Abstract
Nowadays, coffee (Coffea Arabica L.) is among the most significant agricultural products of the world and drinking coffee has become one of the most popular habits in the world. The main contamination of stored coffee beans is related with the mycotoxin produced by the toxigenic fungi belonging the genus Aspergillus. Fungal infection followed by mycotoxin biosynthesis in coffee results in notable financial losses. subsequent mycotoxin biosynthesis in coffee leads to major economic losses. Complications ranging from mild to severe can be caused by the mycotoxins produced by this genus. The aim of this investigation was to determine the effect of menthol and eugenol on Aspergillus parasiticus (CBS 100926T) growth, spore germination, and their potential use as green coffee beans preservative during long-term storage (12 months). The minimum inhibitory concentrations (MICs) values of the menthol and eugenol were recorded to completely inhibit the growth of A. parasiticus in 400 μg/ml and 300 μg/ml, respectively. Both reduced spore germination by 9.33% and 5.66% at 300 μg/ml and 200 μg/ml, respectively. They showed efficacy in fumigated green coffee beans sample during the storage for up to 12 months providing an increase in the protection level of 62.5% for menthol and 73.21% for eugenol against the A. parasiticus contamination. This suggests that menthol and eugenol could be used as good alternatives for decreasing the deteriorations due to the fungal infections in green coffee beans during long-term storage.
Keywords: Green coffee beans, A. parasiticus, Menthol, Eugenol, Fumigation, Antifungal activity
Highlights
-
•
Antifungal activity of menthol and eugenol against the toxigenic strain of Aspergillus parasiticus was assessed.
-
•
Menthol and eugenol decreased fungal growth of A. parasiticus at lower concentrations.
-
•
Menthol and eugenol showed above 50% protection of green coffee beans from A. parasiticus contamination.
-
•
Menthol and eugenol could be used as good alternatives for decreasing the deteriorations due to the fungal infections in green coffee beans during long-term storage.
1. Introduction
Coffee is among the most significant agricultural products of the world and coffee beverage is still considered enjoyable and valuable worldwide. The improvement and preservation of coffee quality are of critical importance, as the amount of sales mainly depends on the coffee quality.
Coffee is one of the most popular products in Algeria. Because all of the coffee is imported, they may become contaminated with toxigenic fungi during processing and storage. The prolonged storage of green coffee causes distinctive quality losses, those are mainly characterized by the cup's typical “flattening and slackening” [1]. These changes apparently are related to a reduction in the aroma potential in the green bean. Green coffee beans are prone to contamination over different processing phases [2,3]. Among all other spoilage agents, filamentous fungi represent the greatest health risk, with their potential to use harmful mycotoxins. In the absence of good practices, studies have shown that mycotoxins are present in a large percentage in coffee beans during storage, and even in the final drink [4]. Environmental conditions such as humidity and high temperatures promote the growth of fungi in stored coffee beans. As a consequence, good hygiene standards and manufacturing practices are strongly advised to decrease contamination risk in processed coffee [5]. The big concern in coffee production currently is climate change [6,7].
The genus Aspergillus contains the majority of the toxigenic fungi found in coffee [8] and the mycotoxins produced by this genus are characterized by their carcinogenicity, nephrotoxicity, immunotoxicity, hepatotoxicity, embryotoxicity, teratogenicity, and mutagenicity, in the literature [9]. Furthermore, the most important stage of strategy to protect the product from infection is the proper storage of green coffee beans after processing. Therefore, enhancement of the quality is only possible by preventing the adverse impacts of fungi contamination and their mycotoxins. Ochratoxin A (OTA) is the most extensively investigated mycotoxin growing in coffee [8,10,11]. However, other studies have also demonstrated the presence of aflatoxins (AFs) [9]. The synergistic effect of combining various mycotoxins is significant, and it occurs frequently in stored foodstuffs [6]. The International Agency for Research on Cancer (IARC) categorized OTA as a feasible carcinogen (group 2B) and AF as human carcinogens based on their carcinogenic effects (group 1). To protect the society from the effects of mycotoxin, developed countries have enacted mycotoxin regulations for both human food and animal feed.
The biological effect of essential oils (EOs) and their bioactive constituents is various. EOs have numerous uses in the food industry and agriculture [12]. Since EO are natural substances, they may also show some effects on fungal growth, sporulation, and even mycotoxin production [13]. As a result, they are proposed as promising and appropriate solutions to the problem of fungi and their mycotoxins contamination not only in coffee, but also in other food products.
There is survey on the occurrence of mycotoxins in the most commonly consumed traditional foods in Algeria and dietary exposure risk assessment [14]. Different food samples of couscous, wheat, nuts, metlou, figs, and Rechta were collected from daily markets, hypermarkets, groceries store, bakeries, cafeterias. Out of 198 samples, 82 (41.4%) were tested positive for at least one mycotoxin. As a result, the study recommended that the AFs concentration limits of authorized in foodstuffs in Algeria must be updated on a regular basis.
This study aims to look into the efficacy of menthol and eugenol on A. parasiticus growth, spore germination, and their potential use as green coffee bean preservatives over a long period of storage.
2. Materials and methods
2.1. Chemicals, solvents and culture media
Dimethyl sulfoxide (DMSO) and media comstituents vis. Potato Dextrose Agar (PDA) medium (Potato, 200 g; Dextrose, 20 g; Agar, 15 g and distilled water 1000 ml) were purchased from Aldrich Sigma (France). Commercial menthol (purity 99%) and eugenol (purity 98%), were purchased from Sigma-Aldrich (France). The components were maintained in glass opaque flasks at 4 °C until use. Green coffee beans (Coffea Arabica L.) were obtained from the Rouiba market in Algiers, Algeria.
2.2. Microorganism
A. parasiticus (CBS 100926T) was provided from Microbial Systems Biology Laboratory (LBSM), Kouba, Algiers and cultured on PDA medium at 28 ± 2 °C for 7 days. Spore inoculum (washing the 7-days culture of A. parasiticus with 20 ml of 0.1% Tween 80 solution) was prepared. Total spores were counted (approx. 1 × 106 spores/ml) using a hemocytometer slide (depth 0.2 mm, 1/400 mm2) under a light microscope (Motic: BA210, China) throughout the study.
2.3. Different menthol and eugenol concentrations and their effects on the inhibition of A. parasiticus
The effect of menthol and eugenol on the mycelial growth of A. parasiticus (CBS 100926T) was assessed using the method as described of José Velázquez-Nuñez et al. [15]. The obtained solution was mixed with the agar medium to produce concentrations ranging from 100 to 400 μg/ml PDA medium. After solidification, 10 μl of the fungal suspension (approx. 1 × 106 spores/ml) were accumulated in the culture medium. The control contained no menthol, eugenol, and their combination. The whole was incubated at 28 ± 2 °C. Mycelium growth was tracked by daily diameter measurement along two straight lines perpendicular to the center. The effectiveness of treatments was assessed at day 7. To determine the percentage of inhibition (I%), the following equation (1) was used:
| I% = (Dcontrol – DTest /Dcontrol) × 100 | (1) |
where DTest: Diameter of the test growth area in mm; Dcontrol: Diameter of the contol growth area in mm.
The minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) were determined for A. parasiticus (CBS 100926T) [16]. First of all, changing concentrations (100–400 μg/ml) of menthol and eugenol were included into 10 ml PDB liquid medium in test tubes. Control samples contained only PDB medium (10 ml). The inoculated tubes (containing 10 μl of spore suspensions) were incubated at 28 ± 2 °C for 7 days. The MIC was determined and then 100 μl of suspensions from the tubes were grown on PDA plates for MFC measurements.
2.4. Different concentrations of menthol and eugenol and their effects on spore germination of A. parasiticus (CBS 100926T)
Spores were collected by rubbing the mycelial surface with 5 ml of sterile distilled water containing 0.1% (v/v) Tween-80 from 7 day cultures of A. parasiticus (CBS 100926T) that had previously been exposed to menthol and eugenol (100–400 g/ml). For the control, the same procedure was used. Fungal spore suspensions were inoculated into fresh PDA medium in depression slides for 24 h at 28 °C. The spore germination percentage was determined [17].
2.5. Antifungal effect of menthol and eugenol vapors of stored coffee beans inoculated with A. parasiticus (CBS 100926T)
The fumigant efficacy of menthol and eugenol against A. parasiticus (CBS 100926T) in green coffee beans was evaluated as previously described by Prakash et al. [16]. The green coffee beans were surface-sterilized with a 1% solution of sodium hypochlorite, rinsed 3 times with sterilized distilled water, and dried. The antifungal effectiveness of menthol and eugenol was estimated by storing 1 kg separately in glass containers for 12 months at 15 °C and 60% relative humidity. Three milliliter of conidial suspension of A. parasiticus were uniformly sprayed on the green coffee beans. Then, menthol and eugenol were applied on sterile cotton swabs attached to the inner surface of the container stoppers and impregnated at their MIC values related to the air container volume. The containers containing the inoculated green coffee beans and the two compounds were tightly closed and sealed with parafilm. An identical group without compounds was served as a control.
For the mycological analysis, the spread-plate method was used. Treated and control samples (10 g each) were mechanically homogenized for 15 min in 250 ml flasks containing 90 ml of sterile Tween water (0.1%). Serial decimal dilutions (up to 10−3) of 0.1 ml (each), was plated and uniformly distributed on freshly prepared PDA. Inoculated Petri dishes were incubated at 28 ± 2 °C for 5 days [18]. The percentage of protection provided by coffee beans was calculated using the number of isolates of A. parasiticus found in treated and control samples, using formula (2):
| (2) |
%P = Protection percentage, Dc = Total number of A. parasiticus isolates from samples of control, Dt = Total number of A. parasiticus isolates from samples in treatment.
2.6. Statistical analysis
All data were expressed as mean ± standard deviations, and experiments carried out in triplicates. Analysis was conducted by one-way analysis of variance (ANOVA) and Tukey's post hoc test (p < 0.05) using STATISTICA software (Version 6).
3. Results and discussion
Menthol and eugenol have been shown to inhibit the growth of A. parasiticus (CBS 100926T) during the incubation period (7 days) as depicted in Fig. 1. Mycelium growth was significantly (p < 0.05) reduced in proportion to concentrations for all treatments, indicating dose-dependent activity, although the growth gradually increased over time. Mycelial growth was delayed by five days for menthol and for eugenol at 300 μg/ml and 200 μg/ml, respectively. On the seventh day, the percent inhibition of mycelia growth was determined, as shown in Fig. 2. The findings revealed that the inhibition percentage of A. parasiticus (CBS 100926T) relative to the control, was in the range of 55.81–82.94% for menthol and 61.89–89.11% for eugenol (p < 0.05).
Fig. 1.
Effect of different concentrations of menthol and eugenol on the growth of A. parasiticus (CBS 100926T) during 7 days. Values are means (n = 3) ± SD.
Fig. 2.
Percent inhibition of mycelial growth of A. parasiticus (CBS 100926T) after 7 days. Values are means (n = 3) ± SD.
Major components of EOs vis. Thymol, eugenol, anethole, menthol, citral, pinenes, cinnamaldhydes, carvacrol, and carvone have been extensively studied concerning their antimicrobial spectrum against different microorganisms, including bacteria and fungi [19,20]. Thus, eugenol is a phenolic component that is mostly taken from buds and leaves of clove and from cinnamon. This component has been thoroughly studied for its antimicrobial properties against a broad range of microorganisms [21]. Also, Prakash et al. [22] found that eugenol, the main constituent of Piper betle, was more potent as a fungal growth inhibitor than the whole EO. According to Abbaszadeh et al. [23], eugenol inhibited the growth of Cladosporium sp. and Aspergillus sp. significantly. On other hand, Soković et al. [24] reported the effecacy of Mentha piperita EO and its constituents against various foodborne fungi. Mentha EO exhibited potent antifungal activity, but less than pure menthol. Mishra et al. [25] reported that A. flavus presented sensibility to menthol on all tested concentrations. The research by Shin et al. [26] showed that thymol and linalool were effective on the significant inhibition of mycelial growth of Botrytis cinerea. In addition, Dammak et al. [27] showed that 1,8-cineole inhibited the mycelial growth of A. carbonarius. Eugenol, D- and l-limonene, α-pinene, nerol against A. niger, A. ochraceus and A. flavus have been also studied for their antifungal activity, by Mihai and Popa [28].
Molecules with a hydroxyl groups (menthol) and a non-localized electron system (eugenol) in the phenolic ring structure exhibited significant antifungal activity [29]. Studies have displayed that the main antimicrobial mechanism involves increased the permeability of cell membranes, which leads to leakage of intracellular components and cell death [30]. According to Hua et al. [31], eugenol causes morphological alterations which occurred in the hyphae and conidiophores of A. ochraceus at 250 μg/ml using scanning electron microscopy. As well, eugenol increased ergosterol production by about 45–85%. Furthermore, eugenol has been revealed to inhibit the H + ATPase system, leading to intracellular acidification and cell death [32,33]. In addition, menthol and eugenol can act directly on the plasma membrane [[34], [35], [36]].
Antifungal activity of menthol and eugenol was also assessed by detecting MIC and MFC using the liquid dilution method. With this method, better conditions for the contact of menthol and eugenol with fungal spores and for their homogeneous diffuse into the medium are assured, as reported by Prakash et al. [16].The MIC values for complete inhibition of growth of A. parasiticus were recorded at 400 μg/ml and 300 μg/ml, respectively. Hua et al. [31] revealed that the MIC of eugenol against A. ochraceus was 600 μg/ml and Ju et al. [30] reported that the MIC of eugenol against A. niger was 250 μg/ml, which indicates that different species may possess different sensitivity towards the same antifungal. Both components were fungicidal in nature and the MFCs against A. parasiticus were higher than the MICs of menthol (>400 μg/ml) and eugenol (300 μg/ml).With such a low MIC value, menthol and eugenol, would require lower doses to inhibit mold infestation in food, allowing them to be formulated as ideal plant-based antimicrobials.
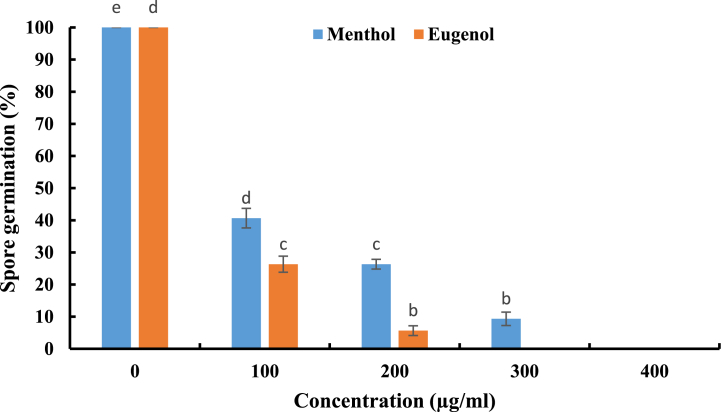
Spore is an important structure for the propagation of fungi. Based on the obtained results, menthol and eugenol showed inhibition of spore germination of A. parasiticus (CBS 100926T) at lower doses tested (Fig. 3). Significant efficacy of menthol and eugenol (p < 0.05) on the spore germination of A. parasiticus (CBS 100926T) was noted, after statistical analysis of the obtained results. Menthol (90.67%), eugenol (94.34%) had highest inhibitory effect on A. parasiticus (CBS 100926T) at 300 μg/ml and 200 μg/ml, respectively. Moreover, both menthol and eugenol exhibited a complete (100%) inhibition at 400 μg/ml.
Fig. 3.
Effect of menthol and eugenol on spore germination of A. parasiticus (CBS 100926T). Values are means (n = 3) ± SD.
The activity of several constituents on spore germination were also investigated. The activity of several constituents on spore germination were also investigated. Fernenol, arundoin, and a combination of stigmasterol and -sitosterol strongly inhibited Colletotrichum gloeosporioides spore germination and subsequent germ tube with 45.8, 62.3, and 86.9 mg/l, respectively [37]. Nishino et al. [38] showed that 1-Phenyl-3-pentanone from an edible mushroom had significantly inhibitory spore germination of some plant-pathogenic fungi 35 ppm. Tian et al. [39] showed that nerol reduced spore germination of A. flavus at 0.8 μl/ml. Thymol has been depicted to inhibit spore germination of fungi such as Rhizoctonia solani, Alternaria mali and Phytophthora capsici [40]. Wang et al. [41] showed that spore germination of A. niger with an increasing concentration of nerol. More recently, Zhou et al. [36] reported that carvacrol and eugenol inhibited R. stolonifer germination.
The efficacy of menthol and eugenol on spores may be as a consequence of enzymes denaturation implicated in spore germination or interference with amino acids involved in germination [42,43].
Menthol and eugenol had great antifungal activity when applied to coffee beans those are artificially contaminated with A. parasiticus. Green coffee beans were fumigated with both compounds as potential natural food preservatives. Menthol and eugenol showed remarkable efficacy in fumigated green coffee beans sample during storage up until 12 months supplying protection from A. parasiticus contamination of 62.5% for menthol and 73.21% for eugenol as presented in Table 1.
Table 1.
Control of A. parasiticus (CBS 100926T) of green coffee beans after storage fumigated with menthol and eugenol.
| Sample | Concentration (μg/ml) |
A. parasiticus isolates |
% protection | |
|---|---|---|---|---|
| Control | fumigation | |||
| Menthol | 400 | 112 | 42 | 62.50 |
| Eugenol | 300 | 112 | 30 | 73.21 |
Aspergillus species reduce food quality and safety by the production of spoilage enzymes that induce discoloration and abnormal flavour, in addition to the synthesis of mycotoxins such as aflatoxins. The most important step of protecting the coffee beans from fungal and mycotoxin contamination is their proper storage after processing [44]. Improving the quality (not only the final product but also during the storage and transportation) and streamlining of coffee processing, is critical to mitigating the negative effects of fungi and mycotoxins contamination. One solution is to use plant-based products, such as EOs and their major constituents.
Natural antifungals are eco-friendly and safer than synthetic chemical fungicides. The preservative effects in food items requires higher concentrations of EOs and their major compounds [14,45,46], which can be explained by the formation of food lipids an envelope around microorganisms to protect them antimicrobial agents [47]. As previously reported, greater concentrations of EOs are needs in foodstuffs than in laboratory media, possibly because the lower water content of coffee beans compared to culture media can hinder the transport of antimicrobial molecules to the active site in the microbial cell [35]. According to the study of Hlebová et al. [48], the use of EOs appears to be an effective method for protecting stored coffee from harmful fungi without compromising the organoleptic properties (aroma and taste) of coffee beverages.
Although previous research has shown that the use of vapors is an appropriate method technique for controlling food contamination because it leaves no remaining components [46], their high volatility is the critical issue regarding their applications and shelf life. The microencapsulation method is a suitable technique to solve the problem. Encapsulation provide effective protection of natural compounds against chemical reactions and undesirable interactions with other food components. In addition, it improves solubility, decreases migration and preserves the stability of bioactive compounds during food processing and storage [[49], [50], [51], [52]].
4. Conclusion
The use of natural sources to control the contamination in food commodities and prolong their shelf life have received a raised attention, recently. Biologically active natural components possess the possibility to take the replace of synthetic fungicides. The EOs are also among the plant extracts alternatives that are applicable against the fungal contamination, thereby prolonging shelf life. Although most of the EOs have been presented to provent postharvest fungi under in vitro conditions, the studies on the in vivo effectiveness and practical activity of most of EOs have been very limited. The present study revealed menthol and eugenol can be effectively used to reduce fungal contamination caused by A. parasiticus during long-term storage. Furthermore, menthol and eugenol may be commercialized for the treatment of postharvest diseases, and possibly for other agricultural products.
Funding
This research was supported by project PRFU/ALG-MICO (MESRS Grant No: D00L01UN150120180002)- Algeria. Funding for open access charge: Universidade de Vigo/CISUG.
Author contribution statement
Yamina Ben Miri: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Ahmed Nouasri: Performed the experiments. Amina Benabdallah, Abderrahim Benslama, Zeynep Tacer-Caba: Analyzed and interpreted the data; Wrote the paper. Affaf Laassami, Djamel Djenane: Contributed reagents, materials, analysis tools or data. Jesus Simal-Gandara: Conceived and designed the experiments; Wrote the paper. Data availability statement: Data will be made available on request. Declaration of interest's statement: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Dellafiora L., Dall'Asta C., Galaverna G. Toxicodynamics of mycotoxins in the framework of food risk assessment—an in silico perspective. Toxins. 2018;10(52) doi: 10.3390/toxins10020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batista L.R. Toxigenic fungi associated with processed (green) coffee beans (Coffea arabica L.) Int. J. Food Microbiol. 2003;85:293–300. doi: 10.1016/s0168-1605(02)00539-1. [DOI] [PubMed] [Google Scholar]
- 3.Heintz M.M., Doepker C.L., Wikoff D.S., Hawks S.E. Assessing the food safety risk of ochratoxin A in coffee. A toxicology-based approach to food safety planning. J. Food Sci. 2021;86(11):4799–4810. doi: 10.1111/1750-3841.15938. [DOI] [PubMed] [Google Scholar]
- 4.Casas-Junco P.P., Ragazzo-Sánchez J.A., Ascencio-Valle F.J., Calderón-Santoyo M. Determination of potentially mycotoxigenic fungi in coffee (Coffea arabica L.) from Nayarit. Food Sci. Biotechnol. 2018;27(3):891–898. doi: 10.1007/s10068-017-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viegas C., Gomes B., Oliveira F., Dias M., Cervantes, Pena P., Gomes A.Q., Caetano L.A., Carolino E., de Andrade E.T. Microbial contamination in the coffee industry: an occupational menace besides a food safety concern? Int. J. Environ. Res. 2022;19 doi: 10.3390/ijerph192013488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paterson R.R.M., Lima N., Taniwaki M.H. Coffee, mycotoxins and climate change. Int. Food Res. J. 2014;61:1–15. [Google Scholar]
- 7.Poltronieri P., Rossi F. Challenges in specialty coffee processing and quality assurance. Rev Challenges. 2016;7:19. [Google Scholar]
- 8.Pakshir K., Dehghani A., Nouraei H., Zareshahrabadi Z., Zomorodian K. Evaluation of fungal contamination and ochratoxin A detection in different types of coffee by HPLC-based Method. J. Clin. Lab. 2021;35 doi: 10.1002/jcla.24001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Ghouti M.A., AlHusaini A., Abu-Dieyeh M.H., Elkhabeer M.A., Alam M.M. Determination of aflatoxins in coffee by means of ultra-high performance liquid chromatography-fluorescence detector and fungi isolation. Int. J. Food Microbiol. 2020;85:293–300. [Google Scholar]
- 10.Geremew T., Abate D., Landschoot S., Haesaert G., Audenaert K. Occurrence of toxigenic fungi and ochratoxin A in Ethiopian coffee for local consumption. Food Control. 2016;69:65–73. [Google Scholar]
- 11.Leitão A.L. Occurrence of ochratoxin A in coffee: threads and solutions—a mini-review. Beverages. 2019;5:36. [Google Scholar]
- 12.Reddy D.N. In Natural Bio-Active Compounds. Springer; Singapore: 2019. Essential oils extracted from medicinal plants and their applications; pp. 237–283. [Google Scholar]
- 13.Singh B.K., Tiwari S., Dubey N.K. Essential oils and their nanoformulations as green preservatives to boost food safety against mycotoxin contamination of food commodities: a review. J. Sci. Food Agric. 2021;101:4879–4890. doi: 10.1002/jsfa.11255. [DOI] [PubMed] [Google Scholar]
- 14.Belasli A., Ben Miri Y., Aboudaou M., Aït Ouahioun L., Montañes L., Ariño A., Djenane Dantifungal D. antitoxigenic, and antioxidant activities of the essential oil from laurel (Laurus nobilis L.): potential use as wheat preservative. Food Sci. Nutr. 2020:1–13. doi: 10.1002/fsn3.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.José Velázquez-Nuñez M., Avila-Sosa R., Palou E., López-Malo A. Antifungal activity of orange (Citrus sinensis var. Valencia) peel essential oil applied by direct addition or vapor contact. Food Control. 2013;31:1–4. [Google Scholar]
- 16.Prakash B., Singh P., Kedia A., Dubey N.K. Assessment of some essential oils as food preservatives based on antifungal, antiaflatoxin, antioxidant activities and in vivo efficacy in food system. Food Res. Int. 2012;49:201–208. [Google Scholar]
- 17.Aloui H., Khwaldia K., Licciardello F., Mazzaglia A., Muratore G., Hamdi M., Restuccia C. Efficacy of the combined application of chitosan and Locust Bean Gum with different Citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates. Int. J. Food Microbiol. 2014;170:21–28. doi: 10.1016/j.ijfoodmicro.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Aziz N.H., Youssef Y.A., El-Fouly M.Z., Moussa L.A. Contamination of some common medicinal plant samples and spices by fungi and their mycotoxins. Bot. Bull. Acad. 1998;39:279–285. [Google Scholar]
- 19.Souza E.L.d., Lima E.d.O., Freire K.R.d.L., Sousa C.P.d. Inhibitory action of some essential oils and phytochemicals on the growth of various moulds isolated from foods. Braz Arch Biol. 2005;48:245–250. [Google Scholar]
- 20.Ben Miri Y., Benabdallah A., Taoudiat A., Mahdid M., Djenane D., Tacer-Caba Z., Topkaya C., Jesus Simal-Gandara J. Potential of essential oils for protection of Couscous against Aspergillus flavus and aflatoxin B1 contamination. Food Control. 2023;145 [Google Scholar]
- 21.Da Cruz Cabral L., Pinto F.V., Patriarca A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013;166:1–14. doi: 10.1016/j.ijfoodmicro.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Prakash B., Shukla R., Singh P., Kumar A., Mishra P.K., Dubey N.K. Efficacy of chemically characterized Piper betle L. essential oil against fungal and aflatoxin contamination of some edible commodities and its antioxidant activity. Int. J. Food Microbiol. 2010;142:114–119. doi: 10.1016/j.ijfoodmicro.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Abbaszadeh S., Sharifzadeh A., Shokri H., Khosravi A.R., Abbaszadeh A. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J. Med. Mycol. 2014;24:51–56. doi: 10.1016/j.mycmed.2014.01.063. [DOI] [PubMed] [Google Scholar]
- 24.Soković M.D., Vukojević J., Marin P.D., Brkić D.D., Vajs V., Van Griensven L.J. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules. 2009;14(1):238–249. doi: 10.3390/molecules14010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra P.K., Singh P., Prakash B., Kedia A., Dubey N.K. Assessing essential oil components as plant-based preservatives against fungi that deteriorate herbal raw materials. Int. Biodeterior. Biodegrad. 2013;80:16–21. [Google Scholar]
- 26.Shin M.H., Kim J.H., Choi H.W., Keum Y.S., Chun S.C. Effect of thymol and linalool fumigation on postharvest diseases of table grapes. MYCOBIOLOGY. 2014;42(3):262–268. doi: 10.5941/MYCO.2014.42.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dammaka I., Hamdib Z., El Euchc S.K., Zemni H., Mliki A., Hassouna M., Lasram S. Evaluation of antifungal and anti-ochratoxigenic activities of Salvia officinalis, Lavandula dentata and Laurus nobilis essential oils and a major monoterpene constituent 1,8-cineole against Aspergillus carbonarius. Industrial Products. 2019;128:85–93. [Google Scholar]
- 28.Mihai A.L., Popa M.E. In vitro Activity of natural antimicrobial compounds against Aspergillus Strains. Agriculture and Agricultural Science Procedia. 2015;6:585–592. [Google Scholar]
- 29.Safaei-Ghomi J., Ahd A.A. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Phcog. Mag. 2010;6(23):172. doi: 10.4103/0973-1296.66930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ju J., Xie Y., Yu H., Guo Y., Cheng Y., Chen Y., Ji L., Yao W. Synergistic properties of citral and eugenol for the inactivation of foodborne molds in vitro and on bread. LWT--Food Sci. Technol. 2020;122 [Google Scholar]
- 31.Hua H., Xing F., Selvaraj J.N., Wang Y., Zhao Y., Zhou L., Liu X., Liu Y. Inhibitory effect of essential oils on Aspergillus ochraceus growth and ochratoxin A production. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmad A., Khan A., Kumar P., Bhatt R.P., Manzoor N. Antifungal activity of Coriaria nepalensis essential oil by disrupting ergosterol biosynthesis and membrane integrity against Candida. Yeast. 2011;28(8):611–617. doi: 10.1002/yea.1890. [DOI] [PubMed] [Google Scholar]
- 33.Rajput S.B., Karuppayil S.M. Vol. 2. Springer plus; 2013. p. 26. (Small Molecules Inhibit Growth, Viability and Ergosterol Biosynthesis in Candida Albicans). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nogueira J.H.C., Gonçalez E., Galleti S.R., Facanali R., Marques M.O.M., Felício J.D. Ageratum conyzoides essential oil as aflatoxin suppressor of Aspergillus flavus. Int. J. Food Microbiol. 2010;137:55–60. doi: 10.1016/j.ijfoodmicro.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Tian J., Huang B., Luo X., Zeng H., Ban X., He J., Wang Y. The control of Aspergillus flavus with Cinnamomum jensenianum Hand.-Mazz essential oil and its potential use as a food preservative. Food Chem. 2012;130:520–527. [Google Scholar]
- 36.Zhou D., Wang Z., Li M., Xing M., Xian T., Tu K. Carvacrol and eugenol effectively inhibit Rhizopus stolonifer and control postharvest soft rot decay in peaches. J. Appl. Microbiol. 2017;124:166–178. doi: 10.1111/jam.13612. [DOI] [PubMed] [Google Scholar]
- 37.Yenjit P., Issarakraisila M., Intana W., Chantrapromma K. Fungicidal activity of compounds extracted from the pericarp of Areca catechu against Colletotrichum gloeosporioides in vitro and in mango fruit. Postharvest Biol. Technol. 2010;55(2):129–132. [Google Scholar]
- 38.Nishino S., Parada R.Y., Ichiyanagi T., Maekawa N., Shimomura N., Otani H. 1-Phenyl-3-pentanone, a volatile compound from the edible mushroom Mycoleptodonoides aitchisonii active against some phytopathogenic fungi. J. Phytopathol. 2013;161:515–521. [Google Scholar]
- 39.Tian J., Zeng X., Zeng H., Feng Z., Miao X., Peng X. Investigations on the antifungal effect of nerol against Aspergillus flavus causing food spoilage. Sci. World J. 2013;(3) doi: 10.1155/2013/230795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi Y.S., Kim K.Y., Jang D.Y., Uhm D.Y., Kim T.J., Jung B.J. Plant essential oils and their antifungal activity. Korean. J. Pestic. Sci. 2006;10:201–209. [Google Scholar]
- 41.Wang Y., Zeng X., Zhou Z., Xing K., Tessema A., Zeng H. Inhibitory effect of nerol against Aspergillus Niger on grapes through a membrane lesion mechanism. Food Control. 2015;55:54–61. [Google Scholar]
- 42.Songsamoe S., Matan N., Matan N. Effect of UV-C radiation and vapor released from a water hyacinth root absorbent containing bergamot oil to control mold on storage of brown rice. J. Food Sci. Technol.-Mysore. 2016;53:1445–1453. doi: 10.1007/s13197-015-2146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gemeda N., Woldeamanuel Y., Asrat D., Debella A. Effect of essential oils on Aspergillus spore germination, growth and mycotoxin production: a potential source of botanical food preservative. Asian Pac. Trop. Biomed. 2014;4:S373–S381. doi: 10.12980/APJTB.4.2014C857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viani R. Proceedings of the Advances in Experimental Medicine and Biology. Vol. 504. Springer; Boston, MA, USA: 2002. Effect of processing on ochratoxin A (OTA) content of coffee; pp. 189–193. [DOI] [PubMed] [Google Scholar]
- 45.Kedia A., Prakash B., Mishra P.K., Dubey N.K. Antifungal and antiaflatoxigenic properties of Cuminum cyminum (L.) seed essential oil and its efficacy as a preservative in stored commodities. Int. J. Food Microbiol. 2014;1(7):168–169. doi: 10.1016/j.ijfoodmicro.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Tian J., Ban X., Zeng H., He J., Huang B., Wang Y. Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta Celak. Int. J. Food Microbiol. 2011;145:464–470. doi: 10.1016/j.ijfoodmicro.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 47.Prakash B., Mishra P.K., Kedia A., Dubey N.K. Antifungal, antiaflatoxin and antioxidant potential of chemically characterized Boswellia carterii Birdw essential oil and its in vivo practical applicability in preservation of Piper nigrum L. fruits. LWT--Food Sci. Technol. 2014;56(2):240–247. [Google Scholar]
- 48.Hlebová M., Hleba L., Medo J., Uzsakova V., Kloucek P., Bozik M., Haščík P., Čuboň J. Antifungal and antitoxigenic effects of selected essential oils in vapors on green coffee beans with Impact on Consumer Acceptability. Foods. 2021;10(12):2993. doi: 10.3390/foods10122993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carneiro H.C.F., Tonon R.V., Grosso C.R.F., Hubinger M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2020;115(4):443–451. [Google Scholar]
- 50.Bouzidi H., Lakhlef Z., Hellal Z., Djenane D. Le conditionnement des fraises fraîches sous "micro atmosphère" à base d’huiles essentielles combinées : effet durant le stockage. Nat. Technol. J. 2019;11(2):35–50. [Google Scholar]
- 51.García-Díaz M., Patiño B., Vázquez C., Gil-Serna J. A novel niosome-encapsulated essential oil formulation to prevent Aspergillus flavus growth and aflatoxin contamination of maize grains during storage. Toxins. 2019;11:646. doi: 10.3390/toxins11110646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z., Lin S., An S., Liu L., Hu Y., Wan L. Preparation, characterization and antiaflatoxigenic activity of chitosan packaging films incorporated with turmeric essential oil. Int. J. Biol. Macromol. 2019;131:420–434. doi: 10.1016/j.ijbiomac.2019.02.169. [DOI] [PubMed] [Google Scholar]