Abstract
In recent years, semiconductors have aroused great interest in connecting, observing and influencing the behavior of biological elements, and it is possible to use semiconductor-cell compound interfaces to discover new signal transduction in the biological field. Among them, III-V nitride semiconductors, represented by gallium nitride (GaN), are used as substrates to form semiconductor-biology interfaces with cells, providing a platform for studying the effects of semiconductors on cell behavior. The interfaces between GaN substrate and cells play an important role in detecting and manipulating cell behaviors and provide a new opportunity for studying cell behavior and developing diagnostic systems. Hence, it is necessary to understand how the properties of the GaN substrate directly influence the behavior of biological tissues, and to create editable biological interfaces according to the needs. This paper reviews the synergism between GaN semiconductors and biological cells. The electrical properties, persistent photoconductivity (PPC), nanostructures, and chemical functionalization of GaN on the promotion of cell behaviors, such as growth, adhesion, differentiation, and signal transduction, are emphatically introduced. The purpose of this study is to provide guidance to explore the detection and regulation methods of cell behavior based on semiconductors and promote the application of them in the field of bioelectronics, such as biochips, biosensors, and implantable systems.
Keywords: Semiconductor, GaN, Surface, Cell, Interface, Bioelectronics
1. Introduction
Studies in the latest several decades have made it obvious that besides structured metal and organic materials, semiconductors can impact cellular systems by affecting bioelectric and biomechanical components, enabling them to perform intricate functions. The field of bio-integrated electronics, optoelectronics, and microelectromechanical systems has showcased immense potential in capturing or manipulating optical, electrical, and mechanical signals within biological systems, ranging from individual cells to the entire body. For instance, gallium nitride (GaN) semiconductors have been used for the brain-computer interface to generate optical and electrical stimulations [1]. Within the realm of cell biology, the research on the effects of GaN on behaviors of various cells has been reported, which has shown its great potential in detecting and manipulating cell behaviors and developing various bioelectronics systems. Therefore, semiconductors have become an important part of biophysical tools and biomedical devices, and the semiconductor-biology interface, as the core of device applications, is particularly critical.
GaN, being a prominent member among third-generation semiconductor materials, possesses the characteristic of being a wide band gap semiconductor. As we know, GaN has many advantages, such as high electron mobility, large trapped surface charge density, good physical and chemical stability, and excellent biocompatibility, which show a great potential application prospect for GaN to be used in semiconductor-biology interfaces [2]. For example, GaN has been recognized as a new and excellent sensing material. The utilization of GaN-based biosensors has been observed in the detection of antibodies against various types of cancer, as well as in monitoring the action potentials of cardiomyocytes [[3], [4], [5]], sodium fluxes of nerve cells [6], and so on. In addition, excellent stability and biocompatibility of GaN in a liquid environment provide the basic conditions for research and application in biological tissues [7]. Some studies have shown that semiconductors (including GaN) can regulate the behaviors of biological molecular cells, such as quantifying different types of (bio)molecules, cells, and bacteria [8,9]. And the development of GaN-based acoustic tweezers, which have potential applications in manipulating particles and biological cells [10]. However, the underlying mechanisms through which semiconductors can influence cellular and subcellular biological processes are still not fully understood, making them elusive.
In this review, first, we will provide a concise overview of the composition and characteristics of GaN semiconductors. And then, we will discuss the effects of different surface properties of GaN semiconductors, including surface electrical properties, polarity, surface nanostructures, surface functionalization, and persistent photoconductivity, on cell behavior, such as growth and proliferation, adhesion and differentiation, apoptosis and migration, cell junction fusion, and expression of genetic material. Finally, we put forward the potential applications and research prospects of the GaN-biology interface, which can offer some references for further research.
2. Structure and properties of GaN
GaN films usually can be grown using various methods, such as hydride vapor phase epitaxy (HVPE), metal-organic chemical vapor deposition (MOCVD) and molecular beam epitaxy (MBE), showing a smooth surface at the atomic level [11]. Because the positive and negative charge centers of the Ga atom and the N atom do not coincide, a polarized electric field up to about 3 MV/cm is formed on the surface. Thus, the Ga-face of GaN carries a negative bound charge, while the N-face exhibits an equivalent positive bound polarization charge. In addition, the doping type of GaN also affects its surface electrical properties. When GaN is n-type doped, its surface will be negatively charged. On the contrary, when GaN is p-type doped, its surface will be positively charged [12].
The chemical property of GaN is very stable and almost insoluble in water, acid, and alkali solutions at room temperature and pressure, except for an electrochemical environment [13,14]. Under high-temperature conditions, concentrated phosphoric acid, concentrated sulfuric acid or molten caustic soda will etch the GaN surface [[15], [16], [17]]. Specific surfaces can be constructed by physical or chemical modification of GaN surfaces, which provides a way to form semiconductor-biology interfaces with different properties and applications. Especially, GaN exhibits excellent biocompatibility and non-toxic characteristics, positioning it as a highly promising choice for various biomedical applications [18,19]. A research study discovered that when exposed to aqueous environments, functionalized and etched GaN surfaces displayed remarkable stability and released an insignificant amount of Ga into the solution, even in the presence of hydrogen peroxide [18]. Another study evaluated the biocompatibility of GaN through cell viability, adhesion and growth and found that there was no cytotoxicity of GaN and the cells showed good adhesion and extensibility on GaN [20].
3. The effects of GaN on cell behaviors
GaN surfaces possess several unique properties, including electrical conductivity, persistent photoconductivity (PPC), nanostructures, and functionalizable surfaces. As shown in Fig. 1, each of these properties can profoundly influence various cellular behaviors, including cell differentiation, proliferation, migration, and adhesion. Firstly, the electrical conductivity of GaN surface can affect cell behavior by promoting proliferation. Conductive surfaces can improve cellular attachment and spreading, leading to enhanced cellular interactions and increased cellular activity. Secondly, the PPC of GaN surface can provide noninvasive electrical stimulation to enhance cell adhesion, change the concentration of intracellular calcium ions and even trigger a cell wall integrity pathway response. Thirdly, GaN's nanostructures allow for the creation of complex topographies that can regulate cellular adhesion and differentiation and protein adsorption. Finally, surface functionalization of GaN with bioactive molecules can enhance the biocompatibility of the material, promoting cellular adhesion, proliferation, and differentiation. Functionalizing GaN surfaces can also be used to create specific binding sites for cells and biomolecules, allowing for precise control over cell growth and adhesion.
Fig. 1.
The schematic of the effects of electrical conductivity, persistent photoconductivity (PPC), nanostructures, and functionalizable surface of GaN on cell behaviors.
3.1. Surface electrical properties of GaN
The surface electrical characteristics of GaN are primarily influenced by multiple factors. These factors encompass band bending at the surface, the type of doping (n-type and p-type), spontaneous polarization arising from an asymmetric distribution of positive and negative charge centers, and piezoelectric polarization induced by epitaxial growth. These effects collectively contribute to the overall surface electrical properties of GaN.
GaN has the ability to deliver electrical stimulation, which can facilitate cell adhesion, proliferation, growth, migration, and differentiation [21,22]. Tai-Horng Young et al. has demonstrated the successful use of GaN to culture rat cerebellar granule neurons in vitro, establishing GaN's excellent biocompatibility and non-toxicity towards neuronal cells [23]. During a 6-day culture period, it was observed that GaN facilitated the response of cerebellar granule neurons, leading to enhanced cell differentiation and neuritic growth. As shown in Fig. 2(a), when cultured on the surface of n-type GaN, cerebellar granule neurons exhibited the formation of neural process networks with near-normal adhesion and neurite development. Fig. 2(b) illustrates that culturing cerebellar granule neurons on the surface of p-type GaN led to a reduction in neurite formation but an observable increase in cell aggregation. In the case of neurons cultured on the surface of Si (as shown in Fig. 2(c)), a similar cellular behavior was observed. Neuron clusters were observed when neurons were cultured separately on the surface of p-type GaN and Si. As is shown in Fig. 2(d), for tissue culture polystyrene (TCPS), the shape of cell aggregates is closer to a circle than that on the surface of Si and p-type GaN. However, neurons cultured on GaN showed a very dense neural process network compared to Si and TCPS substrates, indicating that GaN can promote nerve cell to grow, adhere, and even differentiate. They speculated that the mechanism is that Ga atoms on the surface of GaN exhibit electron deficiency due to the polarity induced by the Ga–N bond, resulting in significant Lewis acidity and reactivity towards various Lewis bases for the outer Ga atoms. This provides an environment conducive to the growth of neurons for the negatively charged sulfuric acid and carboxylic acid groups in glycosaminoglycans.
Fig. 2.
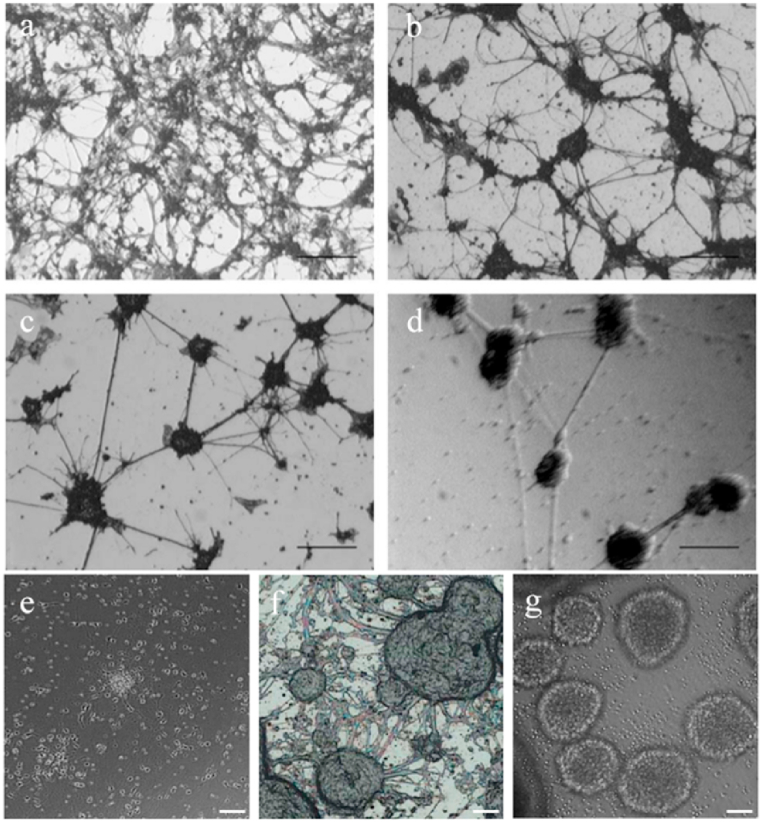
Different types of substrates have different effects on cell growth. The optical microscope photographs of cerebellar granule neurons on (a) n-type GaN, (b) p-type GaN, (c) Si, and (d) TCPS for culturing 3 days (scale bar = 100 μm) [23]. The optical microscope photographs of neurospheres on (e) PDL, (f) GaN, and (d) TCPS for culturing 7 days (scale bar = 50 μm) [24].
Then Chi-Ruei Chen et al. [24] used GaN to induce and culture neural stem cells/progenitor cells (NSPC). As shown in Fig. 2(e and f), cells on GaN grew more neural networks than poly-d-lysine (PDL) and TCPS, and cell proliferation was more obvious. This result demonstrated that GaN could significantly improve cell survival, promote NSPC to differentiate into neurons and astrocytes and induce NSPC to differentiate into mature neurons. In addition, the study indicated that the inhibition of glycogen synthase kinase-3b (GSK-3b) activity facilitated neuronal differentiation when using GaN as a substrate. They speculated that the surface electrical properties of GaN could affect NSPC signal transduction, thus promoting the differentiation of NSPC.
Chi-Ruei Chen et al. [25] used n- and p-type GaN substrates to prove that cerebellar granule neurons cultured for a long time on GaN are larger and can survive better than on silicon or tissue-cultured polystyrene, and can promote and induce neuronal aggregation and axonal growth. In their research, it was discovered that GaN exhibited a strong ability to activate Akt phosphorylation within the first 20 h of culture. Additionally, GaN demonstrated simultaneous promotion of cell adhesion, induction of neurite outgrowth in both serum-dependent and serum-independent interactions, and maintenance of a neuron-friendly environment for axonal growth and synaptogenesis with sustained benefits over an extended period. Therefore, GaN is expected to replace and solve the problem of poor biocompatibility of neural chips that stimulate and collect signals from cultured neurons due to silicon substrate.
In addition, it is possible to regulate the surface polarity of GaN by manipulating the magnitude and direction of spontaneous polarization (SP) and piezoelectric polarization (PE) [26]. To confirm the modulation of GaN film polarity, researchers often employ Annular Bright-Field (ABF) and High-Angle Annular Dark Field (HAADF) scanning transmission electron microscopy (STEM) [27]. Chenguang Zhang et al. [27] have investigated to control the direction and magnitude of SP and PE of GaN layer to enhance bone regeneration. The results showed that endogenous electrical stimulation can promote and induce the differentiation and proliferation of bone cells. They also revealed that surfaces with Ga-polarity, carrying a negative charge, have a substantial positive impact on the growth, proliferation, adherence, and osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs), compared with positively charged N-polar surfaces. Their research was consistent with the reported studies that BMSCs can be attracted and directed towards the defect area via galvanotaxis effects through the induction of an electric field [28,29]. They speculated that the surface charge of GaN may be the electrical microenvironment for the osteogenic behavior of the protein. The electrical stimulation-induced osteogenic differentiation process may involve critical roles of the FA, ECM-receptor interaction, TGFb, and PI3K-Akt signaling pathways. In addition, different concentrations of Ga ions also play a potential role in regulating osteoblast osteoblast-induced ossification, which can increase the activity of alkaline phosphatase and promote the formation of calcium nodules, which provides a promising strategy for the treatment of osteoporosis [30].
In conclusion, GaN has been used in monitoring cellular electrical signals recently [7,31], which has the capability to concurrently stimulate, manipulate, and record cellular bioelectrical activities in both laboratory and living organisms [32,33]. The studies presently demonstrated the potential of GaN as a new generation of electronic biomaterials and devices for the treatment of neurological diseases and the development of neuron chip systems.
3.2. Persistent photoconductive effects of GaN
It is widely known that electrical signals play an important role in biological cells, tissues, or organs. For example, electrical stimulus can promote and direct the orientation of biological nerve processes. In vitro studies published recently have made use of many materials to promote the electrical stimulation of organisms [35]. As the most widely used semiconductor with photoelectric properties, silicon provides a way to study the specific behavior of cells [36]. However, silicon semiconductor needs a light source to continuously irradiate the surface to generate persistent surface charges. In addition, its surface is easy to be oxidized to form a dense silicon dioxide layer, which will also affect the interface charge transport. By utilizing semiconductor materials with persistent photoconductivity (PPC), the requirement for a light source and special conditions during stimulation can be eliminated, showing potential application prospects in the influence of cell behavior [37,38].
GaN is one of the semiconductors with persistent photoconductivity, which can produce instantaneous photoinduced current under ultraviolet illumination, showing an increase in conductivity and can last for hours and days [39,40]. Both n- and p-type GaN have persistent photoconductivity. Studies have shown that the surface potential of n- and p-type GaN is respectively positive and negative when it is not illuminated by light. After being exposed to light, the surface potential of both n-type and p-type GaN increases due to the accumulation of minority carriers, resulting in a higher surface photovoltage. Specifically, the positive and negative charges of n-type and p-type GaN increase correspondingly. The results show that, compared with p-type GaN, the conductivity and surface potential of the n-type GaN exhibit more significant changes under illumination [34].
Patrick J. Snyder et al. [39] have investigated modulating the behavior of PC12 cells by combining three parameters of GaN surface roughness in different regions, surface functional groups, and conductivity with or without ultraviolet illumination. It was found that the metabolic rate and proliferation of rough surfaces were considerably greater when compared to smooth surfaces. By manipulating the GaN pattern size, roughness, and surface chemistry, the adhesion of PC12 cells can be altered when the GaN becomes more conductive through exposure to ultraviolet light. It is proved that the PPC in GaN can enhance cell adhesion, which is consistent with the surface roughness and the existence of specific functional groups and is beneficial to the biological interface. In addition, it has been reported that during the observation of the behavior of PC12 cells cultured in vitro with n-type GaN with PPC, changes in the concentration of intracellular calcium ions were found [34]. As shown in Fig. 3, the PPC of GaN can guide PC12 cells at specific surface locations, indicating that PPC can be non-invasive stimulation of PC12 cells. However, the exact mechanism and details of PPC to stimulate neuronal and neurotypical cells are still unknown.
Fig. 3.
The persistent photoconductive effect of GaN promotes the adsorption and stimulation of cells. Illustration of the noninvasive in vitro stimulation approach employed for PC12 cells [34].
The effects of GaN PPC on biological behavior were further investigated in a separate report by Patrick J. Snyder et al. [41] The n-type GaN with different doping levels and combined with phosphonic acid groups was used to observe the effects on the survival rate, intracellular reactive oxygen species, and calcium concentration of PC12 cells. It was found that PPC could enhance the chemical functionalization of phosphonic acid groups on the GaN surface by increasing the surface potential. And the sample doped with a high carrier concentration can better cause the change of calcium ions. Moreover, the synergistic effect of chemical functionalization and PPC can result in the surface passivation of GaN. This process helps shield the Ga and N atoms near the surface, minimizing the generation of reactive oxygen species (ROS). Consequently, it enhances direct cellular charge transfer from the material to the cell, leading to an additive effect that promotes cell stimulation. In addition, n-type Ga polar GaN film was employed to induce the reaction of Baker's yeast (the model organism of synthetic biology). It was found that surface chemistry and semiconductor surface charge directly affected the population of cells and the dimensions of yeast clusters. PPC could cause surface charge to accumulate, which induced a cellular response in the cell wall integrity pathway, resulting in the reduction of chitin produced by yeast [42]. Of course, in addition to cells, bacteria also have corresponding behavioral responses to the GaN for PPC, such as Gram-negative bacteria Escherichia coli [43,44]. On the surface of GaN with different surface potentials, the structure of Escherichia coli including pili, flagella, reactive oxygen species, and membrane potential changed to some extent, and bacterial adhesion was also enhanced. In short, GaN-induced responses can lead to changes in signal pathways, and these signal pathways can be further studied by monitoring the changes in ion fluxes, which also indicates that GaN has a potential prospect in bioelectronic communication. Moreover, cutting-edge research has leveraged the exceptional photoelectric properties of GaN in unlabeled and real-time cell sensing, achieving unprecedented levels of sensitivity and responsiveness. This novel approach enables the determination of intercellular and intracellular kinetics by precisely detecting cell adhesion and morphological changes, providing invaluable insight into cellular behavior and functions [45].
Therefore, the PPC of GaN semiconductors can be used to realize electrical stimulation of biological cells without electrodes or external current input. In addition, dose-dependent stimulation can be achieved by changing the type of GaN substrate, combined with substrate surface roughness and chemical functionalization, which is a unique bioelectronic interface for future bioelectronic devices. It provides an excellent prospect and application potential to detect and control cell behavior.
3.3. Surface nanostructures of GaN
In cell culture, the most commonly used substrate material is relatively flat and smooth plastic or glass. However, with the development of cell culture technology and mechanical biology research, it has been found that the surface-specific morphology at the nanometer or micrometer level plays a key role in cell behavior. For example, just changing the surface morphology, elasticity, or roughness of the substrate can induce different proliferation and differentiation of stem cells [46,47].
Different surface nanostructures of GaN can also be achieved through a variety of etching methods, such as dry etching [48], wet etching [49], and photoelectrochemical etching [50], etc. GaN surface nanostructures can regulate the hydrophilicity and hydrophobicity which can be determined by measuring the contact angle and then affecting the biological behaviors of cells on the surface [51].
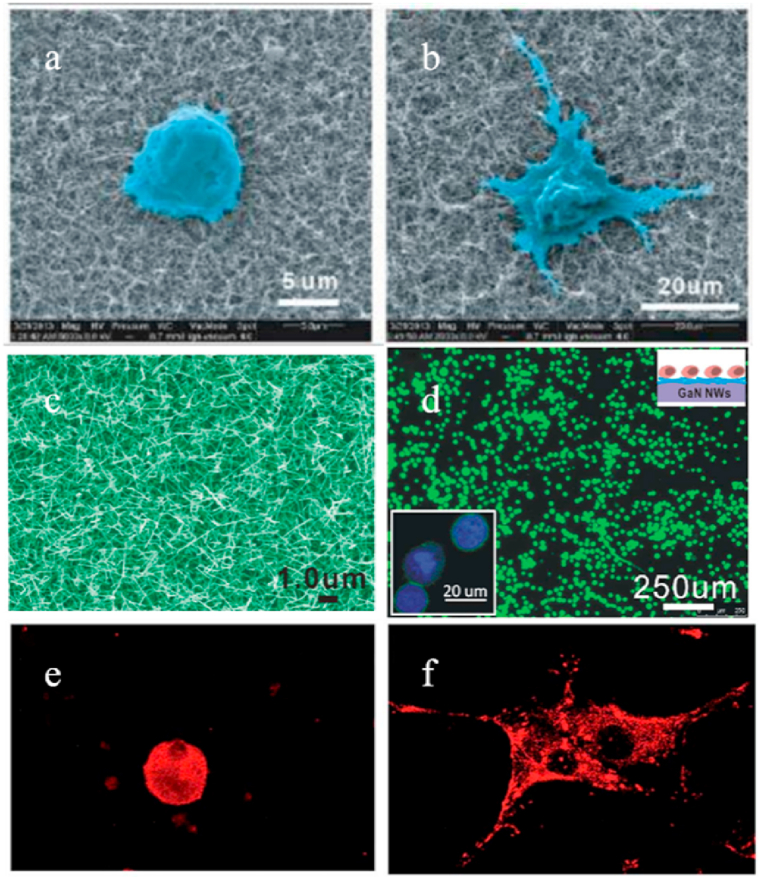
Lauren E. Bain et al. [52] fabricated different GaN surfaces to modify the interfaces between GaN and cells. As shown in Fig. 4(a–g), as-grown planar, unidirectionally polished, and etched nanoporous GaN and vertical GaN nanowire array were used to culture PC12 cells, respectively. They discovered that cells exhibited a higher affinity for roughened surfaces compared to flat, as-grown surfaces. Moreover, the surface roughness influenced the morphology of cellular adhesion and differentiation in neurotype cells. It was also found that the different nanostructure of the GaN surface will influence the cell differentiation morphology although they have similar RMS roughness. For example, a significant portion of the cell population underwent differentiation on the polished surfaces, which have unidirectionally aligned troughs with barriers on either side, exhibited the characteristic elongated and slender extensions typically observed during neuronal development. In contrast, surfaces with a more irregular distribution of surface features, such as the etched surface, showed a higher degree of cell spreading. This can be attributed to the wider range of contact points available on these surfaces. Afterward, they continued their investigation by studying the interactions between common serum proteins and nanotextured GaN surfaces. These surfaces exhibited lateral steppes, as-grown spiral hillocks, as well as mechanically polished and photochemically etched features [53]. Fig. 4(h and i) clearly illustrated the contrasting surface morphologies. The hillock and etched surfaces exhibited more random and disordered features, whereas the lateral and polished surfaces displayed unidirectional ridges. They found that GaN with regular surface nanostructures, such as unidirectional surface features of lateral step and polished surface, appeared to enhance protein accessibility, resulting in a higher occurrence of protein extension events compared to the less ordered surface features of the hillock and etched GaN surfaces. This study indicated that surface nanostructures can affect the adsorption behavior of proteins on semiconductor materials. The mechanism may be related to the ordered or disordered steric hindrance related to the surface characteristics, which promotes the binding of proteins onto the surface with unidirectional characteristics of intermolecular spacing affected by the proximity of parallel ridges. In addition, GaN microrods with special structure can be internalized into cells because of their high aspect ratio, and excellent laser signals can be observed under intracellular conditions, which would hold the potential for applications such as cell labeling and tracking [54].
Fig. 4.
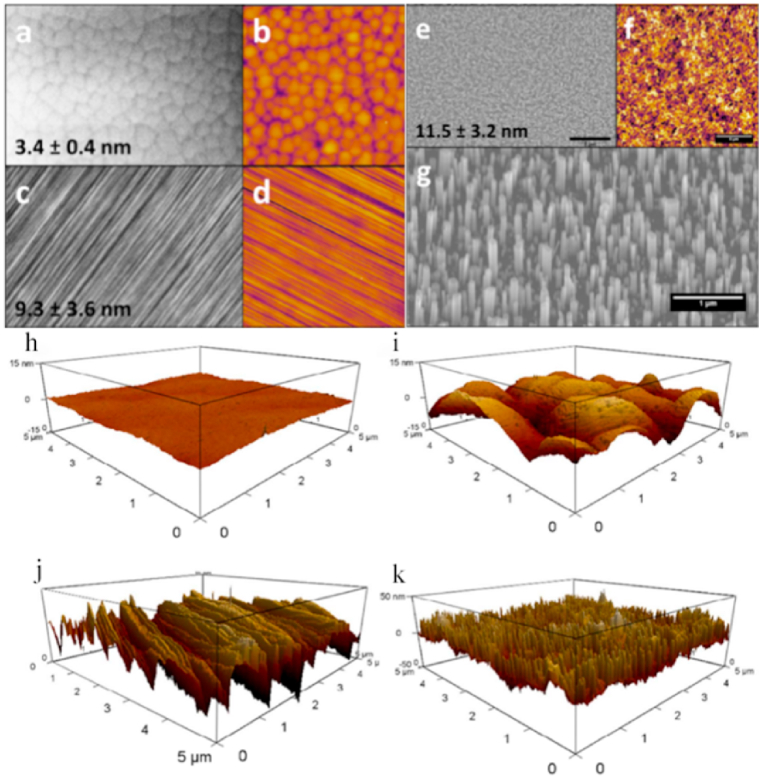
GaN can be etched into different nanostructures by different methods. (a) and (b) for planar surface, (c) and (d) for polished surface, and (e) and (f) for etched surfaces. (a), (c), (e), and (g) were SEM images. (b), (d), and (f) were AFM images. The values listed at the bottom left of the SEM images were RMS roughness. (g) The substrate is positioned at an angle To accurately represent the aspect ratios of the nanowires [52]. Four GaN surface morphologies were demonstrated using 3D AFM. (h)lateral steppes GaN, (i) spiral hillocks GaN, (j) mechanically polished GaN, and (k) photochemically etched GaN. Height range 30 nm in (h–j), 100 nm in (d) [53].
Compared with planar GaN, GaN nanowires (NWs) with lateral confinement below 100 nm have high aspect ratio, high surface area and single crystallinity, which shows its uniqueness in regulating cell behavior. For example, by adjusting the hydrophilicity and hydrophobicity of GaN NWs, the adhesion of cells and proteins can be adjusted. Jingying Li et al. studied the use of photo-responsive GaN NWs with a diameter of about 50 nm to regulate protein binding and cell adhesion [55]. They realized the transition from superhydrophobicity to super hydrophilicity of GaN NWs through ultraviolet illumination. The level of superhydrophilicity exhibited by the GaN NWs was determined by the duration of UV irradiation under ambient conditions, through which the behaviors of protein adsorption and cell adhesion can be modulated as shown in Fig. 5(a and b). Additionally, they observed that the quantity of protein adsorbed on the GaN NW surface was greater compared to that on the flat GaN surface due to a higher roughness for the GaN NW surface. Afterward, they successfully created a bifunctional peptide-aptamer conjugated modified NW platform for capturing, separating, and releasing cells based on the high specific surface area of GaN NWs. Fig. 5(c) displayed a representative SEM image showcasing a network of GaN NWs with an average diameter of approximately 50 nm covering the entire substrate. The fluorescence microscopy image (Fig. 5(d)) indicated that more MCF-7 cells can be captured on the GaN NWs than on that flat surface. The selective and efficient capture of cancer cells is achieved by combining NWs, surface-binding peptides, and cell-targeted aptamers. The platform can be recycled to capture and release specific cells. It has a potential application prospect in cell separation and detection [56].
Fig. 5.
SEM photographs of NIH 3T3 cells for culturing 48 h on GaN NWs surfaces (a) before and (b) after UV irradiation [55]. (c) The SEM photograph of the GaN NWs; (d) The fluorescence microscope photograph showing MCF-7 cells captured on the biofunctionalized GaN NWs depicted in (c) [56]. The confocal images illustrating the distribution of vinculin (red) in NIH 3T3 cells for culturing 48 h on GaN NWs (e) and flat surfaces (f) [57]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Rong Yang et al. successfully fabricated large-scale GaN NW network using the graphene oxide (GO) assisted CVD method [57]. They realized precise control over the morphology of GaN NWs by adjusting the ratio of GO to catalysts during the fabrication process. Unlike the vertical GaN nanowire array prepared by Lauren E. Bain et al., these GaN NWs with different nanostructures showed different superhydrophobic properties, and the adhesion of cells on them was also greatly reduced. Fig. 5(e) and (f) showed fluorescence images illustrating NIH 3T3 cells on both GaN NW and flat surfaces. The cultured cells were subjected to vinculin staining. On the hydrophobic GaN NW surface (Fig. 5(e)), cells appeared rounded and exhibited poor spreading, with no visible focal adhesions. This observation indicates that the nanostructure of the surface inhibited the formation of actin stress fibers and focal adhesions, resulting in weak cell-substrate adhesion, as supported by the low expression of vinculin. Conversely, on the hydrophilic flat GaN surface (Fig. 5(f)), cells displayed extensive spreading and well-formed vinculin-labeled focal adhesions.
GaN lateral polarity structures (LPS) structures can also be employed to regulate cell growth, adhesion and migration. Taking neural PC12 cells as an example [58], trends in cell responses to the N polar regions were more significant cells tended to be more responsive to N poles compared with the Ga polar regions. The alternating step height changes of Ga and N polar regions can affect cell growth, improve cell adhesion and promote cell migration along the direction of the LPS features. Patrick J. Snyder et al. [59] also found that the root means square (RMS) roughness values of the N-polar surface were significantly higher than that of the Ga polar surface, and more PC12 cells were attached to the N polar surface. This conclusion is the same as that obtained by the aforementioned Lauren E. Bain et al.
Different nanostructures of GaN can form different biological interfaces with cells, which can regulate the adhesion of cells to a certain extent, so as to realize the capture, separation and release of cells. These findings may provide new approaches for the regulation of biomolecules and cell behaviors at the GaN-biology interface, which will facilitate semiconductor-based diagnostic device design, implantable chips, and cell culture engineering.
3.4. Surface functionalization of GaN
GaN has garnered significant attention as a versatile component for chemical sensors and biosensors, thanks to its potential in various detection principles such as electrochemical, optical, and plasma-based methods. Meanwhile, GaN has good biocompatibility and can form physical or chemical covalent functionalization with a variety of organic molecules, proteins, peptides, and nucleic acids, which opens up fascinating prospects for GaN applications in the biological field. Therefore, it has emerged as a crucial research objective to modify the surface properties of GaN to adapt to different biological applications.
There have been many studies to modify the GaN surface by physical or chemical adsorption using atoms or molecules, such as magnesium [60], NH3 [61], octanethiol [62], hydrogen [63], H2O [64], fluorine (XeF2) [65] and aniline [66]. These substances form a specific interface with GaN to reduce the surface work function of GaN and regulate the interface barrier and passivate the surface, achieving high-performance electronic devices and optoelectronic devices. However, there has been a lack of systematic research on the biological aspects, especially the effects of GaN surface functionalization on cell behaviors. Presently, it has been reported that protein polypeptide [2,67,68] or some chemical groups, such as alkane [69] and phosphonate [[70], [71], [72]], can be used to physically or chemically functionalize GaN surface. For biological applications, the reported research mainly focused on changing the hydrophilicity and hydrophobicity of the GaN surface, controlling surface potential, or forming a GaN-biology interface structure.
The physical method for GaN surface functionalization is to simply drop compound solution onto the GaN surface and then dry it to form a compound layer on the GaN surface by physical adsorption. The chemical functionalization of the GaN surface is a way of forming covalent bonds between the adsorbed molecules and the GaN surface, usually using an olefin metathesis reaction. Previous research has demonstrated that through surface modification of GaN using sodium hydroxide, 3-aminopropyltriethoxysilane, and subsequent bioconjugation with 1-hydroxy collagen (T1HC), the chemically functionalized GaN exhibits remarkable cell culture properties. These properties include complete cell adhesion and viability, achieved by altering the surface morphology and surface chemistry of GaN [73].
However, presently there are few studies on the influence of GaN surface functionalization on cell behavior, mainly focusing on the affinity, adhesion and morphology of PC12 cells to functionalized GaN surfaces. For example, Matthew S. Makowski et al. demonstrated a technique for chemically modifying the GaN surface by employing olefin cross-metathesis with Grubbs first generation catalyst, which facilitated the covalent binding of hexylamine and a peptide [74,75]. The surface of functionalized GaN was stable in the aqueous environment. It was found that the polypeptide functionalized GaN surface exhibited a high affinity to PC12 cells and could further promote cell adhesion and growth. Fibronectin (FN) also has a similar effect [76], and even plays a significant role in using PC12 cells to simulate nerve cell differentiation [2,52,68]. Corey M. Foster et al. investigated the response of PC12 cells on GaN surfaces that were modified with peptides incorporating the IKVAV (isoleucine-lysine-valine-alanine-valine) motif [68]. This particular motif, derived from laminin, was deliberately selected for its ability to enhance cellular adhesion [77]. They compared the behaviors of PC12 cells on the GaN surface modified by peptides with different IKVAV motifs (P1, RP1, P1-IKVAV, IKVAV-P1, IKVAV (6) and IKVAV (19)). Most of the cells that adhered to the covalent functional surface maintained isolated or small monolayer aggregates, indicating that cell adhesion to the matrix could increase the branches of nerve processes. They also proved that GaN can retain the function of polypeptides when it recognizes that polypeptides and PC12 adhesion-promoting peptides IKVAV are part of the same peptide. Nora G. Berg et al. used ionizing radiation to investigate the stability of peptide functionalization in solution after chlorination of GaN surface, and proved that the surface composition of GaN materials would change after being exposed to ionizing radiation in solution, that is, the formation of peroxides [78]. The material was passivated, so biomolecules such as peptides did not dissociate easily from the GaN surface.
In short, the long-term stability of GaN modified by peptides and other biomolecules in the relevant water environment after covalent functionalization can have a stable and long-term special effect on cell behaviors, such as affinity, adhesion and morphology. Chemical functionalization serves as a compelling initial step in establishing the interface between molecular and biomolecule systems with GaN semiconductors, presenting a favorable avenue for exploration. Moreover, biomolecules and their combination with GaN semiconductors can give specific functions to biomedical science and chemical sensing technology, which will have potential functions in chip implantability research after evaluating GaN surface functionalization, and provide opportunities for new nano-texture GaN biomedical devices.
4. Conclusions and outlook
The excellent chemical stability of GaN in complex environments determines its excellent biocompatibility, which provides a necessary condition for the construction of the GaN-biology compound interface and an in-depth study of its effects on the biological behavior of cells. Researchers can change the surface electrical properties by controlling the doping type, doping concentration and polarity of GaN, or prepare various nano-topological structures, so as to realize the regulation of the growth and proliferation, adhesion and migration, apoptosis and differentiation of cells and the cell signal detection. Using the persistent photoconductivity of GaN, non-invasive electrical stimulation of cells can be realized to enhance cell adhesion and cause changes in intracellular calcium concentration. Chemical and biomolecular modification of GaN surface, such as hyaluronic acid (HA), protein, peptides, polysaccharides, and bionutrients or abiotic modifications, like hydrogels, conductive polymers, and even carbon nanotubes, can enhance the regulatory and mediating effects of these substances on biological cells. In addition, charge transfer between GaN-biology interface also has some effects on macromolecules and genetic materials expressed in biological cells, such as peptides, proteins, and nucleic acids, as well as cellular signal pathways.
The GaN-biology compound interface, because its interface interaction can regulate a variety of cell behaviors, shows great potential application value in the fields of biochips, biosensors, and implantable systems. For example, the size, shape, nanostructure, doping concentration, polarity, and other surface characteristics of GaN can be reliably controlled by semiconductor technology, combined with bioactive coating, optical treatment, surface functionalization, and other technologies, to enhance the influence of the interface on cell behavior, provide further support for cell growth and signal transduction, and reduce abnormal reactions in cell tissue. The different interfaces between GaN and various types of biological cells, tissues, and organs are expected to realize implantable biochips, biosensors, biological behavior research, and medical clinical application through this new biological interface. The ideal state in the future is to implant GaN into human tissue as part of the human implantable system to achieve auxiliary repair and treatment of clinical diseases. Therefore, it requires researchers to continue to explore new technologies and methods to develop a feasible GaN-biology compound interface with long-term functions.
The interlaced combination of these characteristics of GaN can also play a superimposed synergistic effect on biological cells, which provides diversity and richness for the semiconductor-biology interface of GaN. In addition, an effective biological interface requires a certain degree of long-term stability. And observing the signal pathways involved in biological cell responses, mutations that occur or may occur, and other types of biological genetic reactions that the biological interface may experience in the future, all need to be studied and identified, which are great challenges to the development of GaN-biology interface.
Although a great quantity of progress has been made in understanding the biological complex interface of GaN, because of the various characteristics of GaN semiconductor materials, there are still more places to be explored and exploited in the study of the effects on cells. For example, the exact mechanism of changing the hydrophilicity of GaN surface by light illumination and the charge transport process between electron-conducting GaN semiconductors and ion-conducting cells need to be further studied. Due to the limitations of the atomic and electronic characterization technology of this special interface and the complex mechanism of biological systems, this field is still very challenging, thus researchers need to be more involved in further research.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
First two authors contributed equally to this work. The authors gratefully acknowledge financial support of this work by the National Natural Science Foundation of China (61674164, 62164001, and 22004020), the Natural Science Foundation of Jiangxi Province (20202ZDB01018), the open project of the Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases Ministry of Education of China (XN201903), the startup funds of high-level talents of Gannan Medical University (QD201906 and QD202011), the school level project of Gannan Medical University (YB201942), and Special Fund for Graduate Innovation of Gannan Medical University (YC2021-S802).
Contributor Information
Weidong Zhao, Email: zhaowd@gmu.edu.cn.
Haijian Zhong, Email: hjzhong2007@gmu.edu.cn.
References
- 1.Ling W., Yu J., Ma N., et al. Flexible electronics and materials for synchronized stimulation and monitoring in multi-encephalic regions. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.202002644. [DOI] [Google Scholar]
- 2.Jewett S.A., Makowski M.S., Andrews B., Manfra M.J., Ivanisevic A. Gallium nitride is biocompatible and non-toxic before and after functionalization with peptides. Acta Biomater. 2012;8:728–733. doi: 10.1016/j.actbio.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 3.Kang B.S., Wang H.T., Lele T.P., Tseng Y., Ren F., Pearton S.J., Johnson J.W., Rajagopal P., Roberts J.C., Piner E.L., Linthicum K.J. Prostate specific antigen detection using AlGaN∕GaN high electron mobility transistors. Appl. Phys. Lett. 2007;91 doi: 10.1063/1.2772192. [DOI] [Google Scholar]
- 4.Chen K.H., Kang B.S., Wang H.T., Lele T.P., Ren F., Wang Y.L., Chang C.Y., Pearton S.J., Dennis D.M., Johnson J.W., Rajagopal P., Roberts J.C., Piner E.L., Linthicum K.J. c-erbB-2 sensing using AlGaN∕GaN high electron mobility transistors for breast cancer detection. Appl. Phys. Lett. 2008;92 doi: 10.1063/1.2926656. [DOI] [Google Scholar]
- 5.Steinhoff G., Baur B., Wrobel G., Ingebrandt S., Offenhäusser A., Dadgar A., Krost A., Stutzmann M., Eickhoff M. Erratum: recording of cell action potentials with AlGaN∕GaN field-effect transistors. Appl. Phys. Lett. 2006;89 doi: 10.1063/1.2219129. [DOI] [Google Scholar]
- 6.Gebinoga M., Silveira L., Cimalla I., et al. Nanosensors for label-free measurement of sodium ion fluxes of neuronal cells. Mater. Sci. Eng., B. 2010;169:182–185. doi: 10.1016/j.mseb.2009.12.053. [DOI] [Google Scholar]
- 7.Yu J., Jha S.K., Xiao L., Liu Q., Wang P., Surya C., Yang M. AlGaN/GaN heterostructures for non-invasive cell electrophysiological measurements. Biosens. Bioelectron. 2007;23:513–519. doi: 10.1016/j.bios.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y.-L., Chu B.H., Chen K.H., Chang C.Y., Lele T.P., Papadi G., Coleman J.K., Sheppard B.J., Dungen C.F., Pearton S.J., Johnson J.W., Rajagopal P., Roberts J.C., Piner E.L., Linthicum K.J., Ren F. Fast detection of a protozoan pathogen, Perkinsus marinus, using AlGaN/GaN high electron mobility transistors. Appl. Phys. Lett. 2009;94 doi: 10.1063/1.3153130. [DOI] [Google Scholar]
- 9.Kang B.S., Wang H.T., Ren F., Pearton S.J. Electrical detection of biomaterials using AlGaN/GaN high electron mobility transistors. J. Appl. Phys. 2008;104:8. doi: 10.1063/1.2959429. [DOI] [Google Scholar]
- 10.Sun C., Wu F., Wallis D.J., Shen M.H., Yuan F., Yang J., Wu J., Xie Z., Liang D., Wang H. Gallium nitride: a versatile compound semiconductor as novel piezoelectric film for acoustic tweezer in manipulation of cancer cells. IEEE Trans. Electron. Dev. 2020;67:3355–3361. doi: 10.1109/TED.2020.3002498. [DOI] [Google Scholar]
- 11.Yan Q., Kioupakis E., Jena D., Van de Walle C.G. First-principles study of high-field-related electronic behavior of group-III nitrides. Phys. Rev. B. 2014;90 doi: 10.1103/PhysRevB.90.121201. [DOI] [Google Scholar]
- 12.Zhong H., Xu K., Liu Z., et al. Charge transport mechanisms of graphene/semiconductor Schottky barriers: a theoretical and experimental study. J. Appl. Phys. 2014;115 doi: 10.1063/1.4859500. [DOI] [Google Scholar]
- 13.Gao F., Liu Q., Shi J., Li S. Recent progress in gallium nitride for photoelectrochemical water splitting. Nanowires-Recent Progress. 2020 doi: 10.5772/intechopen.92848. [DOI] [Google Scholar]
- 14.Morkoç H. John Wiley & Sons; 2009. Handbook of Nitride Semiconductors and Devices, Materials Properties, Physics and Growth. [DOI] [Google Scholar]
- 15.Hsu H.-C., Su Y.-K., Cheng S.-H., Huang S.-J., Cao J.-M., Chen K.-C. Investigation of etch characteristics of non-polar GaN by wet chemical etching. Appl. Surf. Sci. 2010;257:1080–1083. doi: 10.1016/j.apsusc.2010.08.017. [DOI] [Google Scholar]
- 16.Tautz M., Weimar A., Graßl C., Welzel M., Díaz Díaz D. Anisotropy and mechanistic elucidation of wet‐chemical gallium nitride etching at the atomic level. Phys. Status Solidi. 2020;217 doi: 10.1002/pssa.202000221. [DOI] [Google Scholar]
- 17.Visconti P H.D., Reshchikov M.A. Investigation of defects and surface polarity in GaN using hot wet etching together with microscopy and diffraction techniques. Mater. Sci. Eng., B. 2002;93:229–233. doi: 10.1016/S0921-5107(02)00011-9. [DOI] [Google Scholar]
- 18.Jewett S.A., Makowski M.S., Andrews B., Manfra M.J., Ivanisevic A. Gallium nitride is biocompatible and non-toxic before and after functionalization with peptides. Acta Biomater. 2012;8:728–733. doi: 10.1016/j.actbio.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 19.Foster C.M., Collazo R., Sitar Z., Ivanisevic A. Cell behavior on gallium nitride surfaces: peptide affinity attachment versus covalent functionalization. Langmuir. 2013;29:8377–8384. doi: 10.1021/la401503b. [DOI] [PubMed] [Google Scholar]
- 20.Kang Y.-T., Yen T.-J. 2010 3rd International Nanoelectronics Conference (INEC) IEEE; 2010. Evaluating biocompatibility of semiconductive gallium nitride, flat and nano-structured silicon chips by cell viability, adhesion and growth; pp. 811–812. [DOI] [Google Scholar]
- 21.Lane S.W., Williams D.A., Watt F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014;32:795–803. doi: 10.1038/nbt.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson A., Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 23.Young T.H., Chen C.R. Assessment of GaN chips for culturing cerebellar granule neurons. Biomaterials. 2006;27:3361–3367. doi: 10.1016/j.biomaterials.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Chen C.R., Li Y.C., Young T.H. Gallium nitride induces neuronal differentiation markers in neural stem/precursor cells derived from rat cerebral cortex. Acta Biomater. 2009;5:2610–2617. doi: 10.1016/j.actbio.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 25.Chen C.R., Young T.H. The effect of gallium nitride on long-term culture induced aging of neuritic function in cerebellar granule cells. Biomaterials. 2008;29:1573–1582. doi: 10.1016/j.biomaterials.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 26.Lytvyn P.M., Kuchuk A.V., Mazur Y.I., et al. Polarization effects in graded AlGaN nanolayers revealed by current-sensing and Kelvin probe microscopy. ACS Appl. Mater. Interfaces. 2018;10:6755–6763. doi: 10.1021/acsami.7b19160. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C., Wang W., Hao X., Peng Y., Zheng Y., Liu J., Kang Y., Zhao F., Luo Z., Guo J., Xu B., Shao L., Li G. A novel approach to enhance bone regeneration by controlling the polarity of GaN/AlGaN heterostructures. Adv. Funct. Mater. 2020;31 doi: 10.1002/adfm.202007487. [DOI] [Google Scholar]
- 28.Zhao M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009;20:674–682. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima K.I., Zhu K., Sun Y.H., Hegyi B., Zeng Q., Murphy C.J., Small J.V., Chen-Izu Y., Izumiya Y., Penninger J.M., Zhao M. KCNJ15/Kir4.2 couples with polyamines to sense weak extracellular electric fields in galvanotaxis. Nat. Commun. 2015;6:8532. doi: 10.1038/ncomms9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu M., Wang Y., Zhang Y., Cui D., Gu G., Zhao D. Gallium ions promote osteoinduction of human and mouse osteoblasts via the TRPM7/Akt signaling pathway. Mol. Med. Rep. 2020;22:2741–2752. doi: 10.3892/mmr.2020.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinhoff G., Purrucker O., Tanaka M., Stutzmann M., Eickhoff M. AlxGa1–xN—a new material system for biosensors. Adv. Funct. Mater. 2003;13:841–846. doi: 10.1002/adfm.200304397. [DOI] [Google Scholar]
- 32.Kirste R., Rohrbaugh N., Bryan I., Bryan Z., Collazo R., Ivanisevic A. Electronic biosensors based on III-nitride semiconductors. Annu. Rev. Anal. Chem. 2015;8:149–169. doi: 10.1146/annurev-anchem-071114-040247. [DOI] [PubMed] [Google Scholar]
- 33.Ning C Z.L., Tan G. Fourth-generation biomedical materials. Mater. Today. 2016;19:2–3. doi: 10.1016/j.mattod.2015.11.005. [DOI] [Google Scholar]
- 34.Snyder P.J., Reddy P., Kirste R., LaJeunesse D.R., Collazo R., Ivanisevic A. Noninvasive stimulation of neurotypic cells using persistent photoconductivity of gallium nitride. ACS Omega. 2018;3:615–621. doi: 10.1021/acsomega.7b01894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghasemi-Mobarakeh L., Prabhakaran M.P., Morshed M., et al. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Tissue Eng. Regener. Med. 2011;5:e17–e35. doi: 10.1002/term.383. [DOI] [PubMed] [Google Scholar]
- 36.Bareket-Keren L., Hanein Y. Novel interfaces for light directed neuronal stimulation: advances and challenges. Int. J. Nanomed. 2014;9(sup1):65–83. doi: 10.2147/ijn.s51193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeon S., Ahn S.E., Song I., Kim C.J., Chung U.I., Lee E., Yoo I., Nathan A., Lee S., Ghaffarzadeh K., Robertson J., Kim K. Corrigendum: gated three-terminal device architecture to eliminate persistent photoconductivity in oxide semiconductor photosensor arrays. Nat. Mater. 2015;14:452. doi: 10.1038/nmat4236. [DOI] [PubMed] [Google Scholar]
- 38.Sumanth A., Kolla L.G., Rao M.R., Dixit T. A review on realizing the modern optoelectronic applications through persistent photoconductivity. J. Phys. Appl. Phys. 2022;55 doi: 10.1088/1361-6463/ac7f66. [DOI] [Google Scholar]
- 39.Snyder P.J., Kirste R., Collazo R., Ivanisevic A. Persistent photoconductivity, nanoscale topography, and chemical functionalization can collectively influence the behavior of PC12 cells on wide bandgap semiconductor surfaces. Small. 2017;13 doi: 10.1002/smll.201700481. [DOI] [PubMed] [Google Scholar]
- 40.Dang X., Wang C., Yu E., Boutros K., Redwing J. Persistent photoconductivity and defect levels in n-type AlGaN/GaN heterostructures. Appl. Phys. Lett. 1998;72:2745–2747. doi: 10.1063/1.121077. [DOI] [Google Scholar]
- 41.Snyder P.J., Reddy P., Kirste R., LaJeunesse D.R., Collazo R., Ivanisevic A. Variably doped nanostructured gallium nitride surfaces can serve as biointerfaces for neurotypic PC12 cells and alter their behavior. RSC Adv. 2018;8:36722–36730. doi: 10.1039/C8RA06836D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snyder P.J., LaJeunesse D.R., Reddy P., Kirste R., Collazo R., Ivanisevic A. Bioelectronics communication: encoding yeast regulatory responses using nanostructured gallium nitride thin films. Nanoscale. 2018;10:11506–11516. doi: 10.1039/C8NR03684E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iyer D., Gulyuk A.V., Reddy P., Kirste R., Collazo R., LaJeunesse D.R., Ivanisevic A. Behavior of E. coli with variable surface morphology changes on charged semiconductor interfaces. ACS Appl. Bio Mater. 2019;2:4044–4051. doi: 10.1021/acsabm.9b00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gleco S., Noussi T., Jude A., Reddy P., Kirste R., Collazo R., LaJeunesse D., Ivanisevic A. Oxidative stress transcriptional responses of Escherichia coli at GaN interfaces. ACS Appl. Bio Mater. 2020;3:9073–9081. doi: 10.1021/acsabm.0c01299. [DOI] [PubMed] [Google Scholar]
- 45.Hou Y., Jing J., Luo Y., Xu F., Xie W., Ma L., Xia X., Wei Q., Lin Y., Li K.H., Versatile A., Incubator-Compatible Monolithic GaN photonic chipscope for label‐free monitoring of live cell activities. Adv. Sci. 2022;9 doi: 10.1002/advs.202200910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen W., Villa-Diaz L.G., Sun Y., Weng S., Kim J.K., Lam R.H., Han L., Fan R., Krebsbach P.H., Fu J. Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano. 2012;6:4094–4103. doi: 10.1021/nn3004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beduer A., Vieu C., Arnauduc F., Sol J.C., Loubinoux I., Vaysse L. Engineering of adult human neural stem cells differentiation through surface micropatterning. Biomaterials. 2012;33:504–514. doi: 10.1016/j.biomaterials.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 48.Sanders F.H.M., Dieleman J., Peters H.J.B., Sanders J.A.M. Selective isotropic dry etching ofSi3 N 4 overSiO2. J. Electrochem. Soc. 1982;129:2559. doi: 10.1149/1.2123608. [DOI] [Google Scholar]
- 49.Visconti P., Huang D., Reshchikov M.A., et al. Investigation of defects and surface polarity in GaN using hot wet etching together with microscopy and diffraction techniques. Mater. Sci. Eng., B. 2002;93:229–233. doi: 10.1016/S0921-5107(02)00011-9. [DOI] [Google Scholar]
- 50.Jayaprakash R., Kalaitzakis F.G., Kayambaki M., et al. Ultra-smooth GaN membranes by photo-electrochemical etching for photonic applications. J. Mater. Sci. 2014;49:4018–4024. doi: 10.1007/s10853-014-8071-0. [DOI] [Google Scholar]
- 51.Dzięcielewski I., Weyher J.L., Dzwolak W. On the hydrophobicity of modified Ga-polar GaN surfaces. Appl. Phys. Lett. 2013;102 doi: 10.1063/1.4790435. [DOI] [Google Scholar]
- 52.Bain L.E., Collazo R., Hsu S.H., Latham N.P., Manfra M.J., Ivanisevic A. Surface topography and chemistry shape cellular behavior on wide band-gap semiconductors. Acta Biomater. 2014;10:2455–2462. doi: 10.1016/j.actbio.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 53.Bain L.E., Hoffmann M.P., Bryan I., Collazo R., Ivanisevic A. Adsorption and adhesion of common serum proteins to nanotextured gallium nitride. Nanoscale. 2015;7:2360–2365. doi: 10.1039/c4nr06353h. [DOI] [PubMed] [Google Scholar]
- 54.Song M.S., Baek H., Lee K., Yoo D., Chung K., Lee J., Moon H.C., Lee B.H., Park H.Y., Yi G.-C. Intracellular gallium nitride microrod laser. NPG Asia Mater. 2021;13:32. doi: 10.1038/s41427-021-00299-8. [DOI] [Google Scholar]
- 55.Li J., Han Q., Zhang Y., Zhang W., Dong M., Besenbacher F., Yang R., Wang C. Optical regulation of protein adsorption and cell adhesion by photoresponsive GaN nanowires. ACS Appl. Mater. Interfaces. 2013;5:9816–9822. doi: 10.1021/am403070g. [DOI] [PubMed] [Google Scholar]
- 56.Li J., Qi C., Lian Z., Han Q., Wang X., Cai S., Yang R., Wang C. Cell-capture and release platform based on peptide-aptamer-modified nanowires. ACS Appl. Mater. Interfaces. 2016;8:2511–2516. doi: 10.1021/acsami.5b09407. [DOI] [PubMed] [Google Scholar]
- 57.Yang R., Zhang Y., Li J., Han Q., Zhang W., Lu C., Yang Y., Dong H., Wang C. Graphene oxide assisted synthesis of GaN nanostructures for reducing cell adhesion. Nanoscale. 2013;5:11019–11027. doi: 10.1039/C3NR02770H. [DOI] [PubMed] [Google Scholar]
- 58.Bain L.E., Kirste R., Johnson C.A., Ghashghaei H.T., Collazo R., Ivanisevic A. Neurotypic cell attachment and growth on III-nitride lateral polarity structures. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;58:1194–1198. doi: 10.1016/j.msec.2015.09.084. [DOI] [PubMed] [Google Scholar]
- 59.Snyder P.J., Kirste R., Collazo R., Ivanisevic A. Nanoscale topography, semiconductor polarity and surface functionalization: additive and cooperative effects on PC12 cell behavior. RSC Adv. 2016;6:97873–97881. doi: 10.1039/C6RA21936E. [DOI] [Google Scholar]
- 60.Bermudez V.M. Study of the growth of thin Mg films on wurtzite GaN surfaces. Surf. Sci. 1998;417:30–40. doi: 10.1016/S0039-6028(98)00659-1. [DOI] [Google Scholar]
- 61.Bermudez V.M. Chemisorption of NH3 on GaN(0001)-(1×1) Chem. Phys. Lett. 2000;317:290–295. doi: 10.1016/S0009-2614(99)01404-9. [DOI] [Google Scholar]
- 62.Bermudez V.M. Adsorption of 1-octanethiol on the GaN (0001) surface. Langmuir. 2003;19(17):6813–6819. doi: 10.1021/la030032a. [DOI] [Google Scholar]
- 63.Bermudez V.M. Theoretical study of hydrogen adsorption on the GaN (0001) surface. Surf. Sci. 2004;565:89–102. doi: 10.1016/j.susc.2004.06.209. [DOI] [Google Scholar]
- 64.Bermudez V.M., Long J.P. Chemisorption of H2O on GaN (0001) Surf. Sci. 2000;450:98–105. doi: 10.1016/S0039-6028(00)00051-0. [DOI] [Google Scholar]
- 65.Bermudez V.M. Investigation of the initial chemisorption and reaction of fluorine (XeF2) with the GaN (0001) -(1×1) surface. Appl. Surf. Sci. 1997;119:147–159. doi: 10.1016/S0169-4332(97)00190-6. [DOI] [Google Scholar]
- 66.Bermudez V.M. Functionalizing the GaN (0001)-(1×1) surface I. The chemisorption of aniline. Surf. Sci. 2002;499:109–123. doi: 10.1016/S0039-6028(01)01916-1. [DOI] [Google Scholar]
- 67.Estephan E L.C., Cuisinier F.J.G. Tailoring GaN semiconductor surfaces with biomolecules. Phys. Chem. B. 2008;112:8799–8805. doi: 10.1021/jp804112y. [DOI] [PubMed] [Google Scholar]
- 68.Foster C.M., Collazo R., Sitar Z., Ivanisevic A. Cell behavior on gallium nitride surfaces: peptide affinity attachment versus covalent functionalization. Langmuir. 2013;29:8377–8384. doi: 10.1021/la401503b. [DOI] [PubMed] [Google Scholar]
- 69.Dzięcielewski I., Weyher J.L., Dzwolak W. On the hydrophobicity of modified Ga-polar GaN surfaces. Appl. Phys. Lett. 2013;102 doi: 10.1063/1.4790435. [DOI] [Google Scholar]
- 70.Simpkins B.S., Hong S., Stine R., Mäkinen A.J., Theodore N.D., Mastro M.A., Eddy C.R., Pehrsson P.E. Assembly of phosphonic acids on GaN and AlGaN. J. Phys. Appl. Phys. 2009;43 doi: 10.1088/0022-3727/43/1/015303. [DOI] [Google Scholar]
- 71.Wilkins S.J., Paskova T., Ivanisevic A. Modified surface chemistry, potential, and optical properties of polar gallium nitride via long chained phosphonic acids. Appl. Surf. Sci. 2015;327:498–503. doi: 10.1016/j.apsusc.2014.11.179. [DOI] [Google Scholar]
- 72.Snyder P.J., Davis H., Berg N.G., Pearce B., Romanyuk O., Jiricek P., Paskova T., Ivanisevic A. Passivation of semipolar (10-1-1) GaN with different organic adsorbates. Mater. Lett. 2019;236:201–204. doi: 10.1016/j.matlet.2018.10.109. [DOI] [Google Scholar]
- 73.Mishra M., Sharan J., Koul V., Kharbanda O.P., Kumar A., Sharma A., Hackett T.A., Sagar R., Kashyap M.K., Gupta G. Surface functionalization of gallium nitride for biomedical implant applications. Appl. Surf. Sci. 2023;612 doi: 10.1016/j.apsusc.2022.155858. [DOI] [Google Scholar]
- 74.Makowski M.S., Zemlyanov D.Y., Lindsey J.A., Bernhard J.C., Hagen E.M., Chan B.K., Petersohn A.A., Medow M.R., Wendel L.E., Chen D., Canter J.M., Ivanisevic A. Covalent attachment of a peptide to the surface of gallium nitride. Surf. Sci. 2011;605:1466–1475. doi: 10.1016/j.susc.2011.05.015. [DOI] [Google Scholar]
- 75.Makowski M.S., Zemlyanov D.Y., Ivanisevic A. Olefin metathesis reaction on GaN (0001) surfaces. Appl. Surf. Sci. 2011;257:4625–4632. doi: 10.1016/j.apsusc.2010.12.100. [DOI] [Google Scholar]
- 76.Li J., Han Q., Wang X., Yang R., Wang C. Enhanced cell growth on nanotextured GaN surface treated by UV illumination and fibronectin adsorption. Colloids Surf. B Biointerfaces. 2014;123:293–301. doi: 10.1016/j.colsurfb.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 77.Tashiro K., Sephel G.C., Weeks B., et al. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J. Biol. Chem. 1989;264:16174–16182. doi: 10.1016/S0021-9258(18)71604-9. [DOI] [PubMed] [Google Scholar]
- 78.Berg N.G., Nolan M.W., Paskova T., Ivanisevic A. Surface characterization of gallium nitride modified with peptides before and after exposure to ionizing radiation in solution. Langmuir. 2014;30:15477–15485. doi: 10.1021/la5040245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.