Summary
Developing an effective therapy to overcome carbapenemase-positive Klebsiella pneumoniae (CPKp) is an important therapeutic challenge that must be addressed urgently. Here, we explored a Ca-EDTA combination with aztreonam or ceftazidime-avibactam in vitro and in vivo against diverse CPKp clinical isolates. The synergy testing of this study demonstrated that novel aztreonam-Ca-EDTA or ceftazidime-avibactam-Ca-EDTA combination was significantly effective in eliminating planktonic and mature biofilms in vitro, as well as eradicating CPKp infections in vivo. Both combinations revealed significant therapeutic efficacies in reducing bacterial load in internal organs and protecting treated mice from mortality. Conclusively, this is the first in vitro and in vivo study to demonstrate that novel aztreonam-Ca-EDTA or ceftazidime-avibactam-Ca-EDTA combinations provide favorable efficacy and safety for successful eradication of carbapenemase-producing Klebsiella pneumoniae planktonic and biofilm infections.
Subject areas: Health sciences, Medicine, Medical specialty, Immunology, Medical microbiology, Biological sciences, Microbiology
Graphical abstract
Highlights
-
•
Carbapenemase-positive Klebsiella pneumoniae (CPKp) possess therapeutic challenges
-
•
Ca-EDTA paired with aztreonam/ceftazidime-avibactam can eliminate CPKp in vitro
-
•
These combinations reduce bacterial load, but they also protect mice from mortality
-
•
Promising efficacy and safety have been demonstrated with these combinations
Health sciences; Medicine; Medical specialty; Immunology; Medical microbiology; Biological sciences; Microbiology
Introduction
Because of their ability to exchange resistance genes and their multidrug resistance phenotypes, the dissemination of carbapenemase-producing Klebsiella pneumoniae (CPKp) poses a substantial threat to public health.1,2,3,4,5 CPKp are a prominent cause of nosocomial infections associated with high morbidity.3 Metallo-β-lactamases (MBLs) are prevalent among carbapenemase-producing Enterobacteriaceae (CPE) worldwide, and in many countries, they are more common than Klebsiella pneumoniae carbapenemase (KPC).3,6,7 In some places, the increased use of ceftazidime–avibactam has resulted in MBLs eclipsing KPC as the most frequent carbapenemase.3,6,7 The hydrolysis of β-lactams by MBLs is catalyzed by zinc ions, and MBLs are not inhibited by commercially available β-lactamase inhibitors (BLIs), such as diazabicyclooctane (avibactam and relebactam) or cyclic boronic acid (vaborbactam).6,8 Biofilm formation is crucial for CPKp colonization, causing persistence in the host and antibiotic tolerance.2,6,7,9 Treating infections caused by MBL-producing Klebsiella is an important and continuing therapeutic requirement.
Currently, aztreonam is the only monobactam used clinically.10 Aztreonam is not hydrolyzed by MBLs because of its weak and unproductive interaction with MBLs.10,11 Many bacterial strains that produce MBLs also produce other beta-lactamases, such as extended-spectrum β-lactamase (ESBL) or AmpC β-lactamase, which can hydrolyze aztreonam.8,10,11 Moreover, biofilm formation contributes significantly to aztreonam tolerance.2,11,12 Nevertheless, some studies have examined the utility of aztreonam in combination with ceftazidime–avibactam.8,11,13,14 A combination of aztreonam and ceftazidime–avibactam has shown significant antibacterial synergy against NDM-producing K. pneumoniae, other Enterobacterales, and Stenotrophomonas maltophilia, and the combination is finding more widespread use clinically.8,11,13,14 However, only limited data are available on the effectiveness of this treatment for K. pneumoniae biofilm-associated infections. There is a danger that unindicated extensive use of this combination may lead to the development of tolerance and resistance. Because there are no alternatives in the antibiotic treatment armamentarium and reliance on this combination seems unavoidable, reducing the disparity between the risk of resistance and developing new antibiotics requires innovative use of existing therapeutic options. To achieve this, the documented ability of ethylenediamine tetra-acetic acid (EDTA) to improve the potency of various antibiotics against gram-negative pathogens provides an attractive therapeutic opportunity.2,7,9,15 EDTA enhances bacterial outer membrane permeability and releases lipopolysaccharides (LPS), which improves antibiotic potency by facilitating their ability to penetrate bacterial walls, enabling the antibiotics to reach their intended targets.7,9,16,17,18 EDTA has shown antibiofilm activity by disrupting the biofilm matrix, increasing the ability of existing antibiotics to eradicate bacteria in mature biofilms.7,9,16 EDTA has potent bactericidal activity against inner cells of biofilms that have lower metabolic activity.7,9 Our recent studies on EDTA demonstrate potential used of EDTA as adjuvant by disrupting the biofilm matrix and increasing the effectiveness of colstin for eradicating mature biofilms on colistin-resistant K. pneumoniae (ColRkp).7,9 Moreover, the combination of colistin and EDTA has been found to have powerful synergistic effects against XDR and PDR ColRkp isolates. This is because of the EDTA ions' sequestration ability to increase the permeability of the bacterial outer membrane, which sensitizes and synergizes with colistin to increase its effectiveness.7,9 The chelation of EDTA can also enhance the entry of colistin into bacteria by blocking intracellular targets of colistin, such as essential respiratory enzymes, thereby overcoming colistin resistance regardless of the underlying mechanism.7,9,16,17,18,19,20,21,22 Several other studies also demonstrated the ability of Ca-EDTA-imipenem to clear NDM-1-E. coli and IMP/VIM-P. aeruginosa infections.17,18,19,20,21 In our previous study, we utilized EDTA as an antibiotic lock therapy.7,9 However, EDTA can cause renal toxicity, especially in patients with pre-existing kidney disease or those receiving high doses of the drug.18,19 Furthermore, EDTA can bind to crucial minerals, leading to deficiencies resulting in health issues like anemia, cardiac arrhythmias, and neurological disorders. As a result, it is recommended to use intravenous Ca-EDTA, which is less potent but safer for patients.17,18 Therefore, Ca-EDTA could be used as an potential adjuvant in ceftazidime–avibactam or aztreonam combinations to counteract CPKp.
Results
Ca-EDTA combination with aztreonam or ceftazidime/avibactam displays synergistic activity against CPKp clinical isolates
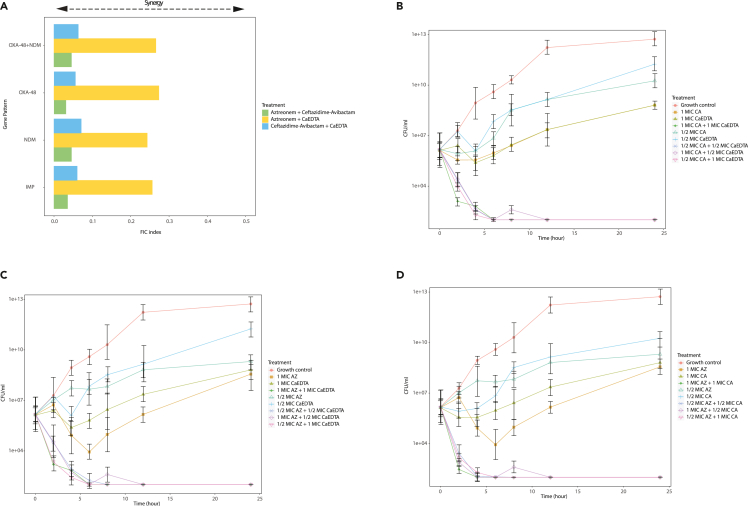
Clinical characteristic, carbapenems susceptibility, phenotype, and gene pattern of CPKp clinical isolates used in this study summarized in Table 1. We did not observe any signs of growth in any planktonic CPKp clinical isolate in the presence of 6–24 mg/mL Ca-EDTA (Table 2). Both Ca-EDTA + ceftazidime–avibactam (0.375 mg mL−1 + 0.5 μg mL−1/) and Ca-EDTA + aztreonam (0.75–0.375 mg mL−1 + 32 μg mL−1) showed potent synergistic activity (FICI ≤0.5) against various CPKp clinical isolates (Figure 1). Compared with ceftazidime–avibactam, aztreonam, or Ca-EDTA alone, Ca-EDTA combined with ceftazidime–avibactam or aztreonam showed a ≥3 log reduction in CFUs within 2 h (Figure 1). It is noteworthy that Ca-EDTA (3–6 mg mL−1) combined with ceftazidime–avibactam (2 μg mL−1) or aztreonam (2 μg mL−1) was able to eradicate mature biofilms derived from CPKp clinical isolates (p < 0.05). Ca-EDTA combined with ceftazidime–avibactam or aztreonam reduced the MBEC by > 1000-fold (Table 2). A similar effect against planktonic cells and biofilms was observed for ceftazidime–avibactam combined with aztreonam (Table 1) (Figure 1).
Table 1.
Clinical characteristic, carbapenems susceptibility, phenotype, and gene pattern of K. pneumoniae clinical isolates (n = 29)
| No. | Specimen | Sex | Age (Years) | Carbapenems susceptibility test |
Phenotype of |
Gene pattern | Pattern of entire of bla gene by sequencing | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMP | MEM | ETP | DOR | Carbapenemases | MBLs | KPC | Biofilm formation category | ||||||
| U17 | Urine | – | – | R | R | R | NT | + | + | – | IMP | IMP-14a | Strong |
| U22 | Urine | – | – | R | R | R | NT | + | + | – | IMP | IMP-14a | Strong |
| U35 | Urine | – | – | R | R | R | NT | + | + | – | IMP | IMP-14a | Strong |
| V17 | Urine | – | – | R | R | R | NT | + | + | – | IMP | IMP-14a | Strong |
| 385 | Urine | Male | 70 | R | R | R | R | + | + | – | IMP | IMP-14a | Strong |
| 593 | Urine | Male | 70 | R | R | R | R | + | + | – | NDM | NDM-1 | Moderate |
| U12 | Urine | Male | – | R | R | R | NT | + | + | – | NDM | NDM-1 | Moderate |
| U27 | Urine | – | – | R | R | R | NT | +/w | + | – | NDM | NDM-1 | Moderate |
| U9 | Urine | – | – | R | R | R | NT | + | + | – | NDM | NDM-1 | Strong |
| 52 | Urine | – | – | R | R | R | NT | +/w | + | – | NDM | NDM-1 | Moderate |
| 383 | Urine | Male | 66 | R | R | R | R | +/w | + | – | NDM | NDM-1 | Strong |
| 480 | Bile | Male | 59 | R | R | R | R | + | + | – | NDM | NDM-1 | Strong |
| 492 | Urine | Female | 91 | R | R | R | R | + | + | – | NDM | NDM-1 | Strong |
| 631 | Urine | Male | 88 | R | R | R | R | + | + | – | NDM | NDM-1 | Moderate |
| 503 | Sputum | Male | 81 | R | R | R | R | + | – | – | OXA-48 | OXA-181 | Moderate |
| 454 | Bile | Male | 73 | R | R | R | R | + | + | – | OXA-48+NDM | OXA-181+NDM-5 | Strong |
| 614 | Endotracheal aspirate | Male | 1 month | R | R | R | R | + | – | – | OXA-48 | OXA-232 | Strong |
| 477 | Bile | Female | 45 | R | R | R | R | + | – | – | OXA-48 | OXA-232 | Strong |
| 500 | Blood | Male | 63 | R | R | R | R | + | – | – | OXA-48 | OXA-232 | Strong |
| 605 | Pus, Wound | Male | 74 | R | R | R | R | + | + | – | OXA-48+NDM | OXA-232+NDM-1 | Strong |
| 601 | Sputum | Male | 89 | R | R | R | R | + | + | – | OXA-48+NDM | OXA-232+NDM-1 | Moderate |
| 628 | Sputum | Male | 83 | R | R | R | R | + | + | – | OXA-48+NDM | OXA-232+NDM-1 | Moderate |
| 460 | Corneal scraping | Male | 67 | R | R | R | R | + | + | – | OXA-48+NDM | OXA-232+NDM-1 | Strong |
| 495–1 | Biopsy. Tissue, Autopsy | Male | 69 | R | R | R | R | + | +/− | – | OXA-48+NDM | OXA-232+NDM-1 | Strong |
| 498 | Urine | Female | 35 | R | R | R | R | + | + | – | OXA-48+NDM | OXA-232+NDM-1 | Moderate |
| 622 | Urine | Male | 88 | R | R | R | R | + | + | – | OXA-48+NDM | OXA-232+NDM-1 | Strong |
| 632 | Urine | Female | 83 | R | R | R | R | + | + | – | OXA-48+NDM | OXA-232+NDM-1 | Strong |
| 478 | Biopsy. Tissue, Autopsy | Male | 69 | I | I | I | S | + | – | – | OXA-48 | OXA-48 | Moderate |
| 633 | Urine | Female | 46 | R | R | R | R | + | – | – | OXA-48 | OXA-48 | Strong |
IMP, Imipenem; MEM, Meropenem; ETP, Ertapenem; DOR, Doripenem; S, Susceptible; I, Intermediate; R, Resistant; NT, not tested; +, positive; -, negative; +/−, CAEDTA disk synergy positive but CAEDTA combination disk negative; +/w, weakly positive.
Table 2.
Susceptibilities of planktonic and biofilms of carbapenemase-producing K. pneumoniae clinical isolates (n = 29) to Ca-EDTA, Aztreonam and Ceftazidime/Avibactam and their combination in vitro
| No of isolates | Gene pattern | Pattern of entire of bla gene by sequencing | Biofilm formation category | ATM (μg/mL) | CZA (μg/mL) | CaEDTA (mg/mL) | ATM +CZA(μg/mL) | ATM (μg/mL) +CaEDTA (mg/mL) | CZA (μg/mL) +CaEDTA (mg/mL) | ATM (μg/mL) | CZA(μg/mL) | CaEDTA | ATM + CZA (μg/mL) | ATM (μg/mL) +CaEDTA (mg/mL) | CZA (μg/mL) + CaEDTA (mg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | IMP | IMP-14a | Strong | 512–128 | 256–64 | 24–6 | 8–0.5/8–0.5 | 32/0.75–0.375 | 0.5/0.375 | 2048–1024 | 4096 | 6 | 4/8 | 2/3 | 2/6 |
| 5 | NDM | NDM-1 | Moderate | 512–128 | 128–64 | 12–6 | 8–0.5/2–0.5 | 32/0.75 | 0.5/0.375 | 2048 | 4096 | 6 | 4/8 | 2/3 | 2/6 |
| 4 | Strong | 512–128 | 256–64 | 24–6 | 8–0.5/8–0.5 | 32/0.75 | 0.5/0.375 | 2048–1024 | 4096 | 12 | 4/8 | 2/3 | 2/6 | ||
| 1 | OXA-48 | OXA-181 | Moderate | 128 | 128 | 12 | 0.5/8 | 32/0.75–0.375 | 0.5/0.375 | 2048 | 4096 | 24 | 4/8 | 2/3 | 2/6 |
| 1 | OXA-48+NDM | OXA-181+NDM-5 | Strong | 128 | 128 | 6 | 8/0.5 | 32/0.375 | 0.5/0.375 | 2048 | 4096 | 6 | 4/8 | 2/3 | 2/6 |
| 3 | OXA-48 | OXA-232 | Strong | 512–128 | 256–64 | 12–6 | 8–0.5/8–0.5 | 32/0.75 | 0.5/0.375 | 2048 | 4096 | 6 | 4/8 | 2/3 | 2/6 |
| 3 | OXA-48+NDM | OXA-232+NDM-1 | Moderate | 512–256 | 256–128 | 12–6 | 8–0.5/8–0.5 | 32/0.75 | 0.5/0.375 | 2048 | 4096 | 6 | 4/8 | 2/3 | 2/6 |
| 5 | Strong | 512–128 | 256–64 | 12–6 | 8–0.5/8–0.5 | 32/0.75–0.375 | 0.5/0.375 | 2048–1024 | 4096 | 6 | 4/8 | 2/3 | 2/6 | ||
| 1 | OXA-48 | OXA-48 | Moderate | 128 | 128 | 6 | 2/0.5 | 32/0.75–0.375 | 0.5/0.375 | 2048 | 4096 | 6 | 4/8 | 2/3 | 2/6 |
| 1 | Strong | 128 | 128 | 6 | 0.5/8 | 32/0.375 | 0.5/0.375 | 2048 | 4096 | 12 | 4/8 | 2/3 | 2/6 |
ATM Aztreonam.
CZA Ceftazidime/Avibactam.
Calcium disodium ethylenediaminetetraacetate (Ca-EDTA).
Minimal inhibitory concentrations (MIC, μgmL−1) for planktonic cells.
Minimal biofilm eradication concentrations (MBEC, μgmL−1) for mature biofilms.
Figure 1.
Synergistic and Time-kill effect on carbapenemase-producing K. pneumoniae clinical isolates (n = 29)
(A) Synergistic, and Time-kill data for (B) Ca-EDTA + ceftazidime/avibactam, (C) Ca-EDTA +aztreonam, (D) aztreonam + ceftazidime/avibactam. All experiments in (A) and (B) was performed as three biologically independent experiments, and the mean ± s.d. is shown. p values were determined using an unpaired, two-tailed Student’s t test.
Ca-EDTA combination with aztreonam or ceftazidime/avibactam shows potent in vitro activity on mature CPKp biofilms
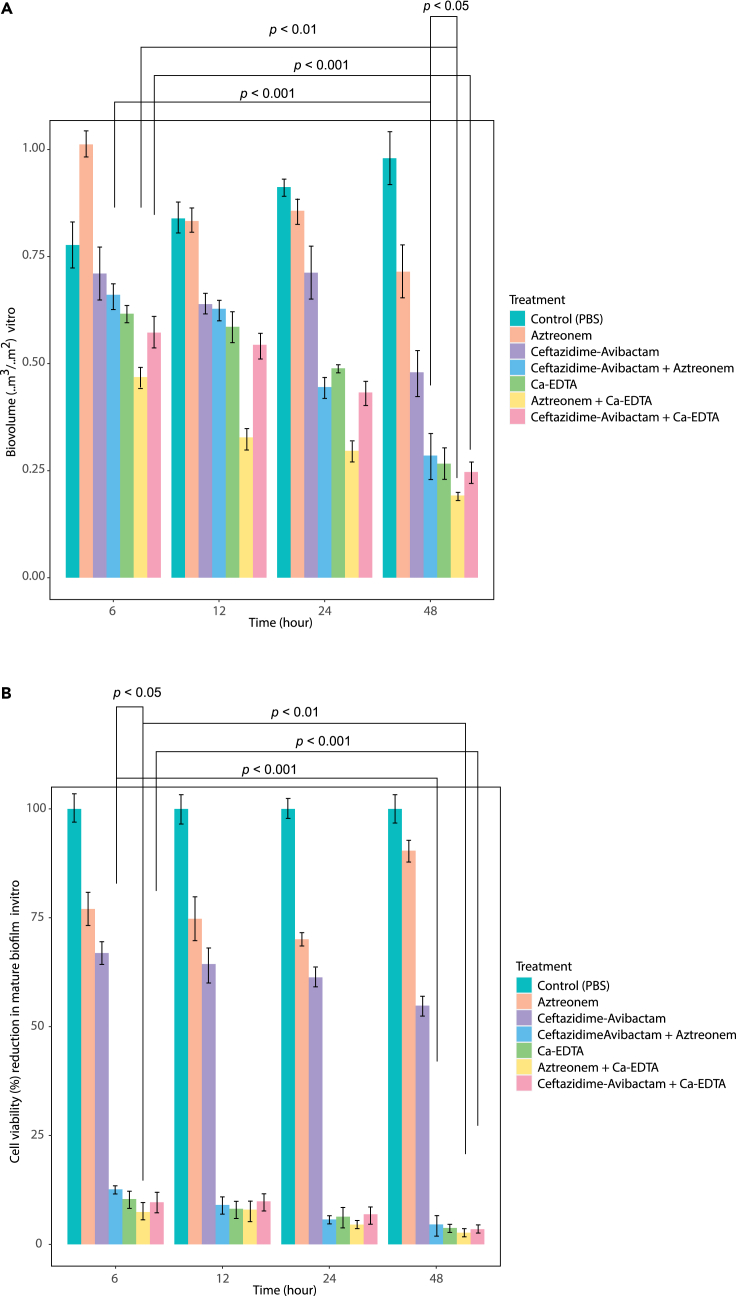
Ca-EDTA (3–6 mg/mL) in combination with aztreonam (2 μg/mL) or ceftazidime/avibactam (2 μg/mL) displayed significant reductions in biofilm biovolume in a time-dependent manner with the most pronounced eradication effects observed within 48 h (p < 0.001) (Figure 2A). Of note was the significant reduction in bacterial cell viability within the biofilm within 6 h (p < 0.001) with the addition of Ca-EDTA to either aztreonam or ceftazidime/avibactam (Figure 2B). Of interest, aztreonam + Ca-EDTA combination displayed a significant (p < 0.05) reduction in bacteria cell viability within the biofilm after 6 h exposure as well as biofilm biovolume reduction at 48 h, when compared to aztreonam + ceftazidime/avibactam combination (Figure 2).
Figure 2.
Effects of Ca-EDTA, aztreonam and ceftazidime/avibactam and their combination in vitro
(A) biofilm biovolume and (B) biofilm cell viability (%) of carbapenemase-producing K. pneumoniae clinical isolates (n = 29) at different time intervals. All experiments in a–b was performed as three biologically independent experiments, and the mean ± s.d. is shown. p values were determined using an unpaired, two-tailed Student’s t test.
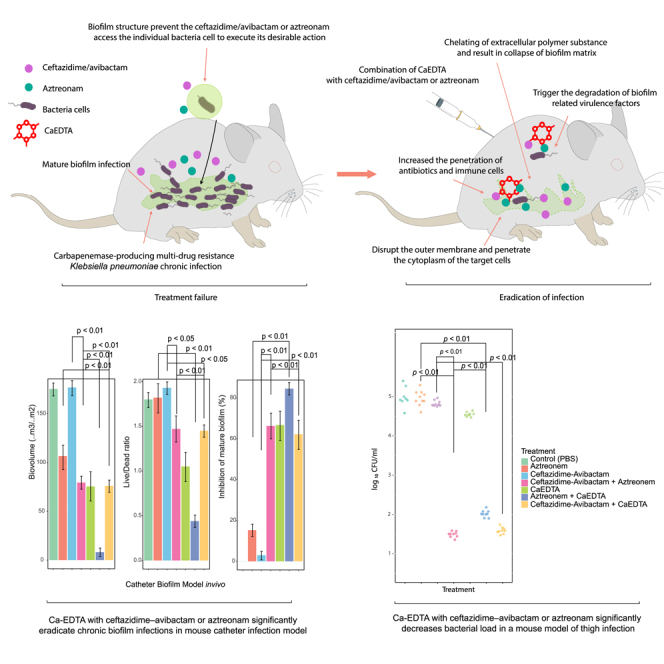
Ca-EDTA combination with aztreonam or ceftazidime/avibactam completely eradicates CPKp catheter-related biofilm infection
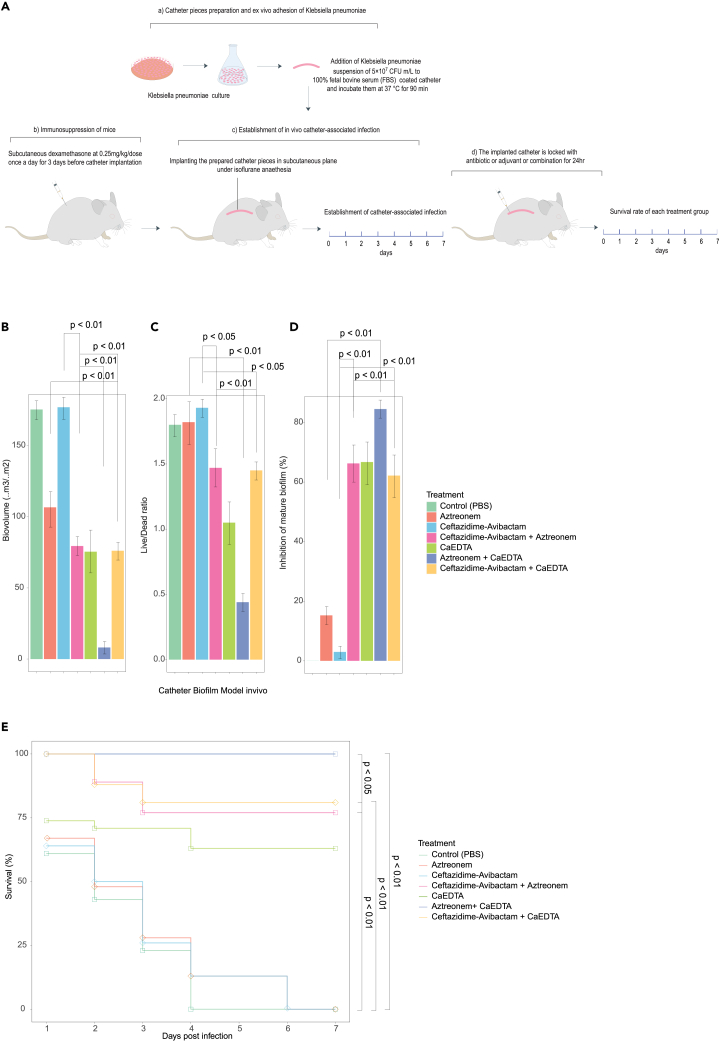
To assess the potential effectiveness of combining Ca-EDTA with either aztreonam or ceftazidime/avibactam for therapeutic purposes in in vivo, we conducted experiments using a murine model of catheter-related biofilm infection caused by CPKp, a significant Gram-negative pathogen commonly associated with such infections. We found a significant (p < 0.01) reduction in biofilm biomass and a lower viable/dead cell ratio (p < 0.01) by adding Ca-EDTA (3–6 mg mL−1) to either ceftazidime–avibactam (2 μg mL−1) or aztreonam (2 μg mL−1) (Figures 3B and 3C). Furthermore, when compared with antibiotic(s) alone, Ca-EDTA combined with ceftazidime–avibactam or aztreonam significantly inhibited biofilm biovolume (p < 0.001) (Figure 3D). However, the Ca-EDTA + aztreonam combination significantly reduced biofilm biomass (p < 0.01), resulted in a lower viable/dead ratio (p < 0.01), and reduced biofilm biovolume compared with the ceftazidime/avibactam + aztreonam combination (Figures 3B–3D). Mice treated with Ca-EDTA combined with ceftazidime–avibactam or aztreonam displayed significantly higher survival rates until day 7 when compared with treatment with antibiotic alone (p < 0.01) or Ca-EDTA alone (p < 0.01) (Figure 3E). Of interest, mice treated with Ca-EDTA + aztreonam had significantly (p < 0.05) higher survival rates (100%) than mice treated with ceftazidime/avibactam + aztreonam (Figure 3E). Confocal microscopic analysis confirmed the significant reduction in biofilm biovolume and viable CPKp cells within biofilms treated with Ca-EDTA combined with ceftazidime/avibactam or aztreonam (Figure 4).
Figure 3.
Effects of Ca-EDTA, aztreonam and ceftazidime/avibactam and their combination on catheter biofilm infections
Effects of PBS (control), aztreonam (16 μg/mL), ceftazidime/avibactam (1 μg/mL) (16 μg/mL), ceftazidime-avibactam + aztreonem (16/16 μg/mL), Ca-EDTA (24 mg/mL), aztreonam -Ca-EDTA (4/12 mg/mL) and ceftazidime/avibactam- Ca-EDTA (4 μg/mL + 12 mg/mL) on carbapenemase-producing K. pneumoniae clinical isolates (n = 7) catheter biofilm infection model in vivo (A) biovolume (B) Live/Dead cell ratio (C) bio-volume inhibition, and (D) survival of mice. Values were measured each of the 10 catheters and represented the as averages. All experiments in A–E were performed as three biologically independent experiments, and the mean ± s.d. is shown. p values were determined using an unpaired, two-tailed Student’s t test.
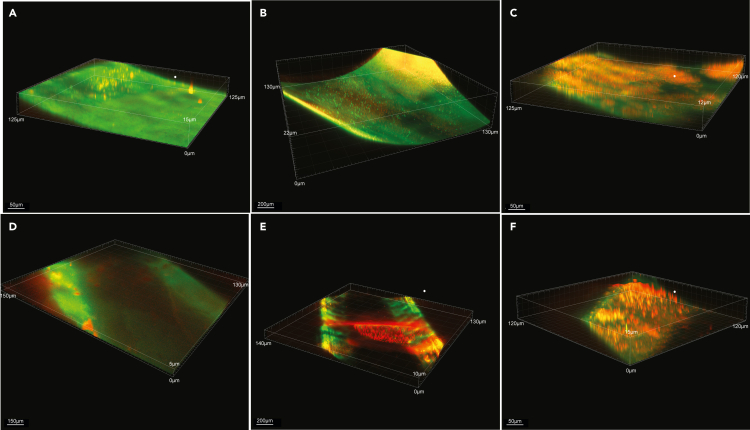
Figure 4.
Confocal images of in vivo catheter biofilm
Confocal imaging analysis (3D) z axis image stack visualizing (40x magnification) of (A) aztreonam (16 μg/mL), (B) ceftazidime/avibactam (1 μg/mL) (16 μg/mL), (C) ceftazidime-avibactam + aztreonem (16/16 μg/mL), (D) Ca-EDTA (24 mg/mL), (E) aztreonam -Ca-EDTA (4/12 mg/mL) and (F) ceftazidime/avibactam- Ca-EDTA (4 μg/mL + 12 mg/mL) on carbapenemase-producing K. pneumoniae clinical isolates (n = 7) catheter biofilm infection model in vivo. One catheter was selected from the 10 catheters after confocal scanning in each treatment group for visualization purposes and live bacteria were stained green and dead bacteria were stained red.
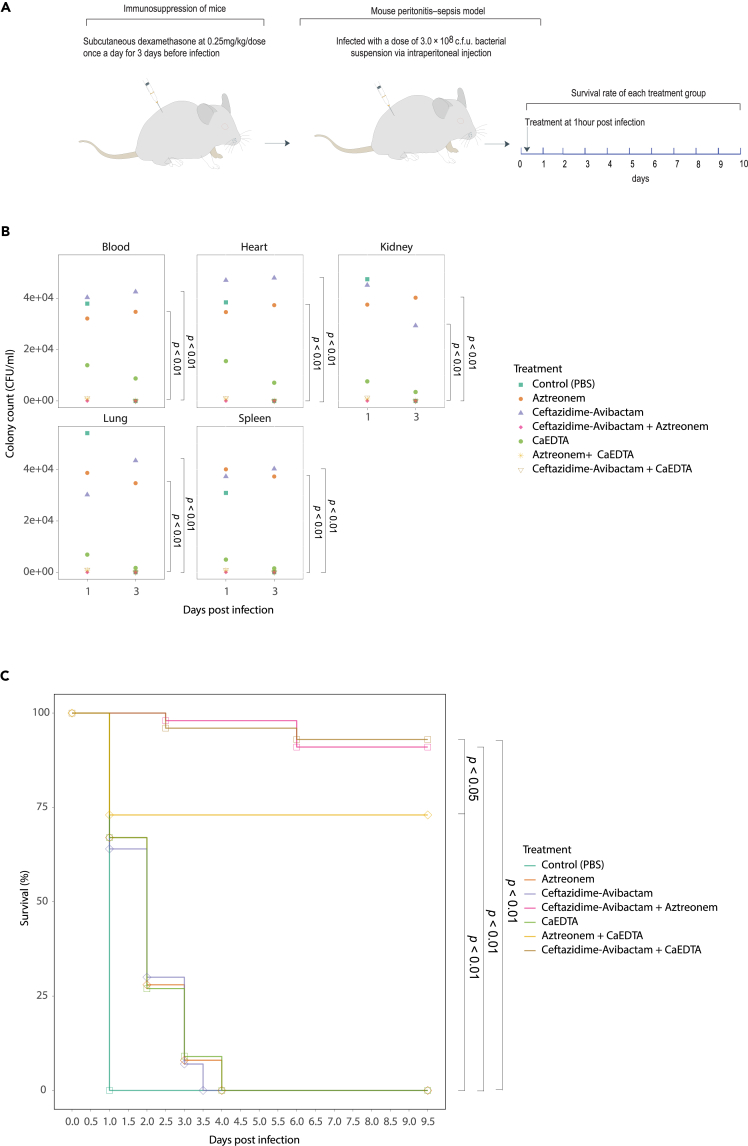
Administration of Ca-EDTA combination with aztreonam or ceftazidime/avibactam showed potent synergistic activities in murine CPKp-associated bacteremia
CPKp is one of the most important Gram-negative pathogens associated with bloodstream infections. Therefore, we conducted experiments using a murine model of bloodstream infections to assess the potential effectiveness of combining Ca-EDTA with either aztreonam or ceftazidime/avibactam for therapeutic purposes. Compared with monotherapy, Ca-EDTA combined with ceftazidime–avibactam or aztreonam significantly reduced the bacterial load on internal organs (blood, heart, kidneys, lungs, and spleen) when administered intraperitoneally (Figure 5B; p < 0.01). Adding Ca-EDTA to either ceftazidime–avibactam or aztreonam significantly improved the survival of treated mice with CPKp-associated bacteremia compared with monotherapy (Figure 5C; p < 0.01). It is noteworthy that mice treated with Ca-EDTA + ceftazidime–avibactam and ceftazidime/avibactam + aztreonam had significantly (p < 0.05) higher survival rates (100%) than mice treated with Ca-EDTA + aztreonam (Figure 5C).
Figure 5.
Effects of Ca-EDTA, aztreonam and ceftazidime/avibactam and their combination on bacteremia
Effects of PBS (control), aztreonam (700 mg/kg), ceftazidime/avibactam (375 mg/kg), ceftazidime-avibactam + aztreonam (375 mg/kg + 700 mg/kg), Ca-EDTA (80 mg/kg/day), aztreonam -Ca-EDTA (700 mg/kg + 80 mg/kg/day) and ceftazidime/avibactam- Ca-EDTA (375 mg/kg + 80 mg/kg/day) on carbapenemase-producing K. pneumoniae clinical isolates (n = 7) mouse bacteremia infection model (A) illustration of a mouse model, (B) bacterial load in internal organs, (C) survival of mice. p values were determined using a two-sided Mann–Whitney U-test. All data were presented as means ± s.d.
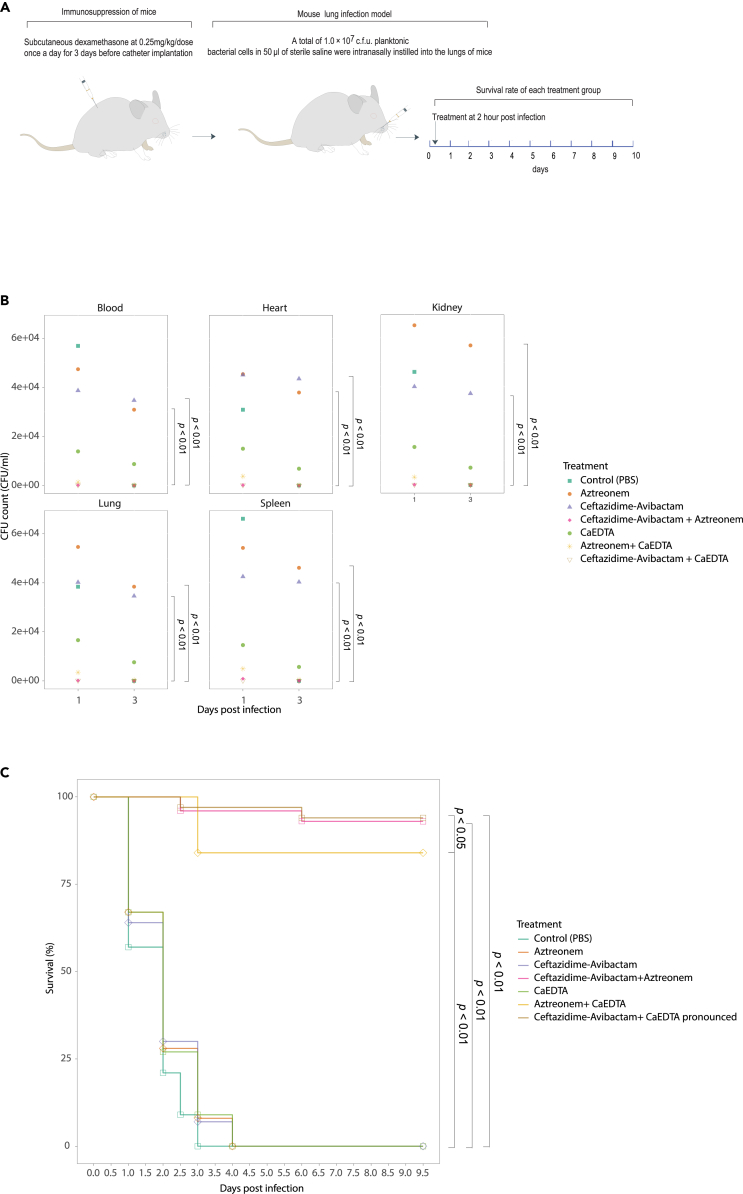
Ca-EDTA combination with aztreonam or ceftazidime/avibactam increases the survival of mice with CPKp-associated pneumonia
To evaluate the possible efficacy of combining Ca-EDTA with either aztreonam or ceftazidime/avibactam for treating pneumonia, we carried out experiments using a mouse model of pneumonia induced by CPKp. CPKp is widely acknowledged as a highly relevant type of Gram-negative pathogen commonly associated with pneumonia. Neither Ca-EDTA, ceftazidime–avibactam, nor aztreonam treatment alone significantly reduced bacterial numbers in the lungs of infected mice (Figure 6B). In contrast, simultaneous administration of Ca-EDTA with either ceftazidime–avibactam or aztreonam significantly improved the clearance of bacteria from their lungs (Figure 6B; p < 0.01). Notably, the effects of Ca-EDTA with ceftazidime–avibactam were striking, with >90% survival for mice treated with this combination (Figure 6C; p < 0.01). Consistent with this observation, the bacterial load was also significantly reduced when mice were treated with Ca-EDTA combined with either ceftazidime–avibactam or aztreonam (Figure 6B; p < 0.01).
Figure 6.
Effects of Ca-EDTA, aztreonam and ceftazidime/avibactam and their combination on pneumonia
Effects of PBS (control), aztreonam (700 mg/kg), ceftazidime/avibactam (375 mg/kg), ceftazidime-avibactam + aztreonam (375 mg/kg + 700 mg/kg), Ca-EDTA (80 mg/kg/day), aztreonam -Ca-EDTA (700 mg/kg + 80 mg/kg/day) and ceftazidime/avibactam- Ca-EDTA (375 mg/kg + 80 mg/kg/day) on carbapenemase-producing K. pneumoniae clinical isolates (n = 7) mouse lung infection model (A) illustration of a mouse model, (B) bacterial load in internal organs, (C) survival of mice. p values were determined using a two-sided Mann–Whitney U-test. All data were presented as means ± s.d.
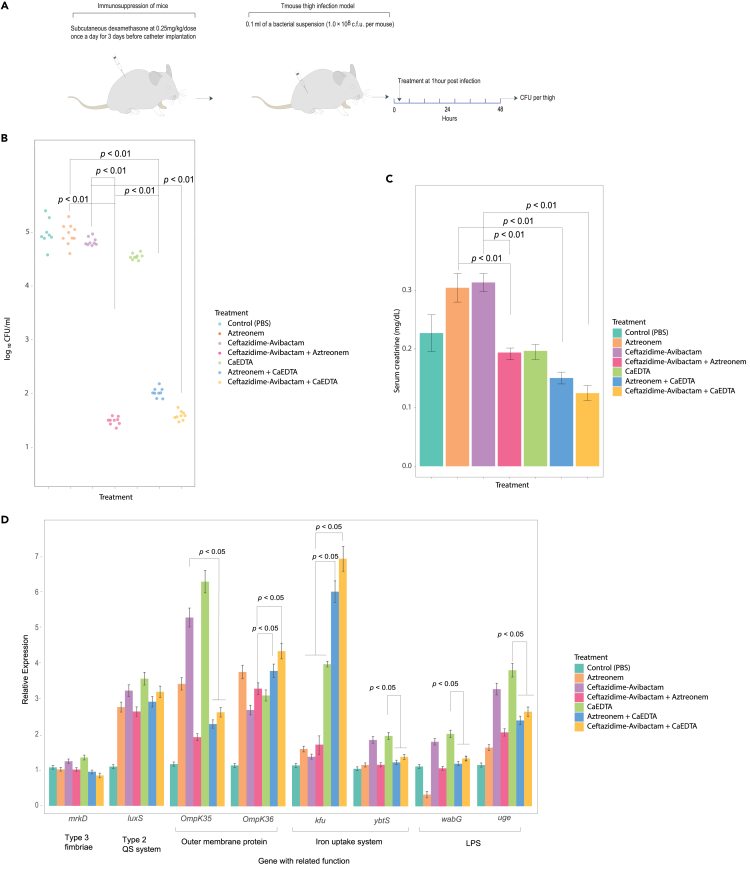
Ca-EDTA with aztreonam or ceftazidime/avibactam combination significantly decreases bacterial load in thigh infection mice and serum creatinine
To evaluate the possible efficacy of combining Ca-EDTA with either aztreonam or ceftazidime/avibactam for treating soft tissue infection, we carried out experiments using a mouse thigh infection model induced by CPKp. In a mouse model of thigh infection, bacterial counts after treatment with Ca-EDTA combined with ceftazidime–avibactam or aztreonam were significantly lower than in mice receiving monotherapy (Figure 7B; p < 0.01). Ca-EDTA + ceftazidime–avibactam and ceftazidime/avibactam + aztreonam significantly reduced the bacterial load more than Ca-EDTA + aztreonam (Figure 7B; p < 0.01). Moreover, infected mice treated with Ca-EDTA + ceftazidime–avibactam or aztreonam showed significantly lower serum creatinine levels than mice treated with monotherapy (Figure 7C; p < 0.05).
Figure 7.
Effects of Ca-EDTA, aztreonam and ceftazidime/avibactam and their combination on thigh infection
Effects of PBS (control), aztreonam (700 mg/kg), ceftazidime/avibactam (375 mg/kg), ceftazidime-avibactam + Aztreonam (375 mg/kg + 700 mg/kg), Ca-EDTA (80 mg/kg/day), aztreonam -Ca-EDTA (700 mg/kg + 80 mg/kg/day) and ceftazidime/avibactam- Ca-EDTA (375 mg/kg + 80 mg/kg/day) on carbapenemase-producing K. pneumoniae clinical isolates (n = 7) mouse thigh infection model (A) illustration of a mouse model, (B) bacterial load in thigh, (C) serum creatinine of mice (D) relative expression of virulence genes. p values were determined using a two-sided Mann–Whitney U-test. All data were presented as means ± s.d.
Ca-EDTA with aztreonam or ceftazidime/avibactam combination altered expressions of virulent genes in vivo
The expression ofmrkD, and luxS was not significantly affected by Ca-EDTA or aztreonam or ceftazidime/avibactam or as combination. The ompK 35 expression was increased significantly (p < 0.05) with Ca-EDTA, aztreonam and ceftazidime/avibactam as compared to Ca-EDTA with aztreonam or ceftazidime/avibactam combination. But the ompK 36 expression level increase significantly (p < 0.05) with Ca-EDTA with aztreonam or ceftazidime/avibactam combination. Expression of kfu was significantly increased (p < 0.05) after giving Ca-EDTA with aztreonam or ceftazidime/avibactam combination as compared to aztreonam or ceftazidime/avibactam or Ca-EDTA alone. Genes—ybtS expression were significantly lower (p < 0.05) in Ca-EDTA with aztreonam or ceftazidime/avibactam combination compared to ceftazidime/avibactam or Ca-EDTA alone. For uge and wabG, their expression levels were significantly increased (p < 0.05) after exposure to Ca-EDTA as compared to Ca-EDTA with aztreonam or ceftazidime/avibactam combination (Figure 7D).
Discussion
The present study demonstrates the effectiveness of combining treatment with ceftazidime–avibactam or aztreonam with Ca-EDTA against CPKp in vitro and associated infections in vivo. Adding Ca-EDTA to the antibiotic treatments reduced the planktonic MICs and biofilm MBEC when ceftazidime–avibactam or aztreonam were used to treat MBL-producing isolates and non-MBL-producing CPKp isolates. We observed similar survival benefits of Ca-EDTA combined with ceftazidime–avibactam or aztreonam in catheter lock therapy after subcutaneous administration. Ca-EDTA + aztreonam combined showed excellent antibiofilm activity compared with Ca-EDTA + ceftazidime–avibactam or ceftazidime–avibactam + aztreonam. Ca-EDTA combined with ceftazidime–avibactam or aztreonam produces similar or even better efficacy against CPKp isolates than current ceftazidime–avibactam + aztreonam treatments.
This study found that CPKp has altered the expression of various virulence genes in response to Ca-EDTA combined with ceftazidime–avibactam or aztreonam, including genes related to lipopolysaccharide (wabG and uge), which protect the bacteria from the host’s immune system and enhance its ability to cause disease. We also found that the bacteria have altered porin genes (ompK 35 and 36) to improve nutrient transport and survival within the host, iron acquisition system genes (kfu and ybtS) to modulate the host’s immune response for systemic survival and dissemination, type 3 fimbria adhesin (mrkD) to bind to abiotic surfaces and develop biofilms, and type 2 quorum-sensing regulatory system gene (luxS) to promote biofilm development by facilitating cell-to-cell communication.7,9 When bacteria are exposed to antimicrobial agents/Ca-EDTA, it creates a stressful environment that can lead to changes in the expression of bacterial genes. Also, the upregulation of kfu was probably due to iron deprivation because of Ca-EDTA sequestering metal ions, including iron. The altered expression of virulence genes observed in this study suggests that CPKp has an adaptive response to survive in a hostile environment caused by the different treatments given to mice. However, further research is needed to investigate the mechanism of virulence gene changes after treatment with Ca-EDTA combined with ceftazidime–avibactam or aztreonam or alone.
The present data suggest a potential therapeutic application of Ca-EDTA to augment antibiotic treatment of infectious diseases caused by CPKp.
EDTA has several antibacterial activities, mainly chelating metal ions from microorganisms.16,19 EDTA can rapidly disperse and eradicate bacteria from biofilms by chelating various divalent cations (e.g., Mg2+ and Ca2+) required to stabilize the biofilm matrix.7,9,16,17,18,19,20,21,22 Exploiting the antibiofilm property of EDTA, several investigators, including those in our group, reported practical uses of this chelator for catheter lock solutions.9,21 EDTA can also deprive LPS of divalent cations, resulting in permeabilization and disorganization of the bacterial outer membrane.16 Combining EDTA with carbapenems augments the post-antibiotic and post-β-lactamase inhibitor effects on MBL-producing Pseudomonas aeruginosa isolates.20 Moreover, adding EDTA can restore the antibacterial activity of colistin in colistin-resistant K. pneumoniae infections. However, the clinical application of EDTA has been limited because of its toxicity.20 The apparent absence of toxicity or mortality in mice treated with higher doses of Ca-EDTA (subcutaneous dose of 80 mg kg−1) observed in the present study and the clinical use of edetate calcium disodium (calcium disodium versenate) to treat lead and other heavy metal poisoning suggests that Ca-EDTA may potentially be used therapeutically to treat CPKp infections.
Owing to a shortage of alternatives, ceftazidime–avibactam and aztreonam combined treatment is increasing markedly.8,11,14 However, there is concern that the increased clinical use of this combination may induce future selective pressure on K. pneumoniae to develop resistance.23 Exposure to several antimicrobial agents creates a stressful environment for bacteria, resulting in altered expression of bacterial genes.7,9,23,24,25,26,27,28 Because CPKp will become resistant to nearly all classes of β-lactamase agents and the scant development of new MBL-stable β-lactam antibiotics or MBL inhibitors, the continued dissemination of CPKp may pose important clinical challenges.6 Our present data demonstrate that Ca-EDTA sensitizes various CPKp clinical isolates to ceftazidime–avibactam and aztreonam. A difference in the synergy between the two combinations was against biofilm, where more effectiveness was observed when Ca-EDTA was combined with aztreonam. This difference is likely attributable to the efficient penetrative ability of aztreonam through the biofilm matrix. However, ceftazidime–avibactam combined with Ca-EDTA still showed efficacy against CPKp biofilms.
Limitations of the study
Our study findings are fully based on in vitro and in vivo mouse experiments and may not 100% reflect the human body environments and associated complexities. Combining the present results with human clinical trials of CPKp infections would clarify the clinical applicability of the findings.
Conclusions
Here, combining Ca-EDTA with ceftazidime–avibactam or aztreonam has demonstrated the potential utility of these combinations to overcome the antibiotic resistance of CPKp. The concentrations of Ca-EDTA used for the experiment were effective and fell within a clinically accessible range.17 We also demonstrated the antibiotic-synergistic effects of Ca-EDTA in vivo. In our murine model of pneumonia caused by CPKp, cotreatment with Ca-EDTA and ceftazidime–avibactam or aztreonam caused an improvement in the survival rate and reduced the bacterial lung load. Moreover, intravenous and subcutaneous administration of Ca-EDTA and antibiotics produced similar effects, suggesting suitable penetration of the chelator into the tissues and biofilm matrix. Further investigation of Ca-EDTA co therapy with ceftazidime–avibactam or aztreonam against infections by CPKp, including pharmacokinetic and safety profiles, is warranted.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| E. coli ATCC 25922 | ATCC | 25922 |
| P. aeruginosa ATCC 27853 | ATCC | 27853 |
| Chemicals, peptides, and recombinant proteins | ||
| Imipenem | Sigma Aldrich | 10160 |
| Meropenem | Sigma Aldrich | M2574 |
| Ertapenem | Sigma Aldrich | SML1238 |
| Doripenem | Sigma Aldrich | SML1220 |
| Ceftazidime–avibactam | GlaxoSmithKline | AVYCAZ |
| Aztreonam | Thermo Fisher Scientific | J62887.03 |
| Phosphate-buffered saline | Thermo Fisher Scientific | 003002 |
| Müller–Hinton agar | Sigma Aldrich | 70191 |
| Müller–Hinton II broth | Sigma Aldrich | 90922 |
| Ca-EDTA | Sigma Aldrich | 304695-78-1 |
| Porcine mucin | Sigma Aldrich | 84082-64-4 |
| Dexamethasone | Thermo Fisher Scientific | A17590.03 |
| Ketamine | Sigma Aldrich | K-113 |
| Isoflurane | Sigma Aldrich | 792632 |
| Fetal bovine serum | Gibco™; Waltham, MA | 1962 |
| Chlorhexidine | Thermo Fisher Scientific | 214980050 |
| Ampicillin sodium powder | Fisher BioReagents™ | BP1760-5 |
| Iodine isopropanol | Sigma-Aldrich | 148938 |
| Ethyl alcohol | Thermo Fisher Scientific | 224095000 |
| SYBR™ Green | Thermo Fisher Scientific | S7563 |
| Critical commercial assays | ||
| PrestoBlue™ HS Cell Viability Reagent | Thermo Fisher Scientific | P50200 |
| Live/Dead Bacterial Viability kit | Thermo Fisher Scientific | L7007 |
| GeneJET RNA Purification Kit | Thermo Fisher Scientific | K0731 |
| STET lysing solution | BD Bioscience | 349202 |
| qScript™ cDNA Synthesis Kit | QuantaBio | 101414-100 |
| Experimental models: Organisms/strains | ||
| C57BL/6 mice | Nomura Siam International (Bangkok, Thailand) | https://nomura-siam.com/en/ |
| Oligonucleotides | ||
| 16srRNA - for AGAGTTTGATCCTGGCTCAG 16srRNA - rev GGTTACCTTGTTACGACTT |
Daikos et al.6 | Daikos et al.6 |
| 16srRNA – for AGAGTTTGATCCTGGCTCAG 16srRNA – rev GGTTACCTTGTTACGACTT |
Wannigama et al.29/Vuotto et al.30 | Wannigama et al.29/Vuotto et al.30 |
|
kfu – for GGCCTTTGTCCAGAGCTACG kfu – rev GGGTCTGGCGCAGAGTATGC |
Wannigama et al.29/Vuotto et al.30 | Wannigama et al.29/Vuotto et al.30 |
|
ybtS – for GACGGAAACAGCACGGTAAA ybtS- rev GAGCATAATAAGGCGAAAGA |
Wannigama et al.29/Vuotto et al.30 | Wannigama et al.29/Vuotto et al.30 |
|
mrkD – for AAGCTATCGCTGTACTTCCGGCA mrkD – revGGCGTTGGCGCTCAGATAGG |
Wannigama et al.29/Vuotto et al.30 | Wannigama et al.29/Vuotto et al.30 |
|
luxS – for AGTGATGCCGGAACGCGG luxS – rev CGGTGTACCAATCAGGCTC |
Wannigama et al.29/Vuotto et al.30 | Wannigama et al.29/Vuotto et al.30 |
|
ompK35 – for GCAATATTCTGGCAGTGGTGATC ompK35 – rev ACCATTTTTCCATAGAAGTCCAGT |
Wannigama et al.29/Vuotto et al.30 | Wannigama et al.29/Vuotto et al.30 |
|
ompK36 – for TTAAAGTACTGTCCCTCCTGG ompK36 – rev TCAGAGAAGTAGTGCAGACCGTCA |
Wannigama et al.29/Vuotto et al.30 | Wannigama et al.29/Vuotto et al.30 |
|
uge – for TCTTCACGCCTTCCTTCACT uge – rev GATCATCCGGTCTCCCTGTA |
Wannigama et al.29/Vuotto et al.30 | Wannigama et al.29/Vuotto et al.30 |
|
wabG- for ACCATCGGCCATTTGATAGA wabG – rev CGGACTGGCAGATCCATATC |
Wannigama et al.29/Vuotto. et al.30 | Wannigama et al.29/Vuotto et al.30 |
| Software and algorithms | ||
| ggplot2 3.3.5 | R software package | https://www.r-project.org |
| PHLIP software | https://sourceforge.net/projects/phlip/ | |
| MATLAB | https://www.mathworks.com/products/matlab.html | |
| Other | ||
| 25-mm catheters | NIPRO | www.nipro.co.jp |
Resource availability
Lead contact
Further information and requests for resources, raw data, and code should be directed to and will be fulfilled by the lead contact, Dhammika Leshan Wannigama (Dhammika.L@chula.ac.th).
Materials availability
The authors confirm that no new reagents were generated in the study.
Experimental model and study participant details
Strains and growth conditions
Without any preference for particular strains, anonymized clinical isolates of CPKp were obtained from a repository held at the Department of Microbiology, King Chulalongkorn Memorial Hospital, after conventional characterization and identification, including 16S rRNA and bla sequencing as described for previous studies.4,7,9,16 Strains had been isolated from 29 infected patients during the course of their standard care for reasons unrelated to the present study. The bacterial isolates were cultured on Müller–Hinton agar plates at 37°C and cryopreserved at −80°C in tryptic soy broth with 15% (v/v) glycerol until their subsequent use in these studies.
Mouse study
After the IACUC of the Faculty of Medicine, Chulalongkorn University approved the procedures to be performed in the present study, we obtained specific-pathogen free, 8-week-old, female C57BL/6 mice from Nomura Siam International (Bangkok, Thailand). The mice were allowed to acclimatize for 1 week in the university animal facility at a maximum of 2 mice per cage before use and were allowed food and water ad libitum. The mice were each weighed, and their health and welfare were monitored closely to determine humane clinical endpoints and for any signs of distress over the experimental period. For all the mouse experiments, we used 7 representative carbapenemase-producing K. pneumoniae clinical isolates (U17; IMP/IMP-14a, U27; NDM/NDM-1, 503; OXA-48/OXA-181, 454; OXA-48+NDM/OXA-181+NDM-5, 500; OXA-48/OXA-232, 628; OXA-48+NDM/OXA-232+NDM-1, 633; OXA-48/OXA-48) randomly selected from among the 29 isolates to represent 7 gene pattern groups, as shown in the Table 2. We culture seven representative isolates individually, and then combine them into a single culture for inoculating the mice.
Catheter-related biofilm infection mouse model
A murine model of catheter-related infection in vivo was used as previously described9,33 (Figure 5A). In brief, mice were immunosuppressed by subcutaneous administration of dexamethasone at a single dose of 0.25 mg kg−1 daily for three days before implanting a catheter, which was maintained in the mice throughout the study. The mice were euthanized under isoflurane anesthesia by removing organs, and samples (blood, heart, lungs, spleen, kidneys, catheters, and tissues surrounding the catheter) were harvested aseptically.
Effects of aztreonam, ceftazidime/avibactam, Ca-EDTA and combinations lock therapy on catheter-related biofilm infection mouse model
We divided 70 mice without selection into a control (no therapy) group and experimental (therapy) groups, including 6 subgroups for a total of 7 groups with 10 mice in each group (Figure 3A). The implanted catheters with catheter-related biofilm infections of CPKp (n = 7) were exposed to phosphate-buffered saline (PBS) (control), Ca-EDTA (24 mg mL−1), ceftazidime–avibactam (1 μg mL−1; 16 μg mL), aztreonam (16 μg mL−1), ceftazidime–avibactam + aztreonam (16 mg mL−1 + 16 μg mL−1), Ca-EDTA + ceftazidime–avibactam (12 mg mL−1 + 4 μg mL−1) and Ca-EDTA + aztreonam (12 mg mL−1 + 4 mg mL−1) After treatment for 24 h, catheters were removed under aseptically, and the treatment efficacy for the various groups was analyzed by confocal laser scanning microscopy (biomass, live/dead ratio, and biovolume inhibition) and bacterial counting.9,34 The experimental endpoint was defined as 10 days post-infection for mice not reaching the clinical endpoint. Mice were also euthanized at various time points post-infection by cervical dislocation under isoflurane anesthesia to determine viable cell counts (CFU mL−1) in the catheters after each treatment.18
In vivo murine bacteremia model
To establish CPKp-associated bacteraemia in vivo, a previously described murine model of bacteraemia obtained through intraperitoneal inoculation of CPKp (n = 7) was used (Figure 5A).7,27,35 Immunocompetent mice were inoculated intraperitoneally with 1 × 106 CFU of a bacterial suspension with 5% (v/v) porcine mucin (Sigma‒Aldrich), and murine CPKp-associated bacteraemia was allowed to develop for 1 h.7,27,35 To evaluate the effects of Ca-EDTA, ceftazidime–avibactam, aztreonam, and their combinations in vivo, infected mice were administered a single intraperitoneal dose of PBS (control), Ca-EDTA (80 mg kg−1), ceftazidime–avibactam (375 mg kg−1), aztreonam (700 mg kg−1), ceftazidime–avibactam + aztreonam (375 mg kg−1 + 700 mg kg−1), Ca-EDTA + ceftazidime–avibactam (80 mg kg−1 + 375 mg kg−1), or Ca-EDTA + aztreonam (80 mg kg−1 + 700 mg kg−1), for a total of 7 groups with 10 mice in each group. All mice were euthanized at 14 h post-infection. Peritoneal fluid was sampled by injecting 2 mL sterile saline solution into the peritoneum, followed by gentle massage and aspiration. Peritoneal samples were then serially diluted and plated on nutrient agar to determine the bacterial load. For survival analysis, the same treatment was repeated once daily, and the survival of mice was monitored until a humane clinical or experimental endpoint was reached. The clinical endpoint was determined using a 5-point body condition score, analyzing weight loss, decreased body temperature, respiratory distress, hampered mobility, and hunched posture. The experimental endpoint was defined as 10 days post-infection for mice not reaching the clinical endpoint.
Murine model of lung infection
To produce a murine model of acute lung infection, CPKp (n = 7) cultured overnight were diluted to an OD of 600 nm of 0.5 in sterile saline (Figure 6A).17,36 Immunocompetent mice were anesthetized with an intraperitoneal injection of ketamine (50 μg mL−1) in sterile saline. We intranasally instilled 1.0 × 107 CFU bacteria suspended planktonically in 50 μL of sterile saline into the lungs of the mice. Therapies were initiated 2 h after the induction of pneumonia. To determine the effects in vivo of Ca-EDTA, ceftazidime–avibactam, aztreonam, and their combinations, infected mice were given a single intraperitoneal dose of PBS (control), Ca-EDTA (80 mg kg−1), ceftazidime–avibactam (375 mg/kg), aztreonam (700 mg/kg), ceftazidime–avibactam + aztreonam (375 mg/kg + 700 mg/kg), Ca-EDTA + ceftazidime–avibactam (80 mg/kg/day +375 mg/kg−1), or Ca-EDTA + aztreonam (80 mg kg−1 + 700 mg kg−1), for a total of 7 groups with 10 mice in each group. For the survival analysis, the same treatment procedure was repeated once daily, and the survival of mice was monitored until the humane clinical or experimental endpoint was reached. The clinical and experimental endpoints were as described above for the model of bacteraemia.
Mouse thigh infection model
We investigated the effects of Ca-EDTA, ceftazidime–avibactam, aztreonam, and their combinations against representative CPKp strains (n = 7) in a murine model of neutropenic thigh infection described previously (Figure 7A).27,37 Mice were immunosuppressed by subcutaneous administration of dexamethasone at 0.25 mg kg−1 once a day for 3 days before infection. Bacteria adjusted to a concentration of approximately 1 × 106 CFU per infection site and suspended in sterile saline were injected into the right and left thighs of 5 mice per treatment group. At 1 h post-infection, mice received either PBS (control), Ca-EDTA (80 mg kg−1), ceftazidime–avibactam (375 mg/kg), aztreonam (700 mg/kg), ceftazidime–avibactam + aztreonam (375 mg/kg + 700 mg/kg), Ca-EDTA + ceftazidime–avibactam (80 mg/kg/day +375 mg/kg−1), or Ca-EDTA + aztreonam (80 mg kg−1 + 700 mg kg−1). At 8 h post-infection, mice were euthanized under isoflurane anesthesia, and their thigh tissue was collected aseptically, weighed, homogenized, diluted serially in PBS, and plated onto solid Luria–Bertani medium (50 μg mL−1). Plates were incubated overnight at 37°C, and colonies were quantified to determine bacterial load. Serum creatinine levels of mice in each treatment group were measured as described previously.9,38
Method details
Antimicrobial agents
Imipenem, meropenem, ertapenem, doripenem, ceftazidime–avibactam, aztreonam, and Ca-EDTA (Sigma‒Aldrich) were used to prepare stock solutions <24 h before use. All agents were dissolved in cation-adjusted Müller–Hinton II broth (MHIIB) (Sigma‒Aldrich), and the solutions were sterilized through a syringe filter with a membrane nominal pore size of 0.22 μm. Serial dilutions of the antibiotic and Ca-EDTA stocks were prepared in MHIIB immediately before use.
Susceptibilities to aztreonam, ceftazidime/avibactam, and Ca-EDTA
We established the susceptibility of planktonic cultures of the various strains to antibiotics using standard techniques (broth microdilution to determine minimal inhibitory concentrations, MICs) according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST criteria for Enterobacteriaceae only)39 and U.S. Clinical and Laboratory Standards Institute (CLSI) guidelines.40 We used E. coli ATCC 25922 and P. aeruginosa ATCC 27853 for quality control. Susceptibility testing was performed for all 29 isolates.
Synergistic activity
We screened the synergistic activity for all 29 isolates with Ca-EDTA, ceftazidime–avibactam, aztreonam, and their combinations against planktonic cultures of CPKp clinical isolates using a checkerboard assay. Synergism was interpreted as follows: fractional inhibitory concentration index (FICI) ≤ 0.5, synergy; 0.5 > FICI ≤1, additive; 1 > FIC index ≤4, no interaction; and FICI >4, antagonism.15
Time-kill assay
The antimicrobial activities of Ca-EDTA + ceftazidime–avibactam + or Ca-EDTA + aztreonam combinations that showed synergistic action toward planktonic cultures of 29 CPKp isolates were confirmed using time–kill assays. Bacteria were cultured with no agent, each agent, or combined agents. We then assessed the viability of bacteria after incubating for 0, 2, 4, 6, 8, 12, and 24 h by counting the number of colonies on solid media to determine the number of colony-forming units (CFU) mL−1. A ≥2 log10 decrease in CFU mL−1 with combined agents compared with single agents was interpreted as synergism; bactericidal activity was defined as a ≥3 log10 decrease in CFU mL−1 when compared with the number of viable bacteria at the initial time point of 0.15
Quantification of biofilm
We quantified biofilm using a crystal violet assay as previously described41,42,43 with all 29 isolates. In brief, we standardized an overnight culture of the strain of interest to an optical density (OD) of 0.02 at 600 nm (5 × 107 CFU mL−1), added 100 μL aliquots in triplicate to flat-bottomed 96-well polystyrene microtiter plates, and incubated these cultures in the plates at 37°C for 24 h. Subsequently, we fixed the adherent biofilms with crystal violet (0.1%) and then released the dye with 30% (v/v) acetic acid. We measured the resulting absorbance at 560 nm using a microtiter-plate-reading spectrophotometer. All experiments were performed in triplicate and repeated three times.
Effects of aztreonam, ceftazidime/avibactam, Ca-EDTA, and combinations for eradication of in vitro biofilms
We determined the minimum biofilm eradication concentrations (MBECs) of Ca-EDTA, ceftazidime–avibactam, aztreonam, and their combinations on 24 h biofilms of CPKp (n = 29) in vitro as described previously.42,43,44 Treatments covered Ca-EDTA alone or combined with single or multiple agents at 37°C for 6, 12, 24, and 48 h. We determined the effects of eradication on biofilm biovolume and cell viability using crystal violet and PrestoBlue assays in vitro, as described previously.29,42,43
Effect of single and combination of aztreonam, ceftazidime/avibactam, Ca-EDTA, and combinations on in vivo virulence gene expression
The expression level of virulence factors related genes (kfu luxS, mrkD, ompK35, ompK36, uge, wabG and ybtS) were determined with Quantitative RT-PCR (qRT-PCR) using the specific primers as described previously.30,45 All primers are listed in key resources table.
Total mRNA were extracted from thigh tissues of control and experimental groups of in vivo mouse thigh infection model that were challenged with various treatments for 8 h. All samples are analyzed in triplicate. Using the housekeeping gene - 16srRNA for normalization, the relative quantification of gene expression for each treatment group was computed using the ΔΔCT (CT is threshold cycle) method to evaluate and compare fold change differences.
Confocal laser scanning microscopy (CLSM) analysis
To conduct the CLSM analysis, we carefully removed supernatants and subsequently stained the biofilms using a Live/Dead Bacterial Viability kit (Invitrogen) according to the protocols specified by the manufacturer. We used PHLIP, a MATLAB-based tool (without connected volume filtration), to calculate descriptive parameters for biofilms (including biovolume, substratum coverage, area-to-volume ratio, spatial spreading, and 3D colocalization) from the integrated total of each slice of a threshold z stack as described previously.42,43,46 We used the “colocalization in 3D″ value and the parameters “red,” “green,” and “total biovolume” (in μm3) generated by the PHLIP software to calculate the various proportions of green (live bacteria) and red and yellow/colocalized (dead bacteria) biovolumes from the stacks analyzed.42,43,46 A biofilm was considered affected by Ca-EDTA, an antibiotic, or their combination when there was a constant increase in the red + yellow (RY) biovolume fraction within the given antibiotic concentration range. This fraction is at least 80% of the total biovolume.
Quantification and statistical analysis
All statistical analyses were conducted using the R statistical package.32 Data were compared by using either an unpaired two-tailed Student’s t test or unpaired two-tailed Mann–Whitney U test. All data are presented as the mean ± SD. Differences were considered significant when p < 0.05.
Acknowledgments
We thank the staff of the bacteriology division, Department of Microbiology at King Chulalongkorn Memorial Hospital, for providing the K. pneumoniae clinical isolates.
Funding: This work was supported by a grant from the 90th Year Anniversary Ratchadapiseksompotch Endowment Fund from the Faculty of Medicine and Graduate School, Chulalongkorn University, Bangkok, Thailand (batch No. 39 (2/61)). This research is funded by Thailand Science Research and Innovation Fund Chulalongkorn University (CU_FRB65_hea(43)_050_30_31). 90th Year Anniversary Ratchadapiseksompotch Endowment Fund from the Graduate School, Chulalongkorn University, Bangkok, Thailand supported Sasipen Sae-Joo. Aye Mya Sithu Shein was supported by Chulalonslgkorn University (Second Century Fund- C2F Fellowship). Dhammika Leshan Wannigama was supported by Chulalongkorn University (Second Century Fund- C2F Fellowship), the University of Western Australia (Overseas Research Experience Fellowship), and Yamagata Prefectural Central Hospital, Yamagata, Japan (Clinical Residency Fellowship). SMS holds an NHMRC Investigator Grant (2007725) and AK is a Rothwell Family Fellow. The sponsor(s) had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
The study protocol was approved by the Institutional Review Board (IRB) of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (Certificate of approval (COA) No. 045/2020, IRB No. 774/63), and experiments were conducted in compliance with national and international ethical guidelines for human research as specified in the Declaration of Helsinki (1964) and its contemporary (2013) amendments, and comparable ethical standards including The Belmont Report, Council for International Organizations of Medical Sciences (CIOMS) Guidelines, and International Conference on Harmonization in Good Clinical Practice (ICH-GCP). For this retrospective study of anonymized clinical isolates, the requirement for informed consent from patients was waived by the IRB.
All protocols involving animals (C57BL/6 mice) conformed to the revised guidelines of the U.S. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council Guide for the Care and Use of Laboratory Animals, Washington, DC: National Academy Press; 1996, and the Animals for Scientific Purposes Act, 2015 (BE 2558), and experiments were performed following the ethical standards as laid down in the Basel Declaration on the use of animals in research. The Institutional Animal Care and Use Committee (IACUC) of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, approved the protocol (Certificate No. 033/2563, Research Project No. 020/2563) and protocols performed by operators licensed by the Thai Institute for Animals for Scientific Purpose Development and National Research Council of Thailand. The present article was prepared in compliance with the ARRIVE 2.0 guidelines for reporting animal research.
For this retrospective study of anonymous clinical isolates, the requirement for informed consent from patients was waived by the Institutional Review Board (IRB) of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (COA No. 045/2020, IRB No. 774/63).
Author contributions
D.L.W.: Conception, investigation, funding acquisition, data curation, formal analysis, and writing the original draft of the manuscript.
A.M.S.S.: Data curation, formal analysis, editing the original draft of the manuscript, and contributed equally to this work as first authors.
C.H.: Formal analysis, supervision, methodology, validation, critical review, editing of the manuscript, and contributed equally to this work as first authors.
P.N.M.: Supervision, critical review, and editing of the manuscript.
P.H.: Formal analysis, supervision, methodology, validation, critical review, editing of the manuscript, and contributed equally to this work as first authors.
P.P.: Critical review and editing of the manuscript.
W.G.F.D.: Critical review and editing of the manuscript.
P.O.: Critical review and editing of the manuscript.
T.S.: Bioinformatics analysis, methodology, and validation.
N.C.: Confocal laser scanning microscopy data acquisition, curation, methodology, and validation.
P.W.: Bioinformatics analysis, methodology, and validation.
S.L.: In-data acquisition, curation, formal analysis, methodology, validation, critical review, and editing of the manuscript.
S.S-J.: Bacteria identification and clinical collection.
S.N.: Bacteria identification and clinical collection.
U.R.: Bacteria identification and clinical collection.
C.T.: Data acquisition and curation.
N.K.: Bacteria identification and clinical collection.
M.L.: Bacteria identification and clinical collection.
T.L.: Conception for mouse model, formal analysis.
R.K.: Conception for mouse model, formal analysis, supervision, critical review and editing of the manuscript.
R.R.: Ca-EDTA preparation and critical review, editing of the manuscript.
N.N.: Critical review, editing of the manuscript.
S.T.: Critical review, editing of the manuscript.
A.L.: Critical review and editing of the manuscript.
N.K.D.R.: Critical review, editing of the manuscript.
V.N.B.: Ca-EDTA preparation and critical review, editing of the manuscript.
S.M.A.H.R.: Critical review, editing of the manuscript.
T.K.: Critical review and editing of the manuscript.
N.H.: Critical review and editing of the manuscript.
R.J.S.: Critical review and editing of the manuscript.
LC.: Critical review, editing of the manuscript.
M.A.: Supervision, critical review and editing of the manuscript.
H.I.: Critical review, editing of the manuscript.
P.G.H.: Supervision, critical review and editing of the manuscript and contributed equally to this work as first authors.
A.K.: Supervision, critical review and editing of the manuscript.
S.MS: Supervision, critical review and editing of the manuscript.
T.C.: Supervision, critical review and editing of the manuscript.
S.A.: Supervision, conception, investigation, funding acquisition, data curation, critical review and editing of the manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper self-identifies as a gender minority in their field of research. One or more of the authors of this paper self-identifies as a member of the LGBTQIA+ community. One or more of the authors of this paper self-identifies as living with a disability. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Published: June 28, 2023
Contributor Information
Dhammika Leshan Wannigama, Email: dhammika.l@chula.ac.th.
Parichart Hongsing, Email: parichart.hon@mfu.ac.th.
Anthony Kicic, Email: anthony.kicic@telethonkids.org.au.
Tanittha Chatsuwan, Email: tanittha.c@chula.ac.th.
Shuichi Abe, Email: abeshu@icloud.com.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Data were visualized using the ggplot2 3.3.5, packages of R program version 4.1.0.31
All statistical analyses were conducted using the R statistical package.32 Data were compared by using either an unpaired two-tailed Student’s t test or unpaired two-tailed Mann–Whitney U test. All data are presented as the mean ± SD. Differences were considered significant when p < 0.05.
References
- 1.Di Tella D., Tamburro M., Guerrizio G., Fanelli I., Sammarco M.L., Ripabelli G. Molecular Epidemiological Insights into Colistin-Resistant and Carbapenemases-Producing Clinical Klebsiella pneumoniae Isolates. Infect. Drug Resist. 2019;12:3783–3795. doi: 10.2147/idr.S226416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shein A.M.S., Hongsing P., Abe S., Luk-In S., Ragupathi N.K.D., Wannigama D.L., Chatsuwan T. Will There Ever Be Cure for Chronic, Life-Changing Colistin-Resistant Klebsiella pneumoniae in Urinary Tract Infection? Front. Med. 2021;8 doi: 10.3389/fmed.2021.806849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okanda T., Haque A., Koshikawa T., Islam A., Huda Q., Takemura H., Matsumoto T., Nakamura S. Characteristics of Carbapenemase-Producing Klebsiella pneumoniae Isolated in the Intensive Care Unit of the Largest Tertiary Hospital in Bangladesh. Front. Microbiol. 2020;11:612020. doi: 10.3389/fmicb.2020.612020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singkham-in U., Muhummudaree N., Chatsuwan T. In Vitro Synergism of Azithromycin Combination with Antibiotics against OXA-48-Producing Klebsiella pneumoniae Clinical Isolates. Antibiotics. 2021;10:1551. doi: 10.3390/antibiotics10121551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wannigama D.L., Dwivedi R., Zahraei-Ramazani A. Prevalence and Antibiotic Resistance of Gram-Negative Pathogenic Bacteria Species Isolated from Periplaneta americana and Blattella germanica in Varanasi, India. J. Arthropod. Borne. Dis. 2014;8:10–20. [PMC free article] [PubMed] [Google Scholar]
- 6.Daikos G.L., Tsaousi S., Tzouvelekis L.S., Anyfantis I., Psichogiou M., Argyropoulou A., Stefanou I., Sypsa V., Miriagou V., Nepka M., et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob. Agents Chemother. 2014;58:2322–2328. doi: 10.1128/aac.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shein A.M.S., Wannigama D.L., Higgins P.G., Hurst C., Abe S., Hongsing P., Chantaravisoot N., Saethang T., Luk-in S., Liao T., et al. High prevalence of mgrB-mediated colistin resistance among carbapenem-resistant Klebsiella pneumoniae is associated with biofilm formation, and can be overcome by colistin-EDTA combination therapy. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-17083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasmin M., Fouts D.E., Jacobs M.R., Haydar H., Marshall S.H., White R., D’Souza R., Lodise T.P., Rhoads D.D., Hujer A.M., et al. Monitoring Ceftazidime-Avibactam and Aztreonam Concentrations in the Treatment of a Bloodstream Infection Caused by a Multidrug-Resistant Enterobacter sp. Carrying Both Klebsiella pneumoniae Carbapenemase–4 and New Delhi Metallo-β-Lactamase–1. Clin. Infect. Dis. 2020;71:1095–1098. doi: 10.1093/cid/ciz1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shein A.M.S., Wannigama D.L., Higgins P.G., Hurst C., Abe S., Hongsing P., Chantaravisoot N., Saethang T., Luk-in S., Liao T., et al. Novel colistin-EDTA combination for successful eradication of colistin-resistant Klebsiella pneumoniae catheter-related biofilm infections. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-01052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shields R.K., Doi Y. Aztreonam Combination Therapy: An Answer to Metallo-β-Lactamase–Producing Gram-Negative Bacteria? Clin. Infect. Dis. 2020;71:1099–1101. doi: 10.1093/cid/ciz1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw E., Rombauts A., Tubau F., Padullés A., Càmara J., Lozano T., Cobo-Sacristán S., Sabe N., Grau I., Rigo-Bonnin R., et al. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J. Antimicrob. Chemother. 2018;73:1104–1106. doi: 10.1093/jac/dkx496. [DOI] [PubMed] [Google Scholar]
- 12.Guerra M.E.S., Destro G., Vieira B., Lima A.S., Ferraz L.F.C., Hakansson A.P., Darrieux M., Converso T.R. Klebsiella pneumoniae Biofilms and Their Role in Disease Pathogenesis. Front. Cell. Infect. Microbiol. 2022;12:877995. doi: 10.3389/fcimb.2022.877995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall S., Hujer A.M., Rojas L.J., Papp-Wallace K.M., Humphries R.M., Spellberg B., Hujer K.M., Marshall E.K., Rudin S.D., Perez F., et al. Can Ceftazidime-Avibactam and Aztreonam Overcome β-Lactam Resistance Conferred by Metallo-β-Lactamases in Enterobacteriaceae? Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/aac.02243-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayol A., Nordmann P., Poirel L., Dubois V. Ceftazidime/avibactam alone or in combination with aztreonam against colistin-resistant and carbapenemase-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2018;73:542–544. doi: 10.1093/jac/dkx393. [DOI] [PubMed] [Google Scholar]
- 15.Vidaillac C., Benichou L., Duval R.E. In vitro synergy of colistin combinations against colistin-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae isolates. Antimicrob. Agents Chemother. 2012;56:4856–4861. doi: 10.1128/AAC.05996-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoki N., Ishii Y., Tateda K., Saga T., Kimura S., Kikuchi Y., Kobayashi T., Tanabe Y., Tsukada H., Gejyo F., Yamaguchi K. Efficacy of calcium-EDTA as an inhibitor for metallo-β-lactamase in a mouse model of Pseudomonas aeruginosa pneumonia. Antimicrob. Agents Chemother. 2010;54:4582–4588. doi: 10.1128/aac.00511-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshizumi A., Ishii Y., Livermore D.M., Woodford N., Kimura S., Saga T., Harada S., Yamaguchi K., Tateda K. Efficacies of calcium-EDTA in combination with imipenem in a murine model of sepsis caused by Escherichia coli with NDM-1 β-lactamase. J. Infect. Chemother. 2013;19:992–995. doi: 10.1007/s10156-012-0528-y. [DOI] [PubMed] [Google Scholar]
- 19.Banin E., Brady K.M., Greenberg E.P. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl. Environ. Microbiol. 2006;72:2064–2069. doi: 10.1128/aem.72.3.2064-2069.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedenić B., Vranes J., Sviben M., Beader N., Kalenić S. Postantibiotic and post-beta-lactamase inhibitor effect of carbapenems combined with EDTA against Pseudomonas aeruginosa strains producing VIM-metallo beta-lactamases. Chemotherapy. 2008;54:188–193. doi: 10.1159/000140461. [DOI] [PubMed] [Google Scholar]
- 21.Raad I., Hanna H., Dvorak T., Chaiban G., Hachem R. Optimal antimicrobial catheter lock solution, using different combinations of minocycline, EDTA, and 25-percent ethanol, rapidly eradicates organisms embedded in biofilm. Antimicrob. Agents Chemother. 2007;51:78–83. doi: 10.1128/aac.00154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherertz R.J., Boger M.S., Collins C.A., Mason L., Raad I.I. Comparative in vitro efficacies of various catheter lock solutions. Antimicrob. Agents Chemother. 2006;50:1865–1868. doi: 10.1128/aac.50.5.1865-1868.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antimicrobial Resistance Collaborators. Ikuta K.S., Sharara F., Swetschinski L., Robles Aguilar G., Gray A., Han C., Bisignano C., Rao P., Wool E., et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phuengmaung P., Somparn P., Panpetch W., Singkham-In U., Wannigama D.L., Chatsuwan T., Leelahavanichkul A. Coexistence of Pseudomonas aeruginosa With Candida albicans Enhances Biofilm Thickness Through Alginate-Related Extracellular Matrix but Is Attenuated by N-acetyl-l-cysteine. Front. Cell. Infect. Microbiol. 2020;10:594336. doi: 10.3389/fcimb.2020.594336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luk-in S., Chatsuwan T., Kueakulpattana N., Rirerm U., Wannigama D.L., Plongla R., Lawung R., Pulsrikarn C., Chantaroj S., Chaichana P., et al. Occurrence of mcr-mediated colistin resistance in Salmonella clinical isolates in Thailand. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-93529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kueakulpattana N., Wannigama D.L., Luk-in S., Hongsing P., Hurst C., Badavath V.N., Jenjaroenpun P., Wongsurawat T., Teeratakulpisan N., Kerr S.J., et al. Multidrug-resistant Neisseria gonorrhoeae infection in heterosexual men with reduced susceptibility to ceftriaxone, first report in Thailand. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-00675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srisakul S., Wannigama D.L., Higgins P.G., Hurst C., Abe S., Hongsing P., Saethang T., Luk-in S., Liao T., Kueakulpattana N., et al. Overcoming addition of phosphoethanolamine to lipid A mediated colistin resistance in Acinetobacter baumannii clinical isolates with colistin–sulbactam combination therapy. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-15386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singkham-in U., Higgins P.G., Wannigama D.L., Hongsing P., Chatsuwan T. Rescued chlorhexidine activity by resveratrol against carbapenem-resistant Acinetobacter baumannii via down-regulation of AdeB efflux pump. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wannigama D.L., Hurst C., Pearson L., Saethang T., Singkham-In U., Luk-In S., Storer R.J., Chatsuwan T. Simple fluorometric-based assay of antibiotic effectiveness for Acinetobacter baumannii biofilms. Sci. Rep. 2019;9:6300. doi: 10.1038/s41598-019-42353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vuotto C., Longo F., Pascolini C., Donelli G., Balice M.P., Libori M.F., Tiracchia V., Salvia A., Varaldo P.E. Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains. J. Appl. Microbiol. 2017;123:1003–1018. doi: 10.1111/jam.13533. [DOI] [PubMed] [Google Scholar]
- 31.RcoreTeam . R Foundation for Statistical Computing; 2021. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 32.RcoreTeam . 2016. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Version 4.2.2. [Google Scholar]
- 33.Kucharíková S., Vande Velde G., Himmelreich U., Van Dijck P. Candida albicans biofilm development on medically-relevant foreign bodies in a mouse subcutaneous model followed by bioluminescence imaging. J. Vis. Exp. 2015:52239. doi: 10.3791/52239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerca N., Gomes F., Pereira S., Teixeira P., Oliveira R. Confocal laser scanning microscopy analysis of S. epidermidis biofilms exposed to farnesol, vancomycin and rifampicin. BMC Res. Notes. 2012;5:244. doi: 10.1186/1756-0500-5-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacNair C.R., Stokes J.M., Carfrae L.A., Fiebig-Comyn A.A., Coombes B.K., Mulvey M.R., Brown E.D. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat. Commun. 2018;9:458. doi: 10.1038/s41467-018-02875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fothergill J.L., Neill D.R., Loman N., Winstanley C., Kadioglu A. Pseudomonas aeruginosa adaptation in the nasopharyngeal reservoir leads to migration and persistence in the lungs. Nat. Commun. 2014;5:4780. doi: 10.1038/ncomms5780. [DOI] [PubMed] [Google Scholar]
- 37.MacNair C.R., Stokes J.M., Carfrae L.A., Fiebig-Comyn A.A., Coombes B.K., Mulvey M.R., Brown E.D. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat. Commun. 2018;9:458. doi: 10.1038/s41467-018-02875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuen P.S.T., Dunn S.R., Miyaji T., Yasuda H., Sharma K., Star R.A. A simplified method for HPLC determination of creatinine in mouse serum. Am. J. Physiol. Renal Physiol. 2004;286:F1116–F1119. doi: 10.1152/ajprenal.00366.2003. [DOI] [PubMed] [Google Scholar]
- 39.EUCAST . version 6. European Committee for Antimicrobial Susceptibility Testing; 2016. Clinical Breakpoints. [Google Scholar]
- 40.CLSI . 30th edition. Clinical and Laboratory Standards Institute; 2020. Performance Standards for Antimicrobial Susceptibility Testing - Twenty-Fifth Informational Supplement. M100 2020. [Google Scholar]
- 41.O'Toole G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011 doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wannigama D.L., Hurst C., Pearson L., Saethang T., Chatsuwan T., Singkham-In U., Luk-In S., Storer R.J. Simple fluorometric-based assay of antibiotic effectiveness for Acinetobacter baumannii biofilms. Sci. Rep. 2019;9:6300. doi: 10.1038/s41598-019-42353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wannigama D.L., Hurst C., Hongsing P., Pearson L., Saethang T., Chantaravisoot N., Chatsuwan T., Singkham-In U., Luk-In S., Storer R.J. A rapid and simple method for routine determination of antibiotic sensitivity to biofilm populations of Pseudomonas aeruginosa. Ann. Clin. Microbiol. Antimicrob. 2020;19:8. doi: 10.1186/s12941-020-00350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jorge P., Grzywacz D., Kamysz W., Lourenço A., Pereira M.O. Searching for new strategies against biofilm infections: Colistin-AMP combinations against Pseudomonas aeruginosa and Staphylococcus aureus single- and double-species biofilms. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Compain F., Babosan A., Brisse S., Genel N., Audo J., Ailloud F., Kassis-Chikhani N., Arlet G., Decré D. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae. J. Clin. Microbiol. 2014;52:4377–4380. doi: 10.1128/JCM.02316-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Müsken M., Di Fiore S., Römling U., Häussler S. A 96-well-plate–based optical method for the quantitative and qualitative evaluation of Pseudomonas aeruginosa biofilm formation and its application to susceptibility testing. Nat. Protoc. 2010;5:1460–1469. doi: 10.1038/nprot.2010.110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Data were visualized using the ggplot2 3.3.5, packages of R program version 4.1.0.31
All statistical analyses were conducted using the R statistical package.32 Data were compared by using either an unpaired two-tailed Student’s t test or unpaired two-tailed Mann–Whitney U test. All data are presented as the mean ± SD. Differences were considered significant when p < 0.05.