Key Clinical Message
Merkel cell carcinoma (MCC) is a rare, aggressive neuroendocrine cancer that primarily affects the elderly, Caucasians, and the immunocompromised. We present a rare case of an immunocompetent young Iranian (non‐Caucasian) female with a small nodule on her left arm. The lesion was initially misdiagnosed as an infected cyst and was treated with antibiotics for 20 days before being surgically removed. Unfortunately, the lump regrew rapidly 2 weeks later, when she had a biopsy, which revealed stage III MCC. She was then treated with adjuvant chemoradiotherapy after a thorough surgical resection of the tumor. Despite the fact that she was in remission after completing chemotherapy courses, she developed neutropenic fever, sepsis and died from septic shock. This case emphasizes the necessity of early clinical diagnosis of MCC and obtaining a biopsy with histopathologic evaluation of rapidly evolving skin lesions suggestive of malignancy.
Keywords: histopathology, immunohistochemistry, Merkel cell carcinoma, recurrence
1. INTRODUCTION
Merkel cell carcinoma (MCC) is a rare, life‐threatening tumor with neuroendocrine features. Due of its extremely low occurrence worldwide, epidemiological data on the disease are limited. 1 Commonly recognized risk factors include fair skin, history of skin cancer, old age, chronic immunosuppression, chronic ultraviolet (UV) light exposure, and Merkel cell polyomavirus (MCPyV) infection. 2 Among these factors, MCPyV and UV exposure play a fundamental role with synergistic effects in the pathophysiology of MCC. 3 Despite being a part of normal skin flora in most individuals, MCPyV DNA can clonally combine with the genome of neoplastic cell precursors at the initial phases of carcinogenesis. 4 , 5 Concurrently, UV exposure elicits antigen‐presenting dendritic cells to produce inflammatory cytokines, leading to local immunosuppression and creating an ideal environment for tumoral growth. 6 Moreover, immune hypersensitivity from UV exposure facilitates the viral tumorigenic process. 2
Merkel cells cannot be derived from the MCC because they lack the ability to proliferate. Merkel cell precursors (perhaps generated from epidermal stem cells or hair follicle stem cells) and pre‐ and pro‐B cells appear to have histopathology, genetics, and molecular characteristics with malignant cells instead. 7
MCC often presents as a single, asymptomatic erythematous or violaceous nodule, often mistaken for cysts or abscesses. It usually originates from the head or neck and generally spares the extremities. 8 Immunohistochemical (IHC) staining is required to validate histopathological findings of small round cells that infiltrate cutaneous or subcutaneous area. 7 Although MCC responds to the combination of excisional surgery, radiotherapy, and chemotherapy, it requires continuous follow‐ups within the first year of diagnosis due to the high recurrence rate. 9
Here, we present a 32‐year‐old Iranian (non‐Caucasian) immunocompetent female with a small nodule on her left arm at the disease onset finally diagnosed as MCC. In this report, we aim to emphasize the significance of early diagnosis and management of this cancer and highlight the complications that a late diagnosis would entail for these patients.
2. CASE REPORT
A 32‐year‐old female patient with an unremarkable medical history presented to the clinic with a small, non‐tender, and erythematous nodule on the dorsolateral aspect of her left arm, which initially appeared 3 months before. Since then, the nodule had slowly darkened and grown to 1 cm × 1 cm in size. Her history and physical examination were insignificant except for multiple warts on the dorsal aspect of the right hand. (Figure 1). Initially, the patient was suspected of having an infectious cyst and was treated with 10 days of antibiotics. However, she was unresponsive to antibiotics and was evaluated by a surgeon, who diagnosed the lesion as an abscess and surgically removed it.
FIGURE 1.

Multiple warts on dorsal aspect of the patient's right hand.
After 2 weeks, a rapidly growing mass measuring 4 cm × 4 cm originated from the incised area. The mass was surgically excised again but reappeared within 1 week, measuring 5 cm × 5 cm, after which the patient was referred to our hospital for further evaluation. A week later, the magnetic resonance imaging (MRI 1 ) of the left arm with and without contrast that was performed in our center showed multiple enhancing lesions at the subdermal region of the posterolateral aspect of the left arm with multiple enlarged axillary lymph nodes, the largest of which measured approximately 17 mm, suggestive of metastasis. The tumor was radically excised with negative surgical margins. Microscopic examination of the lesion demonstrated a neoplastic round cell tumor with prominent foci of necrosis (Figure 2). IHC staining revealed neoplastic cells with positive expression for CD99, Ck20, and NSE (Figure 2), but no LCA, Vimentin, CD3, CD20, and HMB45 expression consistent with MCC (Figure 3). Also, the MCPyV PCR real time of the lesion was positive.
FIGURE 2.
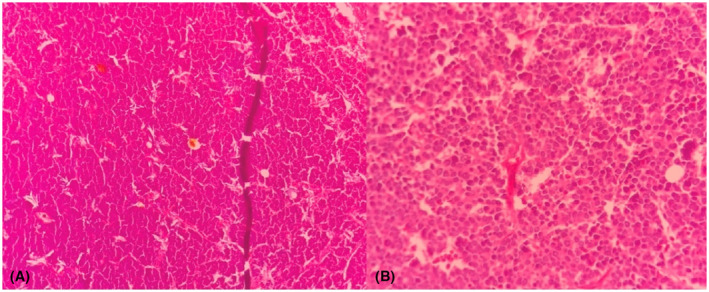
Hematoxylin and eosin (H&E) staining of the cutaneous lesion showing tumoral cells (10×) (A), (40×) (B).
FIGURE 3.
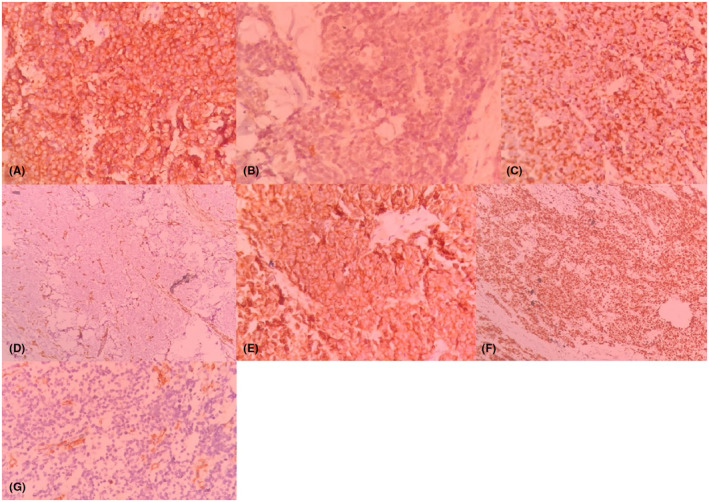
Immunohistochemical staining, the tumoral cells are positive for synaptophysin, (40×) (A), focally positive for S‐100 (40×) (B), positive for AE1/AE3 (40×) (C), negative for Vimentin (10×) (D), positive for CD56 (40×) (E), positive for Ki67 (10×) (F), and negative for CD45 (40×) (G).
High‐resolution computed tomography (HRCT) scan of the chest and abdominopelvic area with and without contrast showed lymphadenopathy 39 mm × 25 mm in the left axillary fossa along with a calcified lymph node (6 mm) in the abdominal cavity beyond the left abdominal muscle. She was diagnosed with stage III MCC and subsequently treated with adjuvant radiation to her left arm and axilla with 45.0 Grays (Gy) of radiation in 35 fractions, followed by six cycles of chemotherapy with etoposide and cisplatin. The patient's post‐treatment PET/CT scan showed no evidence of neoplastic disease and supported remission. However, high‐dose chemotherapy caused frequent myalgias and weakened immune system. Unfortunately, she developed febrile neutropenia and expired due to septic shock 1 month after the last chemotherapy course.
3. DISCUSSION
MCC is a rare, aggressive malignancy with an estimated annual incidence rate of 0.01–0.13 per 100,000 people globally. It predominantly affects the elderly (probably due to chronic UV radiation from the sun exposure), Caucasian males, and chronically immunocompromised patients, especially recipients of organ transplants, those with lymphoproliferative disorders, and untreated HIV infection. 1 Specifically, in the United States, more than 8 in 10 individuals diagnosed with MCC are older than 70 years old, and more than 95% are Caucasian. Furthermore, males are twice more likely to be diagnosed with MCC than females. 10 When MCC occurs in a younger population, it often involves children and is extremely rare in middle‐aged adults. 11
Because of its low incidence, particularly in non‐Caucasian populations, asymptomatic nature, and indistinguishable clinical presentation, MCC has a high rate of misdiagnosis. 12 A retrospective analysis of 195 patients diagnosed with MCC found that <1% were suspected of having MCC on clinical evaluation, leading to a median delay of more than 3 months from the initial appearance of the nodule to biopsy sampling. 13 Similarly, our patient was initially suspected of having an infectious lesion rather than MCC, which significantly delayed her initial presentation until her diagnosis. This case was further complicated by the absence of any risk factors in our patient. The most commonly recognized modifiable risk factors include MCPyV and long‐term UV radiation exposure, with more than half of primary MCC lesions originating from the head and neck region. 14 Despite the presence of MCPyV in her arm lesion and multiple warts on the dorsal aspect of both hands, she had none of the previously mentioned risk factors. Nevertheless, the presence of warts might indicate immunodeficiency.
The clinical presentation of MCC is often variable and nonspecific. MCC often presents as a tender, erythematous red to the violet‐colored lesion on sun‐exposed skin regions, most commonly the head and neck, but less commonly on the trunk or the extremities. 15 The only distinguishing characteristic of MCC is its rapid growth rate. Otherwise, the lesion may or may not have central ulceration and may present with superimposing infection, resulting in its misdiagnosis as an abscess. 2 , 16 Tender or non‐tender painless local or distant lymphadenopathy may also be present in case of lymph‐node metastasis or superimposed infection. 17
Barreira et al. reported a 70‐year‐old immunocompromised woman with painless inguinal lymphadenopathy. Further evaluations revealed a pink plaque in the left knee whose histopathology confirmed MCC with lymph node metastasis. Like our patient, she died due to a high tumor stage and metastasis at the disease onset. However, palliative treatment was indicated for this patient since she was dealing with underlying medical problems such as nephrectomy due to renal tuberculosis and non‐Hodgkin's lymphoma. 18
Similarly, Agut‐Busquet et al. reported a young white woman with a well‐defined subcutaneous mass measuring 3 cm × 2.5 cm in size located in the dorsal aspect of the left arm. Nevertheless, MCC was diagnosed before the tumor spread, resulting in more effective treatment and complete remission 1 year after the diagnosis. 19
Diagnostic imaging, including regional lymph nodes ultrasonography, CT scan, MRI, and PET‐CT scan, are often used for clinical staging and monitoring a patient's prognosis. Early clinical detection is essential, and the possibility of MCC should be considered in patients with rapidly evolving skin lesions unresponsive to antibiotic therapy. However, neither clinical evaluation nor imaging can accurately diagnose MCC, with histopathologic evaluation and IHC studies the gold standard diagnostic approach. 17 Histopathology generally displays small, uniformly rounded blue neoplastic cells with scanty cytoplasm. Even larger pleomorphic cells with increased proliferation rate, broad tissue infiltration, and lymphatic involvement may be detected. MCC‐specific IHC markers should confirm the diagnosis since they distinguish this cancer from other small round cell tumors. The malignant cells show positive immunoreactivity for CK20, CK8, CK18, CK19, synaptophysin, HIP1, P36, TTF1, ASH1, S100B, and CK7, while Vimentin does not stain in the IHC of MCC. 20
Clinical manifestations of patients easily distinguish between basal cell carcinoma (BCC) and metastatic cell carcinoma (MCC). In pathology, BCC neoplastic cells display size variability and stretched nuclei with marked peripheral palisading. Unlike BCC, local lymph node metastasis and intradermal spreading are characteristic of MCC. Nevertheless, atypical cases of these two malignancies share similarities, including the presence of mucin or amyloid in the stroma and peripheral slits located in the tumor borders. Therefore, IHC plays a significant role in differentiating these challenging samples. In contrast to BCC, MCC stains with CK20 and epithelial membrane antigen. 21
Small cell melanoma is a subtype of cutaneous melanoma that displays the intraepidermal pagetoid spread in which round or atypical dendritic melanocytes gather in nests. Despite expressing S100, the presence of keratins and NSE differentiates this skin cancer from MCC. 22 Although lymphoma presents quick indistinct inflammation with prominent small cells in histology, its hematolymphoid markers, such as PAX5, TdT, and immunoglobulins, are not detected in IHC staining of MCC. 23 Also, lymphoma lacks most IHC markers of MCC, such as CD45, CD3, and CD20. 24 Also, MCC and primary cutaneous Ewing sarcoma share similarities. Small tumor cells that may be positive for keratin, CD99, FLI‐1, and NSE may be seen in both types of tumors. CK20 and dot‐like keratin are not found in Ewing Sarcoma while EWSR1 translocation defect is specifically detected in this malignancy. 25
Imaging techniques, including ultrasonography of regional lymph nodes, CT scan, MRI, and PET‐CT scan associated with sentinel lymph node biopsy, are essential for clinical staging, prognosis, and patient follow‐up. The mainstay treatment of MCC is radical surgical excision accompanied by wide‐field adjuvant radiotherapy in patients with lymph node invasion. 7 Chemotherapy indicated for systemic eradication of neoplastic cells often fails to restrain tumor invasion and acts as palliative care. 26 Furthermore, retrospective analyses showing inconsistent result on the effects of post‐operative chemoradiation on patient survival outcomes. Likewise, immune‐check‐point inhibitors against pathways involved in pathogenesis are reserved for advanced‐stage cases unresponsive to chemotherapy. 27
In summary, patients diagnosed with MCC have a range of outcomes, as is the case with all malignancies. The likelihood of survival for people with MCC varies depending on the patient's illness stage at diagnosis. 28 , 29 MCC survival rates vary by illness stage. MCC staging considers initial tumor size, regional nodal basin status, and distant metastatic disease. 30 A more precise prognosis is possible once these indicators are identified in a given patient, and they can help guide patients and doctors as they consider the benefits and dangers of various treatment options. Here, we present a case of MCC with unspecific skin involvement who was misdiagnosed at first and then underwent multiple complicated surgeries. This report focused on the adverse effects of mismanagement in MCC that led to its spread and made all the therapeutic options ineffective. It underlined the consequences of delayed diagnosis in aggressive skin tumors, as higher stages are associated with dismal prognosis despite multidisciplinary approach and patient immunocompetency. Therefore, in rapidly growing and recurrent cutaneous lesions, prompt histopathologic assessment is required to improve the patient's overall survival and minimize side effects.
AUTHOR CONTRIBUTIONS
Fateme Salemi: Conceptualization; investigation; methodology; visualization; writing – original draft; writing – review and editing. Seyed Mohammad Reza Mortazavizadeh: Conceptualization; data curation; investigation; methodology; project administration; resources. Shokouh Taghipour Zahir: Conceptualization; methodology; supervision; writing – review and editing. Soroush Shahrokh: Writing – review and editing.
FUNDING INFORMATION
This case report study was not financially supported by any organization.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
ACKNOWLEDGMENTS
We thank Dr. Henry Ashworth, Medical student at Harvard Medical school for his helpful comments in revising the manuscript.
Salemi F, Mortazavizadeh SMR, Zahir ST, Shahrokh S. Near missed diagnosis of Merkel cell carcinoma in a young immunocompetent woman with a recurrent left‐arm mass: A case report. Clin Case Rep. 2023;11:e7587. doi: 10.1002/ccr3.7587
Endnote
We first met the patient after her last surgery. Unfortunately, the patient did not take any pictures from the arm mass at the disease onset and intervals between surgeries.
DATA AVAILABILITY STATEMENT
The case report data are not publicly available, but it could be available from the corresponding author with a reasonable request.
REFERENCES
- 1. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53‐69. [DOI] [PubMed] [Google Scholar]
- 2. Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3:17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Higaki‐Mori H, Kuwamoto S, Iwasaki T, et al. Association of Merkel cell polyomavirus infection with clinicopathological differences in Merkel cell carcinoma. Hum Pathol. 2012;43(12):2282‐2291. [DOI] [PubMed] [Google Scholar]
- 4. Church CD, Nghiem P. How does the Merkel polyomavirus lead to a lethal cancer? Many answers, many questions, and a new mouse model. J Invest Dermatol. 2015;135(5):1221‐1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong SQ, Waldeck K, Vergara IA, et al. UV‐associated mutations underlie the etiology of MCV‐negative Merkel cell carcinomas. Cancer Res. 2015;75(24):5228‐5234. [DOI] [PubMed] [Google Scholar]
- 6. Lunder EJ, Stern RS. Merkel‐cell carcinomas in patients treated with methoxsalen and ultraviolet a radiation. N Engl J Med. 1998;339(17):1247‐1248. [DOI] [PubMed] [Google Scholar]
- 7. Amaral T, Leiter U, Garbe C. Merkel cell carcinoma: epidemiology, pathogenesis, diagnosis and therapy. Rev Endocr Metab Disord. 2017;18(4):517‐532. [DOI] [PubMed] [Google Scholar]
- 8. Goessling W, McKee PH, Mayer RJ. Merkel cell carcinoma. J Clin Oncol. 2002;20(2):588‐598. [DOI] [PubMed] [Google Scholar]
- 9. McAfee WJ, Morris CG, Mendenhall CM, Werning JW, Mendenhall NP, Mendenhall WM. Merkel cell carcinoma: treatment and outcomes. Cancer. 2005;104(8):1761‐1764. [DOI] [PubMed] [Google Scholar]
- 10. Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49(5):832‐841. [DOI] [PubMed] [Google Scholar]
- 11. Llombart B, Requena C, Cruz J. Update on Merkel cell carcinoma: epidemiology, etiopathogenesis, clinical features, diagnosis, and staging. Actas Dermosifiliogr. 2017;108(2):108‐119. [DOI] [PubMed] [Google Scholar]
- 12. Linjawi A, Jamison WB, Meterissian S. Merkel cell carcinoma: important aspects of diagnosis and management. Am Surg. 2001;67(10):943‐947. [PubMed] [Google Scholar]
- 13. Oh SI, Jin US, Chang H, Kwon ST, Minn KW. A retrospective analysis of eight cases of Merkel cell carcinoma. Arch Craniofac Surg. 2013;14(1):41‐45. [Google Scholar]
- 14. An KP, Ratner D. Merkel cell carcinoma in the setting of HIV infection. J Am Acad Dermatol. 2001;45(2):309‐312. [DOI] [PubMed] [Google Scholar]
- 15. Medina‐Franco H, Urist MM, Fiveash J, Heslin MJ, Bland KI, Beenken SW. Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol. 2001;8(3):204‐208. [DOI] [PubMed] [Google Scholar]
- 16. Xue Y, Thakuria M. Merkel cell carcinoma review. Hematol Oncol Clin. 2019;33(1):39‐52. [DOI] [PubMed] [Google Scholar]
- 17. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78(3):445‐454. [DOI] [PubMed] [Google Scholar]
- 18. Barreira JV, Valejo Coelho MM. Unknown primary Merkel cell carcinoma with cutaneous spread. BMJ Case Rep CP. 2019;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agut‐Busquet E, Yebenes M, Luelmo J. Stony‐hard subcutaneous mass on the arm of a young woman. Actas Dermosifiliogr. 2016;107(2):149‐150. [DOI] [PubMed] [Google Scholar]
- 20. Leroux‐Kozal V, Lévêque N, Brodard V, et al. Merkel cell carcinoma: histopathologic and prognostic features according to the immunohistochemical expression of Merkel cell polyomavirus large T antigen correlated with viral load. Hum Pathol. 2015;46(3):443‐453. [DOI] [PubMed] [Google Scholar]
- 21. Panse G, McNiff JM, Ko CJ. Basal cell carcinoma: CD56 and cytokeratin 5/6 staining patterns in the differential diagnosis with Merkel cell carcinoma. J Cutan Pathol. 2017;44(6):553‐556. [DOI] [PubMed] [Google Scholar]
- 22. Kontochristopoulos GJ, Stavropoulos PG, Krasagakis K, Goerdt S, Zouboulis CC. Differentiation between Merkel cell carcinoma and malignant melanoma: an immunohistochemical study. Dermatology. 2000;201(2):123‐126. [DOI] [PubMed] [Google Scholar]
- 23. Battifora H, Silva EG. The use of antikeratin antibodies in the immunohistochemical distinction between neuroendocrine (Merkel cell) carcinoma of the skin, lymphoma, and oat cell carcinoma. Cancer. 1986;58(5):1040‐1046. [DOI] [PubMed] [Google Scholar]
- 24. Sur M, AlArdati H, Ross C, Alowami S. TdT expression in Merkel cell carcinoma: potential diagnostic pitfall with blastic hematological malignancies and expanded immunohistochemical analysis. Mod Pathol. 2007;20(11):1113‐1120. [DOI] [PubMed] [Google Scholar]
- 25. Fernandez‐Flores A, Suarez‐Penaranda JM, Alonso S. Study of EWS/FLI‐1 rearrangement in 18 cases of CK20+/CM2B4+ Merkel cell carcinoma using FISH and correlation to the differential diagnosis of Ewing sarcoma/peripheral neuroectodermal tumor. Appl Immunohistochem Mol Morphol. 2013;21(5):379‐385. [DOI] [PubMed] [Google Scholar]
- 26. Becker JC, Lorenz E, Ugurel S, et al. Evaluation of real‐world treatment outcomes in patients with distant metastatic Merkel cell carcinoma following second‐line chemotherapy in Europe. Oncotarget. 2017;8(45):79731‐79741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nghiem P, Bhatia S, Lipson EJ, et al. Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first‐line therapy. J Clin Oncol. 2019;37(9):693‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63(5):751‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Santamaria‐Barria JA, Boland GM, Yeap BY, Nardi V, Dias‐Santagata D, Cusack JC. Merkel cell carcinoma: 30‐year experience from a single institution. Ann Surg Oncol. 2013;20:1365‐1373. [DOI] [PubMed] [Google Scholar]
- 30. Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23(10):2300‐2309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The case report data are not publicly available, but it could be available from the corresponding author with a reasonable request.


