Abstract
Objectives
Graves' disease (GD) is the most common cause of thyrotoxicosis. There are many studies that have evaluated bone mineral density (BMD) in Graves’ disease. However, the strength of a bone also depends on its microarchitecture which can be assessed by various techniques. Trabecular bone score (TBS) is a new method for assessing bone microarchitecture that is non-invasive and easily performed.
Methods
The present study was a cross-sectional study that involved 50 patients with active GD and 50 healthy controls. Both groups were subjected to an assessment of biochemical parameters followed by measurement of BMD and TBS on the same dual energy X-ray absorptiometry (DXA) machine.
Results
The mean age of patients with active GD (N = 50) was 31.9 ± 10.9 years while that of controls was 31.2 ± 4.9 years (P = 0.640). The female: male ratio was the same for both groups (F = 31, M = 19). The mean lumbar spine BMD, femoral neck BMD, total hip BMD, and distal radius BMD were significantly reduced in GD when compared to that in controls. The mean absolute lumbar spine TBS in GD was 1.263 ± 0.101 while that in controls was 1.368 ± 0.073 (P < 0.001). On multivariate regression analysis, the factors that predicted TBS were serum thyroxine (T4) and L1-L4 BMD.
Conclusions
Patients with Graves’ disease had reduced bone density at all sites and degraded microarchitecture. Long-term studies are required to understand the pattern of recovery of bone microarchitecture after the restoration of euthyroidism.
Keywords: Graves' disease, Secondary osteoporosis, Bone mineral density, Trabecular bone score, Thyroxine
1. Introduction
Thyroid hormones regulate bone metabolism by influencing the rate of bone turnover or bone remodeling [1]. Untreated thyrotoxicosis can negatively influence bone health by increasing the rate of bone remodeling [1]. This can lead to increased osteoclastic bone resorption, not compensated by osteoblastic bone formation [1]. Thyroid hormones are known to preferentially affect the remodeling of cortical than that of trabecular bone [2].
Graves’ disease (GD) is the most common cause of thyrotoxicosis. Active GD has been known to be a major risk factor for secondary osteoporosis [3,4]. A meta-analysis has reported low bone mineral density (BMD) scores at the lumbar spine, hip, and distal radius in hyperthyroidism [5]. The same study has also reported an increased prevalence of vertebral fractures in hyperthyroid patients [5]. Studies have reported improvement in BMD after the restoration of euthyroidism, even without the use of any anti-osteoporotic drugs [6,7]. However, a few studies have not shown a similar pattern of improvement [8].
Most of these studies have primarily focused on BMD measured on dual-energy X-ray absorptiometry (DXA). The strength of a bone is not determined by BMD alone. A key determinant of bone strength and fragility is its microarchitecture [9]. Trabecular bone score (TBS) has been an emerging tool to assess bone microarchitecture. It is entirely non-invasive and has been shown to be a predictor of skeletal strength and fracture risk, independent of BMD [10]. TBS is a gray-level textural metric that can be extracted from two-dimensional images of lumbar spine DXA [10,11]. TBS is found to correlate with trabecular number, separation, connectivity density, and bone volume fraction [10,11].
Our study is planned to analyze the involvement of bone microarchitecture in addition to BMD, in active GD compared to that in healthy euthyroid controls. Bone microarchitecture was assessed using TBS software installed on the same DXA machine measuring BMD. To the best of our knowledge, there are only 3 previous studies assessing bone microarchitecture with TBS in active GD [[12], [13], [14]].
2. Methods
History was obtained through a pre-designed clinical proforma. Patients were inquired about the symptoms of thyrotoxicosis like palpitations, weight loss, tremors, increased frequency of stools, and heat intolerance. All females included in our study were pre-menopausal. A general examination was done to note height, and weight and calculate body mass index (BMI). Physical examination was done to look for goiter-its consistency, size, and presence of thyroid bruit. An examination was done to look for any evidence of orbitopathy or dermopathy. Patients with Graves’ orbitopathy/dermopathy who were taking glucocorticoids were excluded from the study.
Patients with GD were either treatment-naïve or thyrotoxic despite taking anti-thyroid medicines. None of the patients took calcium, vitamin D, or any other drug that could affect bone metabolism during the study.
Healthy controls (N = 50) matched for age and sex were recruited for the study. They were the patients’ relatives and hospital staff free from any disease, and also not taking any drug. Both patients and controls were subjected to an assessment of biochemical parameters and measurement of BMD and TBS.
2.1. Measurements
Blood samples were drawn in the fasting state. Samples for serum total thyroxine (total T4), free thyroxine (FT4), serum tri-iodothyronine (T3), free-tri-iodothyronine (FT3), thyroid stimulating hormone (TSH) were measured using an electro-chemiluminescence immunoassay (ECLIA, Elecsys-2010, Roche, Germany). The normal ranges for various biochemical parameters were as follows: T4 as 65.6–181.5 nmol/L, FT4 as 11.9–21.8 pmol/L, T3 as 1.2–3.1 nmol/L, FT3 as 3.1–6.7 pmol/L, and TSH as 0.27–4.2 μIU/mL.
Samples for serum calcium (Ca), serum inorganic phosphorus (PO4), and serum alkaline phosphatase (ALP) were measured using Hitachi 917, Roche, Germany (normal ranges as 2.1–2.6 mmol/L, 0.8–1.5 mmol/L, and 80–240 IU/L, respectively). The intra-assay and inter-assay coefficients of variation were 3.5–5.0%. Serum intact parathyroid hormone (iPTH) was measured by chemiluminescence (CLIA, Elecsys-2010, Roche, Germany; minimum detection limit = 0.1 pmol/L, normal range as 1.5–6.8 pmol/L). Serum 25-hydroxy [25(OH)] vitamin D was measured by CLIA (LIAISON, DiaSorin, Inc., Stillwater, MN, USA, normal range as 50–125 nmol/L) with a coefficient of variation of 2.9–5.5%.
After obtaining their biochemical parameters, patients and controls were subjected to assessment of BMD at the lumbar spine, femoral neck, total hip, and distal non-dominant forearm. BMD was measured on DXA (Discovery A 84,023, Hologic Inc, Marlborough, MA, USA), as per the guidelines of the International Society of Clinical Densitometry. TBS was measured using TBS iNsight software (version 3.0.2.0, Medimaps, Merignac, France) installed on the same DXA machine. Vertebrae with syndesmophytes, osteophytes, and fractured vertebrae were excluded from the analysis. T scores and Z scores for BMD and TBS were not considered in the final analysis due to a lack of normative data for the Indian population.
2.2. Statistical analysis
Statistical analysis was done using IBM SPSS Statistics© 23 (Armonk, New York, USA). Normative data are expressed as mean ± standard deviation. Non-normative data are expressed as median with interquartile range (IQR 25th-75th). Parametric tests (independent sample t-test) were used to test the significance between two independent means. Wilcoxon sign rank test and Mann-Whitney U test were used to test the significance between non-parametric variables (paired and unpaired variables respectively). Univariate and multivariate linear regression analysis was used to test the association of various parameters with TBS in both groups. A value of P < 0.05 was considered significant.
3. Results
3.1. Demographic and biochemical parameters
The comparison of demographic and biochemical parameters between GD and healthy controls is shown in Table 1. The mean age of patients with active GD (N = 50) was 31.9 ± 10.9 years while that of controls was 31.2 ± 4.9 years (P = 0.640). The female: male ratio was the same for both groups (F = 31, M = 19). The median duration of symptoms in GD was 15 months (IQR 6–30 months). There was a significant difference in the BMI between the 2 groups [mean BMI of 20.8 ± 4.0 kg/m2 in GD vs 25.7 ± 4.4 kg/m2 in controls (P < 0.001)].
Table 1.
Comparison of demographic and biochemical parameters between Graves’ disease (N = 50) and healthy controls (n-50).
| Parameter | Graves' disease (N = 50) | Healthy controls (N = 50) | P-value |
|---|---|---|---|
| Age, yr | 31.9 ± 10.9 | 31.2 ± 4.9 | P = 0.640 |
| Sex, F:M | 31:19 | 31:19 | |
| BMI, kg/m2 (N: 18.5–24.9) | 20.8 ± 4.0 | 25.7 ± 4.4 | P < 0.001 |
| Serum Ca, mmol/L (N: 2.1–2.6) | 2.3 ± 0.2 | 2.3 ± 0.1 | P = 0.067 |
| Serum PO4, mmol/L (N: 0.8–1.4) | 1.4 ± 0.3 | 1.1 ± 0.2 | P < 0.001 |
| Serum ALP, IU/L (N: 80–240) | 436. 5(IQR 307.7–574.0) | 220. 0 (181.5–288.5) | P < 0.001 |
| Serum 25(OH)D, nmol/L (N:50–125) | 45.4 (IQR 35.2–67.6) | 35.9 (IQR 24.7–47.9) | P = 0.074 |
| Serum iPTH, pmol/L (N: 1.6–6.9) | 4.9 (IQR 2.6–6.7) | 5.2 (IQR 3.6–6.8) | P = 0.378 |
| Serum T4, nmol/L (N:65.6–181.5) | 240.7 ± 63.1 | 104.3 ± 20.5 | P < 0.001 |
| Serum FT4, pmol/L (N:11.9–21.8) | 60. 4 (IQR 37.3–99.1) | 15.6 (IQR 12.1–20.1) | P < 0.001 |
| Serum T3, nmol/L (N:1.2–3.1) | 5.9 ± 2.4 | 1.6 ± 1.2 | P < 0.001 |
| Serum FT3, pmol/L (N:3.1–6.7) | 21.3 (IQR 9.6–30.5) | 4.6 (IQR 3.6–5.9) | P < 0.001 |
| Serum TSH, mIU/L (N:0.4–4.2) | <0.001 | 3.3 ± 0.2 | |
| Serum T Bil, μmol/L (N: 5.1–17.1) | 10.2 (IQR 6.8–15.3) | 8.5 (IQR 6.4–10.2) | P = 0.022 |
| Serum ALT (SGPT), IU/L (N: 4–36) | 34.0 (IQR 22.0–59.0) | 24.0 (IQR 15.0–33.7) | P = 0.002 |
| Serum AST (SGOT), IU/L (N: 8–33) | 33.0 (IQR 23.0–45.0) | 24.0 (IQR 18.7–29.2) | P = 0.001 |
| Serum urea, mmol/L (N: 1.8–7.1) | 3.6 (IQR 2.6–4.8) | 3.5 (IQR 2.9–3.8) | P = 0.332 |
| Serum creatinine, μmol/L (N: 53.0–97.2) | 44.2 (IQR 35.3–53.0) | 61.8 (IQR 53.0–70.7) | P < 0.001 |
| Serum T protein, g/L (N: 60.0–83.0) | 73.0 (IQR 70.5–76.2) | 75.5 (IQR 72.0–77.2) | P = 0.019 |
| Serum albumin, g/L (N:35.0–50.0) | 45.0 (IQR 40.0–49.0) | 48.0 (IQR 46.0–50.2) | P = 0.001 |
Data are expressed as mean ± standard deviation or median [Interquartile range (IQR)].
BMI, body mass index;, Ca, calcium; PO4, phosphorus; ALP, alkaline phosphatase; 25(OH)D-25-hydroxy-vitamin D; iPTH, intact parathyroid hormone; T4, thyroxine; FT4, free thyroxine; T3, tri-iodothyronine; FT3, free tri-iodothyronine; TSH, thyroid stimulating hormone; T Bil, total bilirubin; ALT, alanine transaminase; AST, aspartate transaminase.
The mean serum T4 level [normal range (N): 65.6–181.5 nmol/L] in GD was 240.7 ± 63.1 nmol/L versus 104.3 ± 20.5 nmol/L in controls (P < 0.001). The mean serum T3 level [N: 1.2–3.1 nmol/L] was 5.9 ± 2.4 nmol/L in GD versus 1.6 ± 1.2 nmol/L (P < 0.001) in controls. The values for free hormones were as follows: FT4 [N: 11.9–21.8 pmol/L], 60. 4 (IQR 37.3–99.1) pmol/L in GD vs 15.6 (IQR 12.1–20.1) pmol/L in controls (P < 0.001); FT3 [N: 3.1–6.7 pmol/L], 21.3 (IQR 9.6–30.5) pmol/L in GD vs 4.6 (IQR 3.6–5.9) pmol/L in controls (P < 0.001). Serum TSH [N: 0.4–4.2 mIU/L] was less than 0.001 mIU/L in all patients with GD while that in controls was 3.3 ± 0.2 mIU/L.
The mean serum Ca [N: 2.1–2.6 mmol/L] was 2.3 ± 0.2 mmol/L in GD vs 2.3 ± 0.1 mmol/L in controls (P = 0.067). The mean serum PO4 [N: 0.8–1.4 mmol/L] was 1.4 ± 0.3 mmol/L in GD vs 1.1 ± 0.2 mmol/L in controls (P < 0.001). The median serum ALP [N: 80–240 IU/L] was higher in GD, 436.5 (IQR 307.7–574.0) IU/L compared to that in controls, 220.0 (IQR 181.5–288.5) IU/L (P < 0.001), suggestive of a ‘high bone turnover state’ in GD. The median serum 25(OH) Vit D level [N: 50–125 nmol/L] in GD was 45.4 (IQR 35.2–67.6) nmol/L while that in controls was 35.9 (IQR 24.7–47.9) nmol/L (P = 0.074). The median serum iPTH [N: 1.6–6.9 pmol/L] in GD was 4.9 (IQR 2.6–6.7) pmol/L vs 5.2 (IQR 3.6–6.8) pmol/L in controls (P = 0.378). Significant differences were also noted in liver function tests, that is, serum total bilirubin [N: 5.1–17.1 μmol/L]: 10.2 (IQR 6.8–15.3) μmol/L in GD vs 8.5 (IQR 6.4–10.2) μmol/L in controls (P = 0.022); serum alanine transaminase (ALT) [N: 4–36 IU/L]: 34.0 (IQR 22.0–59.0) IU/L in GD vs 24.0 (IQR 15.0–33.7) IU/L in controls (P = 0.002); serum aspartate transaminase (AST) [N: 8–33 IU/L]: 33.0 (IQR 23.0–45.0) IU/L in GD vs 24.0 (IQR 18.7–29.2) IU/L in controls (P = 0.001); total protein [N: 60.0–83.0 g/L]: 73.0 (IQR 70.5–76.2) g/L in GD vs 75.5 (IQR 72.0–77.2) g/L in controls (P = 0.019) and serum albumin [N:35.0–50.0 g/L]: 45.0 (IQR 40.0–49.0) g/L in GD vs 48.0 (IQR 46.0–50.2) g/L in controls (P = 0.001). A significant difference was also noted in serum creatinine [N: 53.0–97.2 μmol/L] between GD, 44.2 (IQR 35.3–53.0) μmol/L and controls, 61.8 (IQR 53.0–70.7) μmol/L (P < 0.001).
3.2. Bone mineral density
The comparison of densitometric parameters between GD and controls is shown in Table 2. The mean lumbar spine BMD (L1-L4 BMD) in GD was 0.843 ± 0.146 g/cm2, while that in controls was 0.964 ± 0.096 g/cm2 (P < 0.001). The mean femoral neck (FN) BMD in GD was 0.684 ± 0.116 g/cm2 while that in controls was 0.801 ± 0.105 (P < 0.001). The total hip (TH) BMD in GD was 0.769 ± 0.121 g/cm2 while that in controls was 0.919 ± 0.095 g/cm2 (P < 0.001). The distal radius (DR) BMD in GD was 0.588 ± 0.122 g/cm2 while that in controls was 0.697 ± 0.064 (P < 0.001). When we calculated the percentage differences, we found that in GD compared to healthy controls; L1-L4 BMD was reduced by 12.5%, FN BMD by 14.6%, TH BMD by 16.3%, and DR BMD by 15.6%.
Table 2.
Comparison of densitometric parameters between Graves’ disease (N = 50) and healthy controls (N = 50).
| Parameter | Graves' disease (N = 50) | Healthy controls (N = 50) | P-value |
|---|---|---|---|
| L1-L4 BMD, g/cm2 | 0.843 ± 0.146 | 0.964 ± 0.096 | P < 0.001 |
| FN BMD, g/cm2 | 0.684 ± 0.116 | 0.801 ± 0.105 | P < 0.001 |
| TH BMD, g/cm2 | 0.769 ± 0.121 | 0.919 ± 0.095 | P < 0.001 |
| Distal radius BMD, g/cm2 | 0.588 ± 0.122 | 0.697 ± 0.064 | P < 0.001 |
| L1-L4 TBS | 1.263 ± 0.101 | 1.368 ± 0.073 | P < 0.001 |
Data are expressed as mean ± standard deviation.
L1-L4, lumbar spine; BMD, bone mineral density; FN, femoral neck; TH, total hip; TBS, trabecular bone score.
3.3. Trabecular bone score
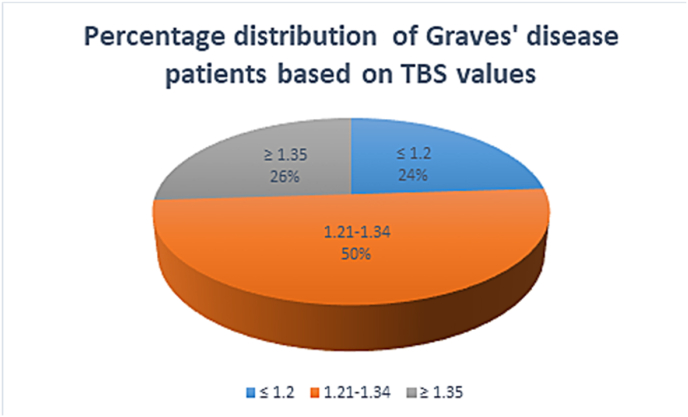
The mean absolute lumbar spine trabecular bone score (L1-L4 TBS) in GD was 1.263 ± 0.101 while that in controls was 1.368 ± 0.073 (P < 0.001). Bone microarchitecture has been divided into 3 categories based upon TBS values: TBS ≤ 1.2-fully degraded microarchitecture; TBS 1.21–1.34-partially degraded microarchitecture; TB ≥ 1.35 –normal microarchitecture. In our study, 50% (N = 25) of the patients had partially degraded microarchitecture, 26% (N = 13) had normal microarchitecture and 24% (N = 25) had fully degraded microarchitecture (Fig. 1).
Fig. 1.
Percentage distribution of patients with Graves' disease based on trabecular bone score (TBS) values.
3.4. Linear regression analysis
Linear regression analysis was applied to look for an association between various parameters and TBS in GD and controls (Table 3). Univariate analysis was applied when an individual variable was tested. On univariate analysis, the parameters: age [Beta regression co-efficient (β) = −0.213, P = 0.033], BMI (β = 0.275, P = 0.006), serum T4 (β = −0.554, P < 0.01), serum TSH (β = 0.314, P = 0.002), L1-L4 BMD (β = 0.733, P < 0.001), FN BMD (β = 0.619, P < 0.001), TH BMD (β = 0.651, P < 0.001) and DR BMD (β = 0.555, P < 0.01) were found to have significant association. On multivariate analysis, after adjustment of all these parameters, only 2 were found to retain a significant statistical association with TBS, that is, serum T4 (β = −0.356, P = 0.001) and L1-L4 BMD (β = 0.545, P < 0.001).
Table 3.
Univariate and multivariate linear regression models predicting TBS in Graves’ disease and healthy controls.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| Beta regression coefficient (β) (CI) | Univariate p | Beta regression coefficient (β) (CI) | Multivariate p | |
| Age | −0.213 (−0.005-0.000) | P = 0.033 | −0.092 (−0.003- 0.000) | P = 0.165 |
| BMI | 0.275 (0.002–0.010) | P = 0.006 | −0.120 (−0.006-0.001) | P = 0.127 |
| Serum T4 | −0.554 (−0.012–−0.006) | P < 0.001 | −0.356 (−0.009–−0.003) | P = 0.001 |
| Serum FT4 | −0.215 (−0.023- 0.004) | P = 0.161 | ||
| Serum T3 | −0.218 (−0.033- 0.005) | P = 0.145 | ||
| Serum FT3 | −0.147 (−0.06- 0.002) | P = 0.401 | ||
| Serum TSH | 0.314 (0.006–0.023) | P = 0.002 | −0.113 (−0.012-0.002) | P = 0.157 |
| 25(OH)D | −0.118 (−0.003- 0.001) | P = 0.328 | ||
| iPTH | −0.027 (−0.001- 0.000) | P = 0.830 | ||
| L1-L4 BMD | 0.733 (0.445–0.649) | P < 0.001 | 0.545 (0.260–0.553) | P < 0.001 |
| FN BMD | 0.619 (0.380–0.638) | P < 0.001 | 0.053 (−0.220-0.309) | P = 0.741 |
| TH BMD | 0.651 (0.389–0.626) | P < 0.001 | 0.061 (−0.249-0.344) | P = 0.752 |
| DR BMD | 0.555 (0.358–0.665) | P < 0.001 | 0.058 (−0.114-0.220) | P = 0.529 |
Data are expressed as beta regression coefficient (β), with 95% confidence interval (CI).
BMI, body mass index; T4, thyroxine; FT4-free thyroxine; T3, tri-iodothyronine; FT3, free tri-iodothyronine; TSH, thyroid stimulating hormone; 25(OH)D, 25-hydroxy-vitamin D; iPTH, intact parathyroid hormone; BMD, bone mineral density; TBS, trabecular bone score; L1-L4, lumbar spine; FN, femoral neck; TH, total hip; DR, distal radius.
4. Discussion
The present study compared biochemical and densitometric parameters between Graves’ disease and healthy controls. The diagnosis of GD was made according to the guidelines of the ATA [15]. The ATA, 2016 guidelines state that in patients who have moderate-severe hyperthyroidism with symmetric goiters and recent onset orbitopathy, further tests are not required [15]. In patients with hyperthyroidism, who lack these features, further tests in the form of TSHR antibodies or radio-active iodine uptake (RAIU), or Technetium-99-thyroid scans are required [15]. In our institute, due to logistic issues, TSHR antibodies could not be done; rather Technetium-99-thyroid scans were done and the scans with diffusely increased uptake in bilateral lobes of the thyroid were diagnosed with GD [15]. Yoshihara et al [16] used artificial intelligence and logistic regression models for the prediction of GD among normal subjects and those with thyrotoxicosis. The study found that age, serum creatinine, total cholesterol, ALP, and total protein were strong predictors of GD in normal subjects, and serum ALP, creatinine, total protein, gamma-glutamyl transferase (GGT), and white blood cells (WBC) were strong predictors of GD in patients with thyrotoxicosis. In our study, we had data on serum ALP, creatinine, total protein of GD and controls. However, we did not have data on total cholesterol, GGT, and WBC to satisfy the prediction model.
The present study found a reduction in bone density at all the sites, that is, at the lumbar spine, femoral neck, total hip, and distal radius, in GD compared to that in healthy controls. Previous studies have shown that there is a reduction of bone density up to 10–20% in overt hyperthyroidism [[17], [18], [19]] which was similar to that observed in our study. GD is known to preferentially affect cortical bone more than trabecular [2]. However, in our study, the percentage reduction in distal radius BMD was almost similar to that in the hip or lumbar spine.
The recovery of bone density after the restoration of euthyroidism has been found to be variable. Two previous studies using single photon absorptiometry showed a reduction in bone density up to 12–28% in patients with overt hyperthyroidism that normalized after treatment with anti-thyroid drugs [19]. One previous study from our institute reported a reduction in bone density that did not normalize after the restoration of euthyroidism in the vitamin D-deficient population [8].
The present study also found a significant deterioration in bone microarchitecture in GD, compared to that in healthy controls. There are several techniques to assess bone microarchitecture such as biopsy of invasively obtained bone specimens, micro CT, micro MRI, and high-resolution peripheral quantitative CT (HRpQCT). However, these may not be feasible in routine clinical practice due to high radiation exposure or its invasive nature. Trabecular bone score (TBS) is a non-invasive technique that can be installed on DXA machines and is found to correlate with bone microarchitecture parameters such as trabecular number, connectivity, and density. Previously, there have been very few studies assessing bone microarchitecture using TBS in Graves' disease. Ock et al [12] included 30 patients with GD (M: F = 17:13) and followed up for 20.7 ± 8.5 months after initiation of anti-thyroid drugs. They found an improvement in lumbar spine BMD and TBS [TBS = 1.377 (IQR 1.299–1.422) at baseline to TBS = 1.390 (IQR 1.327–1.430) after treatment (P = 0.038)]. Rymuza et al [14] included 15 patients with Graves’ orbitopathy who received 12 pulses of methylprednisolone and found that TBS values decreased in 5 out of 15 patients (33%). The mean TBS value decreased by 2.4% than the baseline (P < 0.05). We, however, excluded patients with orbitopathy who had received glucocorticoids since the latter are known to influence bone metabolism.
In the current study, on univariate analysis, the following parameters were found to have a significant association with TBS: age, BMI, serum T4, serum TSH, L1-L4, FN, TH and DR BMD. It is interesting to note that GD may cause significant weight loss which in itself can lead to low bone mass. However, 1 previous study from Rotterdam found that thyroid hormones affected bone density independent of weight in a large sample of the elderly Caucasian population [20].
In the present study, we performed multivariate analysis after adjusting for age, BMI, T4, TSH, L1-L4, FN, TH, and DR BMD. Subsequently, only 2 parameters, serum T4 (β = −0.356, P = 0.001) and L1-L4 BMD (β = 0.545, P < 0.001) were found to have a statistically significant association with TBS. Thus, deterioration in bone microarchitecture can be attributed to the direct effects of thyroid hormones.
It was, however, observed that TBS did not have a statistically significant association with the bioactive hormones, FT4 and FT3. FT4 and FT3 were measured by ECLIA, rather than liquid chromatography-mass spectrometry (LC-MSMS). The free hormone immunoassays may not be as robust as free hormone measurement by LC-MSMS [21,22]. Measurement of T3, FT3, and FT4 by the immunoassay methods (commonly used by the majority of laboratories) is problematic, especially at the low or high end of the range, and does not correlate with both log TSH and patients’ clinical conditions. We did not get a significant association of TBS with FT4 or FT3 possibly due to assay limitations [21,22].
Thyroid hormones (T3 and T4) are known to influence bone health. The T3 receptor has been found in rat and human osteoblast cell lines and also in osteoclasts, derived from osteoclastoma [23]. In addition, TSH is also found to be an independent regulator of bone health [24]. TSH receptors are present in osteoblast and osteoclast precursors [25]. Isolated suppression of TSH with normal T3 and T4 has been found to be associated with poor bone density in many studies [26,27].
Once the bone is fully formed, it undergoes a continuous process of remodeling to remove old, micro-damaged bone and replace it with a newer and mechanically stronger bone to preserve bone strength. It is a tightly coupled process where bone resorption occurs at the same rate as new bone formation occurs. Each remodeling cycle takes approximately around 200 days or 5 months. In overt hyperthyroidism, the process of remodeling is hastened, with the uncoupling of resorption and formation. The increased remodeling rate may be attributed to the direct effects of thyroid hormones and suppression of TSH.
Due to increased bone turnover, there may be hypercalcemia with hypercalciuria leading to net negative calcium balance. Studies have observed hypercalcemia in up to 8% of patients with Graves’ disease [28]. In the present study, however, the mean serum calcium was within normal limits in GD.
Vitamin D deficiency is also very common in the Indian population, with prevalence rates varying from 70 to 100% [29]. In the present study, both the patients and the healthy control groups had a median 25(OH) vitamin D level below the lower limit of normal. Vitamin D deficiency can contribute to overall poor bone health, due to impaired intestinal calcium absorption from the gut, and increased calcium resorption from bone (due to secondary hyperparathyroidism).
GD is also a state of excess cytokines. Kuzma et al [13] studied the association of the chemokine serum fractalkine with bone health in GD and in healthy controls. They found that serum fractalkine levels had a positive correlation with serum FT4 levels and a negative correlation with TBS values. TBS was the lowest in active GD compared to cured Graves’ and healthy controls.
Overt or subclinical hyperthyroidism is an important risk factor for fracture [30,31]. In 1 study, it was found that TSH <0.1 mIU/L was associated with a 4.5-fold increased risk of vertebral fracture and 3.6 fold increased risk of hip fracture [32]. Another cross-sectional study found a 31% fracture risk (for the forearm, hip, and vertebral bone) in patients with TSH < 0.10 mIU/L [33]. In the present study, fractured vertebrae were not included in the final analysis of BMD and TBS. However, as our study showed low bone density and degraded microarchitecture in the patients, it is prudent for the clinician to educate patients about fracture prevention measures. Also, patients should be adequately provided with bone protective measures like calcium and vitamin D supplements.
Our study had a few limitations. We did not measure bone turnover markers osteocalcin, or C- or N-telopeptide, bone-specific ALP and Tracp-5b due to logistic issues. We also did not follow up with the patients to assess the pattern of recovery of BMD and TBS after the achievement of euthyroidism.
5. Conclusions
Our study showed that patients with active Graves' disease had a significant decline in bone health. Both the bone density and trabecular bone score were significantly reduced in GD compared to that in healthy controls. The factors that predicted bone microarchitecture were serum T4 levels and lumbar spine BMD. Long-term studies are required to predict the recovery of bone microarchitecture in active Graves’ disease after the restoration of euthyroidism.
CRediT author statement
Hiya Boro: Conceptualization, Data curation, Writing-Original draft. Rakhi Malhotra: Conceptualization, Data curation, Writing-Review & editing. Suraj Kubihal: Conceptualization, Data curation, Writing-Review & editing. Saurav Khatiwada: Conceptualization, Data curation, Writing-Review & editing. Vinay Dogra: Conceptualization, Data curation, Writing-Review & editing. Velmurugan Mannar: Conceptualization, Data curation, Writing-Review & editing. Ashok Kumar Ahirwar: Formal analysis. Vandana Rastogi: Formal analysis.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
The authors would like to acknowledge Dr. Ravinder Goswami, Professor, Department of Endocrinology, AIIMS, New Delhi for providing valuable input for the work. The authors also acknowledge Mr. Sushil Chugh, DXA technician for conducting the BMD and TBS of the patients and controls. ORCID Hiya Boro: 0000-0001-7868-8026. Rakhi Malhotra: 0000-0002-5173-9193. Suraj Kubihal: 0000-0001-9824-510X. Saurav Khatiwada: 0000-0003-1236-164X. Vinay Dogra: 0000-0003-2108-3290. Velmurugan Mannar: 0000-0003-3075-0112. Ashok Kumar Ahirwar: 0000-0003-3371-378X. Vandana Rastogi: 0000-0002-5848-2554.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Gorka J., Taylor-Gjevre R.M., Arnason T. Metabolic and clinical consequences of hyperthyroidism on bone density. Internet J Endocrinol. 2013;2013 doi: 10.1155/2013/638727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy P.A., Harinarayan C.V., Sachan A., Suresh V., Rajagopal G. Bone disease in thyrotoxicosis. Indian J Med Res. 2012;135:277–286. [PMC free article] [PubMed] [Google Scholar]
- 3.Jódar E., Martínez-Díaz-Guerra G., Azriel S., Hawkins F. Bone mineral density in male patients with L-thyroxine suppressive therapy and Graves' disease. Calcif Tissue Int. 2001;69:84–87. doi: 10.1007/s002230020041. [DOI] [PubMed] [Google Scholar]
- 4.Lucidarme N., Ruiz J.C., Czernichow P., Léger J. Reduced bone mineral density at diagnosis and bone mineral recovery during treatment in children with Graves' disease. J Pediatr. 2000;137:56–62. doi: 10.1067/mpd.2000.106219. [DOI] [PubMed] [Google Scholar]
- 5.Vestergaard P., Mosekilde L. Hyperthyroidism, bone mineral, and fracture risk--a meta-analysis. Thyroid. 2003;13:585–593. doi: 10.1089/105072503322238854. [DOI] [PubMed] [Google Scholar]
- 6.Karga H., Papapetrou P.D., Korakovouni A., Papandroulaki F., Polymeris A., Pampouras G. Bone mineral density in hyperthyroidism. Clin Endocrinol. 2004;61:466–472. doi: 10.1111/j.1365-2265.2004.02110.x. [DOI] [PubMed] [Google Scholar]
- 7.Langdahl B.L., Loft A.G., Eriksen E.F., Mosekilde L., Charles P. Bone mass, bone turnover, body composition, and calcium homeostasis in former hyperthyroid patients treated by combined medical therapy. Thyroid. 1996;6:161–168. doi: 10.1089/thy.1996.6.161. [DOI] [PubMed] [Google Scholar]
- 8.Jyotsna V.P., Sahoo A., Ksh S.A., Sreenivas V., Gupta N. Bone mineral density in patients of Graves disease pre- & post-treatment in a predominantly vitamin D deficient population. Indian J Med Res. 2012;135:36–41. doi: 10.4103/0971-5916.93422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeman E., Delmas P.D. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 10.Hans D., Goertzen A.L., Krieg M.A., Leslie W.D. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26:2762–2769. doi: 10.1002/jbmr.499. [DOI] [PubMed] [Google Scholar]
- 11.Pothuaud L., Carceller P., Hans D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone. 2008;42:775–787. doi: 10.1016/j.bone.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Ock S.Y., Chung Y.S., Choi Y.J. Changes in bone mineral density and trabecular bone score in Graves' disease patients after anti-thyroid therapy. Osteoporos Sarcopenia. 2016;2:175–179. doi: 10.1016/j.afos.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kužma M., Vaňuga P., Binkley N., Ságová I., Pávai D., Blažíček P., et al. High serum fractalkine is associated with lower trabecular bone score in premenopausal women with Graves' disease. Horm Metab Res. 2018;50:609–614. doi: 10.1055/a-0633-2814. [DOI] [PubMed] [Google Scholar]
- 14.Rymuza J., Pelewicz K., Przedlacki J., Miśkiewicz P. Therapy with intravenous methylprednisolone pulses is associated with loss of bone microarchitecture in trabecular bone score -assessment among patients with moderate-to-severe Graves' orbitopathy: a pilot study. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.893600. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross D.S., Burch H.B., Cooper D.S., Greenlee M.C., Laurberg P., Maia A.L., Rivkees S.A., Samuels M., Sosa J.A., Stan M.N., Walter M.A. American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016. 2016;26:1343–1421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 16.Yoshihara A., Yoshimura Noh J., Inoue K., Taguchi J., Hata K., Aizawa T., et al. Prediction model of Graves' disease in general clinical practice based on complete blood count and biochemistry profile. Endocr J. 2022;69:1091–1100. doi: 10.1507/endocrj.EJ21-0741. [DOI] [PubMed] [Google Scholar]
- 17.Krølner B., Jørgensen J.V., Nielsen S.P. Spinal bone mineral content in myxoedema and thyrotoxicosis. Effects of thyroid hormone(s) and antithyroid treatment. Clin Endocrinol. 1983;18:439–446. doi: 10.1111/j.1365-2265.1983.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen H.E., Mosekilde L., Charles P. Bone mineral content in hyperthyroid patients after combined medical and surgical treatment. Acta Radiol Oncol Radiat Phys Biol. 1979;18:122–128. doi: 10.3109/02841867909128198. [DOI] [PubMed] [Google Scholar]
- 19.Diamond T., Vine J., Smart R., Butler P. Thyrotoxic bone disease in women: a potentially reversible disorder. Ann Intern Med. 1994;120:8–11. doi: 10.7326/0003-4819-120-1-199401010-00002. [DOI] [PubMed] [Google Scholar]
- 20.van der Deure W.M., Uitterlinden A.G., Hofman A., Rivadeneira F., Pols H.A., Peeters R.P., et al. Effects of serum TSH and FT4 levels and the TSHR-Asp727Glu polymorphism on bone: the Rotterdam Study. Clin Endocrinol. 2008;68:175–181. doi: 10.1111/j.1365-2265.2007.03016.x. [DOI] [PubMed] [Google Scholar]
- 21.Welsh K.J., Stolze B.R., Yu X., Podsiadlo T.R., Kim L.S., Soldin S.J. Assessment of thyroid function in intensive care unit patients by liquid chromatography tandem mass spectrometry methods. Clin Biochem. 2017;50:318–322. doi: 10.1016/j.clinbiochem.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stockigt J.R. Free thyroid hormone measurement. A critical appraisal. Endocrinol Metab Clin N Am. 2001;30:265–289. doi: 10.1016/s0889-8529(05)70187-0. [DOI] [PubMed] [Google Scholar]
- 23.Harvey C.B., Bassett J.H., Maruvada P., Yen P.M., Williams G.R. The rat thyroid hormone receptor (TR) Deltabeta3 displays cell-, TR isoform-, and thyroid hormone response element-specific actions. Endocrinology. 2007;148:1764–1773. doi: 10.1210/en.2006-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe E., Marians R.C., Yu W., Wu X.B., Ando T., Li Y., et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 25.Tsai J.A., Janson A., Bucht E., Kindmark H., Marcus C., Stark A., et al. Weak evidence of thyrotropin receptors in primary cultures of human osteoblast-like cells. Calcif Tissue Int. 2004;74:486–491. doi: 10.1007/s00223-003-0108-3. [DOI] [PubMed] [Google Scholar]
- 26.Grimnes G., Emaus N., Joakimsen R.M., Figenschau Y., Jorde R. The relationship between serum TSH and bone mineral density in men and postmenopausal women: the Tromsø study. Thyroid. 2008;18:1147–1155. doi: 10.1089/thy.2008.0158. [DOI] [PubMed] [Google Scholar]
- 27.Lee W.Y., Oh K.W., Rhee E.J., Jung C.H., Kim S.W., Yun E.J., et al. Relationship between subclinical thyroid dysfunction and femoral neck bone mineral density in women. Arch Med Res. 2006;37:511–516. doi: 10.1016/j.arcmed.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Mosekilde L., Eriksen E.F., Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin N Am. 1990;19:35–63. [PubMed] [Google Scholar]
- 29.G R., Gupta A. Vitamin D deficiency in India: prevalence, causalities and interventions. Nutrients. 2014;6:729–775. doi: 10.3390/nu6020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wejda B., Hintze G., Katschinski B., Olbricht T., Benker G. Hip fractures and the thyroid: a case-control study. J Intern Med. 1995;237:241–247. doi: 10.1111/j.1365-2796.1995.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 31.Cummings S.R., Nevitt M.C., Browner W.S., Stone K., Fox K.M., Ensrud K.E., et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 32.Bauer D.C., Ettinger B., Nevitt M.C., Stone K.L. Study of Osteoporotic Fractures Research Group. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med. 2001;134:561–568. doi: 10.7326/0003-4819-134-7-200104030-00009. [DOI] [PubMed] [Google Scholar]
- 33.Svare A., Nilsen T.I., Bjøro T., Forsmo S., Schei B., Langhammer A. Hyperthyroid levels of TSH correlate with low bone mineral density: the HUNT 2 study. Eur J Endocrinol. 2009;161:779–786. doi: 10.1530/EJE-09-0139. [DOI] [PubMed] [Google Scholar]