Summary
Surveillance of e-cigarette use among different population groups is important for the timely implementation and evaluation of tobacco regulatory policies. In this review, we identified 13 nationally representative, repeatedly conducted epidemiologic surveys that assess e-cigarette use among U.S. youth and/or adults and have been instrumental in e-cigarette surveillance. These surveys included National Youth Tobacco Survey, Youth Risk Behavior Surveillance System, Monitoring the Future Survey, International Tobacco Control Policy Evaluation Project (ITC) Youth Tobacco and Vaping Survey, Behavioral Risk Factor Surveillance System, National Health Interview Survey, Tobacco Use Supplement of the Current Population Survey, Health Information National Trends Survey, Tobacco Products and Risk Perception Surveys, ITC Four Country Smoking and Vaping Survey, National Health and Nutrition Examination Survey, National Survey on Drug Use and Health, and Population Assessment of Tobacco and Health. These surveys vary in scope and detail, with their unique strengths and the regulatory questions that can be answered using each survey data. We also highlighted the gaps in these surveys and made recommendations for improvement.
Keywords: E-cigarette, Surveillance, United States, Surveys, Youth, Adults
Background
Smoking remains the leading cause of preventable deaths worldwide and in the United States (U.S.), accounting for nearly 1 in 5 deaths each year in the U.S.1,2 Over the last several years, there have been appreciable gains in tobacco control as the smoking rates among U.S. youths and adults have significantly decreased.3,4 Surveillance of population behavior related to tobacco product use has been instrumental in reducing tobacco use, as it provides information used by local, state, and national governmental and nongovernmental organizations to effectively identify needs, and design and implement policies. Ongoing surveillance, therefore, remains critical for the timely implementation and evaluation of new tobacco regulatory policies.5
Under the Tobacco Control Act, the U.S. Food and Drug Administration (FDA) has regulatory authority over the manufacturing, marketing, and distribution of all tobacco products to safeguard population health.6 Similarly, states and local governments also have the authority to regulate tobacco products as they deem appropriate for the protection of public health.7 The FDA and other regulatory bodies rely on high-quality, up-to-date, population-representative data to carry out these duties effectively. Additionally, if repeatedly collected with the same methodology or among the same population, such data can provide reliable information on changes in tobacco-related population behavior with time and can be used to evaluate the impact of policy changes.
Electronic cigarettes have significantly changed the tobacco landscape since their introduction into the U.S. market in the mid-2000s.8 While their use among adults who smoke combustible cigarettes is reported to aid smoking cessation and reduce the number of cigarettes smoked per day,9,10 their uptake by youth, young adults, and other tobacco-naïve persons is an ongoing concern.11 Under the 2016 “Deeming Rule”, e-cigarettes are considered tobacco products; hence, their manufacturing, marketing, and sales are regulated by the FDA.12 As part of its regulatory role, the FDA has required the Premarket Tobacco Product Application (PMTA) for all e-cigarettes that were introduced after 2007 and are currently on the U.S. market, in order to issue marketing authorization that would allow them to continue their sales in the U.S.13 While e-cigarette companies are responsible for providing data supporting their applications, independent epidemiologic surveillance can provide a comprehensive, independent, and reliable tool to help weigh the associated net benefits and drawbacks of e-cigarettes. For example, the utilization of e-cigarettes among persons who smoke combustible cigarettes to aid smoking cessation versus their use by tobacco-naive individuals, can be assessed and carefully weighed using epidemiologic surveillance. Moreover, understanding the usage patterns of e-cigarettes and the risks associated with such patterns is essential for protecting public health while providing the needed information on use differences among key populations in the U.S., including youth and other at-risk groups. Therefore, it is vital to monitor e-cigarette use nationwide, particularly among groups at higher risk of use such as youth and persons who identify as lesbian, gay, bisexual, or transgender, a group that has historically been targeted by tobacco companies, contributing to inequitably high tobacco use.14, 15, 16
Several national- and state-level epidemiologic surveys include questions assessing various domains of e-cigarette use, such as intention to use, current use, and preferred flavors. Data from these surveys can provide estimates of e-cigarette use by sociodemographic characteristics to help monitor trends and investigate inequities in e-cigarette use among different population subgroups. Additionally, such data increase the understanding of the population's perceptions and intentions regarding various tobacco products. Such insights may help predict future use behaviors and further guide preemptive regulatory actions, policies, and interventions to protect public health.17 Furthermore, some of the surveys, particularly those with built-in cohorts, can be used to longitudinally assess health effects and outcomes, including mortality, associated with tobacco product use. However, some of these surveys may be underused or may not be readily used by tobacco regulatory researchers due to the lack of awareness of the domains of e-cigarette use assessed in each survey. In addition, questions assessing e-cigarette use in each of these surveys differ in scope and detail and can range from a few questions that assess ever and current e-cigarette use to a comprehensive set of questions assessing various domains of e-cigarette use, including brands, flavors, and nicotine concentration. Furthermore, questions used to assess some of the domains are not consistent across surveys. For example, while some surveys assess frequency of use by assessing the number of days within the past 30-days during which participants have used e-cigarettes, others only ask if participants now use e-cigarettes every day, some days, or not at all. Thus, the extent to which these surveys successfully capture the different domains of e-cigarette use among their target population may differ. On the other hand, some questions when asked, are usually consistent across surveys; example of this is the preferred flavors of e-cigarette used in the past 30 days.
To provide a resource for the tobacco regulatory science community, we sought to identify and review repeatedly and regularly conducted, nationally representative surveys that assess e-cigarette use among youth and adults in the U.S. and catalog the relevant e-cigarette domains available in these surveys. We also present the prevalence of e-cigarette usage among youth across years using published data from the youth surveys.
Methods
We identified and retrieved information on available nationally representative and repeatedly conducted epidemiologic surveys that assess e-cigarette use prevalence among U.S. adolescents and youth (generally <18/19 years) and/or adults (generally ≥18 years). Nationally representative as used in our inclusion criteria refers to surveys that the nature of their sampling design allows for surveys weights, based on known key population demographics, to be applied to make the data representative of its target population, e.g., adults in the U.S. We first examined PubMed and Google/Google Scholar to identify the surveys that assess e-cigarette use epidemiology among youth and adults in the U.S. Search terms included “e-cigarette”, “vape”, “electronic cigarettes”, “tobacco”, “United States”, “adolescents”, “youth”, and “adults”. We supplemented this step by communicating with colleagues and experts from the tobacco regulatory science community. As a quality check, we searched ICPSR, a data archiving platform, for e-cigarette-related publications and variables to ensure that most surveys that assess e-cigarette use in the U.S. were screened.18 Details of our search strategy are stated under the section “Search Strategy and Selection Criteria”. We excluded surveys that were not nationally representative, those that were conducted at only one-time point, those that by the nature of sampling cannot be used to provide e-cigarette use prevalence estimates, and surveys that ended on or before the 2015 introduction of JUUL–a preeminent e-cigarette brand that has played a crucial role in the youth e-cigarette epidemic.19 Additionally, surveys with proprietary nontransparent methodologies were not included in this review. A complete list of surveys that were screened but excluded from review and the reasons for exclusion have been presented in Supplementary Tables S1 and S2.
For the eligible nationally representative, repeatedly conducted surveys, we gathered and reported information on the institutions responsible for funding or conducting the survey, methods of data collection, and how these methods have changed recently, given the COVID-19 pandemic. We also reported the target population and approximate sample size, the year since e-cigarette questions were included in the survey, and the frequency of survey administration.
To provide a resource for the tobacco regulatory science community, we gathered and cataloged the specific questions used to assess the various e-cigarette use domains, including the age of initiation and frequency of use, type of device, brand, and the use of flavors, reasons for e-cigarette use, the sources of e-cigarette acquisition, and the use of cannabis in e-cigarettes. We also presented the questions used to assess other salient domains, including the curiosity and intentions of individuals who do not use e-cigarettes to try e-cigarettes in the future. Importantly, we reviewed the preambles used in these surveys to examine how they best capture emerging brands and types of e-cigarettes, such as disposable e-cigarettes.
In addition, we also qualitatively analyzed the strengths of each survey, the limitations that may be addressed, and critical regulatory questions that can be answered using such survey data. Finally, to aid in the comparisons of the youth surveys, we presented the prevalence estimates of past-30-day e-cigarette use as well as measures of frequent use among U.S. youth using published data from the different nationally representative youth surveys that have publicly available data.
Findings
We identified 13 nationally representative, repeatedly conducted epidemiologic surveys that assess e-cigarette use among U.S. youth and/or adults. These included the National Youth Tobacco Survey (NYTS),20 Youth Risk Behavior Surveillance System (YRBSS),21 Monitoring the Future Survey (MTF),22 International Tobacco Control Policy Evaluation Project (ITC) Youth Tobacco and Vaping Survey—US Study,23 Behavioral Risk Factor Surveillance System (BRFSS),24 National Health Interview Survey (NHIS),25 Tobacco Use Supplement of the Current Population Survey (CPS-TUS),26 Health Information National Trends Survey (HINTS),27 Tobacco Products and Risk Perception Surveys (TPRPS),28,29 International Tobacco Control Policy Evaluation Project (ITC) Four Country Smoking and Vaping Survey (4CV)–US Study,30 National Health and Nutrition Examination Survey (NHANES),31 National Survey on Drug Use and Health (NSDUH),32 and Population Assessment of Tobacco and Health (PATH).33 Another survey, beside the 13 discussed in this paper, that met our inclusion criteria, but has not been discussed further is the Truth Longitudinal Cohort (TLC), an online cohort created and maintained by the Truth Initiative, an organization with a mission statement/agenda, to evaluate the 2014 relaunch of the antitobacco mass campaign.34 Additionally, communication with the authors of the TLC confirms that the cohort is not designed to provide precise population based prevalence estimates. A second survey that has also not been further reviewed is the Altria Client Services Underage Tobacco Use Survey (UTUS), which is designed and conducted by the tobacco company, Altria.35,36 Information about the TLC and UTUS and reasons for exclusion have been presented in Supplementary Table S2.
Table 1 shows information on the funding and conducting institution, methodology, and target population of these surveys. Most of the surveys are funded by the Centers for Disease Control and Prevention (CDC) in conjunction with the Food and Drug Administration (FDA) or other agencies such as the National Institute on Drug Abuse (NIDA), Substance Abuse and Mental Health Services Administration (SAMHSA), and the National Cancer Institute (NCI).
Table 1.
General information on the surveys used to assess E-cigarette use among youth and adults in the United States.
| Survey | Funding Institution | Conducting Institution | Method of data collection | Population | First Inclusion of E-cigarette Questions | Periodicity | Sample Size |
|---|---|---|---|---|---|---|---|
| National Youth Tobacco Survey (NYTS) | FDA and CDC | FDA and CDC | School-based Self-administered In-person offline tablet-based but in 2021, due to COVID-19, was fully online so that eligible participants could access from classrooms, at home, or from other remote learning environments. |
Middle school (Grades 6–8) High school (Grades 9–12) |
2011 | 1999, 2000, 2002, 2004, 2006, 2009, and annually from 2011 | ∼15,000–20,000 |
| Youth Risk Behavior Surveillance System (YRBSS) | CDC | National by the CDC School-based state, territorial, tribal, and large urban school district surveys conducted by education and health agencies |
School-based Self-administered anonymously by using a computer-scannable questionnaire booklet |
High school (Grades 9–12) in public and private schools | 2015 | Biennially during the spring of odd-numbered years | ∼14,517 |
| Monitoring the Future Survey (MTF) | National Institute on Drug Abuse | University of Michigan | Paper and pencil questionnaires in schools; changed to use of electronic tablets in 2019. The 8th and 10th grade questionnaires are completely anonymous, and in 12th grade they are confidential (name and address information gathered separately from the 12th grade questionnaire to permit the longitudinal follow- up of random subsamples of participants) |
8th, 10th, and 12th grade students | 2014 | Annually | ∼50,000 |
| International Tobacco Control Policy Evaluation Project (ITC) Youth Tobacco and Vaping Survey—US Study | Partly by the US National Institutes of Health | University of Waterloo | Online Web Panel | 16–19-year-old individuals recruited from Nielsen Consumer Insights Global Panel. | 2017 | Annually, with additional semi-annual waves | ∼4500 |
| Behavioral Risk Factor Surveillance System (BRFSS) | Primarily funded by the CDC; various state programs may fund additional modules or questions | State-health departments with technical and methodological assistance from CDC | Telephone interview | Noninstitutionalized adults ≥18 years | 2016 | Annually But e-cigarette questions are part of the core module in 2016, 2017, and 2021 and as optional model in 2018 and 2020. No e-cigarette data in 2019 |
∼400,000 sample adult respondents nationally per year |
| National Health Interview Survey (NHIS) | CDC Sponsored content are questions funded by other federal agencies or other centers within CDC about topics of interest to the sponsor. |
Designed by the CDC's National Center for Health Statistics Administered by U.S. Census Bureau |
Computer-assisted personal interviewing. Face-to-face interviews are conducted in respondents' homes, but follow-ups to complete interviews may be conducted over the telephone | Residents of households and noninstitutional group quarters 18+ years for tobacco questions |
2014 | Annually | ∼87,500 persons in 35,000 households |
| Tobacco Use Supplement of the Current Population Survey (CPS-TUS) | National Cancer Institute | U.S. Census Bureau | Computerized document that is administered by Census Bureau field representatives through personal and telephone interviews | Civilian, noninstitutionalized population ages 18 years and older if the household is eligible for the supplement, the interviewer attempts to interview everyone (18 years or older) in the household |
2014/2015 | Every 3–4 years (up to 2018–2019 data available) | ∼ 240,000 individuals within a given survey period. |
| Health Information National Trends Survey (HINTS) | National Cancer Institute | National Cancer Institute and Westat | Previously by random digit dialing landline telephone alone or with mail questionnaire. Now self-administered mail questionnaire | Adults 18 years or older in the U.S. civilian noninstitutionalized population. | 2012 | Biennial | ∼3000–6000 |
| The Tobacco Products and Risk Perception Surveys (TPRPS) | FDA Center for Tobacco Products | Georgia State University Tobacco Center of Regulatory Science (TCORS) | Online Web Panel | Noninstitutionalized U.S. adults (18 years or older) drawn from Gfk's KnowledgePanel | 2014 | Annual | ∼6000 |
| International Tobacco Control Policy Evaluation Project (ITC) Four Country Smoking and Vaping Survey—US Study | Partly by the US National Cancer Institute | University of Waterloo with support from the Medical University of South Carolina | Online Web Panel | Adults (18 years or older) drawn from GfK KnowledgePanel and other opt-in partner panels. | 2016 | Biennial | ∼2500–2800 |
| National Health and Nutrition Examination Survey (NHANES) | CDC | CDC's National Center for Health Statistics | Health interviews are computer-based in respondent's home; Health measurements are in-person in specially designed and equipped mobile centers | Noninstitutionalized civilian resident all ages | 2013 | Annually but data released every two years | ∼5000–10,000 |
| National Survey on Drug Use and Health (NSDUH) | Substance Abuse and Mental Health Services Administration (SAMHSA) and supervised by the SAMHSA's Center for Behavioral Health Statistics and Quality. | Directed by SAMHSA Conducted by Research Triangle Institute (RTI) International |
Formerly: Personal interviews and self-enumerated answer sheets Currently: Audio Computer-Assisted Self-interviewing (ACASI) Computer-Assisted Personal Interviewing (CAPI)-Interviewers read less-sensitive questions to respondents and enter the respondents' answers on the laptop. English/Spanish versions available. |
Adolescents and adults (≥12 years) | 2020 | Annually | ∼70,000 |
| Population Assessment of Tobacco and Health (PATH) | A collaboration between the U.S. Food and Drug Administration (FDA) Center for Tobacco Products and the National Institutes of Health (NIH) National Institute on Drug Abuse (NIDA) | Westat, an independent research firm in Maryland | Telephone/field interview Audio Computer-Assisted Self-Interviewing Computer-assisted personal interview Computer-assisted telephone interview |
U.S. civilian, noninstitutionalized population aged ≥12 years. Shadow youth (aged 9–11 years) |
2014 | Biannually | ∼45,971 at wave 1 (plus 7207 “shadow youth”) 14,098 replenishment sample added at wave 4. Total at wave 4: 52,731 |
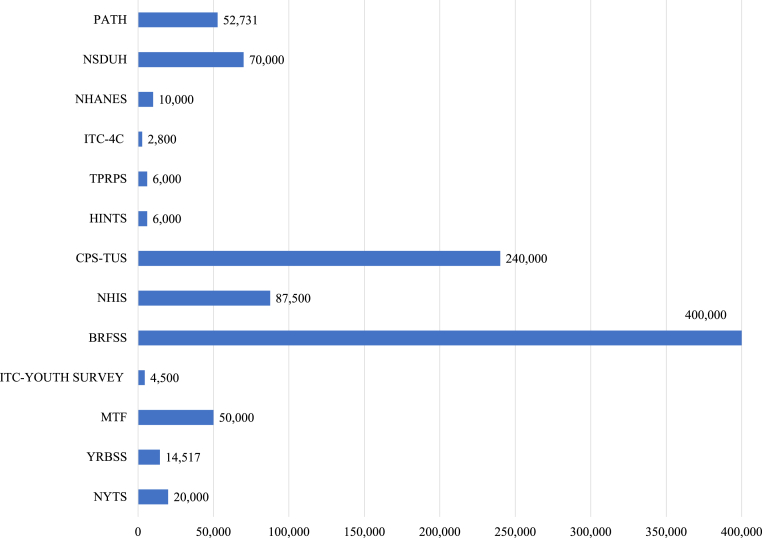
Four surveys (NYTS, YRBSS, MTF, and ITC- Youth Survey) assess e-cigarette use predominantly among youth. Three of these surveys (NYTS, YRBSS, and MTF) have been school-based and self-administered with either offline tablets or computer-scannable questionnaire booklets. However, in 2021 due to the COVID-19 pandemic, the NYTS switched to fully online so that eligible participants could access the survey from classrooms, at home, or in other remote learning environments (Table 2).37 The ITC Youth Survey recruits youth aged 16–19 years from Nielsen Consumer Insights Global Panel and partner panels. Of these four youth surveys, data on e-cigarette use are available annually for the NYTS (since 2011), MTF (since 2014), and ITC Youth Survey (since 2017, with two additional semi-annual waves), and biennially on odd-numbered years for the YRBSS (since 2015). MTF survey has the largest sample size of approximately 50,000 participants, followed by the NYTS (∼15,000–20,000), the YRBSS (∼14,000) and the ITC Youth Survey (∼4500) (Table 1; Fig. 1).
Table 2.
Recent changes in methodology of the surveys used to assess E-cigarette use among youth and adults in the United States.
| Survey | Methodology |
|---|---|
| National Youth Tobacco Survey (NYTS) | Paper and pencil questionnaires since inception in 1999, began using electronic data collection methods (tablets) starting in 2019 (In-person offline tablet-based) but in 2021, due to COVID-19, was fully online so that eligible participants could access from classrooms, at home, or from other remote learning environments. |
| Youth Risk Behavior Surveillance System (YRBSS) | The CDC developed one-time, online Adolescent Behaviors and Experiences Survey (ABES), which was conducted during January–June 2021 to assess student behaviors and experiences during the COVID-19 pandemic among high school students. For the State-based YRBS, some states e.g., Indiana completed the 2021 survey (January–April 2021) and Wisconsin (September 2012–December 2021) while Florida withdrew from the YRBS |
| Monitoring the Future Survey (MTF) | Early termination of field efforts in 2020 resulting in small sample size; 2021 resumed with a change to all web-based surveys. Students completed the surveys on their personal or school-provided device. |
| International Tobacco Control Policy Evaluation Project (ITC) Youth Tobacco and Vaping Survey—US Study | No documented change |
| Behavioral Risk Factor Surveillance System (BRFSS) | Because the BRFSS is a telephone survey and employs computer-assisted telephone interviews, the data collection methodology did not significantly change during COVID-19. |
| National Health Interview Survey (NHIS) | Approximately half of the original sample allocated for the last five months of 2020 was replaced with sample adults who completed the 2019 NHIS sample adult interview. The reinterviewing of Sample Adults from 2019 using the 2020 NHIS questionnaire also provided an opportunity to assess changes over time in health outcomes measured on both survey years and assess measures during the pandemic. |
| Tobacco Use Supplement of the Current Population Survey (CPS-TUS) | No documented change |
| Health Information National Trends Survey (HINTS) | No documented change |
| The Tobacco Products and Risk Perception Surveys (TPRPS) | Last survey in 2018 |
| International Tobacco Control Policy Evaluation Project (ITC) Four Country Smoking and Vaping Survey—US Study | No documented change |
| National Health and Nutrition Examination Survey (NHANES) | The 2019–2020 data collection cycle was suspended in March 2020 due to the inability to perform in-person exams on account of rising COVID-19 cases. Therefore, the 2019-early 2020 data is not nationally representative. The 2021–2022 sampling strategy decreased the number of households screened (from ∼13,000 to ∼7000), leading to fewer in-person encounters for field staff. The reduction in the number of households screened did not change the targeted total examined sample of ∼10,000 persons across the 30 primary sampling units. Additionally, eligible survey participants scheduled a telephone appointment to complete the home interview. |
| National Survey on Drug Use and Health (NSDUH) | Changed to web-based in 2020 |
| Population Assessment of Tobacco and Health (PATH) | No documented change |
Fig. 1.
Approximate sample sizes of the various nationally representative surveys that assess E-cigarette use among youth and adults in the United States.
The BRFSS, NHIS, CPS-TUS, HINTS, TPRPS, and ITC-4CV assess e-cigarette use only among noninstitutionalized U.S. adults aged 18 years or older. The BRFSS has been the largest, continuously conducted survey among U.S. adults, with an annual sample size of approximately 400,000 respondents (Fig. 1). While data on e-cigarette use are available annually from 2016 (except in 2019), questions on e-cigarette use in the BRFSS questionnaire were part of the core module (2016, 2017, 2021) and hence used by all participating states/territories, or part of the optional module (2018, 2020), giving states the flexibility to include in their state survey based on their priorities. Because the BRFSS is a telephone-based survey that uses the computer-assisted telephone interview system, the methodology did not significantly change during the COVID-19 pandemic (Table 2). While data collection was briefly interrupted in some areas, the CDC reports that the shortfalls in the monthly completion during the initial COVID-19-related shelter-in-place were made up by the end of the data collection year, with all states meeting the minimum requirements to be included in the 2020 data.38 The CPS-TUS, another nationally representative survey, is conducted periodically (every 3–4 years) by the U.S. Census Bureau as part of its Current Population Survey, with ∼240,000 respondents in each survey period. HINTS is an NCI-sponsored nationally representative survey with extensive information on the knowledge, attitudes, and perceptions of e-cigarette use. The TPRPS was conducted by the Georgia State University Tobacco Center of Regulatory Science with participants drawn from the Growth from Knowledge (GfK) KnowledgePanel.39 The ITC-4CV, which is partly funded by the U.S. NCI, also recruits participants from GfK KnowledgePanel and other opt-in partner panels, with ∼2500–2800 biennially.30
The NHANES, NSDUH, and PATH surveys assess e-cigarette use in both youth and adults. NHANES is unique in that it combines interviews with physical examinations and laboratory tests, including serum and urinary cotinine and hydroxycotinine, which are nicotine metabolites.40 All participants aged ≥12 years are asked questions about tobacco use, including e-cigarette use. Similarly, the PATH study includes participants aged ≥12 years and has data on some biomarkers of tobacco exposure. Additionally, the PATH study, which has a sample size of approximately 49,000 participants at baseline, is unequaled as a national longitudinal study with almost ten (10) years of follow-up.41 The NSDUH is conducted annually with about 70,000 participants (Fig. 1).
Presented in Table 3 and Supplementary Table S3 are the domains, preambles, and exact questions used to assess e-cigarette use in each of the 13 surveys. All the surveys provide a preamble that introduces the respondents to what e-cigarettes are. Of the four youth surveys, NYTS and YRBSS provide names of common e-cigarette brands such as JUUL, Vuse, and Blu, in addition to listing the common e-cigarette terminologies such as vapes, vape pens, e-cigars, e-hookahs, hookah pens, and mods. The MTF survey does not provide names of common e-cigarette brands. In their preambles, only the ITC Youth Survey out the four youth surveys mentions disposable e-cigarettes. The surveys do not state Puff Bar, the most common disposable e-cigarette used by middle and high school students, in their introductory preamble.42
Table 3.
Domains of E-cigarette use included in the surveys used to assess E-cigarette use among youth and adults in the United States.
| Domains | NYTS | YRBSS | MTF | ITC, US-Youth | BRFSS | NHIS | CPS-TUS | HINTS | TPRPS | ITC, US-Adult | NHANES | NSDUH | PATH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preamble | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Preamble includes disposable e-cigarettes | ✓ | ✓ | ✓ | ✓ | |||||||||
| Lifetime or ever use | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Age at initial use | ✓ | ✓ | ✓ | ✓ | |||||||||
| Frequency of use | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Type or brand used | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Flavors | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Nicotine content | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Accessibility and source | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Details about cost | ✓ | ✓ | ✓ | ||||||||||
| Reason for e-cigarette use | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Curiosity and intentions to use | ✓ | ✓ | ✓ | ✓ | |||||||||
| Vaping other substances beside nicotine e.g., cannabis, flavorings | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Vaping policy at home or workplace | ✓ | ||||||||||||
| Secondhand e-cigarette aerosol exposure | ✓ | ✓ | |||||||||||
| Addiction/dependence | ✓ | ✓ | ✓ | ✓ | |||||||||
| Quitting e-cigarettes | ✓ | ✓ | ✓ | ✓ | |||||||||
| Sources of information on e-cigarettes | ✓ | ✓ | ✓ | ||||||||||
| Knowledge about content, addictiveness, and harmfulness of e-cigarettes | ✓ | ✓ | ✓ | ✓ | |||||||||
| Other tobacco products assessed by survey |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Strengths | Comprehensively assesses several domains of e-cigarette use in both middle and high school students. | Has additional information on a wide array of other high-risk behaviors including substances misuse. | The 12th grade questionnaire permits longitudinal follow- up of random subsamples of participants. | Very extensive in assessing the various domains of e-cigarette use with additional information on the effects of COVID-19 on vaping habits and e-cigarette accessibility. | The largest repeatedly conducted survey among US adults. It also state-level information and rural/urban residence. | Can be linked to the Medical Expenditure Panel Survey to assess healthcare costs associated with e-cigarette use | Information on secondhand exposure to e-cigarette vapor. | Has information on where participants access information on e-cigarette and other tobacco products. | Comprehensively assesses several domains of e-cigarette use among adults including e-cigarette quit intentions and attempts. | Very extensive in assessing the various domains of e-cigarette use with additional information on perceptions regarding e-cigarette-related laws | Can be linked to the Centers for Medicare and Medicaid Services data to study changes in healthcare utilization and expenditures | Has questions on mental health and psychosocial stressors, hence the association of e-cigarette use with these measures can be assessed | Longitudinal design, with about ten years of follow-up data therefore allowing study of tobacco product transitions and health effects |
Among the adult surveys, CPS-TUS and TPRPS include disposable e-cigarettes as part of the e-cigarette examples in their preambles, but BRFSS, NHIS, HINTS, and ITC-4CV do not. Of the three surveys assessing e-cigarette use among youth and adults (NHANES, NSDUH, and PATH), only the PATH study mentions disposable e-cigarettes as part of its preamble. A hand card used in the NHANES to show the different types of e-cigarettes does not include disposable e-cigarettes.43,44
The NYTS, YRBSS, MTF, and the ITC Youth Survey assess ever and current use (typically defined as past 30-day use) of e-cigarettes as well as the sources or access to e-cigarettes. Additionally, the NYTS and ITC Youth Survey comprehensively assess several other domains of e-cigarette use, including type, brand, flavors, reasons for use, and the use of cannabis in e-cigarettes. The NYTS and ITC Youth Survey also assess curiosity and future intentions to use e-cigarettes among respondents who have never used an e-cigarette. Since 2017, the question used to assess the prevalence of past-30-day e-cigarette use in the MTF survey has been modified to three questions with each assessing the prevalence of e-cigarette use for vaping specific substances (nicotine, marijuana, and flavorings).
The BRFSS, NHIS, and HINTS mainly assess ever and current use of e-cigarettes. On the other hand, the CPS-TUS, TPRPS, and ITC-4CV are very comprehensive in assessing e-cigarette use among adults. HINTS and ITC-4CV have questions that extensively examine participants' sources of e-cigarette-related information and their perception of the harms of e-cigarettes.
In addition to assessing the device type and e-cigarette flavors, the CPS-TUS also evaluates the sources and cost of e-cigarettes, reasons for e-cigarette use, and e-cigarette-related policies/rules at the workplace and home. The TPRPS and ITC-4CV assess addiction and changes in e-cigarette use patterns. The NHANES and NSDUH mainly assess ever and current e-cigarette use. The NHANES also assesses secondhand vape exposure, while the NSDUH assesses vaping other substances besides nicotine. The PATH study is very comprehensive and has detailed questions on various domains of e-cigarette use, including frequency of use in the past month, the number of times per day e-cigarettes are used, and the average number of puffs per session. The ITC-4CV is also very comprehensive and includes questions on nicotine strength, quit intentions and attempts, healthcare provider recommendations, opinions on vaping laws, and effects of e-cigarette advertisements.
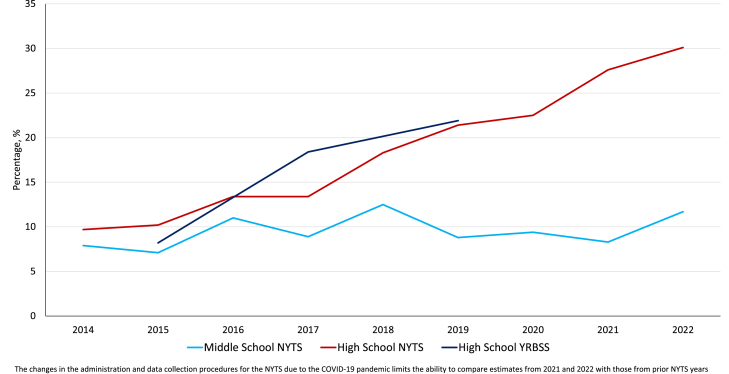
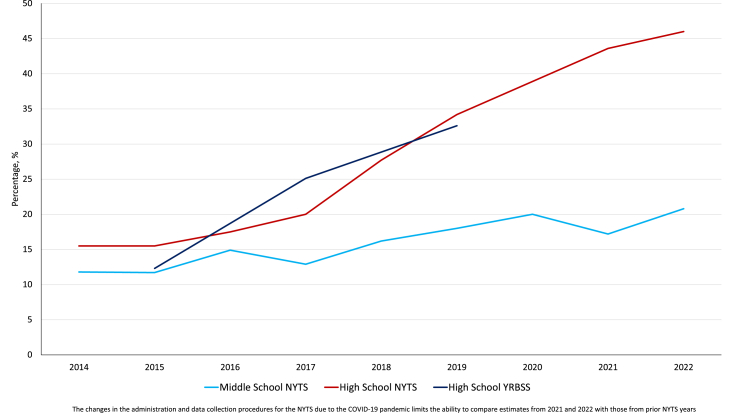
In Table 4, we present the estimates of e-cigarette use among middle and high school students in the U.S. as assessed by the NYTS and YRBSS to examine their comparability of e-cigarette use prevalence estimates in terms of trends. We also present estimates from MTF survey (Table 5) for 8th, 10th, and 12th graders in the U.S., and PATH Youth and ITC Youth Vaping data (Table 6). Of particular importance are the trends in the measures of frequent e-cigarette use among those who reported past 30-day use, i.e., proportion of current users who use their devices frequently or daily. Of those who reported past 30-day use, the proportion who use their devices frequently or daily has been steadily increasing as shown by data from the four surveys (Table 4, Table 5, Table 6; Fig. 2, Fig. 3).
Table 4.
Table with the Estimated Current, Frequent, and Daily E-cigarette use Prevalence among Youth in the United States as Estimated by the National Youth Tobacco Survey and the Youth Risk Behavior Surveillance System.
| Current use | Daily use | Frequent use (≥20 days) | Daily use among current users | Frequent use among current users (≥20 days) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| National youth tobacco survey | Middle school | High school | Middle school | High school | Middle school | High school | Middle school | High school | Middle school | High school |
| 2011 | 0.6 (0.4–0.9) | 1.5 (1.2–2.0) | – | – | – | – | – | – | – | – |
| 2012 | 1.1 (0.9–1.5) | 2.8 (2.3–3.5) | – | – | – | – | – | – | – | – |
| 2013 | 1.1 (0.8–1.5) | 4.5 (3.8–5.3) | – | – | – | – | – | – | – | – |
| 2014 | 3.9 (3.0–5.0) | 13.4 (11.2–16.1) | 0.3 (0.2–0.5) | 1.3 (0.9–1.9) | 0.5 (0.3–0.7) | 2.1 (1.5–2.9) | 7.9 (5.4–11.4) | 9.7 (7.5–12.5) | 11.8 (8.2–16.7) | 15.5 (12.7–18.8) |
| 2015 | 5.3 (4.6–6.2) | 16.0 (14.1–18) | 0.4 (0.3–0.6) | 1.7 (1.3–2.1) | 0.7 (0.5–0.9) | 2.5 (2.0–3.2) | 7.1 (5.1–9.8) | 10.2 (8.4–12.4) | 11.7 (8.6–15.8) | 15.5 (12.9–18.4) |
| 2016 | 4.3 (3.7–4.9) | 11.3 (9.9–12.9) | 0.5 (0.3–0.7) | 1.6 (1.3–2.0) | 0.7 (0.5–0.9) | 2.1 (1.7–2.5) | 11.0 (7.2–16.6) | 13.4 (11.0–16.2) | 14.9 (10.6–20.7) | 17.5 (14.9–20.4) |
| 2017 | 3.3 (2.8–3.9) | 11.7 (9.7–13.9) | 0.4 (0.2–0.6) | 1.6 (1.2–2.3) | 0.5 (0.4–0.7) | 2.5 (1.8–3.4) | 8.9 (5.8–13.4) | 13.4 (10.1–17.6) | 12.9 (9.1–18.1) | 20.0 (15.8–25.0) |
| 2018 | 4.9 (4.2–5.8) | 20.8 (18.8–22.9) | 0.7 (0.5–1.0) | 4.0 (3.4–4.7) | 0.9 (0.7–1.2) | 6.0 (5.1–7.0) | 12.5 (9.4–16.5) | 18.3 (16.0–21.0) | 16.2 (12.8–20.3) | 27.7 (24.7–30.9) |
| 2019 | 10.5 (9.4–11.8) | 27.5 (25.3–29.7) | 0.9 (0.7–1.2) | 5.9 (4.9–7.0) | 1.9 (1.5–2.4) | 9.4 (8.0–10.9) | 8.8 (6.9–11.2) | 21.4 (19.0–24.0) | 18 (15.2–21.2) | 34.2 (31.2–37.3) |
| 2020 | 4.7 (3.6–6.0) | 19.6 (17.2–22.2) | 0.4 (0.3–0.7) | 4.4 (3.5–5.6) | 0.9 (0.7–1.2) | 7.6 (6.2–9.3) | 9.4 (5.6–15.2) | 22.5 (19.0–26.4) | 20 (16.0–24.8) | 38.9 (35.2–42.6) |
| 2021a | 2.8 (2.2–3.4) | 11.3 (9.7–13.0) | 0.2 (0.15–0.35) | 3.1 (2.5–3.8) | 0.5 (0.3–0.7) | 4.9 (4.1–5.9) | 8.3 (5.6–12.0) | 27.6 (24.3–31.2) | 17.2 (12.8–22.6) | 43.6 (39.0–48.2) |
| 2022a | 3.3 (2.6–4.2) | 14.1 (12.4–16.0) | – | – | – | – | 11.7 (8.0–16.7) | 30.1 (26.6–33.9) | 20.8 (15.8–26.8) | 46.0 (41.6–50.4) |
| Youth Risk Behavior Surveillance System | Middle School | High School | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | – | 24.0 (21.9–26.3) | – | 2.0 (1.6–2.5) | – | 3.0 (2.4–3.6) | – | 8.2 (6.8–9.8) | – | 12.3 (10.6–14.2) |
| 2017 | – | 13.2 (11.5–15.1) | – | 2.4 (2.0–3.0) | – | 3.3 (2.6–4.2) | – | 18.4 (15.7–21.4) | – | 25.1 (21.3–29.5) |
| 2019 | – | 32.7 (30.4–35.1) | – | 7.2 (6.1–8.4) | – | 10.7 (9.4–12.1) | – | 21.9 (19.6–24.4) | – | 32.6 (30.0–35.2) |
The changes in the administration and data collection procedures for the NYTS due to the COVID-19 pandemic limits the ability to compare estimates from 2021 to 2022 with those from prior NYTS years.
Table 5.
Table with the estimated current, frequent, and daily E-cigarette use prevalence among youth in the united states as estimated by the monitoring the future survey.
| Years | Current use |
Daily use |
Frequent use (≥20 days) |
Daily use among current users |
Frequent use among current users (≥20 days) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8th | 10th | 12th | 8th | 10th | 12th | 8th | 10th | 12th | 8th | 10th | 12th | 8th | 10th | 12th | |
| 2014 | 8.7 (7.7–9.7) | 16.3 (15.0–17.6) | 17.2 (16.3–18.2) | – | – | – | 0.9 (0.6–1.3) | 1.9 (1.5–2.4) | 2.9 (2.4–3.3) | – | – | – | 10.3 (7.4–14.2) | 11.7 (9.3–14.7) | 16.6 (14.3–19.1) |
| 2015 | 9.4 (8.5–10.6) | 13.9 (12.8–15.0) | 16.2 (15.3–17.2) | – | – | – | 1.1 (0.8–1.5) | 2.3 (1.8–2.8) | 3.1 (2.7–3.6) | – | – | – | 11.6 (8.4–15.7) | 16.3 (13.4–19.7) | 19.3 (16.9–22.0) |
| 2016 | 6.3 (5.6–7.1) | 10.4 (9.4–11.5) | 12.3 (11.5–13.2) | – | – | – | 0.8 (0.6–1.1) | 1.3 (1.0–1.7) | 2.1 (1.7–2.5) | – | – | – | 12.7 (9.2–17.3) | 12.2 (9.4–15.7) | 16.8 (14.3–19.6) |
| 2017 | 6.8 (6.0–7.7) | 10.7 (9.7–11.9) | 15.4 (13.7–17.3) | – | – | – | 1.1 (0.8–1.6) | 2.2 (1.7–2.7) | 3.2 (2.5–4.1) | – | – | – | 16.8 (12.5–22.2) | 20.1 (16.4–24.6) | 20.6 (16.2–25.9) |
| 2018 | – | – | 25.6 (23.4–27.9) | – | – | – | – | – | 9.0 (7.7–10.4) | – | – | – | – | – | 35.1 (30.7–39.8) |
| 2019 | 9.6 (8.9–10.4) | 20.5 (19.5–21.6) | 25.5 (24.4–26.6) | – | – | – | 2.0 (1.7–2.4) | 7.0 (6.4–7.7) | 11.7 (10.9–12.6) | – | – | – | 21.0 (17.9–24.4) | 34.2 (31.6–36.9) | 46.1 (43.6–48.6) |
| 2020a | 10.9 (9.3–12.6) | 21.6 (19.8–23.4) | 25.9 (23.4–28.5) | 0.8 (0.4–1.5) | 3.1 (2.4–3.9) | 5.1 (4.1–6.3) | 1.7 (1.1–2.7) | 6.2 (5.1–7.5) | 8.0 (6.6–9.7) | 7.0 (3.7–12.9) | 14.3 (11.4–17.8) | 19.7 (15.9–24.2) | 15.9 (10.5–23.4) | 28.5 (24.1–33.4) | 31.0 (26.0–36.5) |
| 2021 | 7.4 (6.8–8.0) | 13.6 (12.8–14.4) | – | 1.0 (0.8–1.2) | 2.5 (2.2–2.9) | – | 1.7 (1.4–2.0) | 4.2 (3.7–4.7) | – | 13.3 (11.0–16.2) | 18.6 (16.3–21.0) | – | 22.7 (19.6–26.2) | 30.6 (27.8–33.6) | – |
Question assessing e-cigarette use was changed from general e-cigarette use to nicotine vaping in 2017.
Due to COVID-19, participant recruitment was halted leading to small number of participants (25% of usual sample size)—large confidence intervals.
Table 6.
Table with the estimated current, frequent, and daily E-cigarette use prevalence among youth in the United States as estimated by the population assessment of tobacco and health survey and ITC youth tobacco and vaping survey.
| Waves | Current use | Daily use | Frequent use (≥20 days) | Daily use among current users | Frequent use among current users (≥20 days) |
|---|---|---|---|---|---|
| Population assessment of tobacco and health | |||||
| Wave 1 (2013–14) | 3.12 [2.82–3.44] | 0.2 (0.09–0.23) | 0.36 (0.27–0.48) | 4.79 (3.06–7.41) | 11.52 (8.70–15.11) |
| Wave 2 (2014–15) | 3.60 (3.26–3.98) | 0.30 (0.21–0.42) | 0.49 (0.37–0.65) | 8.48 (6.09–11.70) | 13.89 (10.64–17.93) |
| Wave 3 (2015–16) | 4.09 (3.71–4.50) | 0.27 (0.18–0.39) | 0.69 (0.54–0.88) | 6.89 (4.71–9.96) | 17.81 (14.15–22.18) |
| Wave 4 (2016–17) | 7.25 (6.53–8.05) | 0.48 (0.31–0.73) | 1.10 (0.84–1.45) | 6.85 (4.51–10.27) | 15.70 (12.08–20.16) |
| Wave 5 (2018–19) | 18.34 (16.45–20.40) | 3.65 (2.80–4.75) | 6.40 (5.24–7.80) | 20.44 (15.94–25.84) | 35.87 (30.20–41.96) |
| ITC youth tobacco and vaping surveya | |||||
| 2017 | 11.1 | – | 2.2 | – | – |
| 2018 | 15.7 | – | 3.8 | – | – |
| 2019 | 18.5 | – | 6.7 | – | – |
Estimates were generated using public use data files for youth aged 12–17 years and cross-sectional weights provided by PATH.
Estimates from the ITC Youth Tobacco and Vaping Survey were obtained from published article (Reference45).
Fig. 2.
Trends in E-cigarette daily-to-current use ratio among middle and high school students in the United States.
Fig. 3.
Trends in E-cigarette frequent (≥20 days)-to-current use ratio among middle and high school students in the United States.
Discussion
Our review identified 13 nationally representative surveys that conduct ongoing assessments of e-cigarette use among U.S. youth and/or adults. The epidemiologic surveys such as those reviewed in this report have been instrumental in monitoring e-cigarette and other tobacco products use among different population subgroups. We submit that our paper will serve as an essential resource for members of the tobacco regulatory science community who may be unfamiliar with the many resources available for studying e-cigarette use prevalence and trends. Below, we describe the strengths of the surveys, limitations, and challenges and make recommendations to improve the surveys.
Strengths
All the surveys reviewed are repeatedly conducted, albeit at different frequencies. They can therefore be used for repeated cross-sectional studies to monitor changes in the patterns of e-cigarette use among different population subgroups. Among the surveys, a strength of the NYTS, TPRPS, PATH, and the ITC Youth Survey is their assessment of curiosity and intentions to use among individuals who have never used e-cigarettes. This is particularly important as intentions and susceptibility to use may predict future behavior.46, 47, 48 While not all intentions are carried out, actions are mainly controlled by intentions, as described by the theory of planned behavior.46 Therefore, it is imperative to assess whether the appeal of e-cigarettes is increasing among nonusers so that preemptive policies and interventions can be implemented.
The YRBSS, MTF survey, and NSDUH assess other high-risk behaviors, including binge drinking, cannabis use, and use of other substances of abuse. These surveys have therefore been used to determine whether e-cigarette use clusters with other high-risk behaviors.49, 50, 51, 52 The YRBSS and NSDUH also ask questions on mental health and psychosocial stressors such as bullying and sexual assault and can therefore be used to assess the association of youth e-cigarette use with psychosocial stressors.53 It is crucial to identify stressors that may lead to initiation and continued use of e-cigarettes among youth and other vulnerable populations, as mitigating such stressors may reduce tobacco use among vulnerable populations.54
NHIS and NHANES leverage linkage with other datasets. A unique strength of the NHIS is the linkage to the Medical Expenditure Panel Survey (MEPS), enabling the assessment of the healthcare costs associated with e-cigarette use. The MEPS prospectively follows a representative sample drawn from the prior year's NHIS for two calendar years to assess their healthcare engagement and costs.55 Linking the MEPS to the NHIS data can be used to study the real-world health-related cost implications of e-cigarette use, as has been done for smoking.56, 57, 58, 59 Unique to the NHANES dataset is its linkage to the Centers for Medicare and Medicaid Services (CMS) data to study changes in healthcare utilization and expenditures in specialized populations.60 Both the NHIS and NHANES can also be linked to the national death index (NDI) to study mortality associated with use of e-cigarettes.
The NHANES and CPS-TUS assess exposure to secondhand vapor or e-cigarette aerosol. Although the health implications of secondhand cigarette smoke have been elucidated, those associated with secondhand e-cigarette aerosols are still largely unknown. Few recent studies have reported that secondhand exposure to emissions from e-cigarettes may be associated with adverse health outcomes, including bronchitis symptoms and asthma exacerbations.61,62 Thus, data on the burden and the health effects of secondhand exposure to e-cigarettes are needed.
Continued surveillance of the sources of e-cigarette acquisition, particularly among youth and young adults, is crucial. Understanding the changing sources of e-cigarettes among youth is necessary to inform regulatory approaches that limit youth access to e-cigarettes.63 The NYTS, YRBSS, CPS-TUS, TPRPS, PATH, and the ITC Youth Survey assess the access to e-cigarette use. Therefore, data from these surveys can be used to study the changing sources of e-cigarette acquisition among different population subgroups.53 Such data, particularly among youth, can be used to inform policies and regulatory approaches that further limit youth access to e-cigarettes.
A key strength of the BRFSS, NHIS, CPS-TUS, and NSDUH is the ability to obtain state-level estimates from these surveys. States can therefore use such data to monitor e-cigarette use behaviors and to evaluate state-level policies. Additionally, since e-cigarette-related policies vary from state to state, such state-level data can be used to evaluate the impact of different policies on e-cigarette and other tobacco product use.64
The PATH study, with about ten years of follow-up data to date and with data on outcomes such as cardiovascular diseases, has been instrumental in investigating the long-term health effects associated with e-cigarette use, including incident respiratory and cardiovascular disease.65, 66, 67, 68, 69 Because temporal associations can be established from such longitudinal data, causal inferences may be made between e-cigarette use and adverse health effects. Additionally, tobacco use transitions, e.g., cigarette to e-cigarette, e-cigarette to cigarette, cigarette to dual use, etc., and their associated health effects can be studied using the PATH data due to its longitudinal design.70, 71, 72
Gaps, limitations, and recommendations
Despite their unique strengths, some gaps and limitations exist in the design, methodology, and questions used in these surveys that may limit their utility in guiding the timely implementation and evaluation of tobacco policies. We outline a few below and recommend measures to fill these gaps.
Timely or near real-time surveillance of E-cigarette use
The e-cigarette landscape and epidemiology are rapidly changing. However, most epidemiologic surveys, including some of the surveys reviewed in this study, have long gaps between data collection and availability and hence lag real-time events by about a year or two. For example, the BRFSS typically takes approximately a year between completion of data collection and data release. This limitation is inherent in most traditional epidemiologic surveys since survey conduct, from design to data cleaning and release, takes time. Thus, it becomes challenging to use these data sources to monitor, in real-time, the changing patterns of e-cigarette use. The consequence is a significant delay in implementing and evaluating e-cigarette-related policies as regulatory authorities such as the FDA rely on survey data, among other data sources, to inform and evaluate policies. Such delays may lead to unintended consequences such as the youth's skyrocketing use of disposable e-cigarettes and the class action lawsuit against the FDA for delays in taking action.73
E-cigarette retail sales data can complement epidemiology surveys in monitoring e-cigarette use and for evaluating policies. Such sales data have several inherent strengths such as timeliness and ability to obtain weekly data, thereby allowing for timely assessment of changes in e-cigarette use/purchase trends.74,75 A challenge with sales data is its inability to capture purchases from the internet, which accounts for a considerable proportion of U.S. e-cigarette sales.74,75 Other data sources that can complement survey data in monitoring changes in tobacco use or interest include internet search data such as Google Trends and data curated from social media platforms such as Twitter, Instagram, and Reddit. Infodemiology, as it is often referred to, uses changes in internet searches or online communication patterns as early indicators of changes in population interest, behavior, or health.76 Google search query data have been used to monitor the popularity of e-cigarettes and how their terminology has changed over time.77 Such data also have been used to assess the impact of various population-level policies.78,79 Data from social media platforms have been extracted and analyzed using artificial intelligence, machine learning, and other innovative methods to increase understanding of perceptions, attitudes, and behaviors of people regarding e-cigarettes.80,81 Because infodemiology data can be accessed in real-time, they can be used to monitor changes in population search behaviors–a proxy for actual behavior in near-real-time.82,83 Also, such data are free from recall and social desirability bias and less costly than conventional epidemiologic surveys.76 Thus, such data may complement traditional epidemiologic survey data in the timely monitoring of changes in population behavior as has been used in prior studies.84,85
Preambles
The preambles used to introduce e-cigarette questions in surveys are very important. The preambles for some of the surveys, such as PATH, have changed over the years to include detailed description of e-cigarettes, different types, and brands. While some surveys extensively describe e-cigarettes in their preambles and provide a wide array of different types and brands as examples, other surveys only provide a generic description of e-cigarette devices. Survey respondents may not consider their vaping products, e.g., disposables, as e-cigarettes and may incorrectly indicate nonuse if the products they use are not described in the preamble. Indeed, a recent study found that querying about general e-cigarette use instead of asking about specific e-cigarette devices/brands underestimated the true prevalence of e-cigarette use.86 In the study mentioned above, 35.8% of youth endorsed lifetime e-cigarette use when asked, “Have you ever tried an e-cigarette, even one or two puffs?” However, when asked about the lifetime use of five different e-cigarette devices, 51.3% reported ever using e-cigarettes.86 Thus, it is crucial that the preambles used in these surveys be detailed, including both the standard (e.g., e-cigarette) and colloquial (e.g., vape device) terminologies, different brands, and types, to best capture the actual burden of e-cigarette use. This can be extended to surveys that assess other emerging tobacco products like heated tobacco products; preambles should be comprehensive, with examples of different brands, in order to capture the true prevalence of these products. Also, while there is the need for updated and comprehensive preambles for some of the surveys, they should be anchored to prior preambles to maintain comparability.
Comparability, completeness, and consistency of questions used to assess E-cigarette use
The nationally representative surveys described in our review vary in scope and depth of their assessment of e-cigarette use. Even when assessing the same domain of e-cigarette use, e.g., prevalence, the different surveys use slightly different questions and thus capture different aspects of the prevalence, producing estimates that may vary. For example, while some surveys ask about general e-cigarette use, others specify nicotine vaping or the use of non-nicotine e-cigarettes. Moreover, because e-cigarette use is intermittent and less stable than smoking, it is difficult to quantify cumulative or episodic exposure; additional new approaches are needed to clearly document use patterns, including intermittent use and polyuse.
Questions assessing the domains of e-cigarette use continue to evolve. For example, since 2017, the question used to assess the prevalence of past-30-day e-cigarette use in the MTF survey has been modified to three questions with each assessing the prevalence of e-cigarette use for vaping specific substances (nicotine, marijuana, and flavorings). While such changes could potentially limit the comparability across survey years, they are important to effectively assess e-cigarette use in this rapidly evolving e-cigarette landscape. This can inform other surveys with questions that assess other emerging tobacco products such as oral nicotine and heated tobacco products; the evolution of questions should be timely and comprehensive to best capture the evolving landscape of these products.
The PhenX Toolkit, a publicly available online resource funded by several National Institute of Health divisions, provides recommended standard questions that can be used to access the various domains of e-cigarette use.87 For example, in assessing e-cigarette flavor preference among youth, five (5) standardized questions are recommended: “Was the first e-cigarette you used flavored to taste like menthol, mint, clove, spice, fruit, chocolate, alcoholic drinks, candy, or other sweets?” “What flavor was that first e-cigarette? If multiple flavors were mixed, choose all that apply.” “In the past 30 days [were/was] any of the e-cigarettes, cartridges, or e-liquids you used flavored to taste like menthol, mint, clove, spice, fruit, chocolate, alcoholic drinks, candy, or other sweets?” “Which flavors have you used in the past 30 days? Choose all that apply.” and “Some e-cigarettes come in flavors like menthol, mint, clove, spice, fruit, chocolate, alcoholic drinks, candy, or other sweets. Are flavored e-cigarettes easier to use, about the same, or harder to use than unflavored e-cigarettes?” Most of the surveys reviewed do not incorporate most of the questions included in the PhenX toolkit. An alternative source of comprehensive, standardized, and reliable cross-survey questions for assessing the domains of e-cigarette use may be set up through deliberations among tobacco control experts.88 If standardized questions are used across the different surveys, cross-study meta-analyses will be facilitated, increasing the collective scientific impact of individual studies.87,88
Timeliness of incorporating new questions
The e-cigarette landscape is very dynamic. However, it may take several years for new questions to be incorporated into these surveys or existing questions modified to best capture recent domains of e-cigarette use. For instance, despite the current popularity of disposable e-cigarettes, most of the surveys reviewed, including the BRFSS, do not have questions that precisely assess disposable e-cigarette use among its target population. Another example would be the lack of questions assessing the use of synthetic nicotine-containing products despite reports that such products are rapidly emerging.89 Additionally, despite the ongoing debate about whether e-cigarettes may help persons who smoke to quit, most surveys do not evaluate the use of e-cigarettes for cessation among individuals who smoke. To best capture the rapidly changing e-cigarette landscape and provide regulatory authorities with timely evidence to guide policy implementation, incorporating newer questions and modifying existing questions should be done promptly. While we acknowledge that modifying these questions are important and should be done in a timely manner, it is also important to mention that such changes should be linked and anchored to prior questions to maintain some comparability. Also, for most national surveys, it is unclear how independent researchers in tobacco regulatory science can contribute or submit questions for future survey iterations. Such a process should be made clear and easy to facilitate the sharing of innovative ideas.
Other challenges
Other challenges with repeatedly conducted surveys such as those explored in the review include changes in survey methodology that limit comparison between survey years. For example, the changes in the administration and data collection procedures for the NYTS due to the COVID-19 pandemic limits the ability to compare estimates from 2021 with those from prior NYTS.90 Another challenge worth mentioning is the attrition and resampling challenges with longitudinal studies like the PATH Study.
Considerations and conclusions
The importance of timely surveillance in tobacco control cannot be overemphasized, especially for novel tobacco products like e-cigarettes, which create a dynamic and rapidly changing landscape. In this review, we identified 13 nationally representative surveys that conduct ongoing assessments of e-cigarette use among youth and adults in the U.S. We have highlighted some of their unique strengths. While each of the surveys has its particular strengths, some questions remain: Should we continue to rely on 13 different surveys, or is there a rationale to make one master survey that assesses, in detail, e-cigarette use among the U.S. population? We would argue that different surveys would encourage innovation and complement each other in capturing different domains of e-cigarette use. Also, with a single survey, respondents may be required to answer several questions, which may negatively impact the response and completion rates.91 Furthermore, there is a role for national surveys that evaluate e-cigarette use in specific populations that have been structurally marginalized such as the lesbian, gay, bisexual, transgender, queer, or questioning persons, youth not enrolled in schools, institutionalized persons, etc.
We have also identified limitations and gaps that can be filled to improve these surveys. Some suggestions include improvement in near real-time surveillance of e-cigarette use by complementing conventional epidemiologic surveys using novel data such as Google Trends and machine learning of social media platforms; timeliness in incorporating new questions or modifying existing questions to better understand the rapidly changing e-cigarette landscape; improvement in survey preambles so that they can comprehensively capture the true prevalence of e-cigarette use; and standardization of questions used across surveys. Despite these gaps, it is essential to highlight that the U.S. has some of the more robust nationally representative surveys compared to other countries. Therefore, it is vital that the tobacco regulatory science community learn about these surveys, their strengths and weaknesses, and the opportunities they provide to enhance our understanding and knowledge synthesis for policymaking and tobacco regulation. Moreover, it is equally important to realize that most of these surveys present self-reported data that are not independently verified, which represents a major limitation. Traditional longitudinal cohorts with in-person exams are needed to provide more rigorous use pattern data as well as data related to specific, clinically verified health outcomes.
While a major strength of this paper includes comprehensively reviewing 13 nationally representative surveys, we excluded some surveys that have important information on e-cigarette use but did not completely meet all the inclusion criteria. Such surveys include state-specific or region-specific surveys (e.g., the Oregon Healthy Teens Survey), surveys from nonrandom/convenient sample of participants that are not nationally representative, opinion polls or online surveys with proprietary nontransparent methodologies such as the Social Science Research Solutions Opinion Panel, and surveys by tobacco companies, e.g., Altria Client Services UTUS.
Search strategy and selection criteria.
References for this review were identified via PubMed and Google/Google Scholar searches with terms including “e-cigarette”, “vape”, “tobacco”, “electronic cigarettes”, “United States”, “adolescents”, “youth”, and “adults”. Only papers published in English were reviewed. We reviewed all abstracts displayed in PubMed for our search terms to obtain information on surveys that were used for the various studies. We additionally queried ICPSR, a data archiving platform, for e-cigarette-related publications and variables using the terms “e-cigarettes”, “vape”, and “electronic cigarettes”. The final reference list was generated based on our inclusion criteria and relevance to the scope of this review.
Contributors
EB: Conceptualization, Methodology, Data curation, Writing- Original draft preparation; JE: Conceptualization, Methodology, Data curation; OHO: Writing- Review and editing; ET: Writing- Review and editing; MM: Writing- Review and editing; NO: Review and editing; ADO: Writing- Review and editing; JL: Data curation; APD: Writing- Review and editing; ACS: Writing- Review and editing; GAH: Writing- Review and editing, Supervision; EJB: Writing- Review and editing, Supervision; RMR: Conceptualization, Writing- Review and editing, Funding acquisition; AB: Conceptualization, Writing- Review and editing, Funding acquisition; OE: Writing- Review and editing; MJB: Conceptualization, Methodology, Writing- Writing- Review and editing, Supervision, Funding acquisition.
Declaration of interests
We declare no competing interests.
Acknowledgment
This work was supported, in part, by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) Center for Tobacco Products (CTP) [HL120163]. The funding institutions played no role in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the paper for publication. This paper's content is solely the authors' responsibility and does not necessarily represent the official views of the NIH or the FDA.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100528.
Appendix A. Supplementary data
References
- 1.Health Effects of Cigarette Smoking | CDC. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm
- 2.Samet J.M. Tobacco smoking. Thorac Surg Clin. 2013;23:103–112. doi: 10.1016/j.thorsurg.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Tobacco | CDC winnable battles final report | CDC. https://www.cdc.gov/winnablebattles/report/tobacco.html
- 4.Meza R., Jimenez-Mendoza E., Levy D.T. Trends in tobacco use among adolescents by grade, sex, and race, 1991-2019. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . 2001. Surveillance and evaluation data resources for comprehensive tobacco control programs. [Google Scholar]
- 6.Family smoking prevention and tobacco control Act–an overview | FDA. https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/family-smoking-prevention-and-tobacco-control-act-overview
- 7.Tobacco Control Legal Consortium . 2009. Federal regulation of tobacco: impact on state and local authority.https://publichealthlawcenter.org/sites/default/files/fda-3.pdf [Google Scholar]
- 8.U.S. Department of Health and Human Services . E-Cigarette use among youth and young adults: a report of the surgeon general. Atlanta, GA. 2016. Chapter 1. Introduction, conclusions, and historical background relative to E-cigarettes.https://www.ncbi.nlm.nih.gov/books/NBK538680/pdf/Bookshelf_NBK538680.pdf [Google Scholar]
- 9.Hajek P., Phillips-Waller A., Przulj D., et al. A randomized trial of E-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629–637. doi: 10.1056/nejmoa1808779. [DOI] [PubMed] [Google Scholar]
- 10.El Dib R., Suzumura E.A., Akl E.A., et al. Electronic nicotine delivery systems and/or electronic non-nicotine delivery systems for tobacco smoking cessation or reduction: a systematic review and meta-analysis. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-012680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youth E-cigarette use remains serious public health concern amid COVID-19 pandemic | CDC online newsroom | CDC. https://www.cdc.gov/media/releases/2021/p0930-e-cigarette.html
- 12.Food and Drug Administration D of H and HS . 2016. Deeming tobacco products to be subject to the federal food, drug, and cosmetic act, as amended by the family smoking prevention and tobacco control act; restrictions on the sale and distribution of tobacco products and required warning statements for toba.https://www.govinfo.gov/content/pkg/FR-2016-05-10/pdf/2016-10685.pdf [PubMed] [Google Scholar]
- 13.Market and distribute a tobacco product | FDA. https://www.fda.gov/tobacco-products/products-guidance-regulations/market-and-distribute-tobacco-product
- 14.Al Rifai M., Mirbolouk M., Jia X., et al. E-Cigarette use and risk behaviors among lesbian, gay, bisexual, and transgender adults: the behavioral risk factor surveillance system (BRFSS) survey. Kans J Med. 2020;13:318. doi: 10.17161/kjm.vol13.13861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acosta-Deprez V., Jou J., London M., et al. Tobacco control as an LGBTQ+ issue: knowledge, attitudes, and recommendations from LGBTQ+ community leaders. Int J Environ Res Public Health. 2021;18:5546. doi: 10.3390/ijerph18115546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruskin E.P., Greenwood G.L., Matevia M., Pollack L.M., Bye L.L. Disparities in smoking between the lesbian, gay, and bisexual population and the general population in California. Am J Public Health. 2007;97:1496. doi: 10.2105/ajph.2006.090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Center for Chronic Disease Prevention and Health Promotion (US) Office on smoking and health . 2012. Preventing tobacco use among youth and young adults: a report of the surgeon general. Atlanta. [PubMed] [Google Scholar]
- 18.Search/compare variables. https://www.icpsr.umich.edu/web/pages/ICPSR/ssvd/
- 19.Besaratinia A., Tommasi S. The consequential impact of JUUL on youth vaping and the landscape of tobacco products: the state of play in the COVID-19 era. Prev Med Rep. 2021;22 doi: 10.1016/j.pmedr.2021.101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Youth Tobacco Survey (NYTS) | CDC https://www.cdc.gov/tobacco/data_statistics/surveys/nyts/
- 21.YRBSS | youth risk behavior surveillance system | data | adolescent and school health | CDC. https://www.cdc.gov/healthyyouth/data/yrbs/index.htm
- 22.Welcome to the MTF website. http://www.monitoringthefuture.org/
- 23.P01 project 2–ITC project. https://itcproject.org/projects/p01-program-project-4cv-survey-2021-2026/p01-project-2/
- 24.CDC–BRFSS. https://www.cdc.gov/brfss/index.html
- 25.NHIS–National Health Interview Survey https://www.cdc.gov/nchs/nhis/index.htm
- 26.The tobacco use supplement to the current population survey | division of cancer control and population sciences (DCCPS) https://cancercontrol.cancer.gov/brp/tcrb/tus-cps
- 27.Health Information National Trends Survey | HINTS https://hints.cancer.gov/
- 28.Weaver S.R., Majeed B.A., Pechacek T.F., Nyman A.L., Gregory K.R., Eriksen M.P. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int J Public Health. 2016;61:177. doi: 10.1007/s00038-015-0761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weaver S.R., Kemp C.B., Wesley Heath J., Pechacek T.F., Eriksen M.P. Use of nicotine in electronic nicotine and non-nicotine delivery systems by US adults, 2015. Public Health Rep. 2017;132:545. doi: 10.1177/0033354917723597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.United States of America–ITC Project. https://itcproject.org/countries/united-states-america/
- 31.NHANES–national health and nutrition examination survey homepage. https://www.cdc.gov/nchs/nhanes/index.htm
- 32.National Survey on Drug Use and Health https://nsduhweb.rti.org/respweb/homepage.cfm
- 33.Population assessment of tobacco and health (PATH) study [United States] restricted-use files. https://www.icpsr.umich.edu/web/NAHDAP/studies/36231
- 34.Cantrell J., Hair E.C., Smith A., et al. Recruiting and retaining youth and young adults: challenges and opportunities in survey research for tobacco control. Tob Control. 2018;27:147–154. doi: 10.1136/tobaccocontrol-2016-053504. [DOI] [PubMed] [Google Scholar]
- 35.Cheng H.G., Vansickel A.R., Largo E.G. Awareness and use of tobacco products among underage individuals: findings from the altria client services underage tobacco use survey 2020-2022. BMC Public Health. 2023;23:662. doi: 10.1186/s12889-023-15610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Underage tobacco use survey–altria science. https://sciences.altria.com/library/underage-tobacco-use-survey?src=altria
- 37.Gentzke A.S., Wang T.W., Cornelius M., et al. Tobacco product use and associated factors among middle and high school students — national youth tobacco survey, United States, 2021. MMWR Surveill Summ. 2022;71:1–29. doi: 10.15585/mmwr.ss7105a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention . 2021. Behavioral risk factor surveillance system–comparability of data BRFSS 2020. [Google Scholar]
- 39.The GfK Group GfK methodology. file:///Users/ellenboakye/Downloads/GfKMethodology–April 2016 (2).pdf
- 40.NHANES 2017-2018 laboratory variable list. https://wwwn.cdc.gov/Nchs/Nhanes/Search/variablelist.aspx?Component=Laboratory&Cycle=2017-2018
- 41.PATH (population assessment of tobacco and health) study–home. https://pathstudyinfo.nih.gov/
- 42.Park-Lee E. Notes from the field: E-cigarette use among middle and high school students — national youth tobacco survey, United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1387–1389. doi: 10.15585/mmwr.mm7039a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Center for Health Statistics N . 2015. 2015-2016 NHANES sample person questionnaire hand cards. [Google Scholar]
- 44.Questionnaires 2017-2018–OMB 0920-0950. https://omb.report/icr/202001-0920-015/doc/98124701
- 45.Hammond D., Rynard V.L., Reid J.L. Changes in prevalence of vaping among youths in the United States, Canada, and england from 2017 to 2019. JAMA Pediatr. 2020;174:797–800. doi: 10.1001/jamapediatrics.2020.0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ajzen I. Springer; Berlin, Heidelberg: 1985. From intentions to actions: a theory of planned behavior. [DOI] [Google Scholar]
- 47.Nicksic N.E., Barnes A.J. Is susceptibility to E-cigarettes among youth associated with tobacco and other substance use behaviors one year later? Results from the PATH study. Prev Med (Baltim) 2019;121:109. doi: 10.1016/j.ypmed.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bold K.W., Kong G., Cavallo D.A., Camenga D.R., Krishnan-Sarin S. E-cigarette susceptibility as a predictor of youth initiation of E-cigarettes. Nicotine Tob Res. 2018;20:140. doi: 10.1093/ntr/ntw393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demissie Z., Jones S.E., Clayton H.B., King B.A. Adolescent risk behaviors and use of electronic vapor products and cigarettes. Pediatrics. 2017:139. doi: 10.1542/peds.2016-2921/60303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang Y.P., Seo Y.S. E-cigarette use and concurrent risk behaviors among adolescents. Nurs Outlook. 2021;69:302–310. doi: 10.1016/j.outlook.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Miech R.A., O'Malley P.M., Johnston L.D., Patrick M.E. E-cigarettes and the drug use patterns of adolescents. Nicotine Tob Res. 2016;18:654–659. doi: 10.1093/ntr/ntv217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCabe S.E., West B.T., McCabe V.V. Associations between early onset of E-cigarette use and cigarette smoking and other substance use among US adolescents: a national study. Nicotine Tob Res. 2018;20:923. doi: 10.1093/ntr/ntx231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirbolouk M., Boakye E., Obisesan O., et al. E-cigarette use among high school students in the United States prior to the COVID-19 pandemic: trends, correlates, and sources of acquisition. Prev Med Rep. 2022;29 doi: 10.1016/j.pmedr.2022.101925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holliday E., Gould T.J. Nicotine, adolescence, and stress: a review of how stress can modulate the negative consequences of adolescent nicotine abuse. Neurosci Biobehav Rev. 2016;65:173. doi: 10.1016/j.neubiorev.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Medical Expenditure Panel Survey Background https://www.meps.ahrq.gov/mepsweb/about_meps/survey_back.jsp
- 56.Wang Y., Sung H.Y., Lightwood J., Yao T., Max W.B. Healthcare utilisation and expenditures attributable to current e-cigarette use among US adults. Tob Control. 2022;0:1–6. doi: 10.1136/tobaccocontrol-2021-057058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tibuakuu M., Okunrintemi V., Jirru E., et al. National trends in cessation counseling, prescription medication use, and associated costs among US adult cigarette smokers. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swedler D.I., Miller T.R., Ali B., Waeher G., Bernstein S.L. National medical expenditures by smoking status in American adults: an application of Manning's two-stage model to nationally representative data. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu X., Bishop E.E., Kennedy S.M., Simpson S.A., Pechacek T.F. Annual healthcare spending attributable to cigarette smoking: an update. Am J Prev Med. 2015;48:326. doi: 10.1016/j.amepre.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.NHANES-CMS linked data tutorial–introduction. https://www.cdc.gov/nchs/tutorials/nhanes-cms/introduction.htm
- 61.Bayly J.E., Bernat D., Porter L., Choi K. Secondhand exposure to aerosols from electronic nicotine delivery systems and asthma exacerbations among youth with asthma. Chest. 2019;155:88. doi: 10.1016/j.chest.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Islam T., Braymiller J., Eckel S.P., et al. Secondhand nicotine vaping at home and respiratory symptoms in young adults. Thorax. 2022;77:663–668. doi: 10.1136/thoraxjnl-2021-217041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaiha S.M., Lempert L.K., Halpern-Felsher B. Underage youth and young adult e-cigarette use and access before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du Y., Liu B., Xu G., et al. Association of electronic cigarette regulations with electronic cigarette use among adults in the United States. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2019.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berlowitz J.B., Xie W., Harlow A.F., et al. E-Cigarette use and risk of cardiovascular disease: a longitudinal analysis of the PATH study (2013–2019) Circulation. 2022;145:1557–1559. doi: 10.1161/circulationaha.121.057369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie W., Kathuria H., Galiatsatos P., et al. Association of electronic cigarette use with incident respiratory conditions among US adults from 2013 to 2018. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dai H., Khan A.S. A longitudinal study of exposure to tobacco-related toxicants and subsequent respiratory symptoms among U.S. Adults with varying E-cigarette use status. Nicotine Tob Res. 2020;22:S61–S69. doi: 10.1093/ntr/ntaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhatta D.N., Glantz S.A. Association of E-cigarette use with respiratory disease among adults: a longitudinal analysis. Am J Prev Med. 2020;58:182. doi: 10.1016/j.amepre.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tackett A.P., Keller-Hamilton B., Smith C.E., et al. Evaluation of respiratory symptoms among youth e-cigarette users. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berlowitz J., Xie W., Goghari A., Stokes A. American Thoracic Society International Conference Meetings Abstracts American Thoracic Society International Conference Meetings Abstracts; 2022. Association of cigarette-e-cigarette transitions with respiratory symptom incidence: a longitudinal analysis of the path study, 2014-2019; p. A5523. [DOI] [Google Scholar]
- 71.Wei L., Muhammad-Kah R.S., Hannel T., et al. The impact of cigarette and e-cigarette use history on transition patterns: a longitudinal analysis of the population assessment of tobacco and health (PATH) study, 2013-2015. Harm Reduct J. 2020;17:1–12. doi: 10.1186/s12954-020-00386-z/tables/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dai H., Benowitz N.L., Achutan C., Farazi P.A., Degarege A., Khan A.S. Exposure to toxicants associated with use and transitions between cigarettes, e-cigarettes, and No tobacco. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2021.47891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Federal judge rules FDA acted illegally in delaying required review of E-cigarettes, cigars–campaign for tobacco-free kids. https://www.tobaccofreekids.org/press-releases/2019_05_16_fda_legal_decision
- 74.Ali F.R.M., Diaz M.C., Vallone D., et al. E-Cigarette unit sales, by product and flavor type—United States, 2014–2020. MMWR Morb Mortal Wkly Rep. 2020;69:1313–1318. doi: 10.15585/mmwr.mm6937e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seaman E.L., Ali F.R.M., Schillo B.A., Vallone D.M., King B.A. Different times call for different measures: using retail sales to monitor the tobacco product landscape. Am J Prev Med. 2022;63:e99–e102. doi: 10.1016/j.amepre.2022.03.028. [DOI] [PubMed] [Google Scholar]
- 76.Eysenbach G. Infodemiology and infoveillance: framework for an emerging set of public health informatics methods to analyze search, communication and publication behavior on the internet. J Med Internet Res. 2009;11(1):e11. doi: 10.2196/jmir.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ayers J.W., Althouse B.M., Allem J.P., Leas E.C., Dredze M., Williams R.S. Revisiting the rise of electronic nicotine delivery systems using search query surveillance. Am J Prev Med. 2016;50:e173–e181. doi: 10.1016/j.amepre.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boakye E., Dzaye O., Erhabor J., et al. Impact of the Food and Drug Administration enforcement policy on flavored e-cigarettes on the online popularity of disposable e-cigarettes: analyses of Google search query data. BMC Public Health. 2022;22:1937. doi: 10.1186/s12889-022-14367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghosh A., e-Roub F., Krishnan N.C., Choudhury S., Basu A. Can google trends search inform us about the population response and public health impact of abrupt change in alcohol policy? A case study from India during the covid-19 pandemic. Int J Drug Policy. 2021;87 doi: 10.1016/j.drugpo.2020.102984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu X., Sun L., Xie Z., Li D. Perception of the Food and Drug administration electronic cigarette flavor enforcement policy on twitter: observational study. JMIR Public Health Surveill. 2022;8(3) doi: 10.2196/25697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lazard A.J., Saffer A.J., Wilcox G.B., Chung A.D., Mackert M.S., Bernhardt J.M. E-Cigarette social media messages: a text mining analysis of marketing and consumer conversations on twitter. JMIR Public Health Surveill. 2016;2(2) doi: 10.2196/publichealth.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.West R., White R.W., Horvitz E. Proceedings of the 22nd International Conference on World Wide Web Lyon: ACM. 2013. From cookies to cooks: insights on dietary patterns via analysis of web usage logs; pp. 1399–1410. [Google Scholar]
- 83.Bento A.I., Nguyen T., Wing C., Lozano-Rojas F., Ahn Y.Y., Simon K. Evidence from internet search data shows information-seeking responses to news of local COVID-19 cases. Proc Natl Acad Sci U S A. 2020;117:11220–11222. doi: 10.1073/pnas.2005335117/suppl_file/pnas.2005335117.sd01.xlsx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dai H., Hao J. Online popularity of JUUL and puff bars in the USA: 2019–2020. Tob Control. 2020;31:7. doi: 10.1136/tobaccocontrol-2020-055727. published online Oct 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dzaye O., Adelhoefer S., Boakye E., Blaha M.J. Cardiovascular-related health behaviors and lifestyle during the COVID-19 pandemic: an infodemiology study. Am J Prev Cardiol. 2021;5 doi: 10.1016/j.ajpc.2021.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morean M.E., Camenga D.R., Bold K.W., et al. Querying about the use of specific E-cigarette devices may enhance accurate measurement of E-cigarette prevalence rates among high school students. Nicotine Tob Res. 2020;22:833. doi: 10.1093/ntr/nty240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.PhenX Toolkit: collections. https://www.phenxtoolkit.org/collections/view/3
- 88.Pearson J.L., Hitchman S.C., Brose L.S., et al. Recommended core items to assess e-cigarette use in population-based surveys. Tob Control. 2018;27:341. doi: 10.1136/tobaccocontrol-2016-053541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jordt S.-E. Synthetic nicotine has arrived. Tob Control. 2023;32:e113–e117. doi: 10.1136/tobaccocontrol-2021-056626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Results from the annual national youth tobacco survey | FDA. https://www.fda.gov/tobacco-products/youth-and-tobacco/results-annual-national-youth-tobacco-survey
- 91.Kost R.G., de Rosa J.C. Impact of survey length and compensation on validity, reliability, and sample characteristics for Ultrashort-, Short-, and Long-Research Participant Perception Surveys. J Clin Transl Sci. 2018;2:31. doi: 10.1017/cts.2018.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.