 20.8 (15.1) | 15.6 (10.5) | 14.1 (9.7) | 9.8 (8.3) |
| Systolic Blood Pressure, mean (SD) | 125.8 (14.3) | 128.3 (15.3) | 126.2 (14.1) | 126.5 (14.4) | 122.9 (13.2) |
| Diastolic Blood Pressure, mean (SD) | 72.2 (8.9) | 72.7, (9.6) | 72.2 (8.3) | 72.6 (9.5) | 71.3 (8.2) |
| CRP, mean (SD) | 3.35 (7.8) | 4.13 (12.5) | 4.1 (8.3) | 3.06 (4.4) | 2.39 (4.2) |
| Glucose, mean (SD) | 97.9 (29.4) | 100.7 (35.7) | 98.3 (25.0) | 98.9 (33.9) | 94.5 (21.4) |
| Insulin, mean (SD) | 86.8 (126) | 88.6 (88.6) | 109.6 (212) | 87.8 (93.9) | 65.9 (67.5) |
| Total Cholesterol, mean (SD) | 195.6 (37.9) | 190.3 (38.1) | 192.0 (35.9) | 194.9 (38.3) | 203.4 (38.0) |
| HDL Cholesterol, mean (SD) | 59.84 (15.1) | 56.85 (14.8) | 58.35 (12.8) | 59.2 (14.5) | 64.0 (16.6) |
| LDL Cholesterol, mean (SD) | 113.4 (33.3) | 109.2 (32.5) | 111.3 (31.6) | 112.8 (34.8) | 118.8 (33.2) |
| Triglycerides, mean (SD) | 113.4 (58.6) | 122.4 (64.0) | 112.5 (48.5) | 116.9 (66.3) | 103.8, (52.8) |
| PA intensity and sitting a | |||||
| Light PAa (hour/day), mean (SD) | 4.61 (1.2) | 3.87 (1.0) | 4.35 (1.1) | 4.88 (1.0) | 5.24 (1.1) |
| MVPAa (min/day), mean (SD) | 45.9 (31.4) | 19.8, (13.1) | 32.6 (16.4) | 47.7 (21.1) | 80.0 (31.7) |
| Sitting timea (hour/day), mean (SD) | 10.6 (1.6) | 11.8 (1.37) | 11.1 (1.3) | 10.2 (1.3) | 9.4 (1.4) |
| MSBD (minutes), mean (SD) | 13.6 (5.4) | 17.5 (6.8) | 14.6 (4.6) | 12.1 (3.7) | 10.8 (3.5) |
Abbreviations: CRP=C-reactive protein; MVPA=moderate-to-vigorous physical activity; MSBD=mean sitting bout duration; PA=physical activity; SD=standard deviation
p-value: chi-sq for categorical variables and trend test for continuous
Variables are adjusted for accelerometer awake wear time using the residuals method
Associations of accelerometer measures of PA and SB with incident MCI and dementia
Over a median follow-up of approximately 4.2 years (interquartile range=2.1–6.3 years) there were 167 (13%) incident MCI, 161 (12%) incident dementia, and 267 (21%) combined incident MCI/probable dementia events. Unadjusted incidence rates for MCI, dementia, and MCI/probable dementia were lower across incremental quartiles of MVPA and steps/day, and generally similar across incremental quartiles of LPA, ST and MSBD (Table 2). No violations of the proportional hazards assumption were observed in the Cox proportional hazards models.
Table 2.
Associations of accelerometer measures of physical activity and sitting with incident mild cognitive impairment and probable dementia among older women in the Objective Physical Activity and Cardiovascular Health (OPACH) study 2012–2020.
| Moderate-to-vigorous intensity physical activity (minutes/day) | |||||||
|---|---|---|---|---|---|---|---|
| Q1 (<23) | Q2 (23–39) | Q3 (39–61) | Q4 (≥61) | HR for 1-SD increment (31.2) | P-trenda | n | |
| MCI events [rateb] | 45 [36.8] | 52 [42.0] | 44 [31.0] | 26 [18.3] | |||
| Model 1c | 1 (ref) | 1.14 (0.76–1.71) | 0.91 (0.60–1.38) | 0.61 (0.37–1.00) | 0.81 (0.67–0.98) | 0.030 | 1,284 |
| Model 2c | 1 (ref) | 1.24 (0.79–1.95) | 0.92 (0.57–1.50) | 0.60 (0.34–1.07) | 0.79 (0.63–0.98) | 0.033 | 977 |
| Model 2Ac | 1 (ref) | 1.26 (0.80–1.97) | 0.91 (0.56–1.48) | 0.64 (0.36–1.13) | 0.80 (0.64–1.00) | 0.047 | 1,042 |
| Dementia events [rateb] | 45 [33.8] | 54 [39.8] | 36 [23.5] | 26 [17.7] | |||
| Model 1c | 1 (ref) | 1.17 (0.79–1.74) | 0.77 (0.49–1.19) | 0.66 (0.40–1.08) | 0.81 (0.66–0.98) | 0.031 | 1,331 |
| Model 2c | 1 (ref) | 1.30 (0.82–2.05) | 0.91 (0.54–1.51) | 0.83 (0.47–1.45) | 0.88 (0.70–1.10) | 0.251 | 1,014 |
| Model 2Ac | 1 (ref) | 1.21 (0.77–1.90) | 0.84 (0.51–1.40) | 0.79 (0.45–1.37) | 0.86 (0.69–1.08) | 0.189 | 1,082 |
| MCI or dementia events [rateb] | 74 [60.7] | 88 [71.9] | 61 [43.1] | 44 [31.1] | |||
| Model 1c | 1 (ref) | 1.17 (0.86–1.60) | 0.75 (0.53–1.06) | 0.61 (0.41–0.89) | 0.78 (0.67–0.90) | 0.001 | 1,274 |
| Model 2c | 1 (ref) | 1.29 (0.91–1.84) | 0.81 (0.55–1.21) | 0.66 (0.43–1.03) | 0.79 (0.66–0.94) | 0.007 | 967 |
| Model 2Ac | 1 (ref) | 1.28 (0.91–1.81) | 0.79 (0.53–1.17) | 0.69 (0.45–1.06) | 0.79 (0.67–0.94) | 0.009 | 1,032 |
| Light intensity physical activity (minutes/day) | |||||||
| Q1 (<226) | Q2 (226–275) | Q3 (275–323) | Q4 (≥323) | HR for 1-SD increment (70.7) | P-trenda | n | |
| MCI events [rateb] | 31 [24.7] | 49 [35.8] | 40 [29.5] | 47 [35.5] | |||
| Model 1c | 1 (ref) | 1.54 (0.98–2.43) | 1.26 (0.78–2.03) | 1.63 (1.03–2.59) | 1.14 (0.98–1.33) | 0.081 | 1,284 |
| Model 2c | 1 (ref) | 1.57 (0.93–2.63) | 1.44 (0.84–2.48) | 1.83 (1.05–3.21) | 1.18 (0.98–1.43) | 0.086 | 977 |
| Model 2Ac | 1 (ref) | 1.56 (0.93–2.63) | 1.41 (0.82–2.43) | 1.89 (1.08–3.28) | 1.19 (0.99–1.44) | 0.064 | 1,042 |
| Dementia events [rateb] | 37 [27.5] | 44 [29.7] | 46 [31.8] | 34 [24.1] | |||
| Model 1c | 1 (ref) | 1.16 (0.74–1.79) | 1.25 (0.81–1.93) | 0.99 (0.62–1.59) | 0.97 (0.83–1.14) | 0.734 | 1,331 |
| Model 2c | 1 (ref) | 1.15 (0.70–1.89) | 1.42 (0.86–2.33) | 1.11 (0.63–1.95) | 0.99 (0.81–1.21) | 0.941 | 1,014 |
| Model 2Ac | 1 (ref) | 1.10 (0.67–1.79) | 1.28 (0.78–2.10) | 1.08 (0.62–1.87) | 0.97 (0.80–1.17) | 0.730 | 1,082 |
| MCI or dementia events [rateb] | 55 [44.4] | 77 [56.4] | 67 [49.7] | 68 [51.5] | |||
| Model 1c | 1 (ref) | 1.36 (0.96–1.93) | 1.18 (0.82–1.69) | 1.30 (0.91–1.86) | 1.06 (0.94–1.20) | 0.343 | 1,274 |
| Model 2c | 1 (ref) | 1.47 (0.99–2.17) | 1.33 (0.88–2.02) | 1.41 (0.91–2.19) | 1.07 (0.92–1.24) | 0.398 | 967 |
| Model 2Ac | 1 (ref) | 1.40 (0.95–2.06) | 1.25 (0.83–1.88) | 1.42 (0.93–2.18) | 1.07 (0.92–1.23) | 0.403 | 1,032 |
| Steps (n/day) | |||||||
| Q1 (<1,867) | Q2 (1,867–2,809) | Q3 (2,809–4,050) | Q4 (≥4,050) | HR for 1-SD increment (1,865) | P-trenda | n | |
| MCI events [rateb] | 54 [50.4] | 49 [36.0] | 40 [28.1] | 24 [16.6] | |||
| Model 1c | 1 (ref) | 0.72 (0.49–1.06) | 0.60 (0.39–0.91) | 0.39 (0.24–0.64) | 0.74 (0.60–0.90) | 0.003 | 1,284 |
| Model 2c | 1 (ref) | 0.64 (0.40–1.01) | 0.60 (0.37–1.00) | 0.33 (0.18–0.61) | 0.67 (0.51–0.87) | 0.003 | 977 |
| Model 2Ac | 1 (ref) | 0.62 (0.40–0.99) | 0.60 (0.37–0.99) | 0.36 (0.20–0.66) | 0.69 (0.53–0.89) | 0.006 | 1,042 |
| Dementia events [rateb] | 53 [43.6] | 49 [33.4] | 37 [24.5] | 22 [14.8] | |||
| Model 1c | 1 (ref) | 0.81 (0.55–1.19) | 0.63 (0.41–0.96) | 0.42 (0.25–0.70) | 0.70 (0.57–0.87) | 0.001 | 1,331 |
| Model 2c | 1 (ref) | 0.84 (0.53–1.31) | 0.68 (0.41–1.13) | 0.48 (0.26–0.88) | 0.72 (0.56–0.92) | 0.010 | 1,014 |
| Model 2Ac | 1 (ref) | 0.83 (0.53–1.29) | 0.65 (0.39–1.08) | 0.48 (0.26–0.87) | 0.71 (0.55–0.91) | 0.008 | 1,082 |
| MCI or dementia events [rateb] | 84 [79.1] | 81 [59.9] | 65 [46.0] | 37 [25.6] | |||
| Model 1c | 1 (ref) | 0.77 (0.57–1.05) | 0.61 (0.44–0.84) | 0.37 (0.25–0.55) | 0.69 (0.59–0.81) | <0.001 | 1,274 |
| Model 2c | 1 (ref) | 0.75 (0.53–1.08) | 0.66 (0.44–0.98) | 0.35 (0.22–0.58) | 0.65 (0.53–0.81) | <0.001 | 967 |
| Model 2Ac | 1 (ref) | 0.73 (0.51–1.03) | 0.64 (0.43–0.94) | 0.38 (0.23–0.61) | 0.67 (0.54–0.82) | <0.001 | 1,032 |
| Sitting time (minutes/day) | |||||||
| Q1 (<569) | Q2 (569–637) | Q3 (637–704) | Q4 (≥704) | HR for 1-SD increment (97.2) | P-trenda | n | |
| MCI events [rateb] | 41 [31.3] | 38 [27.9] | 44 [33.9] | 34 [29.3] | |||
| Model 1c | 1 (ref) | 0.83 (0.53–1.29) | 0.94 (0.61–1.44) | 0.81 (0.51–1.28) | 0.92 (0.78–1.08) | 0.286 | 1,234 |
| Model 2c | 1 (ref) | 0.73 (0.44–1.20) | 0.83 (0.51–1.37) | 0.78 (0.45–1.34) | 0.88 (0.72–1.07) | 0.186 | 945 |
| Model 2Ac | 1 (ref) | 0.75 (0.45–1.23) | 0.86 (0.53–1.41) | 0.78 (0.45–1.33) | 0.89 (0.73–1.07) | 0.220 | 1,005 |
| Dementia events [rateb] | 41 [29.6] | 33 [22.7] | 37 [26.0] | 40 [32.8] | |||
| Model 1c | 1 (ref) | 0.74 (0.47–1.18) | 0.81 (0.52–1.27) | 0.94 (0.60–1.46) | 0.97 (0.82–1.15) | 0.734 | 1,277 |
| Model 2c | 1 (ref) | 0.73 (0.44–1.22) | 0.79 (0.48–1.31) | 0.84 (0.49–1.44) | 0.91 (0.75–1.10) | 0.324 | 978 |
| Model 2Ac | 1 (ref) | 0.70 (0.42–1.17) | 0.79 (0.47–1.31) | 0.90 (0.53–1.52) | 0.94 (0.77–1.14) | 0.521 | 1,041 |
| MCI or dementia events [rateb] | 69 [53.1] | 58 [42.7] | 64 [49.6] | 61 [53.0] | |||
| Model 1c | 1 (ref) | 0.77 (0.54–1.09) | 0.85 (0.60–1.20) | 0.88 (0.62–1.25) | 0.96 (0.85–1.09) | 0.537 | 1,226 |
| Model 2c | 1 (ref) | 0.73 (0.49–1.09) | 0.79 (0.53–1.18) | 0.82 (0.54–1.25) | 0.91 (0.78–1.06) | 0.246 | 937 |
| Model 2Ac | 1 (ref) | 0.73 (0.49–1.08) | 0.80 (0.54–1.19) | 0.84 (0.56–1.26) | 0.93 (0.80–1.08) | 0.356 | 997 |
| Mean sitting bout duration (minutes) | |||||||
| Q1 (<10.1) | Q2 (10.1–12.7) | Q3 (12.7–16.2) | Q4 (≥16.2) | HR for 1-SD increment (5.4) | P-trenda | n | |
| MCI events [rateb] | 36 [30.1] | 37 [27.2] | 43 [32.9] | 41 [32.4] | |||
| Model 1c | 1 (ref) | 0.75 (0.47–1.20) | 0.94 (0.60–1.47) | 0.88 (0.55–1.39) | 0.97 (0.82–1.14) | 0.690 | 1,234 |
| Model 2c | 1 (ref) | 0.88 (0.52–1.47) | 0.99 (0.59–1.65) | 0.88 (0.51–1.51) | 0.92 (0.75–1.11) | 0.378 | 945 |
| Model 2Ac | 1 (ref) | 0.86 (0.52–1.42) | 0.98 (0.59–1.61) | 0.83 (0.49–1.42) | 0.90 (0.74–1.10) | 0.303 | 1,005 |
| Dementia events [rateb] | 33 [26.2] | 33 [22.6] | 41 [29.7] | 44 [31.9] | |||
| Model 1c | 1 (ref) | 0.71 (0.44–1.16) | 0.94 (0.59–1.50) | 0.93 (0.59–1.48) | 1.01 (0.86–1.19) | 0.859 | 1,277 |
| Model 2c | 1 (ref) | 0.67 (0.39–1.13) | 0.88 (0.52–1.46) | 0.90 (0.53–1.53) | 1.00 (0.83–1.21) | 0.979 | 978 |
| Model 2Ac | 1 (ref) | 0.72 (0.42–1.21) | 0.92 (0.55–1.54) | 1.00 (0.60–1.69) | 1.03 (0.86–1.24) | 0.739 | 1,041 |
| MCI or dementia events [rateb] | 54 [45.4] | 57 [42.0] | 71 [55.0] | 70 [55.5] | |||
| Model 1c | 1 (ref) | 0.77 (0.53–1.13) | 1.04 (0.73–1.49) | 1.00 (0.69–1.43) | 1.02 (0.90–1.15) | 0.788 | 1,226 |
| Model 2c | 1 (ref) | 0.83 (0.55–1.26) | 1.02 (0.68–1.53) | 0.96 (0.63–1.47) | 0.97 (0.84–1.13) | 0.704 | 937 |
| Model 2Ac | 1 (ref) | 0.83 (0.55–1.24) | 1.03 (0.69–1.53) | 0.98 (0.65–1.48) | 0.98 (0.85–1.13) | 0.777 | 997 |
| Light intensity steps (n/day) | |||||||
| Q1 (<1,236) | Q2 (1,236–1,693) | Q3 (1,693–2,191) | Q4 (≥2,191) | HR for 1-SD increment (728) | P-trenda | n | |
| MCI events [rateb] | 37 [32.2] | 55 [39.7] | 33 [23.5] | 42 [30.7] | |||
| Model 1c | 1 (ref) | 1.23 (0.81–1.87) | 0.74 (0.46–1.2) | 1.12 (0.71–1.75) | 1.04 (0.88–1.23) | 0.650 | 1,284 |
| Model 2c | 1 (ref) | 1.12 (0.70–1.81) | 0.78 (0.45–1.34) | 1.18 (0.69–2.04) | 1.08 (0.88–1.33) | 0.435 | 977 |
| Model 2Ac | 1 (ref) | 1.07 (0.66–1.72) | 0.79 (0.46–1.35) | 1.15 (0.67–1.97) | 1.08 (0.88–1.32) | 0.460 | 1,042 |
| Dementia events [rateb] | 45 [35.8] | 50 [33.4] | 34 [22.9] | 32 [22.1] | |||
| Model 1c | 1 (ref) | 0.96 (0.64–1.43) | 0.70 (0.44–1.09) | 0.73 (0.46–1.15) | 0.87 (0.73–1.04) | 0.118 | 1,331 |
| Model 2c | 1 (ref) | 0.81 (0.52–1.26) | 0.59 (0.35–0.99) | 0.70 (0.41–1.19) | 0.86 (0.69–1.06) | 0.156 | 1,014 |
| Model 2Ac | 1 (ref) | 0.86 (0.55–1.34) | 0.59 (0.35–0.99) | 0.69 (0.40–1.18) | 0.85 (0.69–1.05) | 0.129 | 1,082 |
| MCI or dementia events [rateb] | 62 [54.2] | 89 [65.0] | 56 [40.1] | 60 [44.1] | |||
| Model 1c | 1 (ref) | 1.22 (0.88–1.69) | 0.76 (0.53–1.10) | 0.92 (0.64–1.32) | 0.93 (0.81–1.06) | 0.280 | 1,274 |
| Model 2c | 1 (ref) | 1.10 (0.76–1.59) | 0.75 (0.49–1.14) | 0.95 (0.62–1.46) | 0.94 (0.80–1.11) | 0.472 | 967 |
| Model 2Ac | 1 (ref) | 1.09 (0.76–1.56) | 0.75 (0.50–1.13) | 0.94 (0.61–1.43) | 0.94 (0.80–1.10) | 0.433 | 1,032 |
| Moderate-to-vigorous intensity steps (n/day) | |||||||
| Q1 (<1,222) | Q2 (1,222–1,670) | Q3 (1,670–2,185) | Q4 (≥2,185) | HR for 1-SD increment (1,502) | P-trenda | n | |
| MCI events [rateb] | 52 [44.0] | 57 [44.3] | 33 [24.0] | 25 [17.1] | |||
| Model 1c | 1 (ref) | 1.00 (0.68–1.46) | 0.61 (0.39–0.96) | 0.47 (0.29–0.76) | 0.75 (0.60–0.94) | 0.011 | 1,284 |
| Model 2c | 1 (ref) | 1.04 (0.68–1.60) | 0.61 (0.36–1.02) | 0.48 (0.27–0.87) | 0.73 (0.56–0.96) | 0.024 | 977 |
| Model 2Ac | 1 (ref) | 1.01 (0.66–1.55) | 0.61 (0.37–1.02) | 0.49 (0.28–0.89) | 0.74 (0.56–0.97) | 0.027 | 1,042 |
| Dementia events [rateb] | 43 [32.5] | 56 [40.1] | 36 [24.6] | 26 [17.3] | |||
| Model 1c | 1 (ref) | 1.25 (0.84–1.87) | 0.88 (0.56–1.38) | 0.66 (0.40–1.09) | 0.87 (0.71–1.07) | 0.181 | 1,331 |
| Model 2c | 1 (ref) | 1.43 (0.90–2.27) | 1.18 (0.70–2.00) | 0.90 (0.50–1.64) | 1.00 (0.80–1.24) | 0.968 | 1,014 |
| Model 2Ac | 1 (ref) | 1.34 (0.85–2.11) | 1.09 (0.65–1.83) | 0.89 (0.49–1.59) | 0.98 (0.79–1.23) | 0.878 | 1,082 |
| MCI or dementia events [rateb] | 76 [64.8] | 93 [73.1] | 56 [40.9] | 42 [28.9] | |||
| Model 1c | 1 (ref) | 1.11 (0.82–1.51) | 0.69 (0.48–0.98) | 0.52 (0.35–0.76) | 0.76 (0.64–0.90) | 0.002 | 1,274 |
| Model 2c | 1 (ref) | 1.25 (0.88–1.77) | 0.82 (0.55–1.24) | 0.61 (0.38–0.98) | 0.80 (0.65–0.97) | 0.026 | 967 |
| Model 2Ac | 1 (ref) | 1.20 (0.85–1.69) | 0.80 (0.54–1.19) | 0.63 (0.40–0.99) | 0.80 (0.65–0.97) | 0.027 | 1,032 |
Abbreviations: MCI=mild cognitive impairment
P-values from Cox models with accelerometer measures in continuous form.
Crude incidence rate per 1000 person-years
Data are hazard ratio (95% confidence interval)
Model 1 adjusted for age, race and ethnicity, and education; Model 2 = Model 1 + smoking status + alcohol use + diabetes + hypertension + RAND-36 physical functioning score + APOE ε4 + BMI
p<0.05 (p=0.047)
Model 2A results were estimated with missing covariate data for women with APOE ε4 data imputed using multiple imputation by chained equations (MICE) from the R mice package.
Higher MVPA and steps/day were associated with lower adjusted risks of incident MCI, probable dementia, and MCI/probable dementia (Table 2). The model 2A HRs (95% CI) comparing women with MVPA in the highest quartile to those with MVPA in the lowest quartile were 0.64 (0.36–1.13; p-trend=0.047) for incident MCI, 0.79 (0.45–1.37; p-trend=0.189) for incident probable dementia, and 0.69 (0.45–1.06; p-trend=0.009) for MCI/probable dementia (Table 2). Associations for steps/day were stronger with HRs (95% CI) comparing women in the highest vs lowest quartiles of 0.36 (0.20–0.66; p-trend=0.006) for incident MCI, 0.48 (0.26–0.87; p-trend=0.008) for incident probable dementia, and 0.38 (0.23–0.61; p-trend<0.001) for incident MCI/probable dementia (Table 2). When distinguishing step intensity, higher amounts of MV steps/day were associated with lower risk of MCI and MCI/probable dementia, but not probable dementia alone (Table 2). LPA, sitting, MSBD, and higher amounts of light intensity steps/day were not significantly associated with risk of MCI, probable dementia, or MCI/probable dementia (Table 2). Further adjustment for vision and hearing impairment did not appreciably change results (data not shown).
Dose-response trajectories derived using cubic spline regressions were linear for all PA and sitting measures for incident MCI, dementia, and MCI/dementia (all P-nonlinear>0.081; Figure 1 and Figure S2). Higher amounts of MVPA were linearly associated with lower risk of MCI, probable dementia, and MCI/probable dementia (Figure 1). Results for steps/day were consistent in direction and stronger in magnitude than for MVPA. Kaplan-Meier curves indicated a steeper gradient in the probability of no probable dementia for MVPA and steps/day quartiles but not for LPA, ST, and MSBD quartiles, which was similar for MCI and MCI/probable dementia (Figures 2, S3, and S4). The overall HR (95% CI) the interquartile range of MVPA (38 minutes/day) was 0.76 (0.58–1.00) for MCI, 0.83 (0.63–1.09) for probable dementia, and 0.75 (0.61–0.93) for MCI/probable dementia (Tables 3, S4, and S5). The overall HR (95%) for the interquartile range increment in steps (2163/day) was 0.65 (0.47–0.88) for MCI, 0.67 (0.50–0.90) for probable dementia, and 0.62 (0.49–0.79) for MCI/probable dementia (Tables 3, S4, and S5).
Figure 1.
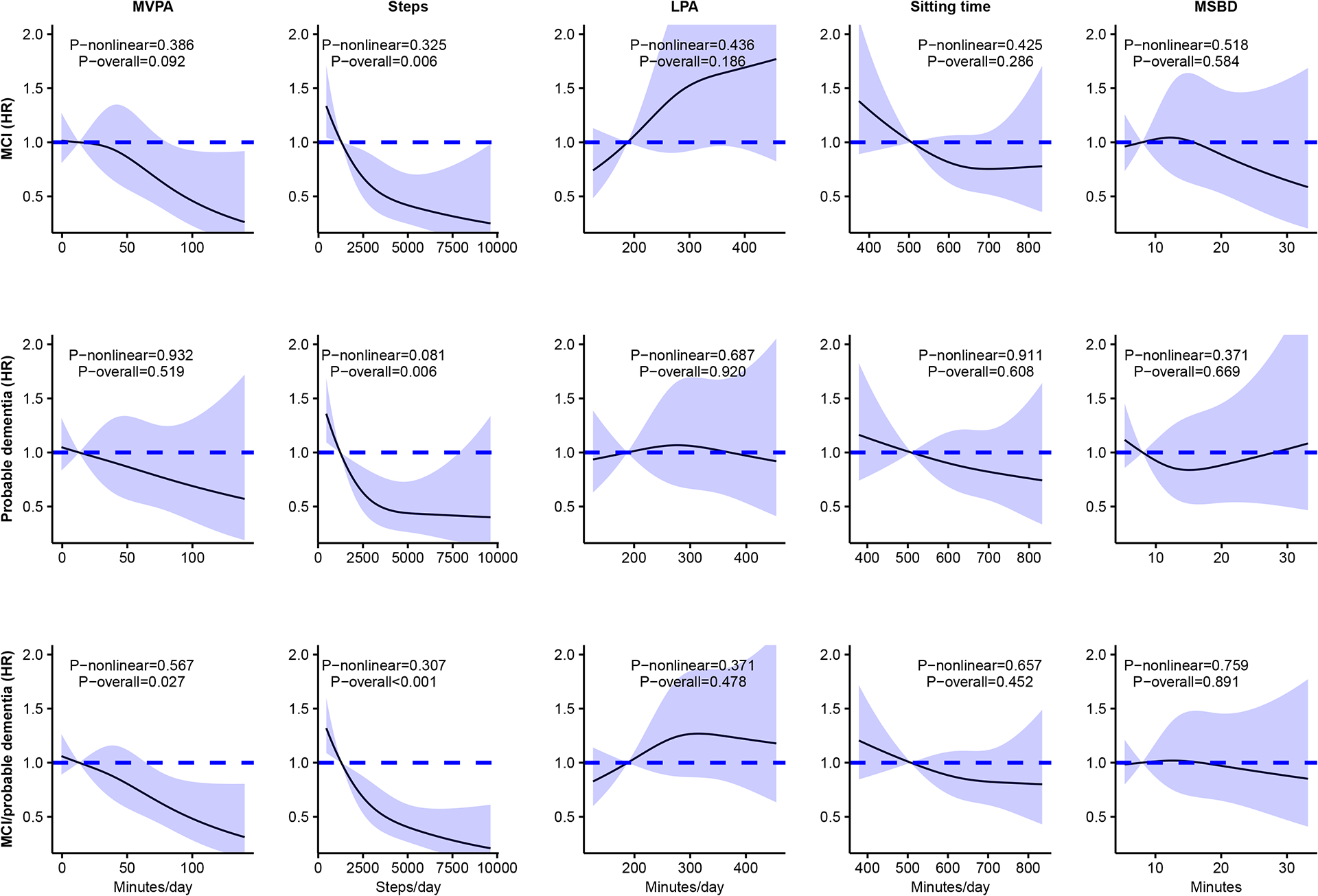
Continuous dose-response associations of accelerometer-measures of MVPA, steps/day, LPA, sitting time, and mean sitting bout duration with incident MCI, probable dementia, and combined MCI or probable dementia.
Abbreviations: HR=hazard ratio; LPA=light intensity physical activity; MVPA=moderate-to-vigorous intensity physical activity; MSBD=mean sitting bout duration. MCI=mild cognitive impairment; BMI=body mass index
Models adjusted for age, race and ethnicity, education, alcohol consumption, smoking status, diabetes, hypertension, RAND-36 physical functioning, BMI, and APOE ε4 carrier status. Results were trimmed at the 1st and 99th percentiles. The reference was set to the 10th percentile
Figure 2.
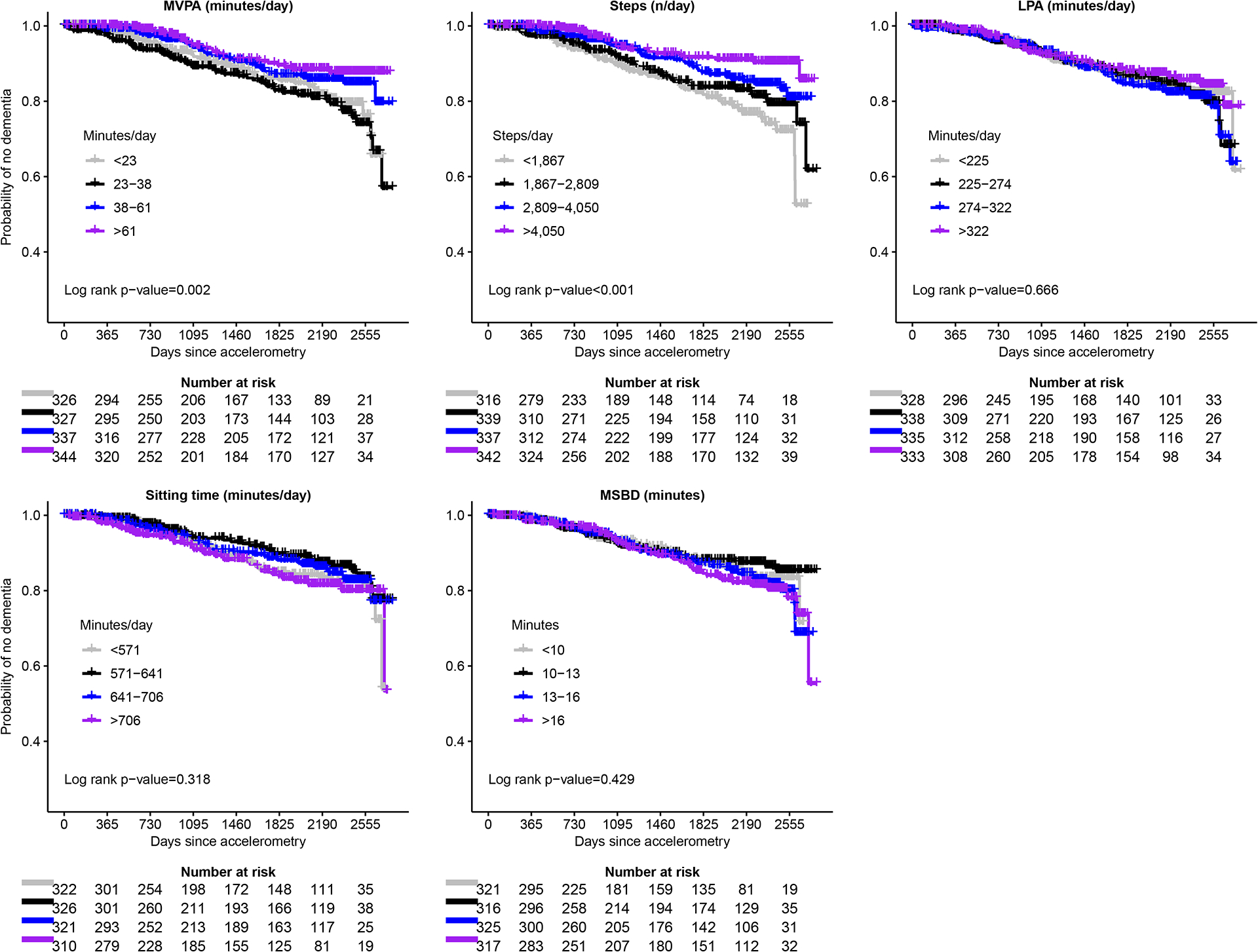
Kaplan-Meier curves for incident probable dementia across quartiles of moderate-to-vigorous physical activity (MVPA), steps/day, light intensity physical activity (LPA), sitting time (ST) and mean sitting bout duration (MSBD).
Abbreviations: HR=hazard ratio; CI=confidence interval; MVPA=moderate-to-vigorous intensity physical activity; LPA=light intensity physical activity; MSBD=mean sedentary bout duration; MCI=mild cognitive impairment; BMI=body mass index
HRs and 95% CIs from forest plots are from models adjusted for age, race and or ethnicity, education, alcohol consumption, smoking status, diabetes, hypertension, RAND-36 physical functioning, BMI, and APOE ε4.
Table 3.
Associations of the interquartile rangea, b in steps/day, MVPA, LPA, sitting time, and mean sitting bout duration with incident probable dementia among older women in the Objective Physical Activity and Cardiovascular Health (OPACH) Study 2012–2020, stratified by baseline characteristics.
| Steps (n/day) | MVPA (minutes/day) | LPA (minutes/day) | Sitting time (minutes/day) | MSBD (minutes) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n events | HR (95% CI) | P-interaction | HR (95% CI) | P-interaction | HR (95% CI) | P-interaction | n* | n events* | HR (95% CI) | P-interaction | HR (95% CI) | P-interaction | |
| Overall association | 1,082 | 143 | 0.67 (0.50–0.90) | 0.83 (0.63–1.09) | 0.96 (0.73–1.24) | 1,041 | 134 | 0.92 (0.71–1.19) | 1.04 (0.84–1.28) | |||||
| Age | 0.689 | 0.492 | 0.605 | 0.992 | 0.25 | |||||||||
| <83 | 494 | 38 | 0.99 (0.59–1.67) | 0.92 (0.56–1.52) | 1.00 (0.58–1.71) | 478 | 37 | 0.70 (0.42–1.16) | 1.23 (0.81–1.86) | |||||
| ≥83 | 588 | 105 | 0.52 (0.35–0.77) | 0.79 (0.56–1.12) | 0.89 (0.65–1.22) | 563 | 97 | 1.04 (0.75–1.42) | 1.01 (0.79–1.30) | |||||
| BMI | 0.668 | 0.396 | 0.756 | 0.548 | 0.595 | |||||||||
| < 30 kg/m2 | 769 | 115 | 0.65 (0.47–0.89) | 0.77 (0.57–1.05) | 1.02 (0.76–1.38) | 742 | 108 | 0.91 (0.68–1.22) | 1.06 (0.82–1.37) | |||||
| ≥ 30 kg/m2 | 313 | 28 | 0.55 (0.18–1.71) | 1.02 (0.42–2.44) | 0.63 (0.29–1.34) | 299 | 26 | 1.24 (0.50–3.07) | 0.99 (0.60–1.61) | |||||
| RAND-36 physical functioning | 0.016 | 0.314 | 0.567 | 0.243 | 0.723 | |||||||||
| < 75 | 564 | 75 | 0.47 (0.28–0.80) | 0.69 (0.44–1.07) | 0.89 (0.60–1.31) | 545 | 70 | 1.07 (0.72–1.59) | 0.99 (0.77–1.27) | |||||
| ≥ 75 | 518 | 68 | 0.72 (0.49–1.05) | 0.94 (0.65–1.34) | 1.04 (0.72–1.50) | 496 | 64 | 0.80 (0.56–1.16) | 0.94 (0.62–1.45) | |||||
| Reynolds risk score | 0.395 | 0.049 | 0.256 | 0.705 | 0.316 | |||||||||
| < 12.2 | 507 | 55 | 0.58 (0.35–0.95) | 0.72 (0.46–1.11) | 0.87 (0.55–1.38) | 495 | 50 | 0.94 (0.61–1.45) | 1.10 (0.68–1.78) | |||||
| ≥ 12.2 | 575 | 88 | 0.69 (0.45–1.05) | 0.88 (0.58–1.34) | 0.98 (0.68–1.39) | 546 | 84 | 0.94 (0.66–1.34) | 1.03 (0.79–1.33) | |||||
| APOE ε4 carrier status | 0.887 | 0.462 | 0.917 | 0.889 | 0.585 | |||||||||
| 0 | 844 | 107 | 0.70 (0.50–0.99) | 0.81 (0.59–1.12) | 1.00 (0.74–1.34) | 814 | 101 | 0.89 (0.65–1.20) | 1.04 (0.82–1.31) | |||||
| 1 or 2 | 238 | 36 | 0.64 (0.34–1.19) | 0.93 (0.53–1.61) | 0.80 (0.44–1.46) | 227 | 33 | 0.97 (0.53–1.78) | 1.01 (0.59–1.70) | |||||
HR=hazard ratio; CI=confidence interval; MVPA=moderate-to-vigorous intensity physical activity; LPA=light intensity physical activity; BMI=body mass index; SPPB=Short Physical Performance Battery; APOE=apolipoprotein E; MSBD=mean sitting bout duration
Accelerometer measures were modeled as continuous variables
Interquartile ranges: steps/day=2,163; MVPA=38 minutes/day; LPA=96 minutes/day; sitting time=134 minutes/day; MSBD=6.1 minutes/day
Models adjusted for age, race and ethnicity, educational attainment, alcohol use, smoking, diabetes, hypertension, RAND-36 physical functioning, BMI, and APOE ε4 carrier status. Model results were estimated with missing covariate data for women with APOE ε4 data imputed using multiple imputation by chained equations (MICE) from the R mice package.
n and n events for models with sitting time and MSBD
Results from sensitivity analyses that restricted the analytic sample to women with ≥4 days of ≥10 hours/day of accelerometer wear were consistent in direction and magnitude with those from main analyses (Table S1). In sensitivity analyses evaluating reverse causation where events from the first 2 years of follow-up were excluded (35 MCI, 43 probable dementia, and 64 MCI/probable dementia events) results were consistent in direction and magnitude with those in the main analyses (Table S2). In sensitivity analyses that accounted for the competing risk of death (n=234, 243, and 226 for incident MCI, probable dementia, or MCI/probable dementia, respectively) results were generally consistent in magnitude and direction with those from the main analyses except for MVPA, which was consistent in direction to the main analyses (Table S3).
Effect modification of associations of accelerometer measures with incident mild cognitive impairment and probable dementia
In stratified analyses, higher amounts of MVPA and steps/day were consistently associated with lower risk of incident probable dementia (Table 3), MCI (Table S4), and MCI/probable dementia (Table S5) across most subgroups, except for the MVPA-probable dementia association, which was non-significant. The steps/day-probable dementia association was stronger among women with a RAND-36 physical functioning score of less than 75 (HR=0.47, 95% CI=0.28–0.80) compared to those with a score of 75 or higher (HR=0.72, 95% CI=0.49–1.05; P-interaction=0.016; Table 3). The MVPA-probable dementia association was stronger among women with an RRS of less than 12.2 (HR=0.72, 95% CI=0.46–1.11) compared to those with an RRS of 12.2 or greater (HR=0.88, 95% CI=0.58–1.34; P-interaction=0.049; Table 3). Otherwise, no evidence of effect modification by age, BMI, RAND-36 physical functioning, RRS, or APOE ε4 alleles for the associations of LPA, sedentary time, and MSBD with incident probable dementia was observed. Results were consistent with those from complete case analysis (Tables S6 for MCI, S7 for probable dementia, and S8 for MCI/probable dementia).
DISCUSSION
In this prospective study of older ambulatory community living women, higher amounts of MVPA and steps/day were associated with lower risk of rigorously adjudicated MCI and probable dementia. Compared to women with less than 23 MVPA minutes/day, those with at least 61 minutes/day had a 36% lower risk of MCI, 21% lower risk of probable dementia, and 31% lower risk of MCI/probable dementia independent of several relevant covariates including physical functioning, BMI, APOE ε4, and CVD risk. Compared to women with less than 1,867 steps/day, those with at least 4,050 steps/day had a 64% lower risk of MCI, 52% lower risk of probable dementia, and 63% lower risk of MCI/probable dementia independent of covariates. LPA, ST, and MSBD were not associated with MCI or probable dementia risk. The inverse multivariable-adjusted associations of MVPA and steps/day with risk of MCI and probable dementia were consistent across cohort subgroups defined by age, BMI, physical functioning, and CVD risk profile, enhancing confidence in the primary findings in the overall cohort.
The present study results have clinical and public health relevance as there is little published information on the amount and intensity of PA needed for a lower dementia risk. Much of older adults’ movement occurs during daily living activities, which is mainly LPA and some MVPA. [36] The present study results showed that higher amounts of accelerometer-measured MVPA and steps were associated with lower risk of MCI and probable dementia. The present findings for steps/day are noteworthy because steps are recorded by a variety of wearable devices increasingly worn by individuals and could be more readily adopted than measures of MVPA volume. [37] Overall, the present study results suggest that more MVPA and steps/day can be encouraged for benefits against MCI and dementia.
Much of the current literature on the associations of PA with dementia is based on self-reported PA data. [4,5,38] In the Atherosclerosis Risk in Communities Study, those with high self-reported leisure-time PA had a lower risk of adjudicated dementia (HR=0.71, 95% CI=0.61–0.86) relative to those with no leisure-time PA. [33] Conversely in the Whitehall II Study, ADRD risk was not lower for participants with high versus low self-reported LPA (0.98, 95% CI=0.73–1.30) or MVPA (HR=1.08, 95% CI=0.82–1.41). [39] In the Honolulu Heart Study, those who reported walking less than 0.25 miles/day had a higher risk of dementia compared to those with >2.0 miles/day (HR=1.93, 95% CI=1.11–3.34). [40] However, self-reported PA and SB measures are at best moderately correlated with accelerometer measures and do not capture the same behaviors or amounts. [12] Few studies have examined accelerometer-measured PA in relation to cognitive outcomes. In the REGARDS study, based on data from hip-worn Actical accelerometers, those with MVPA in higher quartiles had lower odds of cognitive impairment (quartile 2 vs quartile 1 odds ratio=0.64, 95% CI=0.48–0.84), consistent in direction to the present study. [41] In the UK Biobank, participants with at least 1200 MET/min/week of MVPA had an 84% lower risk (HR 95% CI=0.12–0.21) of all-cause dementia relative to those with <300 MET/min/week of MVPA, consistent in direction to and stronger in magnitude than the present study results. [13] Another study in the UK Biobank observed a non-linear positive prospective steps/day-dementia association with the lowest risk observed for 9,826 steps/day (HR=0.49, 95% CI=0.39–0.62), consistent in direction with the present study. In the UK Biobank, accelerometers (Axivity AX3) were worn on the wrist, potentially capturing more non-ambulatory movement than hip-worn accelerometers. Also, dementia was classified using ICD-10 codes from electronic medical records, which have been shown to underestimate dementia cases compared to serial cognitive assessments with rigorous adjudication, potentially explaining differences in estimates of associations compared to the present study, particularly for LPA. [13,15,42,43] The present study extends the published literature by showing that higher amounts of hip-worn accelerometer-measured MVPA and steps/day are significantly associated with a lower risk of rigorously adjudicated MCI and probable dementia among older community-living women. Importantly, we also showed that these associations were consistent in direction among women with both high and lower physical functioning, as results in the English Longitudinal Study of Aging showed that higher physical functioning measured in walking tests is itself prospectively associated with lower dementia risk. [44] The strong inverse associations for steps/day could be due to the GT3X+ normal frequency filter underestimating steps taken at lower speed (i.e., intensity). The GT3X+ low frequency filter captures more steps taken at lower speeds; however, a 2015 study observed large differences in step counts between the normal and low frequency filters; thus, we did not process the GT3X+ steps data using the low frequency filter. [45]
The adverse direction of the LPA-MCI association is unlikely to be causal, lacks biological plausibility, could be due to chance as indicated by the HR 95% CIs, and requires replication in other studies. [13] In OPACH, much of the movement women engaged in was during daily living activities, which is mainly composed of LPA with some MVPA. [46] It is possible that the present findings for the non-significant adverse direction LPA-MCI association could be attributable to changes in behavior associated with agitation resulting in increased time spent in daily living activities prior to MCI classification. [47] Importantly, we did not observe any indication of an association between LPA and dementia. Further studies are needed to examine and characterize the relationship between LPA and dementia.
Few studies have examined SB in relation to MCI and dementia. In the UK Biobank, higher amounts of self-reported sedentary time were associated with higher risk of ICD-10 classified dementia and lower cortical brain volumes. [48] The present study contributes information to this gap by showing that higher amounts of accelerometer-measured ST and MSBD were not associated with higher MCI or probable dementia risk. Overall, the present study contributes important novel information on device-measured PA and sitting in relation to MCI and probable dementia among older women, which is desperately needed as part of an evolving evidence-base aimed at delaying or preventing ADRD in an aging society.
Several biological mechanisms could explain the present study results. Higher PA amounts and intensities can improve cardiorespiratory fitness, which in turn is associated with lower white matter lesion volume and larger brain volume. [49,50] Accelerometer-measured PA is favorably associated with vascular risk factor profiles, suggesting contributions to better cerebral blood flow and lower neuroinflammation levels relevant to dementia and its subtypes including vascular dementia. [50,51] A 12-month randomized moderate intensity walking intervention among older adults increased serum concentrations of brain-derived neurotrophic factor and improved executive function. [52] Sitting is associated with unfavorable cardiometabolic risk factors including waist circumference and insulin, which could result in higher dementia risk in part through CVD. [50,53,54] However, certain activities that involve sitting (e.g., completing puzzles and reading) could be cognitively stimulating and result in brain structure improvements. [5,55] These mechanisms could have contributed to the null associations for ST and MSBD in the present study. Few studies have examined the associations of PA and SB with early AD pathologic markers such as amyloid-beta species and tau protein, and findings are equivocal. [56] Additional research is warranted to better understand how PA and SB influence the heterogenous mechanisms involved in the pathogenesis of MCI, dementia, and dementia subtypes.
We note several limitations in the present study. Accelerometer measures of PA and sitting were collected during older adulthood and the median follow-up was short at 4.2 years, precluding a thorough evaluation of reverse causality. [57] Dementia neuropathology may predate cognitive symptoms by up to 20 years and data from Whitehall II suggested that PA levels declined as early as 10 years before clinical dementia presentation. [39,58] Although results from sensitivity analyses that excluded outcome events during the first 2 years of follow-up were consistent with those from the main analyses, the possibility of reverse causality cannot be fully ruled out. Dementia subtypes (AD, vascular, other) were not classified in WHIMS after 2007, precluding specific examination of associations of PA and sitting with AD. However, AD is the most common cause of dementia, accounting for an estimated 60–80% of dementia cases and many individuals with AD have mixed dementia. [3] There were few Black and Hispanic/Latina women. It is crucial that future studies include populations who disproportionately bear the burden of cognitive decline and dementia. [3] The relatively small sample size in the present study resulted in wide HR 95% CIs and limited power to detect multiplicative interactions in analyses of effect modification. Unmeasured and residual confounding (e.g., self-reported measures of alcohol use and smoking, and clinical conditions) cannot be eliminated in observational studies. Women wore accelerometers for ~7 days, which might not fully capture usual movement and sitting for all women. Strengths of the present study include annual cognitive surveillance with rigorously adjudicated cognitive outcomes in WHIMS and accelerometer measures of free-living PA and sitting in tandem with the application of calibrated cutpoints to classify LPA and MVPA in OPACH, which few studies have available. Extensive health data is available in WHI, allowing for adjustment of several relevant covariates including APOE ε4 and physical functioning. We used MICE to impute missing covariate data to reduce the impact of selection bias and enhance the precision of study results.
The present study showed that higher amounts of MVPA and steps/day were associated with lower incident MCI and MCI/probable dementia risk among community-living older women. Larger studies with accelerometer measures, adjudicated cognitive outcomes, and long follow-up periods are needed to further investigate PA and SB in relation to ADRD, underlying neuropathology, and possible bi-directional associations between PA and brain aging. [59] As we learn more, it is prudent to recommend higher amounts of at least moderate intensity movement and steps for a lower MCI or probable dementia risk.
Supplementary Material
HIGHLIGHTS.
Few studies have examined accelerometer-measured physical activity, including steps, and sitting with incident ADRD.
Moderate-to-vigorous physical activity and steps, but not light physical activity or sitting, were inversely associated with lower ADRD risk.
Among older women, at least moderate intensity physical activity may be needed to reduce ADRD risk
RESEARCH IN CONTEXT.
Systematic review:
The authors reviewed the current literature on the associations of accelerometer measures of physical activity (PA) and sedentary behavior (SB) with Alzheimer’s disease and related dementias (ADRD) using PubMed. Only two studies were identified, where higher amounts of wrist-worn accelerometer-measures of PA and steps were associated with lower risk of ICD-10 defined ADRD. These findings merit replication in a cohort followed carefully for incident adjudicated mild cognitive impairment and dementia. No studies have prospectively examined accelerometer measures of sitting in relation to ADRD.
Interpretation:
Findings show that among older women, moderate-to-vigorous intensity PA, in particularly stepping, is inversely associated with risk of incident mild cognitive impairment (MCI) and probable dementia. Light PA and sitting were not associated with MCI or probable dementia risk.
Future directions:
Future prospective studies of accelerometer-measured PA and SB with ADRD among racially and ethnically diverse study populations and longer follow-up are warranted.
ACKNOWLEDGEMENTS
We thank the WHI participants, staff, and investigators. The short list of WHI investigators can be found at: https://www.whi.org/doc/WHI-Investigator-Short-List.pdf. The full list of WHI Investigators can be found at the following site: https://www.whi.org/doc/WHI-Investigator-Long-List.pdf
FUNDING SOURCES
This work was supported by the National Institute on Aging (P01 AG052352 to A.Z.L.) and the National Heart, Lung, and Blood Institute who provided funding for the OPACH study (grant number R01 HL105065 to A.Z.L.). The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005. Funding also came from a training grant provided by the National Institute on Aging (grant number 5T32AG058529-03 to SN). No funding agency was involved in the design of the study, data collection, analysis, data interpretation, or manuscript writing.
Footnotes
CONFLICTS OF INTEREST
Declarations of interest: none.
REFERENCES
- [1].Nichols E, Steinmetz JD, Vollset SE, Fukutaki K, Chalek J, Abd-Allah F, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022;7:e105–25. 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chêne G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement 2015;11:310–20. 10.1016/J.JALZ.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].2022 Alzheimer’s disease facts and figures. Alzheimers Dement 2022. 10.1002/ALZ.12638. [DOI] [PubMed] [Google Scholar]
- [4].Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet 2020;396:413–46. 10.1016/S0140-6736(20)30367-6/ATTACHMENT/CEE43A30-904B-4A45-A4E5-AFE48804398D/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].National Academies of Sciences E and M, Division H and M, Policy B on HS, Impairment C on PD and C, Downey A, Stroud C, et al. Preventing Cognitive Decline and Dementia. Preventing Cognitive Decline and Dementia 2017. 10.17226/24782. [DOI] [PubMed] [Google Scholar]
- [6].Patterson R, McNamara E, Tainio M, de Sá TH, Smith AD, Sharp SJ, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol 2018;33:811. 10.1007/S10654-018-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ekelund U, Tarp J, Steene-Johannessen J, Hansen BH, Jefferis B, Fagerland MW, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ 2019;366. 10.1136/BMJ.L4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bellettiere J, Lamonte MJ, Evenson KR, Rillamas-Sun E, Kerr J, Lee IM, et al. Sedentary Behavior and Cardiovascular Disease in Older Women: The OPACH Study. Circulation 2019;139:1036–46. 10.1161/CIRCULATIONAHA.118.035312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Young DR, Hivert MF, Alhassan S, Camhi SM, Ferguson JF, Katzmarzyk PT, et al. Sedentary Behavior and Cardiovascular Morbidity and Mortality: A Science Advisory From the American Heart Association. Circulation 2016;134:e262–79. 10.1161/CIR.0000000000000440. [DOI] [PubMed] [Google Scholar]
- [10].Stephen R, Hongisto K, Solomon A, Lönnroos E. Physical Activity and Alzheimer’s Disease: A Systematic Review. The Journals of Gerontology: Series A 2017;72:733–9. 10.1093/GERONA/GLW251. [DOI] [PubMed] [Google Scholar]
- [11].Maasakkers CM, Claassen JAHR, Gardiner PA, Olde Rikkert MGM, Lipnicki DM, Scarmeas N, et al. The Association of Sedentary Behaviour and Cognitive Function in People Without Dementia: A Coordinated Analysis Across Five Cohort Studies from COSMIC. Sports Med 2020;50:403. 10.1007/S40279-019-01186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].LaMonte MJ, Lee I-M, Rillamas-Sun E, Bellettiere J, Evenson KR, Buchner DM, et al. Comparison of Questionnaire and Device Measures of Physical Activity and Sedentary Behavior in a Multi-Ethnic Cohort of Older Women. J Meas Phys Behav 2019;2:82–93. 10.1123/jmpb.2018-0057. [DOI] [Google Scholar]
- [13].Petermann-Rocha F, Lyall DM, Gray SR, Gill JMR, Sattar N, Welsh P, et al. Dose-response association between device-measured physical activity and incident dementia: a prospective study from UK Biobank. BMC Med 2021;19. 10.1186/S12916-021-02172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Anderson G, Cummings S, Freedman LS, Furberg C, Henderson M, Johnson SR, et al. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 1998. 10.1016/S0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- [15].Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, et al. The Women’s Health Initiative Memory Study (WHIMS): A trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials 1998;19:604–21. 10.1016/S0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- [16].Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JAE, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2663–72. 10.1001/JAMA.289.20.2663. [DOI] [PubMed] [Google Scholar]
- [17].LaCroix AZ, Rillamas-Sun E, Buchner D, Evenson KR, Di C, Lee I-M, et al. The Objective Physical Activity and Cardiovascular Disease Health in Older Women (OPACH) Study. BMC Public Health 2017;17:192. 10.1186/s12889-017-4065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Parada H, McDonald E, Bellettiere J, Evenson KR, LaMonte MJ, LaCroix AZ. Associations of accelerometer-measured physical activity and physical activity-related cancer incidence in older women: results from the WHI OPACH Study. Br J Cancer 2020;122:1409. 10.1038/S41416-020-0753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rapp SR, Legault C, Espeland MA, Resnick SM, Hogan PE, Coker LH, et al. Validation of a cognitive assessment battery administered by telephone. J Am Geriatr Soc 2012;60:1616. 10.1111/J.1532-5415.2012.04111.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Welsh KA, Breitner JC, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry Neuropsychol Behav Neurol 1993;6:103–10. [Google Scholar]
- [21].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–8. 10.1001/ARCHNEUR.56.3.303. [DOI] [PubMed] [Google Scholar]
- [22].Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2651–62. 10.1001/JAMA.289.20.2651. [DOI] [PubMed] [Google Scholar]
- [23].Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc 2011. 10.1249/MSS.0b013e3181ed61a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Evenson KR, Wen F, Herring AH, Di C, LaMonte MJ, Tinker LF, et al. Calibrating physical activity intensity for hip-worn accelerometry in women age 60 to 91years: The Women’s Health Initiative OPACH Calibration Study. Prev Med Rep 2015. 10.1016/j.pmedr.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee IM, Shiroma EJ, Kamada M, Bassett DR, Matthews CE, Buring JE. Association of Step Volume and Intensity With All-Cause Mortality in Older Women. JAMA Intern Med 2019;179:1105–12. 10.1001/JAMAINTERNMED.2019.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ngueleu AM, Barthod C, Best KL, Routhier F, Otis M, Batcho CS. Criterion validity of ActiGraph monitoring devices for step counting and distance measurement in adults and older adults: a systematic review. J Neuroeng Rehabil 2022;19:112. 10.1186/S12984-022-01085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, et al. How many steps/day are enough? For older adults and special populations. International Journal of Behavioral Nutrition and Physical Activity 2011;8:80. 10.1186/1479-5868-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Greenwood-Hickman MA, Nakandala S, Jankowska MM, Rosenberg D, Tuz-Zahra F, Bellettiere J, et al. The CNN Hip Accelerometer Posture (CHAP) Method for Classifying Sitting Patterns from Hip Accelerometers: A Validation Study. Med Sci Sports Exerc 2021. 10.1249/MSS.0000000000002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hays RD, Sherbourne CD, Mazel RM. The rand 36-item health survey 1.0. Health Econ 1993. 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- [30].LaMonte MJ, Lewis CE, Buchner DM, Evenson KR, Rillamas-Sun E, Di C, et al. Both Light Intensity and Moderate-to-Vigorous Physical Activity Measured by Accelerometry Are Favorably Associated With Cardiometabolic Risk Factors in Older Women: The Objective Physical Activity and Cardiovascular Health (OPACH) Study. J Am Heart Assoc 2017;6. 10.1161/JAHA.117.007064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ridker PM, Buring JE, Rifai N, Cook NR. Development and Validation of Improved Algorithms for the Assessment of Global Cardiovascular Risk in Women: The Reynolds Risk Score. JAMA 2007;297:611–9. 10.1001/JAMA.297.6.611. [DOI] [PubMed] [Google Scholar]
- [32].Hayden KM, Gaussoin SA, Hunter JC, Manson JAE, Sachs BC, Shadyab AH, et al. Cognitive resilience among APOE ε4 carriers in the oldest old. Int J Geriatr Psychiatry 2019;34:1833–44. 10.1002/GPS.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Palta P, Sharrett AR, Deal JA, Evenson KR, Gabriel KP, Folsom AR, et al. Leisure-Time Physical Activity Sustained Since Mid-life and Preservation of Cognitive Function: the Atherosclerosis Risk in Communities (ARIC) Study Cohort. Alzheimers Dement 2019;15:273. 10.1016/J.JALZ.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].del Pozo Cruz B, Ahmadi M, Naismith SL, Stamatakis E. Association of Daily Step Count and Intensity With Incident Dementia in 78 430 Adults Living in the UK. JAMA Neurol 2022. 10.1001/JAMANEUROL.2022.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 1999. 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- [36].Colbert LH, Matthews CE, Schoeller DA, Havighurst TC, Kim KM. Intensity of physical activity in the energy expenditure of older Adults. J Aging Phys Act 2014. 10.1123/JAPA.2012-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kraus WE, Janz KF, Powell KE, Campbell WW, Jakicic JM, Troiano RP, et al. Daily Step Counts for Measuring Physical Activity Exposure and Its Relation to Health. Med Sci Sports Exerc 2019;51:1206. 10.1249/MSS.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Beckett MW, Ardern CI, Rotondi MA. A meta-analysis of prospective studies on the role of physical activity and the prevention of Alzheimer’s disease in older adults. BMC Geriatr 2015;15:1–7. 10.1186/S12877-015-0007-2/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sabia S, Dugravot A, Dartigues JF, Abell J, Elbaz A, Kivimäki M, et al. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ (Online) 2017;357:1–12. 10.1136/bmj.j2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and Dementia in Physically Capable Elderly Men. JAMA 2004;292:1447–53. 10.1001/JAMA.292.12.1447. [DOI] [PubMed] [Google Scholar]
- [41].Zhu W, Wadley VG, Howard VJ, Hutto B, Blair SN, Hooker SP. Objectively Measured Physical Activity and Cognitive Function in Older Adults. Med Sci Sports Exerc 2017;49:47. 10.1249/MSS.0000000000001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wijndaele K, Westgate K, Stephens SK, Blair SN, Bull FC, Chastin SFM, et al. Utilization and Harmonization of Adult Accelerometry Data: Review and Expert Consensus. Med Sci Sports Exerc 2015;47:2129. 10.1249/MSS.0000000000000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rapp SR, Legault C, Espeland MA, Resnick SM, Hogan PE, Coker LH, et al. Validation of a cognitive assessment battery administered over the telephone. J Am Geriatr Soc 2012;60:1616–23. 10.1111/j.1532-5415.2012.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hackett RA, Davies-Kershaw H, Cadar D, Orrell M, Steptoe A. Walking Speed, Cognitive Function, and Dementia Risk in the English Longitudinal Study of Ageing. J Am Geriatr Soc 2018;66:1670–5. 10.1111/JGS.15312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tudor-Locke C, Barreira T v., Schuna JM. Comparison of step outputs for waist and wrist accelerometer attachment sites. Med Sci Sports Exerc 2015;47:839–42. 10.1249/MSS.0000000000000476. [DOI] [PubMed] [Google Scholar]
- [46].Nguyen S, Bellettiere J, Wang G, Di C, Natarajan L, LaMonte MJ, et al. Accelerometer-Derived Daily Life Movement Classified by Machine Learning and Incidence of Cardiovascular Disease in Older Women: The OPACH Study. J Am Heart Assoc 2022;11:23433. 10.1161/jaha.121.023433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The Trajectory of Gait Speed Preceding Mild Cognitive Impairment. Arch Neurol 2010;67:980–6. 10.1001/ARCHNEUROL.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Huang S-Y, Li Y-Z, Zhang Y-R, Huang Y-Y, Wu B-S, Zhang W, et al. Sleep, physical activity, sedentary behavior, and risk of incident dementia: a prospective cohort study of 431,924 UK Biobank participants. Molecular Psychiatry 2022. 2022:1–12. 10.1038/s41380-022-01655-y. [DOI] [PubMed] [Google Scholar]
- [49].Zhu N, Jacobs DR, Schreiner PJ, Launer LJ, Whitmer RA, Sidney S, et al. Cardiorespiratory fitness and brain volume and white matter integrity: The CARDIA Study. Neurology 2015;84:2347. 10.1212/WNL.0000000000001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Whitaker KM, Gabriel KP, Buman MP, Pereira MA, Jacobs DR, Reis JP, et al. Associations of accelerometer-measured sedentary time and physical activity with prospectively assessed cardiometabolic risk factors: The CARDIA study. J Am Heart Assoc 2019;8:1–11. 10.1161/JAHA.118.010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bliss ES, Wong RHX, Howe PRC, Mills DE. Benefits of exercise training on cerebrovascular and cognitive function in ageing. Journal of Cerebral Blood Flow and Metabolism 2021;41:447–70. 10.1177/0271678X20957807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, et al. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci 2014;8. 10.3389/FNHUM.2014.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bellettiere J, Winkler EAH, Chastin SFM, Kerr J, Owen N, Dunstan DW, et al. Associations of sitting accumulation patterns with cardio-metabolic risk biomarkers in Australian adults. PLoS One 2017;12:e0180119. 10.1371/journal.pone.0180119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wheeler MJ, Dempsey PC, Grace MS, Ellis KA, Gardiner PA, Green DJ, et al. Sedentary behavior as a risk factor for cognitive decline? A focus on the influence of glycemic control in brain health. Alzheimer’s & Dementia : Translational Research & Clinical Interventions 2017;3:291. 10.1016/J.TRCI.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nguyen L, Murphy K, Andrews G. Cognitive and neural plasticity in old age: A systematic review of evidence from executive functions cognitive training. Ageing Res Rev 2019;53:100912. 10.1016/J.ARR.2019.100912. [DOI] [PubMed] [Google Scholar]
- [56].Moniruzzaman M, Kadota A, Akash MS, Pruitt PJ, Miura K, Albin R, et al. Effects of physical activities on dementia-related biomarkers: A systematic review of randomized controlled trials. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 2020;6:e12109. 10.1002/TRC2.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Whitaker KM, Zhang D, Gabriel KP, Ahrens M, Sternfeld B, Sidney S, et al. Longitudinal associations of midlife accelerometer determined sedentary behavior and physical activity with cognitive function: The cardia study. J Am Heart Assoc 2021;10:1–18. 10.1161/JAHA.120.018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li G, Larson EB, Shofer JB, Crane PK, Gibbons LE, McCormick W, et al. Cognitive trajectory changes over 20 years prior to dementia diagnosis: a large cohort study. J Am Geriatr Soc 2017;65:2627. 10.1111/JGS.15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gardiner PA, Reid N, Gebel K, Ding D. Sitting Time and Physical Function in Australian Retirees: An Analysis of Bidirectional Relationships. J Gerontol A Biol Sci Med Sci 2018;73:1675–81. 10.1093/GERONA/GLY008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


