Abstract
Introduction and importance
Adrenal adenomas are benign neoplasms of the adrenal cortex which can be functional or non-functional. The functional tumors manifest as Cushing syndrome. Rarely, they are associated with high levels of metanephrine due to pheochromocytoma. Here, we report an extremely rare case of adrenal adenoma associated with raised metanephrine, but no histopathological evidence of medullary changes.
Case presentation
A 30-year female with diabetes, hypothyroidism, and hypertension was admitted for resistant hypertension, severe refractory hypokalemia, decreased sleep, fearfulness, anxiety, and palpitation. Facial puffiness, ecchymoses on upper and lower limbs, and bilateral pedal edema were present. Laboratory investigation revealed raised 24-hour-urine cortisol (52.5 μmol/dL) and metanephrine (56.00 μmol/24 h), very low ACTH (<5.0 pg/mL), and negative dexamethasone suppression test. MRI brain findings were normal. CT scan of the abdomen revealed a well-defined soft tissue lesion in the right adrenal gland and diffuse osteopenia vertebrae. Hence, diagnosis of Cushing Syndrome secondary to adrenal adenoma, and pheochromocytoma was made, and laparoscopic adrenalectomy was done under pheochromocytoma protocol. The patient improved dramatically, thereafter. Histopathological findings showed adrenocortical adenoma only; no evidence of pheochromocytoma was found.
Clinical discussion
We came across many articles reporting adrenal adenoma associated with high levels of metanephrine due to co-existing pheochromocytomas either in the ipsilateral or contralateral adrenal gland. But, in our case, the adrenal adenoma was associated with high metanephrine levels with no histopathologic features of pheochromocytoma.
Conclusion
Cushing syndrome due to Cortisol secreting Adrenal adenoma can be associated with biochemical and clinical features suggestive of Pheochromocytoma which could resolve after adrenalectomy.
Keywords: Adrenal adenoma, Metanephrine, Pheochromocytoma, Cushing syndrome, Case report
Highlights
-
•
Adrenal adenoma with raised cortisol
-
•
Raised metanephrine with no histopathological evidence of pheochromcytoma.
-
•
Adrenal adenoma be associated with raised metanephrine
1. Introduction
Adrenal adenomas are benign neoplasms of the adrenal cortex which can be functional or non-functional. The functional tumors may manifest as Cushing syndrome due to Adrenocorticotrophic hormone (ACTH) independent or dependent hypersecretion of cortisol [[1], [2], [3]]. Rarely, they are associated with high levels of metanephrine due to co-existing pheochromocytomas either in the ipsilateral or contralateral adrenal gland [[4], [5], [6], [7], [8], [9], [10], [11]]. Pheochromocytomas are rare neuroendocrine tumors that arise from sympathetic adrenomedullary chromaffin tissue, and present with variable symptoms depending on the amount of catecholamine secretion [4]. Here, we report an extremely rare case of adrenal adenoma associated with Cushing Syndrome and raised metanephrine, but no histopathological evidence of pheochromocytoma. This case report has been reported in line with the SCARE Criteria [12].
2. Case presentation
A 30-year-old married non-smoker and non-alcohol consumer female, with a known case of diabetes mellitus for 6 years and hypertension for 2.5 years, presented with a history of increased weight gain, weakness of lower limbs, uncontrolled blood pressure, and blood glucose despite several medications. For the last 2 years, the patient was under consultation with psychiatrist for palpitation, decreased sleep, irritability, and aggressive behavior. She gave no significant family history of diabetes, hypertension, or similar illness.
On physical examination, the patient was ill-looking and obese with a cushingoid face, abdominal striae, and kyphosis. Her face was puffy, and ecchymotic patches were present over the dorsum of bilateral hands, the distal forearm, and the distal legs. Bilateral pedal edema was present. Her pulse was tachycardic (131 beats per minute).
Her biochemical investigations showed features of secondary hypothyroidism. Her HbA1c, 24-hour-urine metanephrine, and 24-hour-free cortisol were highly elevated. ACTH level was towards the lower limit (Table 1). The dexamethasone suppression test revealed no suppression of cortisol levels.
Table 1.
Biochemical findings of the patient.
| S.N. | Tests | Pre-operative result | Post-operative result | Range |
|---|---|---|---|---|
| 1. | Free triiodothyronine (fT3) | 2.35 pmol/L | – | 2.4–6.0 |
| 2. | Free thyroxine (fT3) | 7.90 pmol/L | – | 9.0–19.0 |
| 3. | Thyroxin-stimulating Hormone (TSH) | 0.716 μIU/mL | – | 0.35–4.94 |
| 4. | HbA1c | 10.1 % | – | |
| 5. | 24-Hour-urine metanephrine | 56.00 μmol | 3.26 μmol | (<5.50) |
| 6. | 24-Hour-urine free cortisol | 52.5 μg/dL | 15.00 μg/dL | 3.7–19.4 |
| 7. | Adrenocorticotropin hormone (ACTH) | <5.0 pg/mL | – | <46.0 |
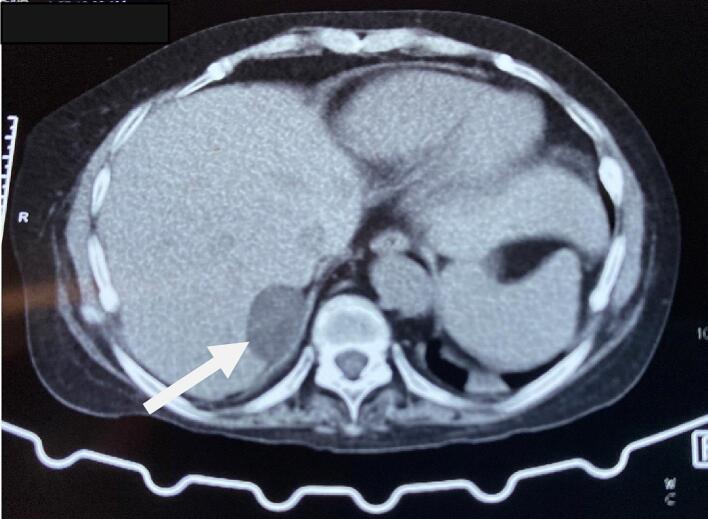
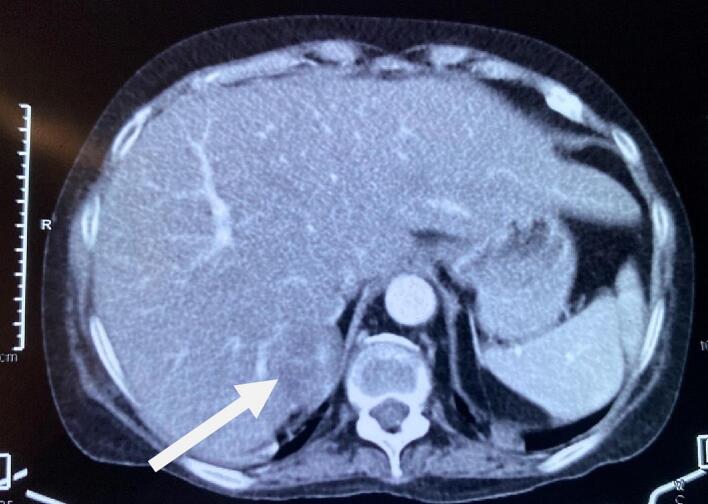
Thus, the patient was initially diagnosed and managed as a case of Cushing syndrome and Pheochromocytoma with resistant hypertension, hypokalemia, Diabetes Mellitus, and hypothyroidism. She was prescribed with Angiotensin receptor blocker, Calcium Channel Blocker, alpha-blocker (Prazosin), spironolactone, potassium chloride, and Insulin. The Computed Tomography (CT) scan of the abdomen revealed a well-defined soft tissue lesion in the right adrenal gland's lateral limb with a high washout likely lipid-poor adenoma (Fig. 1). The visualized spine had diffuse osteopenia with anterior wedging of the D8, D9, D10, D12, and L1 and L4 vertebral bodies. Contrast-enhanced CT (CECT) showed a 3.4 × 2.2 cm enhancing solid cystic lesion abutting the right adrenal gland and segment IV of the liver (Fig. 2).
Fig. 1.
CT abdomen showing well-defined soft tissue lesion in the right adrenal gland (arrow).
Fig. 2.
CECT showing an enhancing solid cystic lesion abutting the right adrenal gland and segment IV of the liver (arrow).
The case was handed over to the urology department for resection of the tumor. Laparoscopic right adrenalectomy following Pheochromocytoma protocol was done under general anesthesia. After the pneumoperitoneum was created, the ports were inserted into the peritoneal cavity. Right adrenal dissection was carried out from the superior pole of the right kidney. The gland was mobilized from the inferior vena cava (medial aspect). Then, dissection was done superiorly from the hepatic surface to separate it from the liver bed. The resected specimen was retrieved via a collecting bag.
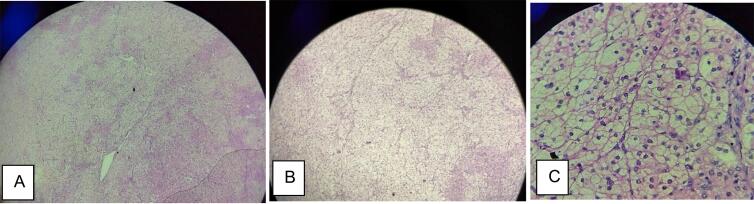
The OT findings were approximately 6 × 4 cm mass present in the right adrenal gland extending superiorly to the liver and medially up to the inferior vena cava. Histopathological finding (Fig. 3) was circumscribed hyperplasia of cortical cells arranged in nests. The cells had a large-sized central bland nucleus and abundant vacuolated eosinophilic cytoplasm. Surrounding adrenal parenchyma was unremarkable. There was no sign of malignancy. Both these findings were consistent with the diagnosis of adrenal adenoma. No microscopic evidence of tumor was found in the medulla. Chromogranin test was not performed due to the poor financial status of the patient.
Fig. 3.
Microscopic views: A. 4× B. 10× C. 40× showing hyperplasia of cortical cells arranged in nests. The cells had large sized central bland nucleus, abundant vacuolated eosinophilic cytoplasm.
She was discharged once her condition was stable and put on regular follow-up. Her 24-hour urine metanephrine and 24-hour-free cortisol level decreased rapidly right adrenalectomy. Further, her symptoms improved dramatically. Her recent follow-up was 7 months after the operation when her 24-hour urine metanephrine and 24-hour-free cortisol levels were 3.26 μmol and 15.00 μg/dL, respectively.
3. Discussion
An extensive literature search was done on PubMed and Google Scholar. There are studies reporting adrenal tumors with clinical and biochemical features of co-existing Cushing Syndrome and Pheochromocytoma. Those tumors have an ACTH or cortisol-secreting adrenal adenoma in the adrenal cortex and pheochromocytoma or medullary hyperplasia in the medulla of the ipsilateral or contralateral gland. All of them are associated with raised cortisol and catecholamines/metanephrine [[4], [5], [6], [7], [8], [9], [10], [11]].
In the presented case, co-existing adrenal adenoma and pheochromocytoma was provisional diagnosis based on clinical features, biochemical findings, and imaging. However, the histopathological examination findings of the resected specimen of the gland were consistent with adrenocortical adenoma only; there was no change in the medulla suggestive of pheochromocytoma or hyperplasia. Further, investigation for exploring molecular markers chromogranin, synaptophysin, and S-100 was not done due to the financial limitation of the patient.
The presented case had a high level of 24-hour-free urine metanephrine level preoperatively that decreased postoperatively. A proposed molecular mechanism responsible for the increased production of catecholamines in the case of isolated adrenal adenoma in animal models says that glucocorticoids can induce Phenyethanolamine N-methyltransferase (PNMT) that converts norepinephrine to epinephrine [13], which is metabolized to metanephrine. But, this is in total contradiction with the finding of a case-control study done on humans which demonstrated chronic endogenous hypercortisolism is not able to change the adrenomedullary PNMT activity, but rather can decrease the basal and stimulated adrenocortical activity [14]. Further, another study done on human subjects showed a negative correlation between the level of cortisol and norepinephrine [15]. Thus, the source of the rise in levels of catecholamines and thus urinary metanephrine could be the adrenocortical adenoma.
4. Conclusion
Cushing syndrome due to Cortisol secreting Adrenal adenoma can be associated with biochemical and clinical features suggestive of Pheochromocytoma which could resolve after adrenalectomy.
Ethical approval
It is exempt/waived at our institution. The ethical review board waives ethical approval for case reports as no patient identification is revealed in doing so and has no harm to the patient.
Funding
N/A
Author contribution
Study concept or design: Amit Kumar Mishra, Naresh Parajuli
Writing the paper: Amit Kumar Mishra, Prahlad Gupta, Radheshyam Yadav, Naresh Parajuli
Proofreading and review: Amit Kumar Mishra, Prahlad Gupta, Radheshyam Yadav, Naresh Parajuli
Guarantor
Amit Kumar Mishra
Naresh Parajuli
Research registration number
N/A
Consent
Written informed consent was obtained from the patient for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflict of interest statement
N/A
Acknowledgments
We acknowledge the help of the patient and her husband in the completion of this study.
References
- 1.Park J.J., Park B.K., Kim C.K. Adrenal imaging for adenoma characterization: imaging features, diagnostic accuracies and differential diagnoses. Br. J. Radiol. 2016;89:20151018. doi: 10.1259/bjr.20151018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mbouché L.O., Epoupa Ngallé F.G., Sando Z., Choukem S.P., Angwafo F.F. The case series of functional adrenal tumors: experience of two tertiary hospitals in Yaoundé, Cameroon. Int. J. Surg. Case Rep. 2020;72:577–583. doi: 10.1016/j.ijscr.2020.05.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar V., Abbas A., Fausto N., Aster J. 8th ed. Saunders Elsevier; Philadelphia: 2010. Robbins and Cotran Pathologic Basis of Disease. [Google Scholar]
- 4.Imaki T., Naruse M., Takano K. Adrenocortical hyperplasia associated with ACTH-dependent Cushing’s syndrome: comparison of the size of adrenal glands with clinical and endocrinological data. Endocr. J. 2004;51:89–95. doi: 10.1507/endocrj.51.89. [DOI] [PubMed] [Google Scholar]
- 5.Park S.Y., Park B.K., Park J.J., Kim C.K. Differentiation of adrenal hyperplasia from adenoma by use of CT densitometry and percentage washout. Am. J. Roentgenol. 2016;206:106–112. doi: 10.2214/AJR.15.14558. [DOI] [PubMed] [Google Scholar]
- 6.Lee M.N., Wan W., Chormanski D.C., Kravchenko M.I. Adrenal adenoma anarchy: a case of an ACTH-secreting Pheochromocytoma. Case Rep. Endocrinol. 2020;2020:1–4. doi: 10.1155/2020/4869467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakai K., Asano M., Hamaguchi M., Taniguchi H., Ukimura O., Fukui M. A cortisol-secreting adrenal adenoma combined with a Micro-Pheochromocytoma: case report and literature review. Clin. Med. Insights Endocrinol. Diabetes. 2023;16 doi: 10.1177/11795514221148556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H., Doppman J.L., Chrousos G.P., Norton J.A., Nieman L.K., Udelsman R. Adrenocorticotropic hormone-secreting pheochromocytomas: the exception to the rule. Surgery. 1995;118:988–995. doi: 10.1016/S0039-6060(05)80104-7. [DOI] [PubMed] [Google Scholar]
- 9.Nijhoff M.F., Dekkers O.M., Vleming L.J., Smit J.W.A., Romijn J.A., Pereira A.M. ACTH-producing pheochromocytoma: clinical considerations and concise review of the literature. Eur. J. Intern. Med. 2009;20:682–685. doi: 10.1016/j.ejim.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Gabi J.N., Milhem M.M., Tovar Y.E., Karem E.S., Gabi A.Y., Khthir R.A. Severe Cushing syndrome due to an ACTH-producing Pheochromocytoma: a case presentation and review of the literature. J. Endocr. Soc. 2018;2:621–630. doi: 10.1210/js.2018-00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel E., Chen Y., Fan X., Liu N.-A., Westreich A.M., Reyes K., Labadzhyan A. A rare case: adrenal corticomedullary mixed tumor with elements of pheochromocytoma, cortical adenoma, and ganglioneuroma cells. AACE Clin. Case Rep. 2023;9:17–20. doi: 10.1016/j.aace.2022.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., Beamish A.J., Noureldin A., Rao A., Vasudevan B., Challacombe B., Perakath B., Kirshtein B., Ekser B., Pramesh C.S., Laskin D.M., Machado-Aranda D., Miguel D., Pagano D., Millham F.H., Roy G., Kadioglu H., Nixon I.J., Mukhejree I., McCaul J.A., Chi-Yong Ngu J., Albrecht J., Rivas J.G., Raveendran K., Derbyshire L., Ather M.H., Thorat M.A., Valmasoni M., Bashashati M., Chalkoo M., Teo N.Z., Raison N., Muensterer O.J., Bradley P.J., Goel P., Pai P.S., Afifi R.Y., Rosin R.D., Coppola R., Klappenbach R., Wynn R., De Wilde R.L., Surani S., Giordano S., Massarut S., Raja S.G., Basu S., Enam S.A., Manning T.G., Cross T., Karanth V.K., Kasivisvanathan V., Mei Z., The S.C.A.R.E. Guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84(2020):226–230. doi: 10.1016/J.IJSU.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Inoue J., Oishi S., Naomi S., Umeda T., Sato T. Pheochromocytoma associated with adrenocortical adenoma: case report and literature review. Endocrinol. Jpn. 1986;33:67–74. doi: 10.1507/endocrj1954.33.67. [DOI] [PubMed] [Google Scholar]
- 14.Calsyn J.D.R., Green R.A., Davis G.J., Reilly C.M. Adrenal Pheochromocytoma with contralateral adrenocortical adenoma in a cat. J. Am. Anim. Hosp. Assoc. 2010;46:36–42. doi: 10.5326/0460036. [DOI] [PubMed] [Google Scholar]
- 15.Kazama Y.-I. Cushing’s syndrome associated with Adrenomedullary hyperplasia. JAMA J. Am. Med. Assoc. 1986;255:2446. doi: 10.1001/jama.1986.03370180072025. [DOI] [PubMed] [Google Scholar]