Abstract
A compartmental, epidemiological, mathematical model was developed in order to analyze the transmission dynamics of Delta and Omicron variant, of SARS-CoV-2, in Greece. The model was parameterized twice during the 4th and 5th wave of the pandemic. The 4th wave refers to the period during which the Delta variant was dominant (approximately July to December of 2021) and the 5th wave to the period during which the Omicron variant was dominant (approximately January to May of 2022), in accordance with the official data from the National Public Health Organization (NPHO). Fitting methods were applied to evaluate important parameters in connection with the transmission of the variants, as well as the social behavior of population during these periods of interest. Mathematical models revealed higher numbers of contagiousness and cases of asymptomatic disease during the Omicron variant period, but a decreased rate of hospitalization compared to the Delta period. Also, parameters related to the behavior of the population in Greece were also assessed. More specifically, the use of protective masks and the abidance of social distancing measures. Simulations revealed that over 5,000 deaths could have been avoided, if mask usage and social distancing were 20% more efficient, during the short period of the Delta and Omicron outbreak. Furthermore, the spread of the variants was assessed using viral load data. The data were recorded from PCR tests at 417 Army Equity Fund Hospital (NIMTS), in Athens and the Ct values from 746 patients with COVID-19 were processed, to explain transmission phenomena and disease severity in patients. The period when the Delta variant prevailed in the country, the average Ct value was calculated as 25.19 (range: 12.32–39.29), whereas during the period when the Omicron variant prevailed, the average Ct value was calculated as 28 (range: 14.41–39.36). In conclusion, our experimental study showed that the higher viral load, which is related to the Delta variant, may interpret the severity of the disease. However, no correlation was confirmed regarding contagiousness phenomena. The results of the model, Ct analysis and official data from NPHO are consistent.
Keywords: Delta variant, Omicron variant, Mathematical modeling, Transmission dynamics, Ct value, Data fitting
1. Introduction
Until May of 2022, 3.6 million confirmed cases and, approximately, 30 thousand deaths due to COVID-19 were recorded, in Greece (Worldometer.info, 2022). In Fig. 1, daily cases and deceased individuals are presented from day zero until May of 2022. During the last months of 2021 and the beginning of 2022 a sharp rise in numbers of daily cases and deaths from the disease was noted (Fig. 1). During this period the dominant variant was Delta (known as B.1.617.2), followed by Omicron (B.1.1.529) (4th and 5th wave) and Omicron sub variants. The main non-pharmaceutical intervention (NPI) measures that were adopted to limit the spread of the virus were the mandatory use of masks, the recommendation for adequate distancing and vaccination. Based on this urgent medical situation, a variety of monoclonal antibodies (mAbs) and vaccines derived from different platforms such as, DNA plasmid based and innovative nucleoside-modified viral messenger RNA encapsulated within nanoparticles. More specifically, lipid ones (LNPs) which had already been developed and commercially exposed worldwide (Tsiambas et al., 2021).
Fig. 1.
Number of daily cases (left axe) and new deaths (right axe) from day zero of pandemic in Greece.
Briefly, it is known that the spike protein of the Delta variant makes it more contagious than the previous variants, with higher viral loads and reduced efficacy of vaccines (Shiehzadegan et al., 2021). The Omicron variant is even more contagious than Delta, despite of the milder severity of the disease. Moreover,it appears that two doses of vaccination are not sufficient enough for adequate protection (Vitiello et al., 2022). Interestingly, differences in mutation/deletion equilibrium in SARS-CoV-2 defined variants – especially Delta and Omicron – which seems to affect the biological behavior of this infectious disease, modifying the corresponding infectivity and mortality rates (Papanikolaou et al., 2022). The purpose of this work is to estimate the parameters related to transmission phenomena and disease severity in patients with Delta and Omicron variants, in Greece, through mathematical methods.
Too many mathematical models had been developed, during the pandemic all over the world, aiming to find the most accurate approximations of infection dynamics. Compartmental models (SEIR), as well as phenomenological models (regression models) and individual-level models (network models), are the main approaches to transmission dynamics of COVID-19 (G. Chowell et al., 2016; Lotfi et al., 2022). Developing a mathematical model is a difficult process due to complex relations between the variables and the unknown parameters which describe them. Some vital factors for valid predictions are the amount of available data, the estimation of the parameters, the factor of re-infection in the immunized population, the multiple mutations of the virus, the undetected cases and asymptomatic population (AlArjani et al., 2022). Models are useful tools to understand the underlying situation, to speculate scenarios and predict the future.
In this study, a SEIR epidemic model was developed to simulate the evolution of the pandemic in Greece. Fitting methods were applied to specify the parameters related to mask usage and observance of social distancing and to analyze scenarios concerning the evolution of the pandemic dependence on these parameters. Model simulations were conducted to calculate hospitalized or deceased individuals depending on adopted or possible NPI measures. These predictions are even more important for countries with a burdened healthcare system, like Greece (Kousi et al., 2021; Siettos et al., 2021). To add, parameters related to Delta and Omicron mutation, such as effective contact rate, hospitalization rate and the possibility of asymptomatic disease were estimated to compare the two variants’ behavior, through a mathematical modeling approach.
2. Material and methods
2.1. Description of the model
The model is an extended SEIR model which is based on the different behaviors of the fully vaccinated population, in terms of how they participate in transmission and their likelihood of hospitalization, compared to the unvaccinated population. The probabilities were calculated using data from the NPHO of Greece when the mutation Delta and Omicron were predominant. In the model, parameters related to the behavior of the population, regarding mask usage and the observance of social distancing were integrated, in order to get more accurate and robust predictions (Wagner et al., 2022).
The population is divided into 11 classes and some subclasses. First class: S (susceptible) class includes people who have not been vaccinated yet and people who have recovered from the disease more than 6 months prior and are again able to infect and be infected (force of infection λs). Second class: LS (less susceptible) class includes the vaccinated individuals and those who have acquired natural immunity, during the last 6 months (Levin et al., 2021). Individuals in this class can infect and be infected (force of infection λu), with reduced probability of illness and hospitalization and also reduced ability to transmit. Third class: E (exposed) class includes exposed individuals in SARS-CoV-2, who are in the incubation period and can transmit the infection. In E class, two subclasses have been created (Ε1, Ε2) to differentiate and parameterize the reduced ability of LS class to infect or become infected compared to S class. Fourth class: Idetected or Id (infected and isolated) class includes all individuals who have symptoms and have been isolated immediately, on onset of symptoms, as well as asymptomatic individuals who have been detected with the disease. Also, in Id class, the subclasses Id,1 and Id,2 have been created to separate the hospitalization rate between unvaccinated (S→E1→Id,1) and vaccinated or natural immunized individuals (LS→E2→Id,2). Fifth class: A (asymptomatic) class includes the infectious asymptomatic individuals or undetected infected individuals with no obvious or mild symptoms. A class consists of A1 and A2, based on the aforementioned reasoning. Sixth class: H (hospitalized) class includes hospitalized individuals. Hospitalized individuals move to R or to D class. Seventh class: R (recovered) includes the recovered individuals. Finally, eight class: D (deceased) includes individuals who have passed away. A simplified flow diagram of the model is described in Fig. 2.
Fig. 2.
Flow diagram of the COVID-19 transmission model.
Amongst the population who is isolated, the cases of infected individuals with obvious symptoms (Id) (without the symptoms that cause secondary infections (p)), the proportion of asymptomatic individuals who have been detected with the virus (kA) and the hospitalized individuals (H) have been taken into account.
Thus, the force of the infection for susceptible ( and less susceptible () individuals is given by the equations:
2.2. Main assumptions of the model
Assumption 1:How long the immunity lasts and to what extent is not fully known (Havervall et al., 2022), but estimations are reported in literature(Andrews et al., 2022; Chemaitelly et al., 2022; Levin et al., 2021). The model does not incorporate the gradual weakening of immunity of vaccinated and recovered individuals, but assumes that recovered individuals have natural immunity for 6months, and after this period they are considered to be susceptible to get infected again (Goldberg et al., 2022; Levin et al., 2021; Thomas et al., 2021). This assumption does not particularly affect the simulation, due to the short-term forecasts.
Assumption 2: We assume that detected infected individuals (Id), hospitalized (H), a proportion of asymptomatic (kA), and a proportion of less susceptible individuals (vaccinated or recently recovered, LS) are isolated and do not contribute to human to human transmission.
Assumption 3: We assume that the infected individuals are isolated from the onset of symptoms and thus do not spread the virus. The groups of the population that spread the virus are a proportion of asymptomatic individuals who are not detected ((1-k)A), infected individuals that cause secondary infections (pI) and exposed individuals (E) during the incubation period. Thus, the possibility of someone being infected is from groups E, (1-k)A, pI and the environment, which is not taken into account in this study.
According to the lemmas in (Acheampong et al., 2022; Deressa & Duressa, 2021), it can be proven that the model has a unique, positive and bounded solution.
2.3. Calculated and fixed parameters
Demographic parameters: The population of Greece in 2021 was 10,370,744 (Macrotrends.net, 2022c). Influx rate (Λ) in our model includes the birth and migration rate in 2021, according to statistics data. The birth rate in Greece, in 2021, was 7.294 per 1,000 population and the migration rate is −1.074 per 1,000 population, so the influx rate is calculated at about 176 individuals per day (Macrotrends.net, 2022a). Because the average lifespan in Greece is approximately 82 years (Macrotrends.net, 2022b), the natural death rate is μ = 1/(82·365) per day.
Mask parameters: To calculate the efficacy of face masks, it has been considered that 3 types of masks are used daily: cloth masks, surgical masks and N95 masks. Cloth masks efficacy varies (Sharma et al., 2020) depending on the material and the way they are constructed, but few studies have been performed to determine their efficacy in COVID-19 transmission (Nanda et al., 2021). The review of (Rizki & Kurniawan, 2020) states that the efficacy of cloth masks ranges between 50 and 95%. Surgical masks and KN95 are generally more effective against virus infection (probably over 95%) (Yuxin Wang et al., 2021). Cloth masks probably offer inferior protection than surgical and N95 masks. A side study reveals that the average efficacy of all type of masks is 79% (Yu Wang et al., 2020) and this value is set to parameterize the model.
Vaccination parameters: In Greece, 4 types of vaccines are available AstraZeneca-University of Oxford, Pfizer-BioNTech, Moderna and Johnson & Johnson (J&J). The effectiveness against variant Delta after complete vaccination is lower in comparison with previous variants (Tregoning et al., 2021) according to a study which reveals that the effectiveness of Pfizer-BioNTech vaccine against the infection is about 88% and AstraZeneca about 67% (Lopez Bernal et al., 2021). Also a study, in Minnesota, reveals that Moderna and Pfizer-BioNtech vaccine effectiveness against infection is significantly reduced (Puranik et al., 2021). J&J vaccine is effective against the Delta variant, but few studies have been done to estimate its effectiveness (Evans & Jewell, 2021). After continuous mutations of the virus, the protection from the vaccines is not as high as expected (Edara et al., 2021; Pegu et al., 2021). Nevertheless, the available vaccines are more efficient in reducing hospital admissions (Mahase, 2021). Since more studies are needed to clarify vaccines efficiency, in our simulation the effectiveness against infection is assumed to be 0.75. Vaccine effectiveness against severe COVID-19 was even lower for the Omicron variant (Higdon et al., 2022; Sohan et al., 2022). Studies reveal that the effectiveness ranged between 55 and 70% (Collie et al., 2021; Kodera et al., 2022). For the simulation, the effectiveness is set, optimistically, at 0.7.
In Greece, Delta variant prevailed during the 4th wave of the epidemic (approximately from mid-summer to the end of 2021) followed by the Omicron variant for the following months. According to official data from NPHO, until 07/03/2021, 3,917,016 people were fully vaccinated (38% of total population) and the rate of vaccination was 2·10−4 per day, after the strict measures that were implemented by the government. At the end of 2021, when the Omicron variant was provoking the next wave, 7,006,801 people had been fully vaccinated (66% of population), while the vaccination rate remained practically constant (data.gov.gr).
Testing parameters: Parameter l is incorporated to represent the sensitivity of detection tests, in asymptomatic population. Self-tests, Rapid Ag tests and PCR tests are available for the detection of the disease and it is assumed that the first two are most frequently used. From 07/03/2021 until 12/27/2021 (Delta period) 32,482,034 Rapid Ag tests and 40,499,614 Self-tests had been performed. That entails about 400,000 tests per day. From 12/28/2021 until 04/18/2022 (Omicron period) 29,341,040 Rapid Ag and 39,293,686 Self-tests were performed. That translates into approximately 620,000 tests per day, an increase of 55% from the previous period. Rapid Ag tests were performed in the mobile units of NPHO and in diagnostic centers, on a daily basis, whereas, it was recommended to use Self tests twice a week. The rate σα is set at 1 to simplify the calculations. Studies reveal that antigen tests have significantly higher sensitivity (70–93%) in detecting the Delta variant compared to the Omicron variant (49–78%) (Bayart et al., 2022; Bekliz et al., 2022; Cocherie et al., 2022; Sun et al., 2022). In the simulations, the highest sensitivity value of each variant is assumed.
Recovery/Death rate: During the five-month period in which the Delta mutation prevailed, there were 40,469 hospital admissions of patients with COVID-19, in the country. 3,963 of those patients were intubated, with 913 of them being discharged, while the rest perished. The total deceased individuals due to COVID-19 during that period was 6,446 people so it turns out that the disease induced death rate of hospitalized patients was 0.17, multiplying by average day of hospitalization. Correspondingly, the recovery rate for hospitalized individuals is 0.83. The percentage remained the same during the Omicron period. Data for Greece was collected from the NPHO and European Center for Disease Prevention and Control (ECDC) (ECDC; NPHO).
The incubation period of the virus is 5.8 days on average, in variant D, thus the rate σu at which exposed individuals become infectious is 1/5.8 per day. In Delta variant the hospitalization rate is 10 times higher among unvaccinated adolescents than among fully vaccinated ones, so f2΄ = f2/10 (Delahoy et al., 2021). Also, the secondary attack rate (SAR) of asymptomatic COVID-19 index cases was 19% and the SAR of symptomatic index cases was 25%. So, it follows that the reduction of infectiousness from asymptomatic population is 24%, so na = 0.76 (Krieg et al., 2022). The calculated and fixed parameters are given in Table 1.
Table 1.
Calculated and fixed parameters of the model.
| Parameter | Value | Description | Source |
|---|---|---|---|
| N | 10,370,744 | population | (Macrotrends.net, 2022c) |
| Λ | 176 per day | influx rate | (Macrotrends.net, 2022a) |
| 2·10−4 per day | rate of vaccination | (govgr) | |
| 1 per day | rate at which A are detected and revert to Idclass | assumed | |
| μ | 1/(82·365) per day | natural mortality rate | (Macrotrends.net, 2022b) |
| emask | 0.79 | efficacy of mask | (Yu Wang et al., 2020) |
| na | 0.76 | modification parameter that reduces infectiousness from A | Krieg et al. (2022) |
| a | 0.17 | proportion of H that pass away | (ECDC; NPHO) |
| v | 1/140–180 per day | rate at which LS revert to S due to immunity weakens | (Andrews et al., 2022; Chemaitelly et al., 2022; Levin et al., 2021) |
| 0.75 (Delta) 0.7 (Omicron) | effectiveness of vaccines or natural immunity | (Collie et al., 2021; Evans & Jewell, 2021; Kodera et al., 2022; Lopez Bernal et al., 2021; Tregoning et al., 2021) | |
| σu | 1/5.8 per day | rate at which E become infectious | Kang et al. (2021) |
| σh | 1/4 per day | rate at which I become H | Zhang et al. (2020) |
| γα | 1/6 per day | recovery rate of A | (Rachaniotis et al., 2021; Sypsa et al., 2021) |
| γi | 1/6 per day | recovery rate of Id | (Rachaniotis et al., 2021; Sypsa et al., 2021) |
| γi/δi | 1/12–18.5 per day | recovery/disease induced death rate of H | Zhao et al. (2021) |
| p | 25% | proportion/probability of quarantined infected that cause secondary attack | Krieg et al. (2022) |
| l | 93% (Delta) 78% (Omicron) | sensitivity of self test diagnosis on asymptomatic individuals | (Bayart et al., 2022; Bekliz et al., 2022; Cocherie et al., 2022; Sun et al., 2022) |
2.4. Fitted parameters
The remaining parameters of the model were approximated by fitting methods, utilizing the real cumulative number of deceased individuals, from NPHO. The number of deaths is more accurate than the number of infected individuals, so the model is not threatened by the underreporting. Most of the fitted parameters are related to the behavior of the population and cannot be fixed or assumed. Thus, genetic algorithmic methods were performed to define the range of these parameters. Subsequently, lsqcurvefit together with Multistart functions were used to capture the global solution, based on least-squares approach. The system of nonlinear differential equations was solved by using the ode45 function. All the above algorithms were performed in Matlab R2020b. The fitting method for Delta period was applied during 08/01/2021-10/20/2021 and for Omicron period during 12/19/2021-03/24/2022, as presented in Fig. 3. The corresponding errors are presented below each graph. Errors were calculated by dividing the model value (fitted value) with the real data. Most errors are at 5%. The fitted parameters are given in Table 2.
Fig. 3.
Cumulative number of deceased individuals in period 08/01/21 to 10/20/21 (Delta variant) (a) and in period 12/19/21 to 03/24/21 (Omicron variant) (b). Green symbols represent real data from NPHO, the magenta line is the fitted curve generated by the model. Below each curve fitting are presented the corresponding errors.
Table 2.
Fitted Parameters of the model.
| Delta Variant |
Omicron Variant |
||||
|---|---|---|---|---|---|
| Parameter | Description | Fitted Value | 95% Confidence Interval | Fitted Value | 95% Confidence Interval |
| β | effective contact rate | 0.4974 | 0.4893–0.5054 | 0.8589 | 0.8561–0.8617 |
| k | proportion of A that are detected (test detection) | 0.1726 | 0.1622–0.1830 | 0.6968 | 0.5190–0.8745 |
| f1 | proportion of E that become Id | 0.3647 | 0.0145–0.7148 | 0.1921 | 0.1920–0.1922 |
| pmask | proportion who wear mask | 0.4515 | 0.4466–0.4563 | 0.6344 | 0.6306–0.6382 |
| pdist | proportion who social distance | 0.6222 | 0.6117–0.6328 | 0.6457 | 0.6389–0.6524 |
| f2 | proportion of Id1that become H1 | 0.038 | 0.0270–0.0480 | 0.0218 | 0.0206–0.0229 |
The calibration of the model is based on strategies that aim to reduce the relative errors and the covariance between the unknown parameters. The quality of fitting is supported from the uniformly distribution of the residuals. Nevertheless, the covariance matrices reveal correlation among the parameters that lead to model uncertainty. The uncertainty associated with the parameter non - uniqueness could be calculated by the bootstrap method (Gerardo Chowell, 2017).
2.5. Viral load method
RT – PCR cycle threshold (Ct) values represent the number of amplification cycles required for the target gene to exceed a threshold level (Bonacorsi et al., 2021). The current knowledge is that Ct cycle is negatively related to viral load and viral load is positively correlated to transmission phenomena and also to the evolution of disease in the patient (Rabaan et al., 2021; Rao et al., 2020; Tom & Mina, 2020). Despite this, there are doubts about whether Ct cycle is a sufficient indicator to predict both the transmission phenomena and disease evolution (Rabaan et al., 2021). In order to check the validity of the above proposal in Greece, the Ct cycles from RT-PCRs of patients with COVID-19 at NIMTS hospital in Athens, were gathered, during the period of October 2020 until May 2022, in the context of a retrospective study. The average Ct values, the number of positive PCR's, the average intubated patients, the number of deceased individuals and the average positive rate during the first waves, the fourth and the fifth wave of pandemic, are given in Table 3. Ct values during Delta and Omicron period are presented in the box plot in Fig. 4. During this period, 894 positive RT-PCR tests were performed and Ct cycles were noted for each patient. RT-PCR kits target regions in N and RdRp genes of SARS-COV-2, which have higher specificity and sensitivity (Mollaei et al., 2020). In order to declare a test as positive, the upper value limit is 35. Approval for the study was acquired from the Bioethics and Deontology Committee of the National and Kapodistrian University of Athens (Protocol number: 564).
Table 3.
Ct values.
| AverageCt value | Number of positive PCRs | Deaths | Average of intubated patients | Positive rateaverage (range) | |
|---|---|---|---|---|---|
| March–July 2021 | 27.26 | n = 321 | 6,436 | 503 per day | 2.12% (0.09–6.65%) |
| August 2021-December 2021 4th wave-Delta variant | 25.44 | n = 118 | 7,605 | 438 per day | 1.29% (0.36–5.85%) |
| January 2022-May 2022 5th wave-Omicron variant | 27.95 | n = 307 | 8,677 | 395 per day | 3.34% (1.89–5.85%) |
Fig. 4.
Boxplot for Ct values in Delta and Omicron variant.
3. Results
3.1. Results of the model fitting
Studies have revealed that the probability of hospitalization is significantly lower in the Omicron variant than in the Delta variant (Peralta-Santos et al., 2022; Veneti et al., 2022; Vitiello et al., 2022).Our model calculated that the risk of hospitalization is reduced by about 42% in the Omicron compared to the Delta variant with the corresponding probabilities of hospitalization being about 2.2% (CI: 2.1–2.3) and 3.7% (CI: 2.7–4.8) respectively. This phenomenon is consistent with the results from Ct values analysis. A meta analysis revealed that asymptomatic infection among the Omicron variant-positive individuals, is significantly higher than with the Delta (Yu et al., 2022). Our applied fitting method showed that 36% of exposed individuals become symptomatically infected with the Delta variant and 19% with the Omicron variant. This implies an increase of about 27% in asymptomatic disease caused by the Omicron variant. Furthermore, the proportion of asymptomatic individuals that were detected during the Omicron period is more than 3 times bigger than those detected during the Delta period. This can be explained by the rapid growth in the number of tests carried out. Moreover, effective contact rate is a parameter related to the transmission of the virus. In the Delta variant, model fitting revealed that β was 0.4974 (CI: 0.4893–0.5054) and in the Omicron variant βwas 0.8589 (CI: 0.8561–0.8617). These values correspond to a 73% increase during the Omicron wave. The above are in agreement with the positive rate that is presented in Table 3.
The next two parameters that were fitted are related to the behavior of the population. The pmask (proportion that wears mask) was 0.4515 (CI: 0.4466–0.4563) and 0.6344 (CI: 0.6306–0.6382) in the Delta and Omicron period, respectively. The higher percentage of people wearing mask (about 41%) is justified by the increased number of daily cases, which intensifies the need for protection and maybe the seasonal differences in temperature. Last parameter is the pdist (proportion that implements social distancing) which remained practically constant between the periods of study. The fitted values were 0.6222% (CI: 0.6117–0.6328) and 0.6457 (CI: 0.6389–0.6524) in the Delta and Omicron period, respectively.
3.2. Sensitivity analysis
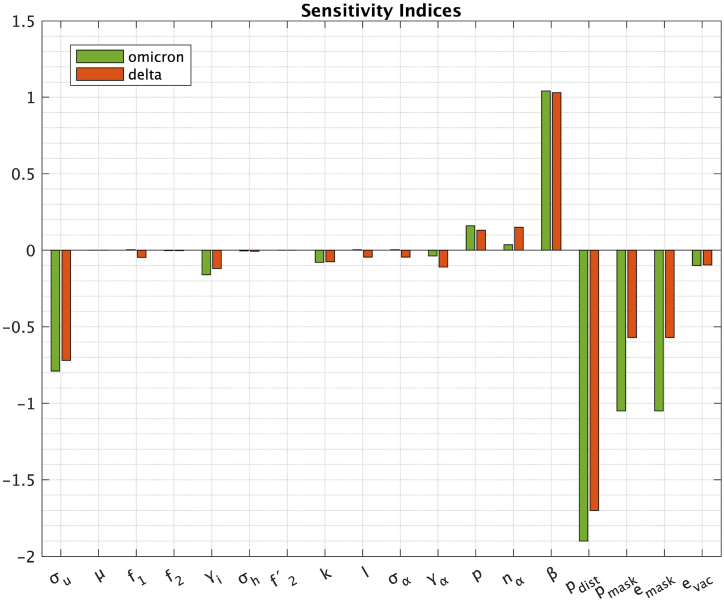
The robustness of the model is evaluated using the sensitivity analysis method, as described by (Chitnis et al., 2008). The method was performed twice, for Delta and Omicron variants on the data from Table 1, Table 2 The model parameters are assessed based on their impact on the reproduction number (R0) and sensitivity indices are presented in Fig. 5. The most impactful parameters identified include the proportion of those who practice social distancing (pdist), the proportion of those who wear masks (pmask), the efficacy of masks (emask), the effective contact rate (β) and the rate at which the exposed become infectious (σu). So, pmask and pdist parameters should be targeted by intervention strategies.
Fig. 5.
Sensitivity indices for Delta (red bars) and Omicron variants (green bars).
3.3. Numerical simulations-3 different scenarios
In order to analyze how effectively the NPI measures performed during the prevalence of the Delta mutation, the potential number of deceased individuals was calculated for different values of the parameters pmask and pdist. The aim of the simulations is to assess the intervention measures of face mask usage and social distancing, separately and combined, during Delta and Omicron period. The model was simulated for two different values of the fitted parameters (pmask and pdist), that are related to population behaviour. In particular, the percentage of population that applies effectively the use of face masks (Mask Usage Scenarios) and adheres to the social distancing guidelines (Social Distancing Scenarios), was set to be 20% more or less than the fitted value. The real data have been used as the baseline scenario. The remaining parameters used in the simulation are noted in the tables. The simulation results indicated that in the quarter during which the Delta variant prevailed, if 20% more of the population used masks, about 610 deaths would have been avoided. If 20% more of the population adhered to the social distancing measures, 1,241 deaths would have been avoided. Furthermore, if both parameters increased simultaneously (Combined Scenarios), then 1,364 deaths would have been avoided. On the other hand, if mask usage was 20% lower, 965 more deaths would have been recorded. If social distancing was 20% lower, 4,504 more deaths would have been recorded. If both measures were lower simultaneously, 8,171 more deaths would have been recorded.
Similarly, the corresponding scenarios were simulated for the next 3-month period, during which the Omicron variant prevailed. The simulation results indicated that in the quarter when Omicron prevailed, if 20% more of the population used mask, about 2,109 deaths would have been avoided. If 20% more of the population adhered to the social distancing measures, 3,072 deaths would have been avoided. If both parameters increased simultaneously, then 3,655 deaths would have been avoided. On the other side of the spectrum, if mask usage was 20% lower, then 2,842 more deaths would have been recorded. If social distancing was 20% lower, then 5,183 more deaths would have been recorded. If both measures were lowered simultaneously, 8,280 more deaths would have been recorded. The above are presented in Fig. 6.
Fig. 6.
Baseline scenario represents the real number of deceased individuals. (a) Two scenarios have been simulated, with increase and decline in mask usage (pm) by 20%, in Delta and Omicron variants. (b) Two scenarios have been simulated, with increase and decline in social distancing (pdist) by 20%, in Delta and Omicron variants. (c) Optimistic scenario corresponds to a 20% increase in pm and pdist. Pessimistic scenario corresponds to a 20% decline in pm and pdist parameters.
3.4. Viral load comparison
The Ct cycle average from all tests, from the beginning of the pandemic until May 2022, was 27.52 (range: 12.32–39.91) and standard deviation (SD) was 6.33. Drawing further observations about the behavior of the Delta and Omicron variants, we focused on the periods when these variants prevailed in Greece. During the period of July ’21 to December ’21, that Delta variant prevailed in the country, the average Ct value from 113 patients was 25.19 (range: 12.32–39.29). During the next period, January ’22-May ’22, when the Omicron variant prevailed, the average Ct value from 303 patients was 28 (range: 14.41–39.36). Compared to the average of all Ct values, measured since the beginning of the pandemic at NIMTS Hospital, the variant's Delta period recorded the lowest values, 2.33 units below the overall average. As for the Omicron variant, the average Ct value was 0.48 units above the overall average. Ct value was significantly different for Omicron cases and Delta cases (25.44 for Delta variant and 27.95 for Omicron variant, p = 0.0005) (Fig. 4). Thus, the Delta variant is associated with higher viral load than the Omicron variant. The conclusion is in agreement with recent publications (Cocherie et al., 2022; Laitman et al., 2022; Sentis et al., 2022).
During these periods we observe the highest numbers of hospitalizations, intubations and deaths, in the country. The number of daily cases is not a reliable marker to explain transmission phenomena (Irons & Raftery, 2021), so the positive rate is chosen to investigate disease transmission in relation with Ct values. According to ECDC data (Table 3), during the Delta variant period, the lowest Ct value and positive rate were recorded (25.44 and 1.29 respectively). Thus, we are not able to confirm a correlation between Ct values and transmission phenomena, as discussed by literature (Che-Kamaruddin et al., 2022).
However, we might confirm the correlation between Ct value and disease severity. During the Delta variant period, the positive rate declined about 40% but the number of deceased individuals increased about 18%, which is related to the severity of the disease. During the Omicron period, the positive rate was increased by 159%, but the number of deceased individuals was increased only by 14%. This phenomenon could be explained by viral load fluctuation. Lower Ct values or higher viral load led to more deaths. Statistical analyses were performed with R Studio.
Τhe above conclusions are simple observations from data collected only at NIMTS hospital and not from the entire country, therefore larger scale studies are required to confirm them. Also, the reader must take into account Ct cycle measurement uncertainties, regarding the collection technique, the type of specimen, the sampling time, the age of the patient etc. (Ade et al., 2021).
4. Discussion
A mathematical model was developed for the transmission dynamics of the Delta and the Omicron variant, in Greece. The model took into account the proportion of vaccinated population, individuals with natural immunity, the secondary attack rate and parameters related to the behavior of population, such as the use of masks and social distancing, in order to simulate mitigation measures to reduce the spread of the variants. Fitting methods were applied to determine important parameters and numerical simulations were carried out to assess the effect of NPI measures on mortality, during the Delta and the Omicron periods.
Our modeling results suggest that during the Omicron period the effective contact rate increased about 73%, the rate of hospitalization decreased by 42% and the possibility of asymptomatic disease increased by 27%. Furthermore, parameters related to the behavior of the population in Greece were also assessed, in particular regarding mask usage and the obedience of social distancing measures. It was estimated that the use of masks was increased by 40% during the period of the Omicron variant, while no significant change was observed in the social distancing parameter. Model simulations revealed that if the percentage of population who used masks and abided by social distancing measures was 20% higher or more efficient, then 1,364 and 3,655 deaths could have been avoided, in just one 80-day period, while Delta and Omicron mutations prevailed.
Significantly increased Ct values were measured during the Delta period compared to the Omicron period, from a total of 746 patients who participated in the study. The period during which the Delta variant prevailed in the country, the average Ct value was calculated as 25.19 (range: 12.32–39.29), whereas during the period when the Omicron variant prevailed, the average Ct value was calculated as 28 (range: 14.41–39.36). During the Delta period, the case fatality ratio (1.1%) was about 3 times higher than the Omicron period (0.4%) and the highest Ct values that were noted throughout the pandemic. So, the Ct statistical analysis did not confirm a correlation between Ct values and transmission phenomena but may indicate a correlation between Ct values and disease severity.
The COVID-19 pandemic has led to the evolution of the modeling of infectious diseases, and in particular of the compartmental models, which are some of the most important types of models. The availability of data is necessary to develop or add compartments for better predictions, despite the impact on the complexity of the model. Assuming that the available data on the population and the pathogen used are correct, this study has developed a model which simulates the transmission dynamics of the Delta and Omicron mutations, in Greece. Data were collected for each mutation separately and the model was parameterized twice for better predictions. Data from NPHO and the retrospective study at NIMTS were used to evaluate the model's results. Future work could include data collection on a larger scale, for more unbiased and accurate results.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Marilena Argyropoulou and Romanos Athanasiou, who performed PCR tests in the NIMTS Hospital and Sylvia Koulouri and Ioannis Stamatiou for the useful advices.
Handling Editor: Daihai He
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- Acheampong E., Okyere E., Iddi S., Bonney J.H.K., Asamoah J.K.K., Wattis J.A.D., Gomes R.L. Mathematical modelling of earlier stages of COVID-19 transmission dynamics in Ghana. Results in Physics. 2022;34 doi: 10.1016/j.rinp.2022.105193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ade C., Pum J., Abele I., Raggub L., Bockmühl D., Zöllner B. Analysis of cycle threshold values in SARS-CoV-2-PCR in a long-term study. Journal of Clinical Virology. 2021;138 doi: 10.1016/j.jcv.2021.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlArjani A., Nasseef M.T., Kamal S.M., Rao B.V.S., Mahmud M., Uddin M.S. Application of mathematical modeling in prediction of COVID-19 transmission dynamics. Arabian Journal for Science and Engineering. 2022;47(8):10163–10186. doi: 10.1007/s13369-021-06419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N., Tessier E., Stowe J., Gower C., Kirsebom F., Simmons R., Gallagher E., Thelwall S., Groves N., Dabrera G., Myers R., Campbell C.N.J., Amirthalingam G., Edmunds M., Zambon M., Brown K., Hopkins S., Chand M., Ladhani S.N., Ramsay M., Lopez Bernal J. Duration of protection against mild and severe disease by covid-19 vaccines. New England Journal of Medicine. 2022;386(4):340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayart J.-L., Degosserie J., Favresse J., Gillot C., Didembourg M., Djokoto H.P., Verbelen V., Roussel G., Maschietto C., Mullier F., Dogné J.-M., Douxfils J. Analytical sensitivity of six SARS-CoV-2 rapid antigen tests for omicron versus delta variant. Viruses. 2022;14(4):654. doi: 10.3390/v14040654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekliz M., Perez-Rodriguez F., Puhach O., Adea K., Melancia S.M., Baggio S., Corvaglia A.-R., Jacquerioz-Bausch F., Alvarez C., Essaidi-Laziosi M., Escadafal C., Kaiser L., Eckerle I. medRxiv; 2022. Sensitivity of SARS-CoV-2 antigen-detecting rapid tests for Omicron variant. 2021-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacorsi S., Visseaux B., Bouzid D., Pareja J., Rao S.N., Manissero D., Hansen G., Vila J. Systematic review on the correlation of quantitative PCR cycle threshold values of gastrointestinal pathogens with patient clinical presentation and outcomes. J Frontiers in Medicine. 2021;8 doi: 10.3389/fmed.2021.711809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che-Kamaruddin N., Teoh B.-T., Tan K.-K., Tan J.-Y., Wong J.-E., Tiong V., Abd-Jamil J., Nor’e S.-S., Khor C.-S., Johari J. 2022. Decrease in RT-PCR Ct values among SARS-CoV-2 positive samples during the emergence of the B. 1.617. 2 (Delta) variant in Malaysia. [DOI] [Google Scholar]
- Chemaitelly H., Ayoub H.H., AlMukdad S., Coyle P., Tang P., Yassine H.M., Al-Khatib H.A., Smatti M.K., Hasan M.R., Al-Kanaani Z., Al-Kuwari E., Jeremijenko A., Kaleeckal A.H., Latif A.N., Shaik R.M., Abdul-Rahim H.F., Nasrallah G.K., Al-Kuwari M.G., Butt A.A.…Abu-Raddad L.J. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Nature Communications. 2022;13(1):3082. doi: 10.1038/s41467-022-30895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis N., Hyman J.M., Cushing J.M. Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. J Bulletin of mathematical biology. 2008;70:1272–1296. doi: 10.1007/s11538-008-9299-0. [DOI] [PubMed] [Google Scholar]
- Chowell G. Fitting dynamic models to epidemic outbreaks with quantified uncertainty: A primer for parameter uncertainty, identifiability, and forecasts. J Infectious Disease Modelling. 2017;2(3):379–398. doi: 10.1016/j.idm.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell G., Sattenspiel L., Bansal S., Viboud C. Mathematical models to characterize early epidemic growth: A review. Physics of Life Reviews. 2016;18:66–97. doi: 10.1016/j.plrev.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocherie T., Bastide M., Sakhi S., Zafilaza K., Flandre P., Leducq V., Jary A., Burrel S., Louet M., Calvez V.J.M.S. Decreased sensitivity of rapid antigen test is associated with a lower viral load of omicron than delta SARS-CoV-2 variant. Microbiology Spectrum. 2022;10(5) doi: 10.1128/spectrum.01922-22. e01922-01922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collie S., Champion J., Moultrie H., Bekker L.-G., Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. New England Journal of Medicine. 2021;386(5):494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- data.gov.gr Στατιστικά εμβολιασμού για τον COVID-19. https://www.data.gov.gr/datasets/mdg_emvolio/ Retrieved from.
- Delahoy M.J., Ujamaa D., Whitaker M., O'Halloran A., Anglin O., Burns E., Cummings C., Holstein R., Kambhampati A.K., Milucky J., Patel K., Pham H., Taylor C.A., Chai S.J., Reingold A., Alden N.B., Kawasaki B., Meek J., Yousey-Hindes K.…Team C.-N.S. Hospitalizations associated with COVID-19 among children and adolescents - COVID-NET, 14 states, march 1, 2020-august 14, 2021. Morbidity and Mortality Weekly Report. 2021;70(36):1255–1260. doi: 10.15585/mmwr.mm7036e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deressa C.T., Duressa G.F. Modeling and optimal control analysis of transmission dynamics of COVID-19: The case of Ethiopia. Alexandria Engineering Journal. 2021;60(1):719–732. doi: 10.1016/j.aej.2020.10.004. [DOI] [Google Scholar]
- ECDC. European Centre for Disease Prevention and Control. Retrieved from https://www.ecdc.europa.eu/en/copyright. Accessed January 24, 2023.
- Edara V.-V., Lai L., Sahoo M.K., Floyd K., Sibai M., Solis D., Flowers M.W., Hussaini L., Ciric C.R., Bechnack S., Stephens K., Mokhtari E.B., Mudvari P., Creanga A., Pegu A., Derrien-Colemyn A., Henry A.R., Gagne M., Graham B.S.…Suthar M.S. Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B.1.617.1 variant. New England Journal of Medicine. 2021;7:664–666. doi: 10.1101/2021.05.09.443299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S.J.W., Jewell N.P. Vaccine effectiveness studies in the field. New England Journal of Medicine. 2021;385(7):650–651. doi: 10.1056/NEJMe2110605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L.S., Ash N., Alroy-Preis S., Huppert A., Milo R. Protection and waning of natural and hybrid immunity to SARS-CoV-2. New England Journal of Medicine. 2022;386(23):2201–2212. doi: 10.1056/NEJMoa2118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- govgr Εμβολιασμός COVID-19. https://emvolio.gov.gr/vaccinationtracker Retrieved from.
- Havervall S., Ng H., Jernbom Falk A., Greilert-Norin N., Månberg A., Marking U., Laurén I., Gabrielsson L., Salomonsson A.-C., Aguilera K., Kihlgren M., Månsson M., Rosell A., Hellström C., Andersson E., Olofsson J., Skoglund L., Yousef J., Pin E.…Thålin C. Robust humoral and cellular immune responses and low risk for reinfection at least 8 months following asymptomatic to mild COVID-19. Journal of Internal Medicine. 2022;291(1):72–80. doi: 10.1111/joim.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higdon M.M., Baidya A., Walter K.K., Patel M.K., Issa H., Espié E., Feikin D.R., Knoll M.D. Duration of effectiveness of vaccination against COVID-19 caused by the omicron variant. The Lancet Infectious Diseases. 2022;22(8):1114–1116. doi: 10.1016/S1473-3099(22)00409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons N.J., Raftery A.E. Estimating SARS-CoV-2 infections from deaths, confirmed cases, tests, and random surveys. Proceedings of the National Academy of Sciences. 2021;118(31) doi: 10.1073/pnas.2103272118. e2103272118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M., Xin H., Yuan J., Ali S.T., Liang Z., Zhang J., Hu T., Lau E.H.Y., Zhang Y., Zhang M., Cowling B.J., Li Y., Wu P. Transmission dynamics and epidemiological characteristics of Delta variant infections in China. Euro Surveillance. 2021;27 doi: 10.1101/2021.08.12.21261991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodera S., Rashed E.A., Hirata A. Estimation of real-world vaccination effectiveness of mRNA COVID-19 vaccines against delta and omicron variants in Japan. Vaccines. 2022;10(3):430. doi: 10.3390/vaccines10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousi T., Mitsi L.-C., Simos J. The early stage of COVID-19 outbreak in Greece: A review of the national response and the socioeconomic impact. Journal of Environmental Research and Public Health. 2021;18(1):322. doi: 10.3390/ijerph18010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg S.J., Schnur J.J., Miranda M.L., Pfrender M.E., Chawla N.V. Symptomatic, presymptomatic, and asymptomatic transmission of SARS-CoV-2 in a university student population, august-november 2020. Public Health Reports. 2022;137(5):1023–1030. doi: 10.1177/00333549221110300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitman A.M., Lieberman J.A., Hoffman N.G., Roychoudhury P., Mathias P.C., Greninger A.L. The SARS-CoV-2 omicron variant does not have higher nasal viral loads compared to the delta variant in symptomatic and asymptomatic individuals. Journal of Clinical Microbiology. 2022;60(4) doi: 10.1128/jcm.00139-22. e00139-00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., Doolman R., Asraf K., Mendelson E., Ziv A., Rubin C., Freedman L., Kreiss Y., Regev-Yochay G. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. New England Journal of Medicine. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G. Effectiveness of covid-19 vaccines against the B. 1.617. 2 (delta) variant. New England Journal of Medicine. 2021;5(5) doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfi R., Kheiri K., Sadeghi A., Babaee Tirkolaee E. An extended robust mathematical model to project the course of COVID-19 epidemic in Iran. Annals of Operations Research. 2022:1–15. doi: 10.1007/s10479-021-04490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrotrends.net . 2022. Greece birth rate 1950-2022.https://www.macrotrends.net/countries/GRC/greece/birth-rate Retrieved from. [Google Scholar]
- Macrotrends.net . 2022. Greece life expectancy 1950-2022.https://www.macrotrends.net/countries/GRC/greece/life-expectancy Retrieved from. [Google Scholar]
- Macrotrends.net . 2022. Greece population 1950-2022.https://www.macrotrends.net/countries/GRC/greece/population Retrieved from. [Google Scholar]
- Mahase E. Delta variant: What is happening with transmission, hospital admissions, and restrictions? BMJ. 2021;373:n1513. doi: 10.1136/bmj.n1513. [DOI] [PubMed] [Google Scholar]
- Mollaei H.R., Afshar A.A., Kalantar-Neyestanaki D., Fazlalipour M., Aflatoonian B. Comparison five primer sets from different genome region of COVID-19 for detection of virus infection by conventional RT-PCR. Iranian Journal of Microbiology. 2020;12(3):185–193. [PMC free article] [PubMed] [Google Scholar]
- Nanda A., Hung I., Kwong A., Man V.C.M., Roy P., Davies L., Douek M. Efficacy of surgical masks or cloth masks in the prevention of viral transmission: Systematic review, meta-analysis, and proposal for future trial. Journal of Evidence-Based Medicine. 2021 doi: 10.1111/jebm.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NPHO. National public health organization https://eody.gov.gr/en/ Retrieved from.
- Papanikolaou V., Chrysovergis A., Ragos V., Tsiambas E., Katsinis S., Manoli A., Papouliakos S., Roukas D., Mastronikolis S., Peschos D. From delta to omicron: S1-RBD/S2 mutation/deletion equilibrium in SARS-CoV-2 defined variants. Gene. 2022;814 doi: 10.1016/j.gene.2021.146134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegu A., O'Connell S., Schmidt S.D., O'Dell S., Talana C.A., Lai L., Albert J., Anderson E., Bennett H., Corbett K.S., Flach B., Jackson L., Leav B., Ledgerwood J.E., Luke C.J., Makowski M., Nason M.C., Roberts P.C., Roederer M.…Doria-Rose N.A. Durability of mRNA-1273 vaccine–induced antibodies against SARS-CoV-2 variants. Science. 2021;373(6561):1372–1377. doi: 10.1126/science.abj4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta-Santos A., Rodrigues E., Moreno J., Ricoca V., Casaca P., Fernandes E., Gomes J., Ferreira R., Isidro J., Pinto M. Omicron (BA. 1) SARS-CoV-2 variant is associated with reduced risk of hospitalization and length of stay compared with delta (b. 1.617. 2) SSRN Electronic Journal. 2022 doi: 10.2139/ssrn.4017381. [DOI] [Google Scholar]
- Puranik A., Lenehan P.J., Silvert E., Niesen M.J.M., Corchado-Garcia J., O'Horo J.C., Virk A., Swift M.D., Halamka J., Badley A.D., Venkatakrishnan A.J., Soundararajan V. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv. 2021 doi: 10.1101/2021.08.06.21261707. [DOI] [Google Scholar]
- Rabaan A.A., Tirupathi R., Sule A.A., Aldali J., Mutair A.A., Alhumaid S., Muzaheed, Gupta N., Koritala T., Adhikari R., Bilal M., Dhawan M., Tiwari R., Mitra S., Emran T.B., Dhama K. Viral dynamics and real-time RT-PCR Ct values correlation with disease severity in COVID-19. Diagnostics. 2021;11(6):1091. doi: 10.3390/diagnostics11061091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachaniotis N.P., Dasaklis T.K., Fotopoulos F., Tinios P. A two-phase stochastic dynamic model for COVID-19 mid-term policy recommendations in Greece: A pathway towards mass vaccination. International Journal of Environmental Research and Public Health. 2021;18(5):2497. doi: 10.3390/ijerph18052497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.N., Manissero D., Steele V.R., Pareja J. A systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infectious Disease and Therapy. 2020;9(3):573–586. doi: 10.1007/s40121-020-00324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki S.A., Kurniawan A. Efficacy of cloth mask in reducing COVID-19 transmission: A literature review. J Kesmas: Jurnal Kesehatan Masyarakat Nasional (National Public Health Journal) 2020 doi: 10.21109/kesmas.v15i2.3893. [DOI] [Google Scholar]
- Sentis C., Billaud G., Bal A., Frobert E., Bouscambert M., Destras G., Josset L., Lina B., Morfin F., Gaymard A. SARS-CoV-2 Omicron variant, lineage BA. 1, is associated with lower viral load in nasopharyngeal samples compared to Delta variant. Viruses. 2022;14(5):919. doi: 10.3390/v14050919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.K., Mishra M., Mudgal S.K. Efficacy of cloth face mask in prevention of novel coronavirus infection transmission: A systematic review and meta-analysis. Journal of Education and Health Promotion. 2020;9 doi: 10.4103/jehp.jehp_533_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiehzadegan S., Alaghemand N., Fox M., Venketaraman V. Analysis of the delta variant B.1.617.2 COVID-19. Clinics and practice. 2021;11(4):778–784. doi: 10.3390/clinpract11040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siettos C., Anastassopoulou C., Tsiamis C., Vrioni G., Tsakris A. A bulletin from Greece: A health system under the pressure of the second COVID-19 wave. Pathogens and Global Health. 2021;115(3):133–134. doi: 10.1080/20477724.2021.1881372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohan M., Hossain M.J., Islam M.R. The SARS-CoV-2 Omicron (B.1.1.529) variant and effectiveness of existing vaccines: What we know so far. Journal of Medical Virology. 2022;94(5):1796–1798. doi: 10.1002/jmv.27574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K.J., Vaeth M.J.E., Robinson M., Elhabashy M., Gupta I., Purekal S., Hammershaimb E.A., Peralta R., Mitchell A., Foyez M., Johnson J.K., Ficke J.R., Manabe Y.C., Campbell J.D., Callahan C.W., Locke C.F., Kantsiper M., Consortium C.C., Siddiqui Z.K. High sensitivity and NPV for BinaxNOW rapid antigen test in children at a mass testing site during prevalent delta variant. Microbiology Spectrum. 2022 doi: 10.1101/2022.01.05.22268788. e00236-00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypsa V., Roussos S., Paraskevis D., Lytras T., Tsiodras S., Hatzakis A. Effects of social distancing measures during the first epidemic wave of severe acute respiratory syndrome infection, Greece. Emerging Infectious Diseases. 2021;27(2):452–462. doi: 10.3201/eid2702.203412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Polack F.P., Zerbini C., Bailey R., Swanson K.A., Xu X., Roychoudhury S., Koury K., Bouguermouh S., Kalina W.V., Cooper D., Frenck R.W.…Jansen K.U. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. New England Journal of Medicine. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom M.R., Mina M.J. To interpret the SARS-CoV-2 test, consider the cycle threshold value. J Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregoning J.S., Flight K.E., Higham S.L., Wang Z., Pierce B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nature Reviews Immunology. 2021;21(10):626–636. doi: 10.1038/s41577-021-00592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiambas E., Chrysovergis A., Papanikolaou V., Mastronikolis N., Ragos V., Batistatou A., Peschos D., Kavantzas N., Lazaris A.C., Kyrodimos E. Impact of ribosome activity on SARS-CoV-2 LNP–based mRNA vaccines. Frontiers in Molecular Biosciences. 2021;8 doi: 10.3389/fmolb.2021.654866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneti L., Bøås H., Bråthen Kristoffersen A., Stålcrantz J., Bragstad K., Hungnes O., Storm M.L., Aasand N., Rø G., Starrfelt J., Seppälä E., Kvåle R., Vold L., Nygård K., Buanes E.A., Whittaker R. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 Omicron BA.1 variant compared with the Delta variant, Norway, December 2021 to January 2022. Euro Surveillance. 2022;27(4) doi: 10.2807/1560-7917.Es.2022.27.4.2200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello A., Ferrara F., Auti A.M., Di Domenico M., Boccellino M. Advances in the Omicron variant development. Journal of Internal Medicine. 2022;292(1):81–90. doi: 10.1111/joim.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C.E., Saad-Roy C.M., Grenfell B.T. Modelling vaccination strategies for COVID-19. Nature Reviews Immunology. 2022;22(3):139–141. doi: 10.1038/s41577-022-00687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Deng Z., Shi D. How effective is a mask in preventing COVID-19 infection? J Medical devices. 2021;4(1) doi: 10.1002/mds3.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Tian H., Zhang L., Zhang M., Guo D., Wu W., Zhang X., Kan G.L., Jia L., Huo D., Liu B., Wang X., Sun Y., Wang Q., Yang P., MacIntyre C.R. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: A cohort study in beijing, China. BMJ Global Health. 2020;5(5) doi: 10.1136/bmjgh-2020-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worldometer.info . 2022. Coronavirus.https://www.worldometers.info/coronavirus/country/greece/ Retrieved from. [Google Scholar]
- Yu W., Guo Y., Zhang S., Kong Y., Shen Z., Zhang J. Proportion of asymptomatic infection and nonsevere disease caused by SARS-CoV-2 omicron variant: A systematic review and analysis. Journal of Medical Virology. 2022;94(12):5790–5801. doi: 10.1002/jmv.28066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Litvinova M., Wang W., Wang Y., Deng X., Chen X., Li M., Zheng W., Yi L., Chen X., Wu Q., Liang Y., Wang X., Yang J., Sun K., Longini I.M., Jr., Halloran M.E., Wu P., Cowling B.J.…Yu H. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside hubei province, China: A descriptive and modelling study. The Lancet Infectious Diseases. 2020;20(7):793–802. doi: 10.1016/s1473-3099(20)30230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Zha X., Wang N., Li D., Li A., Yu S. Clinical characteristics and durations of hospitalized patients with COVID-19 in beijing: A retrospective cohort study. Cardiovascular Innovations and Applications. 2021;6(1):33–44. doi: 10.15212/CVIA.2021.0019. [DOI] [Google Scholar]