Abstract
A solitary large ulcerated mass is the common morphological feature of diffuse large B‐cell lymphoma (DLBCL) in the large intestine under endoscopy. Here we report a 54‐year‐old man with DLBCL presenting with multiple polypoid lesions in the large intestine, which is an uncommon morphological form of DLBCL.
Keywords: colonic polyps, diffuse large B‐cell lymphoma, endoscopy
The common feature of diffuse large B‐cell lymphoma in the gastrointestinal tract is a solitary large ulcerated mass. Multiple polypoid lesions from the ascending colon to the rectum were found under colonoscopy in a 54‐year‐old man with diffuse large B‐cell lymphoma.
Introduction
Diffuse large B‐cell lymphoma (DLBCL) ranks first among all histological types of gastrointestinal lymphoma. 1 Under endoscopy, DLBCL in the large intestine usually presents as a solitary, large, ulcerated mass. 2 , 3 Here we report a case of DLBCL in the large intestine with a distinct morphology of multiple polypoid lesions.
Case report
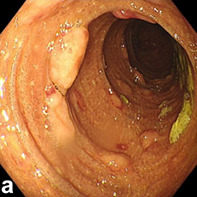
A 54‐year‐old man was admitted to our department complaining of fever and hematochezia for 10 days. He did not suffer abdominal pain or hematemesis. Physical examination revealed cervical and inguinal lymphadenopathies. Blood tests showed a decreased hemoglobin level of 80 g/L (normal value: 130–170 g/L), low platelet count of 35 × 109/L (normal value: 100–300 × 109/L), increased lactate dehydrogenase level of 1001 IU/L (normal value: 120–250 IU/L), and positive Epsten‐Barr virus (EBV) DNA (5.8 × 106 copies/mL). Liver and renal function tests were normal. Computed tomography of the chest and abdomen showed enlarged lymph nodes scattered in the pelvic cavity, retroperitoneum, hepatic hilum and inguinal region, and around the abdominal aorta and iliac vessels. Esophagogastroduodenoscopy showed gastric and duodenal ulcers. Capsule endoscopy found no abnormalities. However, in addition to melanosis coli, colonoscopy revealed multiple polypoid lesions from the descending colon to the rectum (Fig. 1a,b). During colonoscopy, biopsy was performed on polypoid lesions, and the pathology of these lesions revealed DLBCL (Fig. 1c). Immunostaining tested positive for CD20 and a high Ki‐67 proliferation index (60–70%), as well as positive EBV‐encoded RNA (Fig. 1d–f). The cervical lymph node biopsy also confirmed B‐cell lymphoma. Based on these findings, the patient was diagnosed with DLBCL. He thereafter received six cycles of chemotherapy with the R‐CHOP regimen, but eventually died of pneumonia 7 months after diagnosis.
Figure 1.
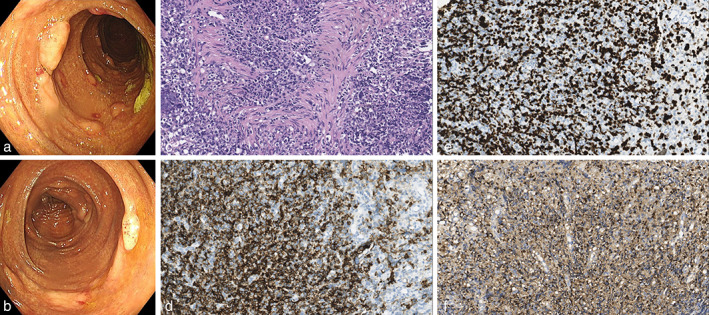
Imaging of colonoscopy and pathology. (a, b) Colonoscopy images showing multiple polypoid lesions. (c–f) Pathology of the intestinal polypoid lesions: (c) HE staining; (d) CD20 staining; (e) Ki‐67 staining; (f) Epstein–Barr virus‐encoded RNA staining. All pathological images are at 400× magnification.
Discussion
Lymphoma is categorized into Hodgkin's lymphoma (10% of all lymphomas) and non‐Hodgkin lymphoma. 4 Extranodal lymphoma refers to lymphomas that occur in organs or tissues beyond the lymph nodes and spleen, and the gastrointestinal tract is the most commonly involved site for extranodal non‐Hodgkin's lymphoma, accounting for 30–40% of all cases. 5 Risk factors for gastrointestinal DLBCL include immune dysfunction, viral infections, and genetic factors. 6 , 7 The symptoms of gastrointestinal DLBCL lymphoma include abdominal pain, weight loss, abdominal mass, as well as gastrointestinal bleeding, perforation, and obstruction. 8 Under endoscopy, DLBCL in the large intestine usually presents as a unique, large, ulcerated mass, whereas multiple polypoid lesions are seldom seen. 2 , 3 When DLBCL presents as multiple polypoid lesions in the gastrointestinal tract, these lesions are not easily detectable by physical examination and abdominal imaging. Therefore, endoscopy has become a pivotal tool for diagnosis in that clinical scenario. The CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen has been the mainstay therapy for DLBCL. 9 As an aggressive lymphoma, DLBCL carries a poor prognosis with a median survival of 15.8 months if untreated. 10 In addition, positive EBV infection might worsen the prognosis of DLBCL. 7 In light of the poor prognosis of DLBCL, it is crucial to promptly confirm the diagnosis of DLBCL, especially for those presenting with multiple polypoid lesions, which would shorten the lengthy duration of diagnosis to initiate the specialized treatment of DLBCL earlier, to improve the clinical outcome.
Acknowledgment
This work was supported by the corporation project of Sichuan University and Zigong (2022CDZG‐26).
Rui Yang and Tian Lan contributed equally to this manuscript.
Declaration of conflict of interest: The authors declare no conflicts of interest.
References
- 1. Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010; 102: 83–87. [PubMed] [Google Scholar]
- 2. Vetro C, Romano A, Amico I et al. Endoscopic features of gastro‐intestinal lymphomas: from diagnosis to follow‐up. World J. Gastroenterol. 2014; 20: 12993–13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Airaghi L, Greco I, Carrabba M et al. Unusual presentation of large B cell lymphoma: a case report and review of literature. Clin. Lab. Haematol. 2006; 28: 338–342. [DOI] [PubMed] [Google Scholar]
- 4. Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non‐Hodgkin lymphoma. Lancet. 2017; 390: 298–310. [DOI] [PubMed] [Google Scholar]
- 5. d'Amore F, Brincker H, Gronbaek K et al. Non‐Hodgkin's lymphoma of the gastrointestinal tract: a population‐based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J. Clin. Oncol. 1994; 12: 1673–1684. [DOI] [PubMed] [Google Scholar]
- 6. Lu YH, Chang ST, Yang SF et al. Primary intestinal diffuse large b‐cell lymphoma in Taiwan showed a relative higher rate of perforation and EBV association. Appl. Immunohistochem. Mol. Morphol. 2016; 24: 541–549. [DOI] [PubMed] [Google Scholar]
- 7. Malpica L, Marques‐Piubelli ML, Beltran BE, Chavez JC, Miranda RN, Castillo JJ. EBV‐positive diffuse large B‐cell lymphoma, not otherwise specified: 2022 update on diagnosis, risk‐stratification, and management. Am. J. Hematol. 2022; 97: 951–965. [DOI] [PubMed] [Google Scholar]
- 8. Li B, Shi YK, He XH et al. Primary non‐Hodgkin lymphomas in the small and large intestine: clinicopathological characteristics and management of 40 patients. Int. J. Hematol. 2008; 87: 375–381. [DOI] [PubMed] [Google Scholar]
- 9. Fisher RI, Gaynor ER, Dahlberg S et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non‐Hodgkin's lymphoma. N. Engl. J. Med. 1993; 328: 1002–1006. [DOI] [PubMed] [Google Scholar]
- 10. Borchmann P, Papadimitrious M, Riou S, Mahlich J, Werner BJB. Survival patients newly diagnosed with diffuse large B‐cell lymphoma (DLBCL)‐Real World Evidence from Germany. Blood. 2022; 140: 7919. [DOI] [PMC free article] [PubMed] [Google Scholar]


