Abstract
Background and Aim
Acute severe ulcerative colitis (ASUC) remains a significant cause of morbidity and healthcare utilization. This study aimed to characterize the total healthcare costs of ASUC, explore factors associated with significant cost over the 12 months following an index admission, and document outcomes including corticosteroid exposure.
Methods
Patients admitted from January 2016 until January 2021 for ASUC to a tertiary inflammatory bowel disease (IBD) center in Australia were identified via retrospective chart review. Costs were calculated over a 12‐month period following index admission.
Results
Seventy‐two patients (30 [42%] female, median age 39 [IQR 27–54] years) were included. The median length of stay of index admission was 6 days (IQR 5–10 days). The median cost of index admission was 7829 AUD, which was driven by the initial length of stay (P < 0.01) and requirement for colectomy (P < 0.01). Median total healthcare cost over the first 12 months was 13 873 AUD (IQR 9684–19 936 AUD), again predominately driven by the length of stay (P < 0.01) and requirement for colectomy (P < 0.01). Median cumulative corticosteroid use over 12 months inclusive of index hospitalization was 1760 mg (IQR 1560–2350 mg). Requirement for inpatient medical salvage therapy with infliximab was associated with increased corticosteroid requirement (P = 0.01).
Conclusion
Healthcare expense related to ASUC remains high, driven predominantly by the length of stay during initial hospitalization and need for colectomy. From a healthcare cost perspective, novel methods to reduce inpatient hospital stay as well as need for colectomy may help reduce the economic and steroid burden of ASUC.
Keywords: acute severe ulcerative colitis, healthcare cost, inflammatory bowel disease, infliximab
This study aimed to characterize the total healthcare costs of ASUC and explore factors associated with this. Overall healthcare expense related to ASUC remains high, driven significanlty by the length of stay during initial hospitalization and need for colectomy.
Background and aim
Acute severe ulcerative colitis (ASUC) remains a significant cause of morbidity, affecting up to 25% of patients with ulcerative colitis (UC). 1 Conventional standard of care for ASUC includes hospitalization for intravenous (IV) corticosteroids, salvage therapy with infliximab or cyclosporin for inadequate response, and colectomy for refractory cases. 2 , 3 The cost of care for patients with active inflammatory bowel disease (IBD) is considerably higher than for those in remission. 4 Characterization of factors associated with cost in the treatment of patients with ASUC has not been previously reported.
Corticosteroid use at diagnosis of UC is high, with over 20% of patients remaining on steroids at 6 months. 5 The adverse effect profile of these drugs necessitates minimization of use beyond initial induction therapy, with alternative strategies for maintenance of remission recommended. As a result, appropriate steroid use is now considered a key performance indicator in the management of UC. 6 There remains a paucity of data regarding repeated steroid use in patients hospitalized with ASUC in the medium to long term.
This study aimed to characterize the total healthcare costs in ASUC, focusing on the primary drivers of cost for both index admission and up to 12 months in a real‐world tertiary center cohort. Furthermore, the cumulative steroid burden during the first 12 months including index hospitalization was explored, alongside factors associated with this.
Methods
We performed a retrospective chart review of all consecutive patients admitted to a single IBD center with ASUC between January 2016 and January 2021. Demographic and clinical information from the index hospitalization and 1 year following it was recorded, including further corticosteroid use (cumulative), re‐admission, colectomy, medical salvage, total biologic exposure, IBD outpatient appointments, IBD nurse helpline utilization, daycase admissions to infusion center, and endoscopic interventions.
Cost data was obtained from a hospital‐based, prospectively maintained database using the International Statistical Classification of Diseases and Related Health Problems (ICD), Australian Classification of Health Interventions (ACHI), and Australian Coding Standards (ACS). Following discharge, we calculated costs of healthcare contact using Australian Medicare Benefits Schedule (MBS) codes for subsequent consultations 7 and Pharmaceutical Benefits Schedule (PBS) costs for the biologics used. 8 We did not bill the cost associated with IBD helpline contacts, nor could we track costs relating to general practitioner contacts. Determinants of length of stay were assessed using multiple logistic regression. Cost of stay was split into quartiles, and then ranked in an ordinal scale with 4 being the top quartile. An ordinal logistic regression was performed to adjust for the impact of variables on the cost. Given the data was collected over a 5‐year period, adjustment for inflation was not done. When comparing the median cost of admission for patients requiring salvage therapy versus without, the Shapiro–Wilk test was used to determine normality of the data, followed by a two‐sided t‐test for significance. P‐values <0.05 were considered significant. All analyses were performed in R studio build 554 (RStudio Team 2020, Boston, MA).
Corticosteroid burden over 12 months was converted into an equivalent oral prednisolone in milligrams, with cumulative burden placed into quartiles and ranked from lowest to highest. The data were analyzed using ordinal logistic regression to estimate the effect of each variable on total corticosteroid exposure to produce odds ratios (ORs) with 95% confidence interval (CI). The parallel regression assumptions were tested to ensure that the relationship between the coefficients and corticosteroid exposure was true for all quartiles. Corticosteroid‐free clinical remission (CFCR) was defined as a partial Mayo score ≤2 in the absence of corticosteroid use.
Results
Seventy‐two patients (30 [42%] female, median age 39 (IQR 27–54 years) were identified with ASUC and included in the study (Table 1). All patients received 100 mg intravenous hydrocortisone q6 hourly from admission, with daily review by IBD and colorectal surgical teams. Non‐responders at day 3 according to the Modified Oxford Criteria were administered infliximab.
Table 1.
Patient characteristics and outcomes
| Characteristics/clinical outcomes | |
|---|---|
| Patients, n | 72 |
| Female, n (%) | 30 (42) |
| Age, median (range) | 39 (18–70) |
| Weight, median, kg | 76 |
| Prior diagnosis, n (%) | 66 (92) |
| Disease extent at diagnosis, n (%) | E3–30 (42) |
| E2–32 (44) | |
| E1–10 (14) | |
| 5‐ASA prior to admission, n (%) | 41 (57) |
| Immunomodulator prior | 15 (21) |
| Biologic prior | 15 (21) |
| CRP on admission, median | 38 |
| Albumin on admission, median | 33 |
| Endoscopic severity on admission, n (%) | M1–3 (4) |
| M2–29 (40) | |
| M3–40 (56) | |
| Median length of stay, n, days (IQR) | 6 (5–10) |
| Infliximab salvage, n (%) | 34 (47) |
| Infliximab at 1 year, n (%) | 32 (44) |
| Vedolizumab at 1 year, n (%) | 12 (17) |
| Colectomy during index admission, n (%) | 4 (6) |
| Colectomy at 1 year, n (%) | 10 (14) |
The median length of stay of index admission was 6 days (IQR 5–10 days). Thirty‐four patients (47%) required medical salvage therapy with infliximab during index admission, with 6 (18%) receiving 10 mg/kg dosing and 12 (35%) requiring a second dose of infliximab during index admission. Completion of induction with infliximab with three doses was administered between 3 and 6 weeks following the initial dose. Twenty‐two (65%) of these patients continued maintenance infliximab therapy after induction. At 6 months, CFSR was seen in 42 patients (58%): 20 patients who received infliximab salvage therapy at index admission, and 22 who did not.
Twelve patients received vedolizumab in the first year following ASUC: 4 patients (6%) switched after infliximab induction and 8 (11%) patients who did not require medical salvage. Two patients experienced infusion‐related adverse events, both mild. Four patients (6%) required colectomy during index admission and 6 more (8%) in the following 12 months. Twenty‐six patients (37%) required re‐admission to hospital (14 due primarily to UC, 2 due to wound infection, and 10 for other indications), with 2 requiring infliximab salvage therapy and 18 requiring more than one re‐hospitalization in the first 12 months after ASUC. Three other patients experienced gastrointestinal infection, all mild and not requiring admission. There was no significant difference in re‐admission, subsequent colectomy, or rate of CFSR between patients who received salvage medical therapy on index admission and those who did not, or between patients who continued maintenance infliximab or ceased after induction. The median number of interactions with the IBD team in the following year was 12. This includes daycase endoscopy (median 2) and infusion (median 2), IBD nursing Helpline utilization (median 2), and outpatient appointments (median 6).
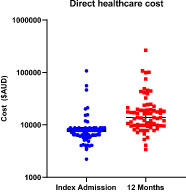
Median cost of index admission was 7829 AUD (IQR 6067–8764 AUD) (Fig 1). On multivariate analyses, this was significantly associated with the initial length of stay (P < 0.01) and the requirement for colectomy (P < 0.01) (Table 2). There was a trend toward higher cost associated with C‐reactive protein (CRP)/albumin ratio (P = 0.13). Prior exposure to biologic therapy (P = 0.30), Mayo 2 (P = 0.61) or 3 (P = 0.30) colitis on index endoscopy (P = 0.30), need for salvage therapy during initial hospitalization ( P = 0.61), and CRP on admission (P = 0.94) were not associated with cost.
Figure 1.

Healthcare cost during index hospitalization and over 12 months.
Table 2.
Predictors of cost of index admission
| Predictors | Odds ratio | 95%CI | P‐value |
|---|---|---|---|
| Length of stay | 1.22 | 1.16–1.58 | <0.01 |
| Need for colectomy | 0.96 | NA | <0.01 |
| Need for infliximab salvage Therapy | 0.75 | 0.75–1.23 | 0.61 |
| CRP on admision | 1.00 | 0.97–1.03 | 0.94 |
| Mayo 2 endoscopic score | 4.19 | 0.29–107 | 0.61 |
| Mayo 3 endoscopic score | 2.12 | 0.29–11.3 | 0.30 |
| Prior biologic exposure | 0.47 | 0.12–1.85 | 0.30 |
| CRP/albumin ratio | 0.72 | 0.27–1.40 | 0.13 |
Median total healthcare cost over the first 12 months was 13 873 AUD (IQR 9684–19 936 AUD). On multivariate analyses, this was again significantly associated with the initial length of stay (P < 0.01) and requirement for colectomy (P < 0.01) (Table 3). There was a trend toward an association between healthcare cost and cumulative steroid burden over 12 months (P = 0.15). Need for salvage therapy during initial hospitalization (P = 0.58), need for re‐hospitalization (P = 0.45), and prior exposure to biologic therapy (P = 0.50) were not associated with cost.
Table 3.
Predictors of cost over 12 months
| Predictors | Odds ratio | 95%CI | P‐value |
|---|---|---|---|
| Length of stay | 1.32 | 1.15–1.54 | <0.01 |
| Need for colectomy | 0.76 | NA | <0.01 |
| Need for infliximab salvage Therapy | 0.74 | 0.23–2.3 | 0.58 |
| Need for re‐admission | 0.88 | 0.60–1.28 | 0.45 |
| Prior biologic exposure | 0.72 | 0.16–3.01 | 0.50 |
| Total steroid exposure | 0.99 | 0.99–1.0 | 0.15 |
Patients requiring medical salvage therapy during initial hospitalization had median costs of 17 170.04 AUD, compared with those who were not treated with rescue medical therapy (13 257.99 AUD, P = 0.06).
Median cumulative corticosteroid use over 12 months inclusive of index hospitalization was 1760 mg (IQR 790 mg) (Fig 2). The requirement for inpatient medical salvage therapy with infliximab was significantly associated with increased corticosteroid requirement (P = 0.01) (Table 4). Corticosteroid use was not associated with the length of inpatient stay (P = 0.36), requirement for colectomy (P = 0.37), need for re‐admission (P = 0.83), or prior biologic therapy (P = 0.61). Parallel regression assumption held true, ensuring the model was accurate and the coefficients had the same effect on each quartile of cumulative corticosteroid burden.
Figure 2.

Cumulative steroid exposure over 12 months from index presentation.
Table 4.
Predictors of corticosteroid use (in quartiles) in the 12 months following admission
| Predictors | Odds ratio | 95% CI | P‐value |
|---|---|---|---|
| Length of stay | 1.02 | 0.97–1.09 | 0.36 |
| Infliximab salvage therapy | 3.91 | 1.43–11.14 | 0.01 |
| Colectomy | 0.44 | 0.07–2.55 | 0.37 |
| Re‐admission | 0.95 | 0.06–1.41 | 0.83 |
| Previous biologic exposure | 6.2 | 0.29–107 | 0.61 |
Discussion
Despite advances in the assessment of severity and medical salvage therapy for patients with ASUC, there remains a paucity of data regarding cumulative real‐world healthcare burden, specifically regarding cost and steroid exposure during the index hospitalization and over the subsequent medium to long term. This real‐world retrospective study shows a significant healthcare cost, predominately driven by the length of stay. Almost half of the patients required infliximab salvage therapy, with 14% requiring colectomy in the first 12 months. Although we saw a trend toward medical salvage associated with increased cost of initial admission, this did not reach significance. Total corticosteroid exposure was associated with the requirement for salvage therapy. Overall, the total healthcare expense over 12 months was primarily driven by the length of stay but not administration of salvage medical therapy during index hospitalization. Hence, strategies to reduce the duration of initial hospitalization may reduce the healthcare burden associated with ASUC.
We have shown that patients presenting with ASUC carry a significant cost burden not only during index admission but also in the following 12 months. As the cost of biologic salvage therapies decreases, 9 the primary driver of cost has become the duration of inpatient stay. In the era of COVID‐19, there is added pressure on hospital systems worldwide relating to challenges with hospital bed availability. Strategies aiming to reduce the duration of stay include ambulatory models of care, focusing on outpatient delivery of daily intravenous corticosteroid and medical assessment, and show similar observational outcomes to the current gold standard. 10 Early initiation of medial salvage, as well as novel options for salvage therapy, are avenues for further research on feasibility, safety, and efficacy.
Median corticosteroid use was 1760 mg over a 1‐year period, equating to 44 days of prednisolone dosed at 40 mg daily. This was associated with the trequirement for medical salvage therapy during initial hospitalization, likely a marker of more severe disease phenotype. Most patients were de‐escalated to oral steroids between days 3 and 5 of admission, in line with hospital procedures, with weaning of prednisolone dose from 40 mg over 4–8 weeks. Hence, this degree of corticosteroid exposure resulted from repeated courses of prednisolone. Previous studies have shown that as little as 30 days of corticosteroid use over a 1‐year period leads to increased risk of sepsis, venous thromboembolism, and fracture. 11 The majority of patients in our cohort experienced a cumulative steroid exposure, placing them at increased risk of multiple adverse health outcomes. This has not previously been documented and is an area that requires further exploration via increased use of steroid‐sparing strategies.
Several limitations of this study must be acknowledged. Data regarding cost were collected retrospectively from a single center in the context of the Australian public healthcare system, and hence may not be applicable across other jurisdictions, with significant variability expected for cost associated with consultations, medications, pathology, radiology services, and hospitalizations. Furthermore, owing to the relatively small numbers, our data may not have been adequately powered to detect significant factors associated with cost, an issue addressible with further multicenter studies in this area.
Conclusion
In conclusion, healthcare expense due to inpatient hospitalization with ASUC, as well as over the first 12 months, is associated with the initial length of hospitalization and the need for colectomy. Median corticosteroid burden is high and is in turn associated with the need for infliximab salvage therapy. Further studies aimed at reduction of steroid burden and length of inpatient stay for patients with ASUC are warranted. The safety and efficacy of early initiation of medical salvage therapy, or exploration of the safety of ambulatory care pathways for patients with ASUC, may address these issues.
Acknowledgment
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Declaration of conflict of interest: JPS has received speaker fees for Jannsen, Takeda, and Abbvie and also an unrestricted research grant from Tillots. MG has served on the Advisory Boards of Pfizer and Pharmacosmos and has received speaker fees, research or travel grants from Abbvie, Celltrion, Dr. Falk, Janssen, and Pfizer.
Author contribution: Robert Gilmore, Jonathan P. Segal, Syeda T. Karim, and Mayur Garg contributed to study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and study supervision. Rachael Cheong, Deborah Farrah, Soleiman Kashkooli, and Sheng Wei Lo contributed to acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Financial support: No specific funding has been received for this work.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Dinesen L, Walsh A, Protic M et al. The pattern and outcome of acute severe colitis. J. Crohns Colitis. 2010; 4: 4–437. [DOI] [PubMed] [Google Scholar]
- 2. Laharie D, Bourreille A, Branche J et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open‐label randomised controlled trial. Lancet (London, England). 2012; 380: 380–1915. [DOI] [PubMed] [Google Scholar]
- 3. Turner D, Walsh C, Steinhart A, Griffiths A. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta‐regression. Clin. Gastroenterol. Hepatol. 2007; 5: 5–110. [DOI] [PubMed] [Google Scholar]
- 4. Jackson B, Con D, Ma R, Gorelik A, Liew D, de Cruz P. Health care costs associated with Australian tertiary inflammatory bowel disease care. Scand. J. Gastroenterol. 2017; 52: 851–6. [DOI] [PubMed] [Google Scholar]
- 5. Okayasu M, Ogata H, Yoshiyama Y. Use of corticosteroids for remission induction therapy in patients with new‐onset ulcerative colitis in real‐world settings. J. Mark. Access. Health Policy. 2019; 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blackwell J, Selinger C, Raine T, Parkes G, Smith MA, Pollok R. Steroid use and misuse: a key performance indicator in the management of ibd. Frontline Gastroenterol. 2021; 12: 12–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mbs online. http://www9.health.gov.au/mbs/fullDisplay.cfm?type=item&q=110&qt=item 2022.
- 8. Care AGDoHaA . Pharmaceutical benefits scheme (pbs)|. https://www.pbs.gov.au/medicine/item/10057H-10067W-10184B-10196P-11389K-11396T-11399Y-11400B-11412P-11423F-11424G-11432Q-11445J-11448M-11449N-11450P-11459D-11461F-11481G-11482H-11483J-11486M-11487N-11488P-11489Q-11490R-11497D-11498E-11514B-11515C-11590B-11595G-11605T-11606W-11796W-11797X-12717H-12751D-4284L-5753T-5754W-5755X-5756Y-5757B-5758C-6397Q-6448J-6496X-9612X-9613Y-9617E-9654D-9674E, 2022.
- 9. Gleeson D, Belinda T, Lopert R, Lexchin J, Moir H. Financial costs associated with monopolies on biologic medicines in Australia. Aust. Health Rev. 2019; 43: 36‐42. [DOI] [PubMed] [Google Scholar]
- 10. Sebastian S, Patel KV, Segal JP et al. Ambulatory care management of 69 patients with acute severe ulcerative colitis in comparison to 695 inpatients: insights from a multicentre UK cohort study. BMJ Open. Gastroenterol. 2022; 9: e000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waljee AK, Rogers MAM, Lin P et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017; 357: j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


