Summary
Background
Of the eight large (>50 cases) US postelimination outbreaks, the first and last occurred in Ohio. Ohio's vaccination registry is incomplete. Community-level immunity gaps threaten more than two decades of measles elimination in the US. We developed a statistical model, VaxEstim, to rapidly estimate the early-phase vaccination coverage and immunity gap in the exposed population during the 2022 Central Ohio outbreak.
Methods
We used reconstructed daily incidence (from publicly available data) and assumptions about the distribution of the serial interval, or the time between symptom onset in successive measles cases, to estimate the effective reproduction number (i.e., the average number of secondary infections caused by an infected individual in a partially immune population). We estimated early-phase measles vaccination coverage by comparing the effective reproduction number to the basic reproduction number (i.e., the average number of secondary infections caused by an infected individual in a fully susceptible population) while accounting for vaccine effectiveness. Finally, we estimated the early-phase immunity gap as the difference between the estimated critical vaccination threshold and vaccination coverage.
Findings
VaxEstim estimated the early-phase vaccination coverage as 53% (95% credible interval, 21%–77%), the critical vaccination threshold as 93%, and the immunity gap as 42% (95% credible interval, 18%–74%).
Interpretation
This study estimates a significant immunity gap in the exposed population during the early phase of the 2022 Central Ohio measles outbreak, suggesting a robust public health response is needed to identify the susceptible community and develop community-specific strategies to close the immunity gap.
Funding
This work was supported in part by the National Institute of General Medical Sciences, National Institutes of Health; the UK Medical Research Council (MRC); the Foreign, Commonwealth and Development Office; the National Institute for Health Research (NIHR) Health Protection Research Unit in Modelling Methodology; Imperial College London, and the London School of Hygiene & Tropical Medicine, Community Jameel; the EDCTP2 programme, supported by the EU; and the Sergei Brin Foundation.
Keywords: Measles, Outbreak-prediction models, Statistical modelling, Vaccination coverage, Critical vaccination threshold, Infectious disease, Measles elimination, United States postelimination measles outbreak, Vaccine-hesitancy, Antivaccination movement, Measles surveillance, Measles complications, Rapid response systems
Research in context.
Evidence before this study
To explore evidence before this study, we searched PubMed, Embase, Google Scholar, WHO, and CDC with the terms ‘‘measles’’ and “vaccination coverage,” or “immunity gap,” or “modeling/modelling,” or “elimination,” or “outbreak,” or “United States,” or “Ohio,” or “Columbus.” Despite high vaccination coverage in the United States, most large postelimination measles outbreaks were associated with community-level immunity gaps. However, few studies assessed the vaccination coverage and immunity gap in an exposed population at the onset of a postelimination measles outbreak. Methods used to estimate vaccination coverage in previous large postelimination measles outbreaks in the United States were time-consuming, resource-intensive, and may have been inaccurate, even when vaccination records were complete. While a 2017–2020 study suggested measles underimmunization in the Columbus area, its participants were too young to estimate the two-dose vaccination coverage required to protect against large measles outbreaks.
Added value of this study
To our knowledge, VaxEstim is the first rapid assessment tool to estimate the vaccination coverage and immunity gap in an exposed population during an outbreak. VaxEstim requires minimal population-level data and may provide more specific estimates than direct methods used in previous large US postelimination outbreaks. VaxEstim is particularly useful when vaccination coverage data are not readily available, such as in Ohio, where registry data is incomplete.
Implications of all the available evidence
The critical component of measles elimination is high vaccination coverage. This study's estimate of a significant measles immunity gap at the onset of the 2022 Central Ohio measles outbreak and previous evidence of underimmunization in the Columbus area indicate that a more robust public health response may be necessary to increase vaccination coverage in the susceptible Central Ohio community and to protect more than two decades of measles elimination in the United States.
Introduction
Measles is a highly contagious virus that can cause lethal disease.1 Although there is no specific antiviral treatment, it is vaccine-preventable.2 Transmission occurs via person-to-person contact or airborne spread of aerosolized droplets up to two hours after an infected individual occupies a confined space.3 The prodromal stage typically begins one to seven days after infection and presents with symptoms that mimic the common cold,3 which can lead to underreporting and misdiagnosis. A characteristic rash, which starts on the face and spreads toward the extremities, typically develops seven to 21 days after infection.3 Infants and young children are most likely to experience complications, including pneumonia and encephalitis, and lifelong disability, such as brain damage, blindness, and hearing loss.4 Most deaths occur in children under five years.4 Measles infects over 90% of susceptible individuals exposed to an infected person within four days of rash onset.3 All suspected or confirmed United States (US) measles cases require immediate notification to the Centers for Disease Control and Prevention (CDC).5
Measles elimination is the absence of endemic transmission in a defined geographical area for at least 12 months in the presence of adequate surveillance.6 Measles is eligible for elimination because it is vaccine-preventable, humans are the only natural hosts,1 and it has no known carrier state.3 The critical component of elimination is population immunity acquired by high vaccination coverage with two doses of measles-containing vaccine; high coverage with one dose is inadequate to protect against large outbreaks.7 In the US, routine immunization with a two-dose series of measles, mumps, and rubella (MMR) at ages 12–15 months and four to six years is recommended for measles prevention.3 MMR vaccine effectiveness (E) is 93% after the first dose and 97% after the second dose in children 12 months or older.8 The US achieved measles elimination in 2000.1 In 2012, all six World Health Organization (WHO) Regions committed to achieving measles elimination, and five Regions set an elimination target date of 2020.7 By 2016, the Americas briefly became the only Region to eliminate measles.9
Between 2017 and 2019, a global measles resurgence increased the risk of international measles importation into eliminated countries.10 The Americas Region lost its measles elimination status in 2018 when Venezuela reestablished endemic transmission. The United Kingdom and several other European Region countries lost their elimination status in 2019, and a prolonged outbreak threatened measles elimination in the US.10 The COVID-19 pandemic presented new challenges to measles elimination. A record-high of approximately 40 million children worldwide missed a measles-containing vaccine dose in 2021, and measles caused an estimated nine million cases and 128,000 deaths — an increase from seven and a half million cases and 60,700 deaths in 2020.4,11
Columbus Public Health declared the 2022 Central Ohio measles outbreak on November 9 and implemented control measures after four unimmunized children with no travel history from a local daycare center were diagnosed with measles.12 By November 22, ten daycares and two schools confirmed a total of 21 measles cases.13 At a November 30 press conference, the health commissioner for Columbus Public Health announced that, with the support of the CDC, they had linked the outbreak to one of four unrelated internationally imported measles cases in Columbus over the summer.14 The first imported case occurred on June 16 112,15; however, the dates of the other three summer cases were not publicly disclosed. Columbus Public Health reported an additional 85 outbreak-related cases between October 22 and December 24.16 While Ohio accounted for most measles cases reported in the US in 2022,17 vaccination coverage data are incomplete because Ohio state law does not require measles vaccination reporting.18 We developed a statistical model, VaxEstim, to estimate measles vaccination coverage and the immunity gap in the exposed population during the early phase of the 2022 Central Ohio measles outbreak.
Methods
This study was determined not to constitute human subjects research by the Nationwide Children's Hospital Institutional Review Board. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for cross-sectional studies. We completed all analyses in Microsoft Excel version 2208 (Microsoft Corporation, Redmond, Washington) and EpiEstim R package version 2.4 (R Foundation for Statistical Computing, Vienna, Austria).
Data sources
We derived all study data from publicly available sources. We obtained daily measles case incidence classified by the date of rash onset from the Measles Public Report by the City of Columbus (Supplementary Fig. S1) sourced from the Ohio Disease Reporting System and published on January 23, 2023 (with a disclaimer that all data are preliminary and subject to change), press releases by the City of Columbus and Ohio Department of Health, and a Freedom of Information Act request from the CDC.15,16,19
We used historical estimates from scientific literature for the distribution of the measles serial interval (SI), defined as the time between symptom onset in successive cases; basic reproduction number (R0), defined as the average number of secondary infections caused by an infected individual in a completely susceptible population, and E. The prior distribution is an estimate of the effective reproduction number before the outbreak.
For our base analysis, we selected a measles SI with a gamma distribution, a mean of 11.1 days, a standard deviation of 2.47 days, and a 95% confidence interval of 6–18 days, derived from US household studies.20,21 Recent studies highlight the importance of using regionally derived R0 estimates.22,23 Therefore, we selected an R0 median of 15.3 derived from the WHO Americas region for our base analysis.22 We used the E of 97% associated with the two-dose measles vaccination coverage required to protect against large outbreaks.8 For the prior distribution, we chose a neutral mean of 1 and a standard deviation of 5 to reflect a large uncertainty around the mean.
Statistical analysis
Primary outcomes
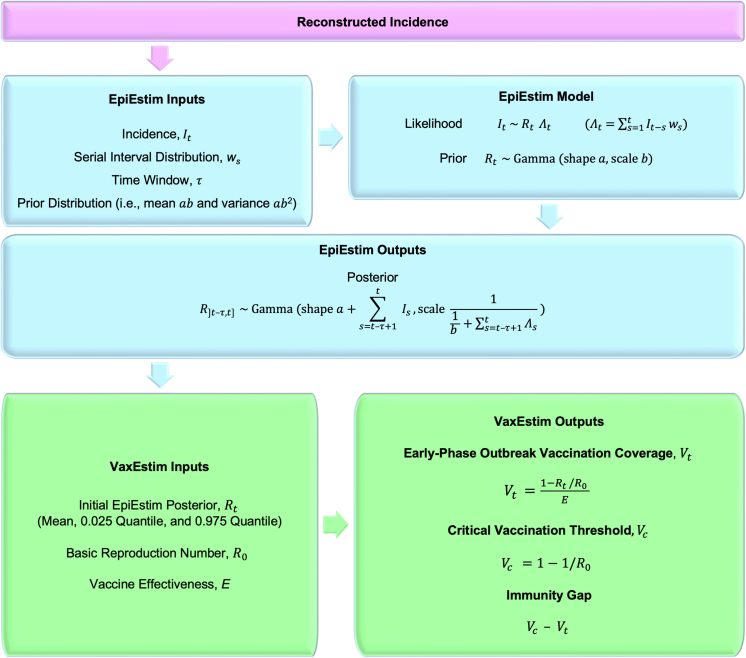
We used our VaxEstim model (Fig. 1) to estimate the vaccination coverage and immunity gap in the exposed population at the onset of the 2022 Central Ohio measles outbreak.
Fig. 1.
VaxEstim model description.
Secondary outcomes
We used EpiEstim (Fig. 1) to estimate average daily Rt values during the outbreak. In a partially immune population, the effective reproduction number at time t (Rt) is observed instead of R0.24 When Rt <1, sustained transmission eventually halts because the average number of secondary infections is less than one.24
Reconstructed incidence
The first identified case in a chain of transmission is the index case. Postelimination index cases must be internationally imported. In the US, these cases are defined as infections resulting from exposure to measles outside the US seven to 21 days before rash onset, a rash developing within 21 days of entering the US, and no known exposure to measles in the US; all other measles cases are defined as US-acquired.5
We used the internationally imported case on June 16 to determine plausible summer index cases (Supplementary Fig. S2). An unknown chain of transmission occurred between the summer index case and the first reported US-acquired case. We reconstructed the minimum incidence required to sustain this chain of transmission by assuming an 18-day (i.e., the longest possible) serial interval between cases. We considered cases separated by a duration within the 95% SI confidence interval (six to 18 days) linked and those outside the interval unlinked and attributable to an earlier unidentified case.
The initial October 22, 24, and 26 US-acquired cases were considered unlinked due to their separation by two and four days. We assumed these cases were linked to an earlier unidentified common ancestor on October 8, separated by 14, 16, and 18 days respectively. We assigned previous US-acquired cases at 18-day intervals from October 8 to June 22. Finally, we linked the June 22 and 16 cases due to their six-day separation. All reported cases between October 26 and December 24 were considered linked. Plausible summer index cases occurred between June 16 and September 20.
EpiEstim
We used the recommended Cori et al. EpiEstim Bayesian approach to estimate real-time Rt (Fig. 1).24,25 The model assumes that the incidence of the disease of interest (It) follows a Poisson process, and the past incidence (It-s) is weighted by the infectivity profile (ws). Specifically, we derived It from our reconstructed incidence data and approximated ws by the measles SI distribution.26 In particular, the mean and 95% credible intervals for Rt were obtained from the gamma posterior distribution.
Rt estimation models are generally sensitive to incidence data; however, a salient feature of EpiEstim is its ability to provide robust Rt estimates amid constant underreporting.27 Underreporting occurred in this outbreak, as evidenced by its unknown index case. We selected September 20 (the latest plausible summer index case) for our base analysis to minimize errors introduced by fluctuations in underreporting.
In EpiEstim, Rt is the average transmissibility over a time window () ending at time t. We selected an optimal of 14 days for the reconstructed incidence time series by balancing the statistical noise associated with small values against the smoothing effect associated with large values (Supplementary Fig. S3).25 EpiEstim uses the following established criteria to determine the optimal time to begin Rt measurement: 1) one elapsed, 2) one SI elapsed, and 3) 12 cases occurred.25 We used EpiEstim to calculate daily Rt values until the last recorded outbreak case on December 24.
VaxEstim
To estimate early-phase vaccination coverage (Vt) in the exposed population at time t during an outbreak, we used the initial EpiEstim Rt value — when immunity was assumed to be primarily conferred by pre-outbreak vaccination — in the following formula:28
Notably, late-phase Vt estimation is unreliable because control measures (such as contact tracing, quarantines, post-exposure prophylaxis, and increased vaccination coverage) affect Rt estimates.5
We calculated the critical vaccination threshold (Vc) required to protect a population from sustained transmission through vaccination alone using the following formula:29
Finally, we compared Vt to Vc to estimate the immunity gap during the early phase of the 2022 Central Ohio measles outbreak.
Role of Funding Source
The funders had no role in the study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit the paper for publication.
Results
Primary outcomes
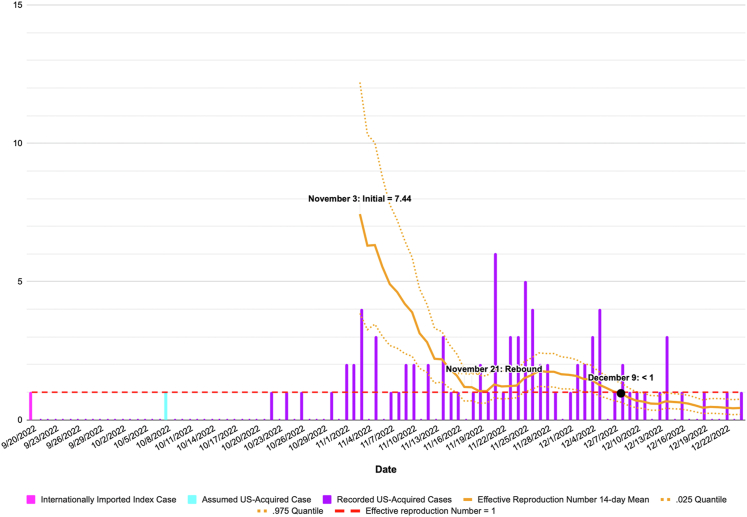
Based on a September 20 index case, the optimal time to begin estimating Rt was November 3, 2022 (day 45). All Rt estimates occurred over a 14-day . The initial mean Rt was 7.44 (95% credible interval, 3.85–12.20). At the median WHO Americas Region R0 of 15.3, the critical vaccination threshold to prevent large outbreaks was 93%, and VaxEstim estimated an early-phase vaccination coverage of 53% (95% credible interval, 21%–77%) and an immunity gap of 42% (95% credible interval, 18%–74%) (Fig. 2).
Fig. 2.
2022 Central Ohio measles outbreak cases and effective reproduction number.
Secondary outcomes
After the initial Rt estimate on November 3, Rt steadily declined, reaching 1.04 on November 19. An Rt rebound occurred between November 21 and November 27 and peaked at 1.77. Following this rebound, Rt continued to decline and fell below 1 on December 8 (Fig. 3).
Fig. 3.
2022 Central Ohio measles outbreak immunity gap for plausible summer index cases.
Sensitivity analyses
Sensitivity analyses varying several of our model's assumptions did not change the statistical significance of our findings. Since the index case for this outbreak was unknown, we examined the impact of different summer index cases on vaccination coverage and the immunity gap (Supplementary Table S1 and Supplementary Fig. S4). We also assessed the vaccination coverage and immunity gap during the early phase of the outbreak over an R0 range from 10.7 to 27.0 derived from WHO Americas Region studies (Supplementary Fig. S5).22,23 Finally, we examined the vaccination coverage and immunity gap for the 95% credible interval around the SI mean (Supplementary Fig. S6).
Discussion
This study suggests a significant immunity gap existed in the exposed population in the early phase of the 2022 Central Ohio measles outbreak. The results were robust for plausible summer index cases between June 16 and September 20. VaxEstim can rapidly estimate early-phase vaccination coverage during an outbreak using EpiEstim's initial incidence-based Rt estimate. EpiEstim's branching process model requires fewer population assumptions than traditional compartmental models and is well suited to the initial stages of an outbreak when the exposed population is challenging to characterize. We used the latest plausible summer index case for our base analysis to minimize EpiEstim errors associated with fluctuations in underreporting. Notably, the 14-day Rt rebound on November 21 suggested increased case reporting occurred between November 7 and 21. At a November 30 press conference, the health commissioner for Columbus Public Health attributed increased case reporting to increased public awareness and contact tracing,14 which likely followed the November 9 outbreak declaration. Based on this data, we considered the early phase of the outbreak to end on November 7.
Despite high national and state measles vaccination coverage,30 larger and longer postelimination measles outbreaks in the US have been associated with community-level immunity gaps,10 underscoring the importance of rapid assessment of community-specific vaccination coverage at the onset of an outbreak. While, EpiEstim's initial Rt measurements generate wide credible intervals, direct vaccination coverage estimates of previous large (>50 cases) postelimination outbreaks in the US are time-consuming and may be less specific to the exposed population. For example, a 2014 Holmes County, Ohio, measles outbreak was the largest in the US since 1992 and lasted four months.31 Researchers acknowledged that their 14% vaccination coverage assessment underestimated measles vaccination coverage in the exposed population due to a review of vaccination records limited to outbreak-affected households.31 A larger 2018–2019 New York City measles outbreak in Brooklyn threatened measles elimination in the US after sustaining endemic transmission for almost 12 months.10 Researchers used data from the Citywide Immunization Registry for children aged 12–59 months in the Williamsburg area of Brooklyn to estimate a baseline vaccination coverage of 79.5% in the exposed population. However, this data was not representative of the 649 outbreak cases, of which 43.4% (272) occurred in individuals 60 months and older and 27.1% (176) occurred outside the Williamsburg area.
Five of the seven previous large postelimination outbreaks in the US were associated with community-level underimmunization in close-knit communities that shared concerns about vaccine safety. Of 383 reported measles cases in nine counties during the 2014 Holmes County, Ohio measles outbreak, 99.2% occurred in the Amish community.31 While the Amish community's persistently low vaccination coverage has been associated with limited access to healthcare, a study found that the primary barrier to immunization amongst Holmes County Amish parents was their concern over the adverse effects of vaccines.32 Of 649 measles cases reported during the 2018–2019 New York City outbreak, 93.4% occurred in Orthodox Jewish communities in two Brooklyn neighborhoods.33 Additional large postelimination outbreaks occurred in the New York Orthodox Jewish community in 2013 and 2018–2019.10,34 Vaccine hesitancy in this community was associated with targeted misinformation campaigns by antivaccination groups, and mothers specifically expressed concerns about vaccine safety and autism.33 Of 75 cases reported during the 2017 Hennepin County measles outbreak, 81.3% were of Somali descent.35 Vaccination coverage by the age of two years in Minnesota-born Somali children declined from 91.1% in 2004 to 42% in 2017 after misinformation about MMR and autism became entrenched within Minnesota's Somali community.35,36 Antivaccination movement leaders have actively undermined efforts to increase vaccination coverage by the Minnesota Department of Health since 2011.35,37 During the 2022 Central Ohio measles outbreak, the health commissioner for Columbus Public Health noted there had not been “a significant increase” in MMR vaccination three weeks into the outbreak,14 and vaccine hesitancy and refusal, predating the COVID-19 pandemic, drove the outbreak.38
While the Ohio Department of Health maintains the Impact Statewide Immunization Information System (ImpactSIIS), vaccine providers are not required to report MMR vaccinations to the state registry, rendering Ohio's vaccination coverage data incomplete. Additionally, Ohio state law permits vaccination exemptions for medical, religious, and philosophical reasons.39 An extensive Columbus area pediatric primary care network study documented an average one-dose measles vaccination rate in children aged 16 months of 72.0% between March 2017 and March 2020 and 62.4% between June and August 2020.40 While this study suggested measles underimmunization in the Columbus area, its participants were too young to estimate the two-dose vaccination coverage required to protect against large measles outbreaks. Public Health Departments may require additional resources to identify the susceptible population and conduct a root cause evaluation of the reasons for underimmunization at the onset of the 2022 Central Ohio measles outbreak. Understanding the characteristics of a susceptible population can inform community-specific strategies to close immunity gaps, including direct measures of vaccination coverage that health departments can use to track the outcome of vaccination campaigns. Transmission occurred rapidly in daycare and school settings during the 2022 Central Ohio measles outbreak. Cases occurred exclusively in children under age 18 years, 94.1% (80) were under six years, and 29.4% (25) were too young to receive a routine first dose of measles-containing vaccine.16 This data suggests that measles vaccination coverage and exemption rates among kindergarten-aged children in affected Central Ohio areas may provide helpful information about the community-specific immunity gap.
Limitations
Despite our study's contributions toward examining the 2022 Central Ohio measles outbreak, we identified several limitations. First, while real-time Rt estimation models can provide timely and specific results, they have constraints. Rt estimates are sensitive to incidence data, and errors in EpiEstim calculations can occur if underreporting is not constant. Additionally, Rt estimates can also vary based on SI and selection. Second, Rt and Vt credible intervals capture stochasticity in the epidemic process; however, they do not account for other sources of uncertainty, such as imperfect observations or super spreading. Third, critical vaccination thresholds assume homogenous mixing. If preferential mixing in an underimmunized community occurred during this outbreak, the critical vaccination threshold required for herd immunity could be higher, and the estimated immunity gap could be wider. Finally, our model assumes immunity was primarily conferred by immunization prior to the outbreak. If significant population immunity was attributed to past infection, the predicted immunity gap could be narrower, and if substantial post-outbreak vaccination occurred by November 3, the estimated immunity gap could be wider.
Conclusions
Our study developed VaxEstim, a rapid assessment tool that can estimate the vaccination coverage and immunity gap at the onset of an outbreak with sparse population data. Our findings of a robust immunity gap in the exposed population at the onset of the 2022 Central Ohio measles outbreak and evidence of longstanding measles underimmunization in the Columbus area suggest that barriers to vaccination exist. Additional research is needed to define the susceptible population, identify the root causes of underimmunization, and inform community-specific approaches to close immunity gaps.
Contributors
Authors contributed as follows: conceptualization (RM, MW, AC, MM), data curation (RM, MW, JM, AC, MM), formal analysis (RM, JM, AC, MM), funding acquisition (AC, MM), investigation (RM, MW), methodology (RM, MW, AC, MM), project administration (RM, JM), resources (RM), software (RM, JM, AC, MM), supervision (RM, MM), validation (RM, MW, JM, AC, MM), visualization (RM, JM, AC), writing (RM, JM, MW, AC, MM), original draft (RM, JM, MM), review and editing (RM, MW, JM, AC, MM).
Data sharing statement
This study used aggregate, deidentified public data shared in the Supplementary Material.
Declaration of interests
Drs. Rosemary A. Martoma, Matthew Washam, Maimuna S. Majumder, and Mr. Joshua C. Martoma declare no competing interests. Dr. Anne Cori has received payment from Pfizer for teaching mathematical modeling of infectious disease transmission and vaccination.
Acknowledgements
Funding: This work was supported in part by grant R35GM146974 from the National Institute of General Medical Sciences, National Institutes of Health. This work was also supported by joint funding from the UK Medical Research Council (MRC) and the Foreign, Commonwealth and Development Office (MR/R015600/1); the National Institute for Health Research (NIHR) Health Protection Research Unit in Modelling Methodology, a partnership between the UK Health Security Agency (UKHSA; formerly Public Health England); Imperial College London, and the London School of Hygiene & Tropical Medicine (NIHR200908), Community Jameel; the EDCTP2 programme, supported by the EU; and the Sergei Brin Foundation.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100533.
Appendix A. Supplementary data
References
- 1.Gastañaduy P.A., Goodson J.L., Panagiotakopoulos L., Rota P.A., Orenstein W.A., Patel M. Measles in the 21st century: progress toward achieving and sustaining elimination. J Infect Dis. 2021;224:S420–S428. doi: 10.1093/infdis/jiaa793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hübschen J.M., Gouandjika-Vasilache I., Dina J. Measles. Lancet. 2022;399:678–690. doi: 10.1016/S0140-6736(21)02004-3. [DOI] [PubMed] [Google Scholar]
- 3.Gastañaduy P., Haber P., Rota P.A., Patel M. In: Epidemiology and Prevention of Vaccine-Preventable Diseases. The Pink Book: Course Textbook 14th Edition. Hall E., Wodi A.P., Hamborsky J., Morelli V., Schillie S., editors. Centers for Disease Control and Prevention; Washington, D.C: 2021. Measles.https://www.cdc.gov/vaccines/pubs/pinkbook/meas.html [Google Scholar]
- 4.World Health Organization [Press release] Nearly 40 million children are dangerously susceptible to growing measles threat. 2022. https://www.who.int/news/item/23-11-2022-nearly-40-million-children-are-dangerously-susceptible-to-growing-measles-threat#:%7E:text=Nearly%2040%20million%20children%20are%20dangerously%20susceptible%20to%20growing%20measles%20threat,-23%20November%202022%26text=Me
- 5.Gastañaduy P.A., Redd S.B., Clemmons N.S., et al. In: Manual for the surveillance of vaccine-preventable diseases. Roush S.W., Baldy L.M., Kirkconnell Hall M.A., editors. Centers for Disease Control and Prevention; Atlanta, GA: 2018. Measles.https://www.cdc.gov/vaccines/pubs/surv-manual/chpt07-measles.html [Google Scholar]
- 6.Papania M.J., Wallace G.S., Rota P.A., et al. Elimination of endemic measles, rubella, and congenital rubella syndrome from the western hemisphere: the US experience. JAMA Pediatr. 2014;168:148–155. doi: 10.1001/jamapediatrics.2013.4342. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . 2012. Global measles and rubella strategic plan: 2012–2020.https://www.who.int/publications/i/item/9789241503396 [Google Scholar]
- 8.Centers for Disease Control and Prevention Measles, Mumps, and Rubella (MMR) Vaccination: what everyone should know. 2021. https://www.cdc.gov/vaccines/vpd/mmr/public/index.html
- 9.Pan American Health Organization [Press release] 2016. Region of the Americas is declared free of measles.https://www3.paho.org/hq/index.php?option=com_content&view=article&id=12528:region-americas-declared-free-measles&Itemid=0&lang=en#gsc.tab=0 [Google Scholar]
- 10.Mathis A.D., Clemmons N.S., Redd S.B., et al. Maintenance of measles elimination status in the United States for 20 years despite increasing challenges. Clin Infect Dis. 2021;75:416–424. doi: 10.1093/cid/ciab979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon M.G., Ferrari M., Antoni S., et al. Progress toward regional measles elimination — worldwide, 2000–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1563–1569. doi: 10.15585/mmwr.mm7045a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Columbus Public Health [Press release] Measles outbreak in local child care facility. 2022. https://www.columbus.gov/publichealth/press/Measles-Outbreak-in-Local-Child-Care-Facility/
- 13.Howard J. As measles outbreak sickens more than a dozen children in Ohio, local health officials seek help from CDC. 2022. https://www.cnn.com/2022/11/17/health/measles-outbreak-columbus-ohio/index.html
- 14.Columbus Public Health [Press conference] Columbus public health and nationwide Children’s Hospital are sharing an update on the current measles outbreak in central Ohio. 2022. https://www.facebook.com/ColumbusPublicHealth/videos/420346483499723
- 15.Ohio Department of Health [Press release] Health director reports first measles case in Ohio. 2022. https://odh.ohio.gov/media-center/odh-news-releases/odh-news-release-06-16-22
- 16.Columbus Public Health . 2023. Measles public report.https://public.tableau.com/app/profile/columbus/viz/MeaslesPublicReport/MeaslesPublicReport [Google Scholar]
- 17.Centers for Disease Control and Prevention Measles cases and outbreaks. 2023. https://www.cdc.gov/measles/cases-outbreaks.html
- 18.Ohio Department of Health Requests for immunization records: Ohio impact statewide immunization information system (ImpactSIIS) https://coronavirus.ohio.gov/static/vaccine/public-instructions-to-access-impactsiis-record.pdf
- 19.Columbus Public Health [Press release] 2022. Columbus public health is investigating a local case of measles.https://www.columbus.gov/publichealth/press/Columbus-Public-Health-is-investigating-a-Local-Case-of-Measles/ [Google Scholar]
- 20.Klinkenberg D., Nishiura H. The correlation between infectivity and incubation period of measles, estimated from households with two cases. J Theor Biol. 2011;284:52–60. doi: 10.1016/j.jtbi.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Gastañaduy P.A., Funk S., Lopman B.A., et al. Factors associated with measles transmission in the United States during the postelimination era. JAMA Pediatr. 2020;174:56–62. doi: 10.1001/jamapediatrics.2019.4357. https://doi:10.1001/jamapediatrics.2019.4357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerra F.M., Bolotin S., Lim G., et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis. 2017;17:e420–e428. doi: 10.1016/S1473-3099(17)30307-9. [DOI] [PubMed] [Google Scholar]
- 23.Delamater P.L., Street E.J., Leslie T.F., Yang Y.T., Jacobsen K.H. Complexity of the basic reproduction number (R(0)) Emerg Infect Dis. 2019;25:1–4. doi: 10.3201/eid2501.171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gostic K.M., McGough L., Baskerville E.B., et al. Practical considerations for measuring the effective reproductive number, Rt. PLoS Comput Biol. 2020;16 doi: 10.1371/journal.pcbi.1008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cori A., Ferguson N.M., Fraser C., Cauchemez S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol. 2013;178:1505–1512. doi: 10.1093/aje/kwt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nash R.K., Nouvellet P., Cori A. Real-time estimation of the epidemic reproduction number: scoping review of the applications and challenges. PLoS Comput Biol. 2022;1 doi: 10.1371/journal.pdig.0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson R.N., Stockwin J.E., van Gaalen R.D., et al. Improved inference of time-varying reproduction numbers during infectious disease outbreaks. Epidemics. 2019;29 doi: 10.1016/j.epidem.2019.100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majumder M.S., Cohn E.L., Mekaru S.R., Huston J.E., Brownstein J.S. Substandard vaccination compliance and the 2015 measles outbreak. JAMA Pediatr. 2015;169:494–495. doi: 10.1001/jamapediatrics.2015.0384. https://doi:10.1001/jamapediatrics.2015.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fine P., Eames K., Heymann D.L. “Herd immunity”: a rough guide. Clin Infect Dis. 2011;52:911–916. doi: 10.1093/cid/cir007. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention Vaccination coverage among young children (0 – 35 Months) 2020. https://www.cdc.gov/vaccines/imz-managers/coverage/childvaxview/interactive-reports/index.html
- 31.Gastañaduy P.A., Budd J., Fisher N., et al. A measles outbreak in an underimmunized amish community in Ohio. N Engl J Med. 2016;375:1343–1354. doi: 10.1056/NEJMoa1602295. https://doi/10.1056/NEJMoa1602295 [DOI] [PubMed] [Google Scholar]
- 32.Wenger O.K., McManus M.D., Bower J.R., Langkamp D.L. Underimmunization in Ohio's amish: parental fears are a greater obstacle than access to care. Pediatrics. 2011;128:79–85. doi: 10.1542/peds.2009-2599. [DOI] [PubMed] [Google Scholar]
- 33.Zucker J.R., Rosen J.B., Iwamoto M., et al. Consequences of undervaccination—measles outbreak, New York City, 2018–2019. N Engl J Med. 2020;382:1009–1017. doi: 10.1056/NEJMoa1912514. https://doi/10.1056/NEJMoa1912514 [DOI] [PubMed] [Google Scholar]
- 34.McDonald R., Ruppert P.S., Souto M., et al. Notes from the field: measles outbreaks from imported cases in orthodox jewish communities — New York and New Jersey, 2018–2019. MMWR Morb Mortal Wkly Rep. 2019;68:444–445. doi: 10.15585/mmwr.mm6819a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minnesota Department of Health Measles, 2017. 2022. https://www.health.state.mn.us/diseases/reportable/dcn/sum17/measles.html
- 36.Gahr P., DeVries A.S., Wallace G., et al. An outbreak of measles in an undervaccinated community. Pediatrics. 2014;134:e220–e228. doi: 10.1542/peds.2013-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dyer O. Measles outbreak in Somali American community follows anti-vaccine talks. BMJ. 2017;357 doi: 10.1136/bmj.j2378. [DOI] [PubMed] [Google Scholar]
- 38.Abbasi J. Amid Ohio measles outbreak, new global report warns of decreased vaccination during COVID-19 pandemic. JAMA. 2022;329:9–11. doi: 10.1001/jama.2022.23241. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention, office for state, tribal, local, and territorial support. State school immunization requirements and vaccine exemption laws. 2022. https://www.cdc.gov/phlp/docs/school-vaccinations.pdf
- 40.Bode S.M., Gowda C., Mangini M., Kemper A.R. COVID-19 and primary measles vaccination rates in a large primary care network. Pediatrics. 2021;147 doi: 10.1542/peds.2020-035576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.