Abstract
The host restriction factor, Serinc5, incorporates into budding HIV particles and inhibits their infection by an incompletely understood mechanism. We have previously reported that Serinc5 but not its paralogue, Serinc2, blocks HIV cell entry by membrane fusion, specifically by inhibiting fusion pore formation and dilation. A body of work suggests Serinc5 may alter the conformation and clustering of the HIV fusion protein, Env. To contribute an additional perspective to the developing model of Serinc5 restriction, we assessed Serinc2 and Serinc5’s effects on HIV pseudoviral membranes. By measuring pseudoviral membrane thickness via cryo-electron microscopy (cryoEM) and order via the fluorescent dye, FLIPPER-TR, Serinc5 was found to increase membrane heterogeneity, skewing the distribution towards a larger fraction of the viral membrane in an ordered phase. We also directly observed for the first time the coexistence of membrane domains within individual viral membrane envelopes. Using a TIRF-based single particle fusion assay, we found that incorporation of exogenous phosphatidylethanolamine (PE) into the viral membrane rescued HIV pseudovirus fusion from restriction by Serinc5, which was accompanied by decreased membrane heterogeneity and order. This effect was specific for PE and did not depend on acyl chain length or saturation. Together, these data suggest that Serinc5 alters multiple interrelated properties of the viral membrane—lipid chain order, rigidity, line tension, and lateral pressure—which decrease the accessibility of fusion intermediates and disfavor completion of fusion. These biophysical insights into Serinc5 restriction of HIV infectivity could contribute to the development of novel antivirals that exploit the same weaknesses.
Graphical Abstract

Serinc5 is a recently described host restriction factor that incorporates into budding HIV particles and inhibits infection at the cell entry step1,2. While it is known to inhibit multiple steps of membrane fusion—hemifusion, fusion pore opening, and fusion pore dilation3,4—the exact mechanism by which it does so remains uncertain. Many studies have focused on the interaction between Serinc5 and the HIV fusion protein, Env, demonstrating that Serinc5 alters Env conformation5,6, antibody binding4,7 and clustering8. However, alterations in Env cannot fully explain the observed fusion defects induced by Serinc5 incorporation. In current models of viral membrane fusion, progression through receptor binding, hemifusion, and early fusion pore formation is driven by rearrangements of the viral fusion protein and membrane deformation9,10. However, the penultimate step, fusion pore dilation, is driven by membrane tension and curvature11. Disruption of fusion pore dilation, as we previously observed with Serinc5-containing HIV pseudoviruses3, suggests that Serinc5 may alter fluid mechanical properties of the viral membrane. Increased viral membrane stiffness would require a larger input of energy to deform the membrane into highly curved intermediates like hemifusion stalks12 and fusion pores13. Changing lipid biophysical properties of the viral membrane (often via cholesterol depletion) has been shown to reduce fusion of HIV and other enveloped viruses14,15,24,16-23.
While it has already been shown that Serinc5 does not change the overall lipid composition of the viral membrane25, membrane organization could be altered by other means, thereby affecting local concentrations of lipids. Model membranes of similar composition to the HIV membrane were shown to support liquid-liquid phase separation26-28. Specifically, areas enriched in saturated phospholipids, cholesterol and sphingolipids form domains with a more ordered packing of lipid acyl chains that coexist with regions of unsaturated phospholipids, less cholesterol and fewer sphingolipids packed in a more disordered manner; these two phases are termed liquid-ordered (Lo) and liquid-disordered (Ld), respectively29. As a consequence of ordered packing, membranes in an Lo phase have different lateral pressure profiles and are several angstroms thicker than membranes in an Ld phase30. Lipid order and packing parameters can be reported by specific fluorescent membrane probes31-33, some of which are also suitable for imaging in cellular and viral membranes. More recently, heterogeneity of membrane thickness was directly observed by cryogenic electron microscopy (cryoEM) in model membranes composed of pure lipids as well as isolated plasma membrane vesicles34,35. Additionally, the host plasma membrane, from which the HIV viral membrane is derived, has an asymmetric lipid distribution, in which the outer leaflet is enriched in sphingomyelin (SM) and phosphatidylcholine (PC), while phosphatidylethanolamine (PE) and phosphatidylserine (PS) are actively sequestered on the inner leaflet. PE and PS are only present in the outer leaflet of the plasma membrane in times of cellular stress36, but are detectable in the outer leaflet of the HIV envelope26,37-39 and thus steady-state asymmetry of plasma membrane lipids is assumed to be lost in viral particles.
Previously, we showed that the impediment to membrane fusion of Serinc5-containing HIV pseudoviruses is overcome by incorporation of the exogenous lipid Atto488-dimyristoyl PE (Atto488-DMPE) and the lipophilic antifungal drug amphotericin B, while fusion of HIV particles containing the non-restricting paralogue, Serinc2, is unaffected by these additions3. These data suggest that Serinc5 restriction may be dependent on the lipid environment of the viral membrane. To address the hypothesis that Serinc5 alters lipid bilayer properties of the HIV envelope, we systematically investigated the effects of Serinc5 incorporation on membrane order of pseudoviral particles and the physical and chemical properties of lipids required to overcome restriction by Serinc5.
Results
Serinc5 increases lipid acyl chain order in the viral membrane
To examine the effects of Serinc incorporation on the order and tension of the HIV lipid bilayer, we labeled HIV pseudovirus particles with the fluorescent membrane dye FLIPPER-TR. While FLIPPER-TR has been described as a reporter of membrane tension, it also reports on acyl chain packing in model or biological membranes. Viral membranes lack the typical sources of tension in cellular membranes (hydrostatic, cytoskeleton interactions, and adhesion) although it is possible that the HIV matrix protein (MA) introduces tension in the viral membrane11. Thus, in the context of viral membranes, FLIPPER-TR may primarily function as a reporter of membrane order and elastic modulus, i.e., membrane biophysical properties that are all inter-related. This push-pull fluorescent probe changes wavelength (red-shifted excitation) and has a longer lifetime in membranes with increased lipid order32,40,41. FLIPPER-TR has been used as a reporter of membrane order not only in lipid model membranes but also in a variety of biomembranes32,40,42. To calibrate the FLIPPER-TR method for application to pseudoviruses, which appear as diffraction limited spots by fluorescence microscopy, we first measured the fluorescence lifetimes of FLIPPER-TR-stained large unilamellar vesicles (LUVs) of comparable size to pseudoviruses (Fig. 1, left and Fig. S1) by fluorescence lifetime imaging microscopy (FLIM). LUVs were made of ternary lipid compositions previously reported to exist as all Lo, all Ld, or co-existing Lo/Ld phases43,44. As expected, we observed longer, shorter, and intermediate average lifetimes for Lo, Ld, and Lo/Ld LUVs, respectively (Fig. 1, left). The measured lifetimes of FLIPPER-TR in all LUVs studied here are shorter than previously reported lifetimes in membrane vesicles with a larger diameter, which may be due to altered pressure profiles and elastic moduli in vesicles with decreasing diameters45,46. The lifetimes of FLIPPER-TR in these LUVs composed of varying ratios of sphingomyelin (SM), cholesterol, and palmitoyl-oleoylphosphatidylcholine (POPC) were also comparable to LUVs with simpler compositions known to exist as all Lo, all Ld, or co-existing Lo/Ld phases (Fig. S2).
Figure. 1. Fluorescence lifetimes of the lipid order sensitive membrane dye, FLIPPER-TR, in liposomes of defined lipid composition and in HIV pseudovirus particles.
Large unilamellar liposomes (LUVs) composed of ternary mixtures of the major lipid components of the HIV envelope in ratios known to exist solely in Lo (35/40/25, SM/chol/POPC), solely Ld (25/5/70, SM/chol/POPC), or as a mixture of Lo and Ld (40/20/40, SM/chol/POPC) phases were used as standards for comparison. LUVs and HIV pseudoviruses were stained with FLIPPER-TR, adhered to a coverslip, and imaged by fluorescence microscopy. The fluorescent puncta were segmented from background by intensity thresholding and the average lifetime of all particles within a field of view are plotted as a point with mean and SD of all data points of identically prepared samples. The mean lifetime in nanoseconds of all data points for a condition is written above each bar. Data are from at least three independent preparations of LUVs and pseudoviruses. Multiple comparisons by Dunnett’s T3 multiple comparisons test: **** p<0.0001, ** p<0.01, * p<0.05, ns not significant.
HIV pseudoviruses were stained with FLIPPER-TR and imaged in the same manner by fluorescence lifetime microscopy. The lifetimes of the dye in pseudovirus membranes without Serincs or with Serinc2 (Fig. 1, right, orange and light blue circles, respectively), were similar to those in LUVs with coexisting Lo and Ld phases whereas the lifetimes in pseudovirus membranes that incorporate Serinc5 were longer, indicating more ordered membranes (Fig. 1, right, dark blue circles). These data show that unrestricted HIV pseudoviral membranes exhibit lipid chain order intermediate between the Lo and Ld extremes of model membranes and further support the hypothesis that Serinc5, but not Serinc2, alters the organization of lipids within the viral membrane to become more ordered.
Viral membrane thickness is heterogeneous and Serinc5 broadens the viral membrane thickness distribution
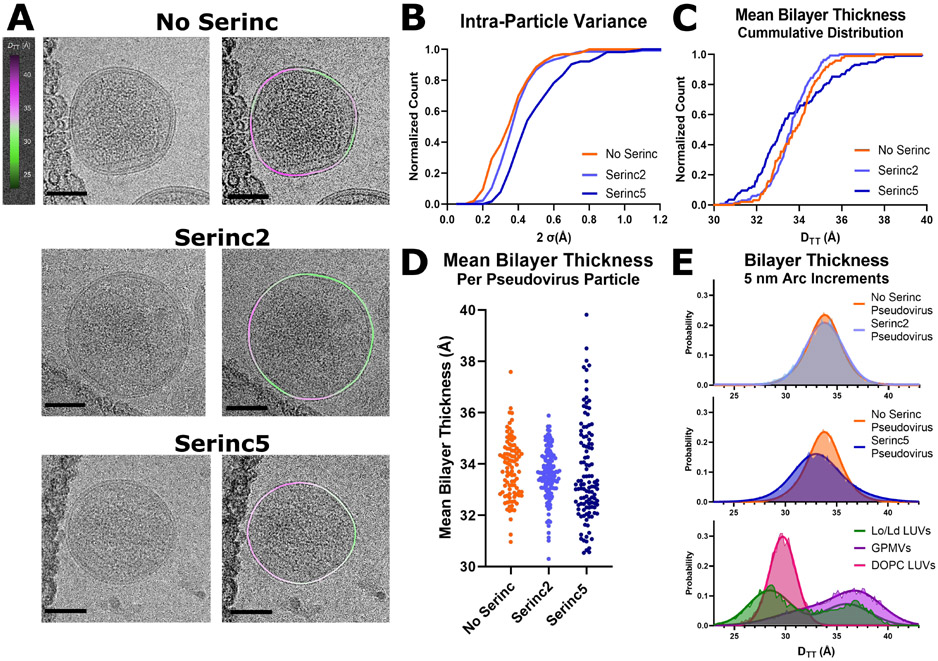
To examine the effects of Serinc5 incorporation on viral membranes at higher resolution, we turned to recently developed techniques for visualizing membrane heterogeneity by cryoEM34. Increased membrane order is usually accompanied by an increase in membrane thickness. However, it is not trivial to measure thickness in biological membrane samples, especially if the membrane itself contains coexisting areas of variable thickness. This problem can be overcome by applying cryoEM to LUVs and biologically derived membranes34,35. To detect membrane thickness variation in the envelopes of HIV pseudovirus particles, we flash-froze particles that incorporated Serinc2, Serinc5, or no Serincs and imaged them by cryoEM (Fig. 2). The membrane envelopes of the particles are characterized by two lines of high density, or “troughs” (Fig. S4C). The trough-to-trough distance (DTT) was previously found to match most closely with the hydrophobic thickness of the bilayer when compared with small-angle X-ray scattering34, suggesting that the trough position corresponds roughly to the glycerol backbone region of the bilayer. DTT of the viral membrane in these projection images was measured in 5 nm segments as described previously34 and example micrographs with DTT heat maps are shown in Fig. 2A and Fig. S3. We also frequently observe a line of density under the thinner portions of the membrane at a distance that is consistent with the matrix (MA) protein of HIV (Fig. S4).
Figure. 2. Serinc5 incorporation increases membrane heterogeneity and widens the thickness distribution of HIV pseudovirus particles as measured by cryoEM.
(A) Example micrographs (left) with membrane thickness (DTT) overlay (right) of HIV pseudoviruses prepared without Serincs (No Serinc), with Serinc2, or with Serinc5. The measured distances between intensity troughs (DTT) for 5 nm segments of the membranes were plotted as a smoothed, colored overlay according to the scale on the left. Scale bars are 50 nm. (B) 95% confidence intervals of the mean bilayer thickness (DTT) of individual HIV pseudovirus particles plotted as a cumulative distribution function. Mean membrane thickness (DTT) of individual HIV pseudoviruses plotted as a cumulative distribution function (C) and as a bee swarm plot (D). Each point represents one pseudovirus particle. Data are combined from at least two independent preparations of pseudoviruses with over 100 viral particles analyzed for each condition. (E) Normalized distributions of membrane thickness (DTT) measurements of 5 nm segments of HIV pseudovirus, LUV, or GPMV membranes with double Gaussian fits. 1-phase LUVs were composed of DOPC/POPG (95/5 molar ratio) and 2-phase LUVs were composed of a 40/20/35/5 ratio of DPPC/chol/DOPC/POPG. LUV and GPMV distributions reproduced with permission from 34.
The majority of particles from all three preparations show intra-particle variations in membrane thickness with contiguous areas of thicker or thinner membrane within any particle cross-section. Cumulative distribution plots of the standard deviation of membrane thickness within individual particles reveals substantially greater intra-particle thickness variance for Serinc5 particles compared to particles with Serinc2 or without Serincs (Fig. 2B). Averaging DTT per particle, the mean bilayer thicknesses were similar for HIV particles with Serinc2 and without Serincs, but the distribution of thicknesses for Serinc5-containing pseudoviruses is much broader, with about 35% of all particles exhibiting thicker and about 65% thinner membranes than the average No Serinc or Serinc2 particle (Fig. 2C, D), reinforcing and expanding on the fluorescence lifetime data shown in Fig. 1. The broader distribution of membrane thicknesses in Serinc5 particles may be attributable to the known variability in Serinc5 incorporation into individual particles4. The distribution of particle sizes is comparable for HIV pseudoviruses with and without Serincs and we detected no relationship between particle size and mean membrane thickness (Fig. S5). When the thicknesses of 5 nm segments of all analyzed pseudoviral membranes are plotted as probability histograms, the distributions of viral particles without Serincs and with Serinc2 are practically identical while the distribution from Serinc5-containing particles is broader, i.e. containing more regions of both thinner and thicker membrane areas (Fig. 2E). Comparing these pseudoviral distributions to previously published distributions of LUV and giant plasma membrane vesicle (GPMV) membranes34, all pseudoviral distributions show a single peak that is more broadly distributed than single phase LUVs, but still not as broadly distributed as GPMVs (Fig. 2E). While the pseudoviral distributions are centered between the Ld and Lo peaks of the two-phase LUVs, the GPMV distribution is skewed towards Lo-like thicknesses. While membrane thicknesses in neither pseudoviral particles nor GPMVs were clearly bimodally distributed as they are in the two-phase LUVs, the broad distributions suggest a higher complexity in the lipid phase behavior of biological compared to model membranes. Broader distributions would also result if domain sizes were smaller and more numerous because out of plane domains of different thicknesses may appear as intermediate thickness in projection images. As domain size approaches the segment length of the analysis (5 nm), the probability increases that a segment contains densities of both phases, resulting in more segments with intermediate thickness and thus a broader unimodal distribution rather than a clearly bimodal distribution.
Enrichment of the HIV pseudoviral membrane with PE reverses Serinc5’s fusion inhibition by decreasing membrane heterogeneity and order.
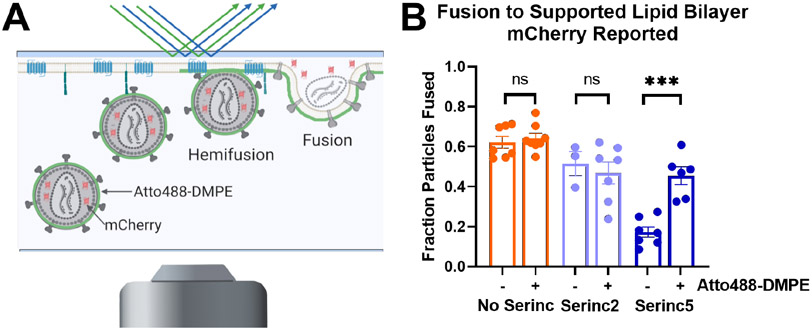
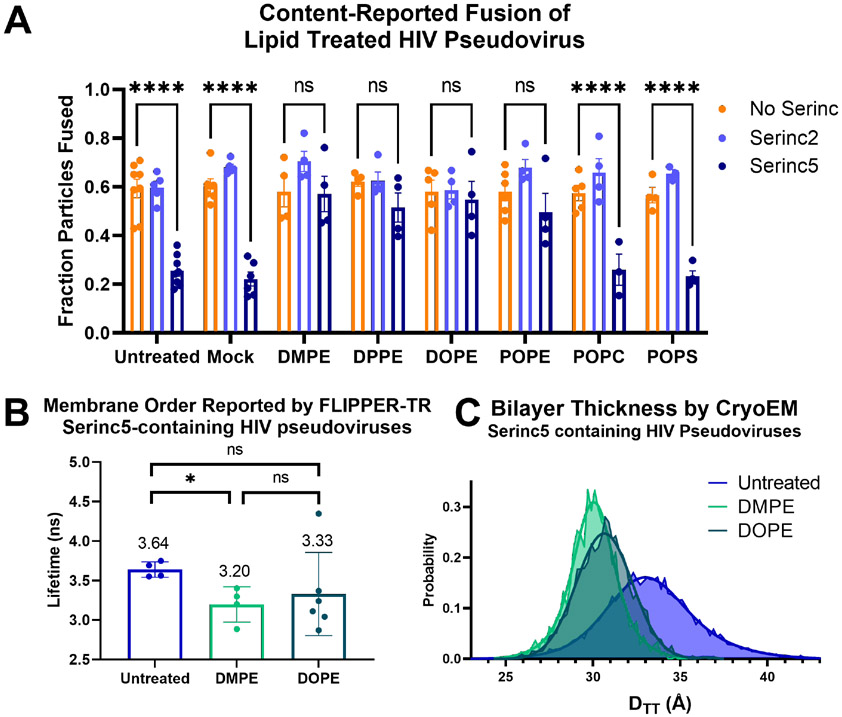
We used a previously described total internal reflection fluorescence (TIRF) microscopy-based, single-particle fusion assay to examine the effects of enrichment of HIV pseudovirus particles with exogenous lipids (Fig. 3A). Briefly, binding of pseudovirus particles engineered to incorporate an mCherry content marker to a supported planar plasma membrane containing CD4 receptor and CCR5 co-receptor produces a sudden appearance of punctate fluorescence in the observed evanescent field. After remaining stably bound for several imaging frames, the particle either fuses, as reported by a decay of fluorescence as the mCherry content diffuses away over multiple frames of recorded video, or becomes unbound from the membrane, reported by a sudden drop in fluorescence intensity back to baseline over a single frame. We have previously established strong concordance between fusion of HIV pseudoviruses in this assay with in vitro infection efficiency, including particles with or without Serincs3. Additionally, we showed that treatment with the polyene antifungal, Amphotericin B, which has complex interactions with membranes, increases infection and fusion of Serinc5-containing HIV pseudoviruses3. Similarly, labeling HIV pseudoviruses with the fluorescent lipid, Atto488-dimyristoylphosphatidylethanolamine (DMPE), increased fusion capability of Serinc5-containing HIV pseudoviruses (Fig. 3B reproduced from ref. 3). DMPE modified at the headgroup with Atto488 is chemically distinct from unmodified DMPE, so we assessed the effects of exogenous unmodified DMPE on fusion of HIV pseudoviruses incorporating Serinc5, Serinc2, or no Serinc (Fig. 4A) and found the same reversal of Serinc5’s inhibition of HIV membrane fusion. Additionally, we compared the effects of acyl chain length and saturation by examining the fusion of pseudoviruses enriched with dipalmitoyl (DP), dioleoyl (DO), and palmitoyl-oleoyl (PO) PE. Both saturated (DMPE and DPPE) and unsaturated (DOPE) lipids increased fusion of Serinc5-containing pseudoviruses with only minimal effect on pseudoviruses without Serincs or with Serinc2. Thus, restoration of fusion of Serinc5-containing pseudoviruses upon enrichment with PE is a function of the PE headgroup independent of acyl chain length or saturation.
Figure. 3. Lipid composition of the viral membrane affects Serinc restriction of HIV membrane fusion.
(A) Diagram of TIRF microscopy-based single HIV pseudovirus particle fusion assay. Pseudovirus binding to a supported planar plasma membrane containing CD4 receptor and CCR5 co-receptor is reported as a sudden appearance of bright puncta that remain stationary for several imaging frames. Fusion is reported by a decrease in fluorescence over several imaging frames as a genetically encoded content marker, mCherry, diffuses away. (B) Incorporation of Atto488-DMPE increases fusion of HIV pseudovirus particles containing Serinc5 but does not affect fusion of particles without Serinc or containing Serinc2. Each data point represents the fraction of particles fused on a separately prepared bilayer. Reproduced from ref. 3, copyright year 2020. ***, p < 0.001; ns, not significant by multiple unpaired t-tests via the Holm-Sidak method. Each condition includes data from at least three distinct preparations of pseudovirus.
Figure. 4. Enrichment of HIV pseudoviruses with PE overcomes restriction of fusion of Serinc5-containing particles by decreasing membrane heterogeneity and order.
HIV pseudoviruses with or without Serincs were enriched with the lipids listed on the X-axis, separated from free lipids, and (A) fusion with supported plasma membranes was assessed by TIRF microscopy-based single-particle membrane fusion. A two-way ANOVA test was conducted to examine the statistical significance of the effects of Serinc incorporation and lipid enrichment on HIV pseudovirus fusion. There was a statistically significant interaction between the two factors, F(14, 88)=4.894, p<0.001. Tukey’s multiple comparisons test showed very significant differences in the means of the comparisons shown above (**** p<0.0001, ns not significant). None of the No Serinc-Serinc2 comparisons were significant. Each data point represents the fraction of particles fused on a separately prepared supported membrane. Each condition includes data from at least three distinct preparations of pseudovirus. (B), Fluorescence lifetimes of FLIPPER-TR in lipid-enriched, Serinc5-containing HIV pseudoviruses. HIV pseudoviruses containing Serinc5 were stained with FLIPPER-TR after enrichment with the lipid listed on the X-axis and imaged by fluorescence microscopy as in Figure 1. Each data point is the result obtained from one field of view within a sample and is plotted with mean and standard deviation. Statistical significance by Welch’s unpaired t-test: * p<0.05, ns not significant. (C) Normalized distributions of membrane thickness (DTT) measurements of 5 nm segments of the same preparation of Serinc5-containing HIV pseudoviruses enriched with the lipid listed in the key as panel (B) and their corresponding double-Gaussian fits. The unenriched Serinc5 distribution is replotted from Figure 2E for comparison.
To further confirm that the PE headgroup is critical to counteract the fusion restriction of Serinc5, we enriched particles with palmitoyl-oleoyl-phosphatidylcholine (POPC) or palmitoyl-oleoyl-phosphatidylserine (POPS) and found that these lipids had no effect on fusion of any pseudoviruses tested (Fig. 4A). While fewer particles incorporated fluorescently labeled PC than PE or PS (Fig. S6A and C) and the amount of PC incorporated was lower than the amount of PE or PS (Fig. S6B), PS incorporation was equal to PE incorporation yet still failed to increase fusion of Serinc5-containing particles, indicating the specificity of PE in overcoming Serinc5-restriction of HIV membrane fusion. Due to their smaller headgroup, negative membrane curvature-inducing and hydrogen bonding capability, PEs are known to alter the lateral pressure profile and hence membrane bending elasticity of lipid bilayers47.
To better understand the mechanisms underlying the reversal of Serinc5’s inhibition of HIV fusion by PE enrichment, we applied the FLIM and cryoEM assays described above to assess membrane order of Serinc5-containing pseudoviruses that had been enriched with PEs of varying chain length and saturation (Fig. 4B-C and Fig. S7). DMPE is a lipid with two saturated 14-carbon chains and DOPE is an unsaturated lipid with two 18-carbon chains with one cis double bond each. Compared to unenriched Serinc5-containing pseudovirus particles, DMPE and DOPE-enriched particles had a clear trend to shorter FLIPPER-TR lifetimes with enrichment with DMPE showing a significant difference from unenriched (Fig. 4B). Imaging the same PE-enriched viral particles by cryoEM revealed there was a marked decrease in membrane thickness (as measured by DTT) for both PEs (Fig. 4C), echoing the decrease in membrane order reported by FLIPPER-TR. Additionally, the PE-enriched Serinc5 particles had much lower variance in membrane thickness than unenriched particles (Fig. S7C). From these data, it appears that enrichment with exogenous PE reverses Serinc5’s increase in membrane order and heterogeneity to restore the particles’ membrane fusion capability.
Discussion
In this study, we sought to better understand the mechanism by which Serincs restrict HIV membrane fusion and entry of HIV particles into cells. We previously established that Serinc5, but not Serinc2, inhibits fusion pore opening and constrict the widening of the fusion “neck” of virus particles fusing with plasma membranes that were derived from HIV receptor and co-receptor expressing cells3. Changes in the function of Env would be expected to primarily affect the early steps of membrane fusion that are dependent on conformational rearrangements of Env. However, the final step, pore enlargement, is thought to occur after all conformational rearrangements of Env are complete and thus is dependent on membrane properties such as curvature and line tension for energy input11. Serinc-dependent changes to the properties of the viral membrane could explain the observed defects in fusion pore dilation with viral envelopes containing Serinc5.
To reveal more insightful details on the mechanism by which Serincs inhibit virus entry, we employed fluorescence lifetime imaging and cryoEM methods to assess the importance of lipid order in the viral membrane for Serinc5’s inhibition of HIV membrane fusion. While HIV pseudoviral membranes with and without the non-restricting isoform, Serinc2, were nearly identical by FLIPPER-TR-reported membrane order (Fig. 1) and cryoEM-measured membrane thickness (Fig. 2B, 2C, 2D and 2E, top panel), pseudoviral membranes that incorporated Serinc5 were more ordered (Fig. 1) and more heterogeneous (Fig. 2B), and had a broader interparticle thickness distribution by cryoEM (Fig. 2C and 2D), with many more regions of increased and decreased membrane thickness than viral envelopes with Serinc2 or no Serincs (Fig. 2E, middle panel). These two techniques report on slightly different properties of membranes and while the direction of the changes they report are consistent, their magnitudes differ, as is common for techniques measuring membrane order48. More specifically, three factors could reconcile the broader thickness distributions observed by cryoEM and the increased lipid order measured by fluorescence lifetime imaging. First, as mentioned above, overlapping areas of Lo and Ld phases that are too small to be resolved in cryoEM projection images will lead to an apparent broadening of the membrane thickness distributions measured by cryoEM. Second, domain sizes may fluctuate in space and time and these fluctuations may be beyond the resolution of cryoEM such that observed thicknesses are blurred over those of fluctuating segments. Third, a more favorable partitioning of FLIPPER-TR into Lo than into Ld domains or perhaps a more complex behavior of the probe at lipid domain interfaces may explain the increased order measured in the Serinc5 particles. Notably, Raghunath et al. failed to detect increased membrane heterogeneity in Serinc5 containing HIV particles with fluorescent membrane order probes that report solvent accessibility, a membrane property that is frequently but not always related to membrane order50. Despite these caveats, we demonstrate with two techniques that increased heterogeneity in the viral membrane only occurred with incorporation of the restricting isoform, Serinc5, and not the non-restricting isoform, Serinc2, which strongly suggests the restriction function of Serinc5 is related to altered lipid order and organization in the viral membrane.
Our observation that PE increases fusion of Serinc5-containing viruses (Fig. 4A) is further demonstration of the importance of altered membrane properties for Serinc5-mediated fusion inhibition. The reversal of Serinc5’s function with PE enrichment is accompanied by a reversal of Serinc5’s effects on viral membrane organization with decreased order reported by FLIPPER-TR (Fig. 4B) and dramatically reduced membrane thickness by cryoEM (Fig. 4C). Again, these two techniques report on slightly different properties of ordered/disordered membranes, so while the magnitude of the effect of PE enrichment is different between the two measures, the overall direction of the change is consistent—PE enrichment reduces membrane order regardless of acyl chain length or saturation. The data strongly suggest that altered membrane order and heterogeneity is essential for Serinc5’s restriction of HIV membrane fusion.
The observation of coexistence of thicker and thinner membrane domains in HIV pseudoviral membranes (Fig. 2 and Fig. S3) is, to our knowledge, the first time that domain coexistence has been directly observed in a viral membrane. Previous bulk measurements of lipid order of HIV particles have shown the membrane to be largely ordered, but that lipid order in HIV particles also depends on the producing cell line51. Phase separation has been observed in supported lipid bilayers made from viral membrane lipid extracts26, but not in intact viral particles. Building on our observation of membrane heterogeneity in the HIV envelope, we note a line of density (Fig. S4) at a distance from the bilayer consistent with an incomplete MA layer of HIV52,53. Prior reconstitution experiments have shown thinning of the bilayer upon MA binding to model membranes54 and similarly, we observe MA density most frequently under thinner portions of the viral membrane, thus recapitulating prior reconstitution data in virio. It is possible that the underlying MA layer introduces tension and further changes the mechanical properties of the viral envelope, however, a more detailed analysis of MA dependent effects on the viral envelope is beyond the scope of the current study.
It has been shown previously that both Env and Serinc5 partition into membrane domains with more ordered lipids5,55 and that Serinc5 disrupts the clustering of Env in the viral membrane8. If clustering in the mature viral envelope is driven by confinement of Env to small, ordered lipid domains, increasing area or fragmentation of ordered domains could reduce clustering, thus decreasing the number of Env trimers available to catalyze fusion56. Additionally, Serincs have been hypothesized to alter Env conformation as demonstrated by multiple methods4-8,57,58. Changes in Env conformation, especially increased exposure of the membrane proximal external region (MPER) of Env, could also be a result of altered membrane thickness and order59.
As a result of increased order, the Serinc5 viral membrane is likely stiffer than a viral membrane without Serincs60, potentially explaining arrest of membrane fusion at highly curved intermediates (Fig. 5). Additionally, changing area fraction and distribution of ordered lipid domains may reflect altered line tension between domains in the viral membrane61. Previous work from our laboratory has shown that in phase separated viral and target membranes, the energy of line tension can drive HIV gp41-mediated fusion, that this effect depends on the size and distribution of ordered domains in the target membrane, and that increasing ordered domain area in a viral particle beyond its optimum would be expected to inhibit fusion62. Applying this simplified model to study the effect of increased ordered domain area in a membrane of viral particles with 110 nm diameter, we found that increasing the fraction of the membrane in an ordered domain first increases and then sharply decreases the contribution of line tension to the free energy change of fusion (Fig. S8). It is well known that the final step of fusion, fusion pore expansion, is dependent on lateral tension in the membrane11,63-66. In a more ordered membrane, the elastic modulus would be expected to change along with altered curvature and line tension, all of which affect the energy profile for membrane fusion47,67. Decreased lateral tension would also be expected to inhibit fusion pore expansion resulting in the increased frequency of early fusion products observed with cinched membranes3.
Figure. 5. Model of energy changes to fusion resulting from Serinc5-induced increase in membrane heterogeneity of the viral envelope.
The free energy change of intermediate steps (orange titles) along the fusion reaction is shown by the blue line. Proposed effects of Serinc5-induced alterations of membrane physical properties are listed in black near each intermediate state. Many of these properties are inter-related and are not easily disentangled. The effect of increased membrane heterogeneity on the contribution of line tension to the energetics of fusion is shown by the red dashed line.
Serinc 3 and 5’s possible functions as lipid binding and/or translocating integral membrane proteins25,57,68 could alter effective lipid concentrations and asymmetry in the viral membrane, and thus alter lipid order. Specifically, Serincs 3 and 5 may alter the distribution of PE across the two leaflets of the viral membrane. PE is known to enhance membrane fusion, especially when preferentially incorporated into the two contacting leaflets of the two fusing membranes69-71. As a cone-shaped molecule, PE induces frustrated negative curvature in the pre-fusion membrane, increasing lateral tension in the headgroup region47. In support of this hypothesis, we showed repletion of PE in the viral outer leaflet can overcome Serinc5’s inhibitory function on fusion (Fig. 4A) and that this was accompanied by larger changes in membrane order that likely promote fusion as well. The fundamental and unifying change underlying the potential hypotheses for Serinc 3 and 5’s mechanism of action is increased viral membrane heterogeneity.
Previously, we hypothesized that Serinc5 would need to cause “broad energetic changes” to the membrane fusion process to accumulate arrested intermediates at every step, as we observed3. With the data presented here, we show that Serinc5 increases viral membrane heterogeneity and that this is necessary for the partial inhibition of HIV fusion. Contextualizing previous observations and hypotheses about Serinc5’s mechanism of action with our data, we posit that alteration of membrane order underlies the other effects of Serinc5 incorporation into the HIV envelope. Following from this, it bears further study whether agents that increase membrane order could be effective anti-HIV therapeutics.
Methods
Cell lines, reagents, and plasmids:
HEK 293T/17 cells (ATCC) were maintained in high glucose Dulbecco’s Minimum Essential Media (Gibco) supplemented with 10% fetal bovine serum (Atlanta Biologicals), 1% antibiotic-antimycotic (Gibco), 1 mM sodium pyruvate (Gibco), and 2 mM glutamine (Gibco). CD4 and CCR5 overexpressing HeLa cells (gift of David M. Rekosh, University of Virginia) were maintained in Iscove’s Modified Dulbecco’s Medium (Gibco) supplemented with 10% fetal bovine serum and 1% antibiotic/antimycotic with 0.5 mg/mL of G418 (Gibco), and 1 μg/mL puromycin. All cells were maintained at 37°C with 5% CO2 atmosphere.
pHIV-luciferase, pHIV-Rev, and pHIV-pack were gifts of Wen Yuan (University of Virginia). pHIV-Env-SF162 was provided by the AIDS Reagent Program. pHIV-imCherry72 was a gift of Gregory Melikian (Emory University). pPBJ5-Serinc2-HA was a gift of Massimo Pizzato (University of Trento) and pPBJ5-Serinc5-HA was a gift of Heinrich Gottlinger (University of Massachusetts Medical School, Worcester).
The following compounds were purchased from Avanti Polar Lipids and used without modification: brain phosphatidylcholine (bPC), egg sphingomyelin (SM), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine (DMPE), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE), 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (POPG), 1-palmitoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphocholine (NBD-PC), 1-palmitoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphoserine (NBD-PS), and 1-palmitoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphoethanolamine (NBD-PE). Cholesterol was purchased from Sigma-Aldrich. 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-PEG3400-triethoxysilane (DPS) was synthesized as described previously73.
HIV pseudovirus preparation:
HIV pseudoviruses were produced as described before by transfection of HEK 293T cells (ATCC) with Lipofectamine 2000 (Invitrogen) and the following amounts of plasmids per 10 cm dish: 13 μg pHIV-luciferase, 5 μg pHIV-pack, 4 μg pHIV-Env-SF16274-76, 4 μg pHIV-imCherry, 1 μg pHIV-Rev and 4μg of pBJ5-Serinc2-HA1 or pBJ5-Serinc5-HA2 as indicated in text. pHIV-pack expresses the major HIV-1 polyproteins, Gag and Pol, while Env and Rev are expressed from separate plasmids. Additionally, a fluorescently tagged version of HIV gag is included to enable reporting of post-fusion content release in the TIRF-based fusion assay and luciferase under the HIV-1 5’ long terminal repeat (LTR) to allow easy titering of pseudovirus preparations. Culture media was changed 4-6 hours after transfection to phenol-red free DMEM supplemented with 10% FBS, 1% antibiotic-antimycotic, 1 mM sodium pyruvate, and 2 mM glutamine. Culture supernatants were harvested 2 days after transfection and cleared by centrifuging 5000xg before passing through a 0.22 μm filter. HIV pseudoviruses were pelleted through a 25% sucrose-HME (20 mM HEPES, 20 mM morpholineethanesulfonic acid [MES], 130 mM NaCl, 1 mM EDTA [pH 7.4]) cushion as previously described77 and resuspended in buffer HME without sucrose. Pseudovirus preparations for cryoEM were further purified by density dependent centrifugation on a discontinuous sucrose gradient composed of 65% sucrose-HME and 25% sucrose-HME spun 151,000xg for 18 hours. Pseudovirus was collected from the 65%/25% sucrose interface, diluted in buffer HME without sucrose, and repelleted through a 25% sucrose cushion. After resuspension in buffer HME without sucrose, the pseudovirus preparation was aliquoted and stored at −80°C. Additionally, the concentration of HIV p24 in each preparation was measured by ELISA78,79 and used to normalize the amount of pseudovirus added to downstream experiments. Quantification of Serinc incorporation into pseudoviral particles prepared in the same manner is shown in supplemental figures 3 and 8 of ref. 3. While Serinc5 incorporates into viral particles more readily than other Serincs, there is no relationship between level of Serinc incorporation and restriction activity80 for Serinc levels above a low threshold3.
Lipid enrichment of viruses:
10−10 moles of the indicated lipid were dried on the bottom of a glass test tube to remove chloroform/methanol solvent and resuspended by vigorous vortexing in buffer HB (20 mM HEPES, 150 mM NaCl [pH 7.4]) to yield a concentration of 1.4 μM. 21 ng of HIV pseudovirus, as measured by p24 ELISA, was mixed with the lipid suspension. The mixture was incubated at room temperature for 2 hours on a rotary spinner. To remove free lipid, the HIV pseudovirus mixture was diluted up to 1.5 mL in buffer HB and pelleted by spinning at 21,000xg for 1 hour at 4°C before final resuspension in buffer HB. Lipid-enriched HIV pseudoviruses were used within 24 hours.
Large unilamellar vesicle preparation:
Chloroform stocks of desired lipids were mixed and the solvent was evaporated under a gentle stream of nitrogen gas. The resulting lipid film was desiccated under vacuum for at least one hour before resuspension in buffer appropriate to the experiment to a final concentration of 1 mM. After vortexing at room temperature, the lipid suspension was subjected to 10 freeze/thaw cycles in liquid nitrogen and warm water before extrusion through two 100 nm polycarbonate membranes (Avestin). Resulting LUVs were stored at 4°C and used within 24 hours of extrusion.
Plasma membrane bleb preparation:
Blebs were produced from HeLa cells overexpressing CD4 and CCR5 by previously published methods19,81. Briefly, when cells reached 90% confluence, they were washed twice with blebbing buffer (10 mM HEPES, 150 mM NaCl, 2 mM CaCl2, pH 7.4) and blebbing was induced by replacing buffer on the cells with 5 mL of 25 mM formaldehyde (J.T.Baker) and 2 mM dithiothreitol (DTT) diluted in blebbing buffer and incubating the cells at 37°C, 5% CO2 for 1 hour. After an hour, blebs were detached from cells by shaking on a radial shaker at room temperature for 1 hour before the supernatant was collected and cleared of large cell debris by centrifuging at 100xg for 10 minutes. Blebs were pelleted at 20,000xg for 1 hour and washed twice in blebbing buffer without DTT or formaldehyde.
TIRF supported lipid bilayer fusion assay:
Supported planar plasma membranes derived from blebs were prepared as previously described19,73,82. Quartz slides were cleaned in piranha solution (95% H2SO4 and 30% H2O2 in a 3:1 ratio) and rinsed in 12 liters of deionized water. Next, a lipid monolayer composed of 4:1 brain phosphatidylcholine and cholesterol (Avanti Polar Lipids) with 3% 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-PEG3400-triethoxysilane was deposited on the quartz slide by the Langmuir-Blodgett method. A chloroform solution of the lipid mixture was applied to a Nima 611 Langmuir-Blodgett trough and after letting the solvent evaporate for 10 minutes, the lipid layer was compressed at a rate of 10 cm2/min to a pressure of 32 mN/m. A cleaned, rinsed, and dried quartz slide was rapidly dipped (68 mm/min) and slowly removed (5 mm/min) from the trough and then dried in a desiccator chamber overnight.
The slide was then assembled into a custom-built microscopy flow cell and plasma membrane blebs diluted in blebbing buffer without DTT or formaldehyde were flowed in to form the outer leaflet of the supported planar plasma membrane. After 1-2 hours at room temperature, the flow cell was washed with multiple volumes of blebbing buffer, then multiple volumes of buffer HB, and transferred to a prism-based TIRF microscope (Zeiss AxioObserver Z1). The sample was excited with a 561nm diode laser (OBIS 561 nm LS, Coherent) at an angle of 72 degrees from normal and emission light was filtered through a dichroic mirror (DC565, Semrock) and a band-pass filter (BP605/50, Semrock). Video was recorded by an EMCCD (DV887ESC-BV, Andor Technology) in frame transfer mode with an exposure time of 0.2 s for 13.3 minutes as a dilution of HIV pseudovirus totaling 21 ng of p24 as measured by ELISA was flowed into the chamber. Laser intensity, shutter, and camera were controlled by a custom LabView program (National Instruments).
Intensities of single particles over time were extracted with a custom-built LabView program and classified as representing binding without fusion or binding with fusion based on the criteria described in3.
FLIM data acquisition and analysis:
HIV pseudoviruses and LUVs were stained with 500 nM FLIPPER-TR (Cytoskeleton Inc.) for 2 hours at room temperature. Pseudoviruses and LUVs were resuspended in buffer HB and added to a poly-L-lysine coated coverslip. After liposomes and pseudoviruses were allowed to settle and adhere to the coverslip for 30 minutes, unbound sample was washed in buffer HB. Samples were imaged on a Leica Stellaris8 microscope with an 80 MHz pulsed white light laser, HyD detector, and FALCON software. Background was subtracted by intensity thresholding and the fluorescence lifetime of all pixels above the intensity threshold within the field of view was fit with two components per the n-component reconvolution with IRF function within Leica LAS-X software. Only the longer component is displayed as this is the more sensitive reporter of membrane order and tension32. For data collected in bulk (Fig. S2), LUVs were stained in the same manner but added to a quartz cuvette instead of a coverslip and spectra were acquired in a Fluorolog-QM 75-22-C (Horiba, Canada) spectrofluorometer with a SuperK Extreme high power super continuum white laser (NKT Photonics) in time-correlated single photon counting mode. Laser repetition rate was 5.5 MHz. Spectra were again fit to a two-component exponential curve with background subtraction of a buffer only spectrum with the integrated PowerFit-10 decay analysis package (Horiba).
CryoEM of HIV pseudovirus particles:
C-Flat 2/2-3C or 1.2/1.3-3C grids (Electron Microscopy Sciences) were glow discharged at 10 mA for 90 seconds. A suspension of HIV pseudovirus in buffer HME was applied and blotted from the grid before freezing in liquid nitrogen cooled ethane. For initial experiments, LUVs composed of 20/40/35/5 Chol/DPPC/DOPC/POPG were spiked into the pseudovirus suspension before freezing to serve as internal standards for quality control. The grids were imaged on a Titan Krios electron microscope operating at 300 kV equipped with a K3/GIF (Gatan) and controlled by EPU software (ThermoFisher Scientific). Magnification was 33,000X, which yielded a pixel size of 2.7 Å. As described in34, the optimal total dose was set at 13.8 e/Å2. Micrographs were motion corrected with MotionCorr283 (10 by 10 patch for 10 iterations with a tolerance of 0.5, dose weighted) before analysis of trough-to-trough distance as described previously34.
Calculation of the contribution of line tension to free energy change of membrane fusion:
The previously described model to calculate the free energy change due to changes in line tension during fusion between phase separated membranes62 was adapted to the parameters relevant to the presented experimental data. Specifically, fusion between a flat membrane and a vesicle of 110 nm diameter with one Lo and one Ld domain each, representing the plasma membrane and HIV viral membrane respectively. Assuming simple geometries without domain fluctuations, the boundary energy of an isolated domain is given by E = γL, where γ is the line tension and L is the circumference of the domain84. Line tension in both membranes is assumed to be 1 pN84 and the negative energy gain from line tension reduction (dE) relative to kBT (−dE/ kBT) was calculated for varying lipid domain sizes and fractions in both membranes. Depending on vesicle size, area fraction of Lo phase membrane, and domain sizes in the target membrane, fusion of the two membranes can result in an energy gain or energy cost from changes in line tension (Fig. S8).
Statistical Analysis:
All tests of statistical significance calculated in GraphPad Prism 9. Specific tests are listed in the figure legends.
Supplementary Material
Acknowledgments
We thank Drs. Barbie Ganser-Pornillos and Kelly Dryden for expert help with the collection of the cryo-EM data acquired at the Molecular Electron Microscopy Core at the University of Virginia, which is supported in part by NIH grant U24 GM116790. Additionally, we thank the lab of Dr. Cliff Stains for assistance with the collection of FLIM spectra.
Funding:
National Institutes of Health grant F30 HD101348 (AEW)
National Institutes of Health grant R01 AI30557 (LKT)
National Institutes of Health grant R01 GM138887 (FAH and MNW)
National Institutes of Health grant R35 GM134949 (IL)
National Institutes of Health grant R01 GM120351 (KRL)
National Institutes of Health grant T32 GM080186 (AEW)
National Institutes of Health grant T32 GM007267 (AEW)
Volkswagen Foundation Grant 93091 (IL)
William Wheless III professorship (MNW)
Footnotes
Supporting Information:
Figures S1-S8 including example fluorescence and cryoEM micrographs and model calculations (PDF)
Competing interests: Authors declare that they have no competing interests.
Data and materials availability: All data, code, and materials used in the analyses available upon request
References
- (1).Rosa A; Chande A; Ziglio S; De Sanctis V; Bertorelli R; Goh SL; McCauley SM; Nowosielska A; Antonarakis SE; Luban J; Santoni FA; Pizzato M HIV-1 Nef Promotes Infection by Excluding SERINC5 from Virion Incorporation. Nature 2015, 526 (7572), 212–217. 10.1038/nature15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Usami Y; Wu Y; Göttlinger HG SERINC3 and SERINC5 Restrict HIV-1 Infectivity and Are Counteracted by Nef. Nature 2015, 526 (7572), 218–223. 10.1038/nature15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ward AE; Kiessling V; Pornillos O; White JM; Ganser-Pornillos BK; Tamm LK HIV-Cell Membrane Fusion Intermediates Are Restricted by Serincs as Revealed by Cryo Electron and TIRF Microscopy. J. Biol. Chem 2020, 295 (45), 15183–15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Sood C; Marin M; Chande A; Pizzato M; Melikyan GB SERINC5 Protein Inhibits HIV-1 Fusion Pore Formation by Promoting Functional Inactivation of Envelope Glycoproteins. J. Biol. Chem 2017, 292 (14), 6014–6026. 10.1074/jbc.M117.777714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Schulte B; Selyutina A; Opp S; Herschhorn A; Sodroski JG; Pizzato M; Diaz-Griffero F Localization to Detergent-Resistant Membranes and HIV-1 Core Entry Inhibition Correlate with HIV-1 Restriction by SERINC5. Virology 2018, 515, 52–65. 10.1016/j.virol.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Staropoli I; Dufloo J; Ducher A; Commere P-H; Sartori-Rupp A; Novault S; Bruel T; Lorin V; Mouquet H; Schwartz O; Casartelli N Flow Cytometry Analysis of HIV-1 Env Conformations at the Surface of Infected Cells and Virions: Role of Nef, CD4, and SERINC5. J. Virol 2019, 94 (6). 10.1128/jvi.01783-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Beitari S; Ding S; Pan Q; Finzi A; Liang C Effect of HIV-1 Env on SERINC5 Antagonism. J. Virol 2017, 91 (4). 10.1128/JVI.02214-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Chen Y-C; Sood C; Marin M; Aaron J; Gratton E; Salaita K; Melikyan GB Super-Resolution Fluorescence Imaging Reveals That Serine Incorporator Protein 5 Inhibits Human Immunodeficiency Virus Fusion by Disrupting Envelope Glycoprotein Clusters. ACS Nano 2020, acsnano.0c02699. 10.1021/acsnano.0c02699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Harrison SC Viral Membrane Fusion. Virology 2015, 479–480, 498–507. 10.1016/j.virol.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).White JM; Whittaker GR Fusion of Enveloped Viruses in Endosomes. Traffic 2016, 17 (6), 593–614. 10.1111/tra.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Kozlov MM; Chernomordik LV Membrane Tension and Membrane Fusion. Current Opinion in Structural Biology. Elsevier Ltd; August 1, 2015, pp 61–67. 10.1016/j.sbi.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Gracià RS; Bezlyepkina N; Knorr RL; Lipowsky R; Dimova R Effect of Cholesterol on the Rigidity of Saturated and Unsaturated Membranes: Fluctuation and Electrodeformation Analysis of Giant Vesicles. Soft Matter 2010, 6 (7), 1472–1482. 10.1039/b920629a. [DOI] [Google Scholar]
- (13).Chernomordik LV; Kozlov MM Mechanics of Membrane Fusion. Nature Structural and Molecular Biology. Nat Struct Mol Biol; July 2008, pp 675–683. 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Domanska MK; Wrona D; Kasson PM Multiphasic Effects of Cholesterol on Influenza Fusion Kinetics Reflect Multiple Mechanistic Roles. Biophys. J 2013, 105 (6), 1383–1387. 10.1016/j.bpj.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Zawada KE; Wrona D; Rawle RJ; Kasson PM Influenza Viral Membrane Fusion Is Sensitive to Sterol Concentration but Surprisingly Robust to Sterol Chemical Identity. Sci Rep 2016, 6, 29842. 10.1038/srep29842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Kasson PM; Pande VS Control of Membrane Fusion Mechanism by Lipid Composition: Predictions from Ensemble Molecular Dynamics. PLoS Comput. Biol 2007, 3 (11), 2228–2238. 10.1371/journal.pcbi.0030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Yang S-T; Kreutzberger AJ; Lee J; Kiessling V; Tamm LK The Role of Cholesterol in Membrane Fusion. Chem. Phys. Lipids 2016, 199, 136–143. 10.1016/j.chemphyslip.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chlanda P; Mekhedov E; Waters H; Schwartz CL; Fischer ER; Ryham RJ; Cohen FS; Blank PS; Zimmerberg J The Hemifusion Structure Induced by Influenza Virus Haemagglutinin Is Determined by Physical Properties of the Target Membranes. Nat Microbiol 2016, 1 (6), 16050. 10.1038/nmicrobiol.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Yang S-T; Kreutzberger AJB; Kiessling V; Ganser-Pornillos BK; White JM; Tamm LK HIV Virions Sense Plasma Membrane Heterogeneity for Cell Entry. Sci. Adv 2017, 3 (6), e1700338. 10.1126/sciadv.1700338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Sun X; Whittaker GR Role for Influenza Virus Envelope Cholesterol in Virus Entry and Infection. J. Virol 2003, 77 (23), 12543–12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Haldar S; Mekhedov E; McCormick CD; Blank PS; Zimmerberg J Lipid-Dependence of Target Membrane Stability during Influenza Viral Fusion. J. Cell Sci 2019, 132 (4). 10.1242/jcs.218321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Biswas S; Yin SR; Blank PS; Zimmerberg J Cholesterol Promotes Hemifusion and Pore Widening in Membrane Fusion Induced by Influenza Hemagglutinin. J. Gen. Physiol 2008, 131 (5), 503–513. 10.1085/jgp.200709932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Lee J; Kreutzberger AJB; Odongo L; Nelson EA; Nyenhuis DA; Kiessling V; Liang B; Cafiso DS; White JM; Tamm LK Ebola Virus Glycoprotein Interacts with Cholesterol to Enhance Membrane Fusion and Cell Entry. Nat. Struct. Mol. Biol 2021, 28 (2), 181–189. 10.1038/s41594-020-00548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Wudiri GA; Schneider SM; Nicola AV Herpes Simplex Virus 1 Envelope Cholesterol Facilitates Membrane Fusion. Front. Microbiol 2017, 8 (DEC). 10.3389/fmicb.2017.02383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Trautz B; Wiedemann H; Lüchtenborg C; Pierini V; Kränich J; Glass B; Kräusslich H-G; Brocker T; Pizzato M; Ruggieri A; Brügger B; Fackler OT The Host-Cell Restriction Factor SERINC5 Restricts HIV-1 Infectivity without Altering the Lipid Composition and Organization of Viral Particles. J. Biol. Chem 2017, 292 (33), 13702–13713. 10.1074/jbc.M117.797332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Huarte N; Carravilla P; Cruz A; Lorizate M; Nieto-Garai JA; Kräusslich H-GG; Jesús P-G; Jose R-I; Nieva JLL Functional Organization of the HIV Lipid Envelope. Sci Rep 2016, 6 (1), 34190. 10.1038/srep34190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Brügger B; Glass B; Haberkant P; Leibrecht I; Wieland FT; Kräusslich HG The HIV Lipidome: A Raft with an Unusual Composition. Proc. Natl. Acad. Sci. U. S. A 2006, 103 (8), 2641–2646. 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Yang S-T; Kiessling V; Simmons JA; White JM; Tamm LK HIV Gp41–Mediated Membrane Fusion Occurs at Edges of Cholesterol-Rich Lipid Domains. Nat. Chem. Biol 2015, 11 (6), 424–431. 10.1038/nchembio.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Feigenson GW Phase Behavior of Lipid Mixtures. Nature Chemical Biology. Nature Publishing Group; 2006, pp 560–563. 10.1038/nchembio1106-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Levental I; Levental KR; Heberle FA Lipid Rafts: Controversies Resolved, Mysteries Remain. Trends in Cell Biology. 2020. 10.1016/j.tcb.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Ashdown GW; Owen DM Imaging Membrane Order Using Environmentally Sensitive Fluorophores. Methods Mol. Biol 2015, 1232, 115–122. 10.1007/978-1-4939-1752-5_10. [DOI] [PubMed] [Google Scholar]
- (32).Colom A; Derivery E; Soleimanpour S; Tomba C; Molin MD; Sakai N; González-Gaitán M; Matile S; Roux A A Fluorescent Membrane Tension Probe. Nat. Chem 2018, 10 (11), 1118–1125. 10.1038/s41557-018-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Niko Y; Didier P; Mely Y; Konishi G; Klymchenko AS Bright and Photostable Push-Pull Pyrene Dye Visualizes Lipid Order Variation between Plasma and Intracellular Membranes. 2016, 6, 18870. 10.1038/srep18870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Heberle FA; Doktorova M; Scott HL; Skinkle AD; Waxham MN; Levental I Direct Label-Free Imaging of Nanodomains in Biomimetic and Biological Membranes by Cryogenic Electron Microscopy. Proc. Natl. Acad. Sci. U. S. A 2020, 117 (33), 19943–19952. 10.1073/PNAS.2002200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Cornell CE; Mileant A; Thakkar N; Lee KK; Keller SL Direct Imaging of Liquid Domains in Membranes by Cryo-Electron Tomography. Proc. Natl. Acad. Sci. U. S. A 2020, 117 (33), 19713–19719. 10.1073/PNAS.2002245117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Lorent JH; Levental KR; Ganesan L; Rivera-Longsworth G; Sezgin E; Doktorova M; Lyman E; Levental I Plasma Membranes Are Asymmetric in Lipid Unsaturation, Packing and Protein Shape. Nat. Chem. Biol 2020, 16 (6), 644–652. 10.1038/s41589-020-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Callahan MK; Popernack PM; Tsutsui S; Truong L; Schlegel RA; Henderson AJ Phosphatidylserine on HIV Envelope Is a Cofactor for Infection of Monocytic Cells. J. Immunol 2003, 170 (9), 4840–4845. 10.4049/jimmunol.170.9.4840. [DOI] [PubMed] [Google Scholar]
- (38).Amara A; Mercer J Viral Apoptotic Mimicry. Nat. Rev. Microbiol 2015, 13 (8), 461–469. 10.1038/nrmicro3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Chua BA; Ngo JA; Situ K; Morizono K Roles of Phosphatidylserine Exposed on the Viral Envelope and Cell Membrane in HIV-1 Replication. Cell Communication and Signaling. BioMed Central Ltd. October 21, 2019, p 132. 10.1186/s12964-019-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Dal Molin M; Verolet Q; Colom A; Letrun R; Derivery E; Gonzalez-Gaitan M; Vauthey E; Roux A; Sakai N; Matile S Fluorescent Flippers for Mechanosensitive Membrane Probes. J. Am. Chem. Soc 2015, 137 (2), 568–571. 10.1021/ja5107018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Licari G; Strakova K; Matile S; Tajkhorshid E Twisting and Tilting of a Mechanosensitive Molecular Probe Detects Order in Membranes. Chem. Sci 2020, 11 (22), 5637–5649. 10.1039/d0sc02175j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Goujon A; Colom A; Straková K; Mercier V; Mahecic D; Manley S; Sakai N; Roux A; Matile S Mechanosensitive Fluorescent Probes to Image Membrane Tension in Mitochondria, Endoplasmic Reticulum, and Lysosomes. J. Am. Chem. Soc 2019, 141 (8), 3380–3384. 10.1021/jacs.8b13189. [DOI] [PubMed] [Google Scholar]
- (43).De Almeida RFM; Fedorov A; Prieto M Sphingomyelin/Phosphatidylcholine/Cholesterol Phase Diagram: Boundaries and Composition of Lipid Rafts. Biophys. J 2003, 85 (4), 2406–2416. 10.1016/S0006-3495(03)74664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Ionova IV; Livshits VA; Marsh D Phase Diagram of Ternary Cholesterol/Palmitoylsphingomyelin/ Palmitoyloleoyl-Phosphatidylcholine Mixtures: Spin-Label EPR Study of Lipid-Raft Formation. Biophys. J 2012, 102 (8), 1856–1865. 10.1016/j.bpj.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Lin CM; Li CS; Sheng YJ; Wu DT; Tsao HK Size-Dependent Properties of Small Unilamellar Vesicles Formed by Model Lipids. Langmuir 2012, 28 (1), 689–700. 10.1021/la203755v. [DOI] [PubMed] [Google Scholar]
- (46).Lipowsky R. Remodeling of Membrane Shape and Topology by Curvature Elasticity and Membrane Tension. Advanced Biology. John Wiley and Sons Inc; January 1, 2022. 10.1002/adbi.202101020. [DOI] [PubMed] [Google Scholar]
- (47).Fan ZA; Tsang KY; Chen SH; Chen YF Revisit the Correlation between the Elastic Mechanics and Fusion of Lipid Membranes. Sci. Rep 2016, 6 (1), 1–10. 10.1038/srep31470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Gupta A; Kallianpur M; Roy DS; Engberg O; Chakrabarty H; Huster D; Maiti S Different Membrane Order Measurement Techniques Are Not Mutually Consistent. Biophys. J 2022, 0 (0). 10.1016/j.bpj.2022.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Raghunath G; Chen Y-C; Marin M; Wu H; Melikyan GB SERINC5-Mediated Restriction of HIV-1 Infectivity Correlates with Resistance to Cholesterol Extraction but Not with Lipid Order of Viral Membrane. Viruses 2022, 14 (8), 1636. 10.3390/v14081636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).M’Baye G; Mély Y; Duportail G; Klymchenko AS Liquid Ordered and Gel Phases of Lipid Bilayers: Fluorescent Probes Reveal Close Fluidity but Different Hydration. Biophys. J 2008, 95 (3), 1217–1225. 10.1529/biophysj.107.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Lorizate M; Brügger B; Akiyama H; Glass B; Müller B; Anderluh G; Wieland FT; Kräusslich HG Probing HIV-1 Membrane Liquid Order by Laurdan Staining Reveals Producer Cell-Dependent Differences. J. Biol. Chem 2009, 284 (33), 22238–22247. 10.1074/jbc.M109.029256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Eells R; Barros M; Scott KM; Karageorgos I; Heinrich F; Lösche M Structural Characterization of Membrane-Bound Human Immunodeficiency Virus-1 Gag Matrix with Neutron Reflectometry. Biointerphases 2017, 12 (2), 02D408. 10.1116/1.4983155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Qu K; Ke Z; Zila V; Anders-Össwein M; Glass B; Mücksch F; Müller R; Schultz C; Müller B; Kräusslich H-G; Briggs JAG Maturation of the Matrix and Viral Membrane of HIV-1. Science (80-. ) 2021, 373 (6555), 700–704. 10.1126/science.abe6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).O’Neil L; Andenoro K; Pagano I; Carroll L; Langer L; Dell Z; Perera D; Treece BW; Heinrich F; Lösche M; Nagle JF; Tristram-Nagle S HIV-1 Matrix-31 Membrane Binding Peptide Interacts Differently with Membranes Containing PS vs. PI(4,5)P2. Biochim. Biophys. Acta - Biomembr 2016, 1858 (12), 3071–3081. 10.1016/j.bbamem.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Schwarzer R; Levental I; Gramatica A; Scolari S; Buschmann V; Veit M; Herrmann A The Cholesterol-Binding Motif of the HIV-1 Glycoprotein Gp41 Regulates Lateral Sorting and Oligomerization. Cell. Microbiol 2014, 16 (10), 1565–1581. 10.1111/cmi.12314. [DOI] [PubMed] [Google Scholar]
- (56).Brandenberg OF; Magnus C; Regoes RR; Trkola A The HIV-1 Entry Process: A Stoichiometric View. Trends in Microbiology. 2015. 10.1016/j.tim.2015.09.003. [DOI] [PubMed] [Google Scholar]
- (57).Leonhardt SA; Purdy MD; Grover JR; Yang Z; Poulos S; McIntire WE; Tatham EA; Erramilli S; Nosol K; Lai KK; Ding S; Lu M; Uchil PD; Finzi A; Rein A; Kossiakoff AA; Mothes W; Yeager M CryoEM Structures of the Human HIV-1 Restriction Factor SERINC3 and Function as a Lipid Transporter. bioRxiv 2022, 2022.07.06.498924. 10.1101/2022.07.06.498924. [DOI] [Google Scholar]
- (58).Kirschman J; Marin M; Chen Y-C; Chen J; Herschhorn A; A. B. S. III; Melikyan GB SERINC5 Restricts HIV-1 Infectivity by Promoting Conformational Changes and Accelerating Functional Inactivation of Env. Viruses 2022, 14 (7), 1388. 10.3390/v14071388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Hollingsworth LR; Lemkul JA; Bevan DR; Brown AM HIV-1 Env Gp41 Transmembrane Domain Dynamics Are Modulated by Lipid, Water, and Ion Interactions. Biophys. J 2018, 115 (1), 84–94. 10.1016/j.bpj.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Steinkühler J; Sezgin E; Urbančič I; Eggeling C; Dimova R Mechanical Properties of Plasma Membrane Vesicles Correlate with Lipid Order, Viscosity and Cell Density. Commun. Biol 2019, 2 (1). 10.1038/s42003-019-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Usery RD; Enoki TA; Wickramasinghe SP; Weiner MD; Tsai WC; Kim MB; Wang S; Torng TL; Ackerman DG; Heberle FA; Katsaras J; Feigenson GW Line Tension Controls Liquid-Disordered + Liquid-Ordered Domain Size Transition in Lipid Bilayers. Biophys. J 2017, 112 (7), 1431–1443. 10.1016/j.bpj.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Yang S-T; Kiessling V; Tamm LK Line Tension at Lipid Phase Boundaries as Driving Force for HIV Fusion Peptide-Mediated Fusion. Nat Commun 2016, 7, 11401. 10.1038/ncomms11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Kliesch TT; Dietz J; Turco L; Halder P; Polo E; Tarantola M; Jahn R; Janshoff A Membrane Tension Increases Fusion Efficiency of Model Membranes in the Presence of SNAREs. Sci. Rep 2017, 7 (1). 10.1038/s41598-017-12348-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Shillcock JC; Lipowsky R Tension-Induced Fusion of Bilayer Membranes and Vesicles. Nat. Mater 2005, 4 (3), 225–228. 10.1038/nmat1333. [DOI] [PubMed] [Google Scholar]
- (65).Staykova M; Holmes DP; Read C; Stone HA Mechanics of Surface Area Regulation in Cells Examined with Confined Lipid Membranes. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (22), 9084–9088. 10.1073/pnas.1102358108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Ryham RJ; Klotz TS; Yao L; Cohen FS Calculating Transition Energy Barriers and Characterizing Activation States for Steps of Fusion. Biophys. J 2016, 110 (5), 1110–1124. 10.1016/j.bpj.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Siegel DP The Gaussian Curvature Elastic Energy of Intermediates in Membrane Fusion. Biophys. J 2008, 95 (11), 5200–5215. 10.1529/biophysj.108.140152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Pye VE; Rosa A; Bertelli C; Struwe WB; Maslen SL; Corey R; Liko I; Hassall M; Mattiuzzo G; Ballandras-Colas A; Nans A; Takeuchi Y; Stansfeld PJ; Skehel JM; Robinson CV; Pizzato M; Cherepanov P A Bipartite Structural Organization Defines the SERINC Family of HIV-1 Restriction Factors. Nat. Struct. Mol. Biol 2020, 27, 78–83. 10.1038/s41594-019-0357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Kreutzberger AJB; Kiessling V; Liang B; Yang S-T; Castle JD; Tamm LK Asymmetric Phosphatidylethanolamine Distribution Controls Fusion Pore Lifetime and Probability. Biophys. J 2017, 113 (9), 1912–1915. 10.1016/J.BPJ.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Churchward MA; Rogasevskaia T; Brandman DM; Khosravani H; Nava P; Atkinson JK; Coorssen JR Specific Lipids Supply Critical Negative Spontaneous Curvature-an Essential Component of Native Ca2+-Triggered Membrane Fusion. Biophys. J 2008, 94 (10), 3976–3986. 10.1529/biophysj.107.123984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Chernomordik LV; Kozlov MM Membrane Hemifusion: Crossing a Chasm in Two Leaps. Cell. Elsevier; November 4, 2005, pp 375–382. 10.1016/j.cell.2005.10.015. [DOI] [PubMed] [Google Scholar]
- (72).Padilla-Parra S; Marin M; Gahlaut N; Suter R; Kondo N; Melikyan GB Fusion of Mature HIV-1 Particles Leads to Complete Release of a Gag-GFP-Based Content Marker and Raises the Intraviral PH. 2013, 8 (8), e71002. 10.1371/journal.pone.0071002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Wagner ML; Tamm LK Tethered Polymer-Supported Planar Lipid Bilayers for Reconstitution of Integral Membrane Proteins: Silane-Polyethyleneglycol-Lipid as a Cushion and Covalent Linker. Biophys. J 2000, 79 (3), 1400–1414. 10.1016/S0006-3495(00)76392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Cheng-Mayer C; Liu R; Landau NR; Stamatatos L Macrophage Tropism of Human Immunodeficiency Virus Type 1 and Utilization of the CC-CKR5 Coreceptor. J. Virol 1997, 71 (2), 1657–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Stamatatos L; Lim M; Cheng-Mayer C Generation and Structural Analysis of Soluble Oligomeric Gp140 Envelope Proteins Derived from Neutralization-Resistant and Neutralization-Susceptible Primary HIV Type 1 Isolates. AIDS Res. Hum. Retroviruses 2000. 10.1089/08892220050058407. [DOI] [PubMed] [Google Scholar]
- (76).Stamatatos L; Wiskerchen M; Cheng-Mayer C Effect of Major Deletions in the V1 and V2 Loops of a Macrophage-Tropic HIV Type 1 Isolate on Viral Envelope Structure, Cell Entry, and Replication. AIDS Res. Hum. Retroviruses 1998. 10.1089/aid.1998.14.1129. [DOI] [PubMed] [Google Scholar]
- (77).Hulseberg CE; Fénéant L; Wijs KMS; Kessler NP; Nelson EA; Shoemaker CJ; Schmaljohn CS; Polyak SJ; White JM Arbidol and Other Low-Molecular-Weight Drugs That Inhibit Lassa and Ebola Viruses. J. Virol 2019, 93 (8), e02185–18. 10.1128/JVI.02185-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Wehrly K; Chesebro B P24 Antigen Capture Assay for Quantification of Human Immunodeficiency Virus Using Readily Available Inexpensive Reagents. Methods 1997, 12 (4), 288–293. 10.1006/METH.1997.0481. [DOI] [PubMed] [Google Scholar]
- (79).Toohey K; Wehrly K; Nishio J; Perryman S; Chesebro B Human Immunodeficiency Virus Envelope V1 and V2 Regions Influence Replication Efficiency in Macrophages by Affecting Virus Spread. Virology 1995, 213 (1), 70–79. 10.1006/viro.1995.1547. [DOI] [PubMed] [Google Scholar]
- (80).Diehl WE; Guney MH; Kyawe PP; White JM; Pizzato M; Luban J Influence of Different Glycoproteins and of the Virion Core on SERINC5 Antiviral Activity. bioRxiv. bioRxiv; September 24, 2019, p 780577. 10.1101/780577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Sezgin E; Kaiser H-J; Baumgart T; Schwille P; Simons K; Levental I Elucidating Membrane Structure and Protein Behavior Using Giant Plasma Membrane Vesicles. Nat. Protoc 2012, 7 (6), 1042–1051. 10.1038/nprot.2012.059. [DOI] [PubMed] [Google Scholar]
- (82).Kalb E; Frey S; Tamm LK Formation of Supported Planar Bilayers by Fusion of Vesicles to Supported Phospholipid Monolayers. Biochim. Biophys. Acta - Biomembr 1992, 1103 (2), 307–316. 10.1016/0005-2736(92)90101-Q. [DOI] [PubMed] [Google Scholar]
- (83).Zheng SQ; Palovcak E; Armache J-P; Verba KA; Cheng Y; Agard DA MotionCor2: Anisotropic Correction of Beam-Induced Motion for Improved Cryo-Electron Microscopy. Nat. Methods 2017, 14 (4), 331–332. 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Kuzmin PI; Akimov SA; Chizmadzhev YA; Zimmerberg J; Cohen FS Line Tension and Interaction Energies of Membrane Rafts Calculated from Lipid Splay and Tilt. Biophys. J 2005, 88 (2), 1120–1133. 10.1529/biophysj.104.048223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.