Abstract
Background and Aim
Biliary obstruction causes bacteriobilia and significant morbidity and high mortality, which necessitates prompt and effective treatment for a good clinical outcome. Hence, the aim of this study was to determine updated knowledge of biliary microbial spectrum, antibiotic sensitivity pattern, and key clinical factors of bacteriobilia.
Methods
This is a prospective study conducted during the period between November 2021 and December 2022 at Ibn Sina specialized hospital, Khartoum, Sudan, on 50 patients diagnosed with obstructive jaundice and symptomatic bacteriobilia who underwent open biliary surgeries electively. Bile samples were aspirated intra‐operatively and cultured, and antibiotic sensitivity tests were performed.
Results
Fifty‐four percent of patients diagnosed with obstructive jaundice who underwent elective open biliary surgeries were males with the ratio (2:1). Forty‐six percent of patients were between 61 and 75 years (elderly). The most frequent cause of obstructive jaundice was migrating biliary stones (48% of cases). Thirty‐two percent of patients were diabetic with bacteriobilia. The predominant isolated bacterial pathogen in this study was Escherichia coli (36% of cases). These biliary pathogens were sensitive to meropenem in 54% of cases and ciprofloxacin in 46%. Eventually, in all patients in this study, biliary bacterial pathogens were found to be resistant to a broad spectrum of antibiotics.
Conclusion
Careful selection of empirical antibiotic therapy based on surveillance of routine bile cultures during biliary tree procedures in patients with high risk of bacteriobilia will potentially help in improving the surgical outcomes and optimizing treatment of acute cholangitis, which is associated with high mortality.
Keywords: antibiotics, bacteriobilia, bile culture, cholangitis, obstructive jaundice, Sudan
This prospective study showed careful selection of empirical antibiotic therapy based on surveillance of routine bile cultures during biliary tree procedures in patients with high risk of bacteriobilia will potentially help in improving the surgical outcomes and optimizing treatment of acute cholangitis, which is associated with high mortality.

Introduction
Bile ducts are sterile in healthy persons. This is related to the anatomic and physiologic barriers that prevent bacterial entry into the bile and limit the translocation of bacterial endotoxins. Anatomic barriers include tight junctions between hepatocytes, Kupffer cells, sphincter of Oddi, and excreted immunoglobins (predominantly IgA); physiological barriers include the composition of bile salts and constant bile flow which flushes any contaminating bacteria. 1 Bacteriobilia (positive bile culture) is defined as the presence of microorganisms in the bile that is detected by at least one positive bile culture and not necessarily be followed by clinical manifestations. The most widely described risk factors of bacteriobilia in the literature are (i) previous biliary tree manipulation such as endoscopic retrograde cholangiopancreatography (ERCP); (ii) common bile duct (CBD) stones with bacteriobilia (up to 30%); (iii) associated comorbidities or a high ASA classification; (iv) male gender; (v) advanced age (>60 years) leading to altered immune response; and (vi) complicated biliary obstruction, which is characterized by fever, palpable gallbladder, elevated serum alkaline phosphatase (ALP), and leukocytosis. 2 , 3 , 4 , 5 , 6 Obstructed biliary tree with stagnant bile and choledocholithiasis are reported as the leading causes for acute (ascending) cholangitis, with a mortality rate of 5–12%. This is related to impaired bile flow with stasis, acute mucosal cytokine production, and increased intra‐biliary pressure. Then, pathogenic bacteria will gain access to biliary ducts through major duodenal papilla, portal venous system, hepatic secretion, and periductal lymphatic routes, which will result in infected bile, thus increasing the rate of complications such as bacteremia, wound infection, and intra‐abdominal sepsis. 7 , 8 , 9 , 10 In 1877, the clinical manifestations of acute cholangitis were described by Charcot as a triad of fever/chills, right upper quadrant or epigastric pain, and jaundice. 10 However, Charcot's triad has low sensitivity (26%) in the diagnosis of acute cholangitis. Hence, new criteria were established by the Tokyo guidelines, which have an overall sensitivity and specificity of 92 and 78%, respectively. These new criteria included laboratory and clinical signs of systemic inflammation, in addition to cholestasis and imaging evidence of biliary obstruction. 11 Persistence of biliary obstruction leads to inaccurate antibiotic concentration and excretion, particularly if associated with high bilirubin, low albumin, and tachycardia. This necessitates prompt biliary decompression and the empirical use of broad‐spectrum antibiotics for systemic infection. If these appropriate treatments are not given, septic shock will lead to significant rise in the mortality rate up to 88–100%. 3 , 8 Furthermore, inappropriate empirical therapy leads to antimicrobial resistance through mechanisms including alteration of the antibiotic active binding site, restriction of antibiotic cell entry, active antibiotic extrusion through an efflux pump, and enzymatic inactivation of antibiotics. 12 , 13 Therefore, identification of the causative microorganism and appropriate choice of antibiotics are essential in the acute cholangitis management and reduction of mortality, especially in the elderly. 14 , 15 Bile cultures provide an opportunity to detect the pathogenic bacteria, antibiotic susceptibility, and resistance profile even in cases with negative blood cultures. Positive rates of bile cultures range between 59 and 93% in acute cholangitis patients. This acceptable high sensitivity rate is achieved by directly obtaining bile from the inflammatory site for microbiological analysis. 12 , 16 Some studies have reported a strong relation between bacteriobilia related to obstructive jaundice and postoperative complications such as wound infection. 17 , 18 The commonly described bacteria associated with biliary tree infections is gram‐negative microorganisms including Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii, and Salmonella typhi. Enterococcus spp. are the most commonly observed gram‐positive microorganisms. 19 , 20 , 21 Gram‐positive bacterial infection has attracted attention recently because it is highly detected in cholangitis of liver transplantation patients. 22 , 23 , 24 Mohammed et al. showed that the consequences of bacteriobilia can be prevented by pre‐operative and postoperative antibiotics that act on the common digestive tract pathogens such as enterobacters and anaerobes. 25 Also, Herzog et al. recommended bile cultures during biliary surgeries for better antibiotic sensitivity and prophylaxis from infective complications. 26 Studies in the literature give no specific data comparing differences of bacteriobilia infection between the countries worldwide. Moreover, there are few studies focused on bile microbiology during biliary tree procedures with high risk of bacteriobilia, and the choice of optimum antibiotic regimens remains a challenge to clinicians due to the lack of agreement on unique regimens for isolated biliary microorganisms. 2 Hence, the aim of this study was to update the knowledge of biliary microbial spectrum, antibiotic sensitivity patterns, and key clinical factors of bacteriobilia that are associated with symptomatic obstructive jaundice, to optimize the therapeutic use of antibiotics in times of increasing antibiotic resistance and reach appropriate empirical therapy for biliary infections to reduce morbidity in Sudanese patients.
Methods
This is a prospective, observational, analytic, cross‐sectional, hospital‐based study conducted during the period between November 2021 and December 2022. The study received no grant funding and was conducted in the gastroenterological surgical department of Ibn Sina specialized hospital, Khartoum, Sudan. Our research complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Before the study commenced, ethical approval was obtained from the local research ethics committee of the Faculty of Medicine and Health Sciences, Omdurman Islamic University (committee meeting no. 191 dated 14 October 2021), to use the Sudanese patients after they have provided written and signed consent to publish this original article for the purpose of research and to collected medical data from the international medical websites and medical literature. No clinical trials were involved in this study. A written consent was taken from each patient. Data in this study, including demographic characteristics, clinical presentation, modalities of investigation, primary disease, comorbidities, risk factors for symptomatic bacteriobilia, prior biliary interventions (ERCP/percutaneous transhepatic biliary drainage [PTBD]), indications for surgical approaches, postoperative complications, days of postoperative hospital stay, and postoperative deaths, were collected through a questionnaire. Also, laboratory variables, such as WBC count, total bilirubin, ALP, serum albumin, and C‐reactive protein, at admission were included. Inclusion criteria were the following: Sudanese patients aged 18 to 75 years or more of either sex who were clinically diagnosed with obstructive jaundice with symptomatic bacteriobilia and acute cholangitis confirmed by trans‐abdominal ultrasound (TUS) and magnetic resonance cholangiopancreatography (MRCP). These patients underwent various procedures ranging from preoperative drainage to simple open cholecystectomy and CBD exploration and surgeries of hepato‐biliary‐pancreatic malignancies. Bile samples were aspirated intraoperatively before stab incision of the CBD using syringe size 10 ml and placed immediately on a sterile 20‐ml propylene bottle. Then, the samples were sent to the microbiology laboratory service as soon as possible (within 10 min) and incubated in a bacteriologic bile culture oven at 37°C for 24 h. No blood samples were taken from the patients, nor were any blood cultures performed in this study. All bile cultures were positive, and bacterial colonies were identified using routine biochemical methods used in a standard clinical microbiology laboratory. The patients were divided according to the culture results into bile‐positive and bile‐negative groups. The microbiological variables were the isolated causative microorganisms, antibiotic use upon admission, prophylactic antibiotic use, and initial antibiotic resistance for microorganisms of the bile cultures. All patients were hemodynamically stable and did not need ICU admission. Therefore, bacterial infection control regimens in the ICU are not discussed in this study. Exclusion criteria included the use of antibiotics within 7 days prior to surgical interventions or at induction of surgery, laparoscopic cholecystectomy, and nonelective surgeries. Also, all patients with incomplete records, those who were admitted outside the time zone of the study, and those with any other comorbidity that precluded general anesthesia (GA) were excluded from this study. The clinical details of all patients included were entered into a spread sheet (Excel 2016 for Windows). This data were statistically analyzed by using the computer program Statistical Package for Social Sciences (SPSS) version 21 (IBM SPSS Statistics for Windows, Armonk, NY, IBM Corp, USA). For comparison between groups, t‐test was conducted. The statistical results were considered significant when the P‐value was <0.05.
Results
Fifty patients were included in this study. These patients were diagnosed with obstructive jaundice and underwent elective open biliary surgeries. Twenty‐seven were males (54%) and 23 were females (46%) (Fig. 1). The patients′ age ranged from 61 to 75 years, representing 23 patients (46%) (Fig. 2). In this study, all patients (100%) were diagnosed clinically with obstructive jaundice. The duration of jaundice was as follows: within 3 months in 22 patients (44%); less than 1 month in 19 patients (38%), and more than 3 months in 9 patients (18%). The causes of jaundice were migrating biliary stones in 24 cases (48%), malignant obstruction in 23 cases (46%), and begin stricture in 3 cases (6%). Moreover, there were different cancers resulting in malignant biliary obstruction: 13 patients had cancer in the pancreatic head; 5 patients had cholangiocarcinoma; and 5 patients had ampullary cancer. In this study, 16 diabetic patients (32%) were diagnosed with obstructive jaundice due to bacteriobilia. Seventeen patients (34%) had bacteriobilia related to a pervious history of cholangitis. Total bilirubin was <10 mg/dl in 19 patients (38%), 10–20 mg/dl in 18 patients (36%), and >20 mg/dl in 13 patients (26%) (Fig. 3). Abdominal ultrasound, abdominopelvic CT scan, and MRCP were carried out in all patients (100%). Preoperative biliary drainage was carried out for 18 patients (36%), 16 had ERCP/stent, and two had PTBD. Also, the standard sterilization method for ERCP‐scope was done through meticulous washing cycles by special disinfectants and autoclaving for 20 min. The duration of biliary drainage ranged from 1 to 6 weeks in 13 patients (26%). The isolated bacterial microorganisms included E. coli in 18 cases (36%), Klebsiella spp. in 7 cases (14%), Pseudomonas aeruginosa in 6 cases (12%), Staphylococcus aureus in 1 case (2%), and C. freundii in 1 case (2%) (Fig. 4). There were no bacterial growth in 17 patients (34%). Eventually, in all patients of this study, biliary bacterial pathogens were found to be resistant to a broad spectrum of antibiotics throughout the study period, and no changes were noticed in the resistance patterns for these antibiotics over time. These included cefixime in 19 patients (38%), ceftriaxone in 13 patients (26%), amoxyclav in 7 patients (14%), cefotaxime in 7 patients (14%), and ceftazidime in 4 patients (8%) (Fig. 5). The results of this study were statistically significant (P‐value < 0.05).
Figure 1.
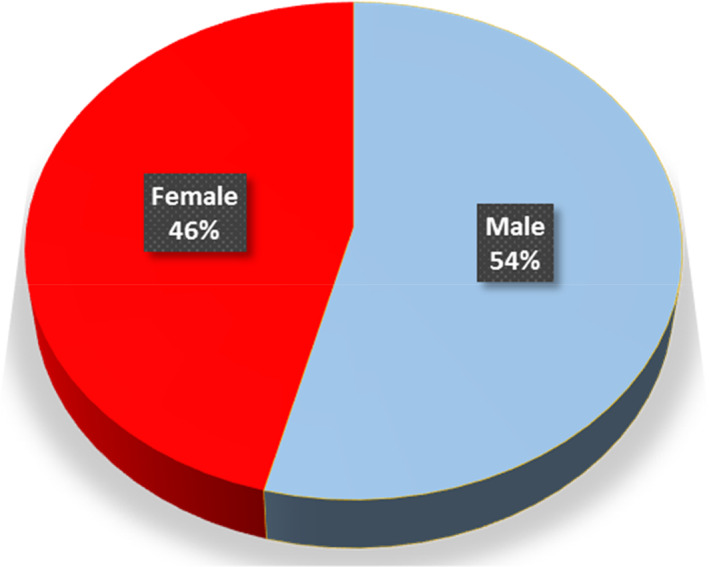
Gender distribution of patients with bacteriobilia.
Figure 2.
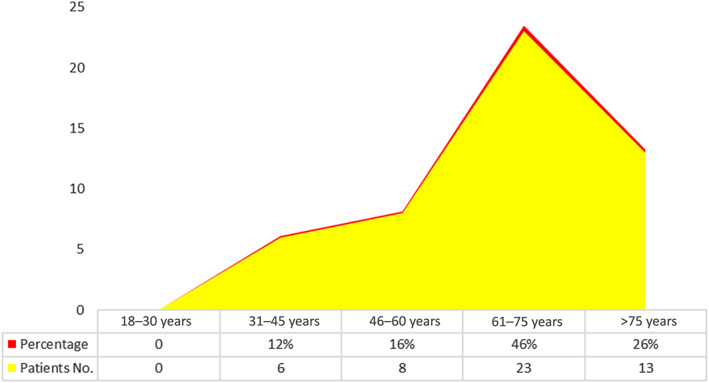
Age distribution of patients with bacteriobilia.
Figure 3.
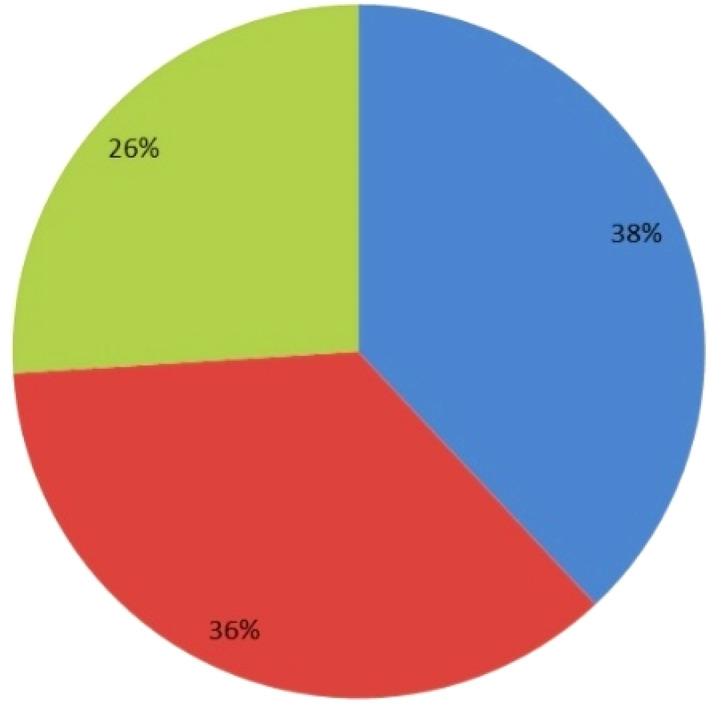
Total bilirubin levels for patients with obstructive jaundice and bacteriobilia. ( ), <10 (n = 19); (
), <10 (n = 19); ( ), 10–20 (n = 18); (
), 10–20 (n = 18); ( ), >20 (n = 13)
), >20 (n = 13)
Figure 4.
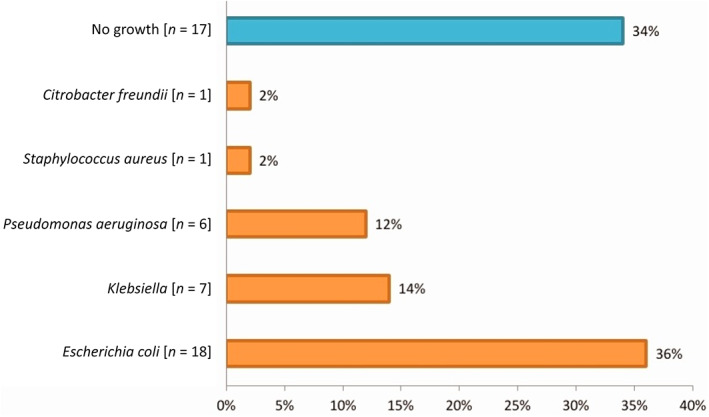
Isolated bacterial microorganisms in bile cultures of patients with obstructive jaundice.
Figure 5.
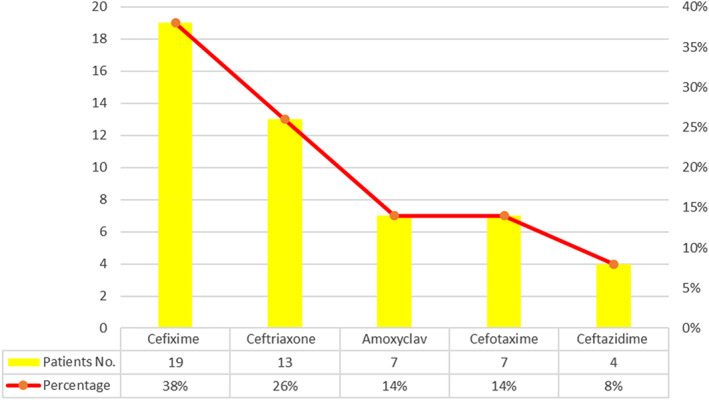
Antibiotic resistance for bacterial pathogens.
Discussion
Normally, bile is sterile because of various anatomical and physiological protective mechanisms keeping it free of organisms. Partial or complete biliary obstruction is common and affects a large portion of the world population, causing increased intrabiliary pressure, bacteremia, impaired intestinal wall barrier function, and significant morbidity and high mortality. 1 The intraoperative bacterial isolation in the bile culture continues to be a subject of debate. Therefore, focusing on the microbiological analysis of pathogenic organisms in bile cultures, identification of the risk factors due to their presence in the bile, antibiotic sensitivity patterns, and resistance profiles may be valuable tools that could lead to appropriate choice of antibiotic therapy in patients with bacterial cholangitis complicating obstructive jaundice. 20 , 27 Our study revealed that symptomatic bacteriobilia is more common in male patients with obstructive jaundice (54 vs 46%) in the ratio 2:1. This is consistent with the literature, with Mehta et al. reporting that positive bile culture is more frequent in males than in females (52 vs 48%). 28 The mean age for bacteriobilia associated with obstructive jaundice was between 61 and 75 years, representing (46%) of cases. This is in agreement with the results of other studies in the literature of reported cases of bacteriobilia in elderly patients above 70 years. 11 , 29 This is related to the altered immune response in the elderly patients. Bacteriobilia was more prevalent in patients with migrating biliary stones (48%). Our results similarly agree with the findings of Kiriyama et al. and Sharma et al. who proposed partial or intermittent nature of benign biliary obstruction facilitating bacterial colonization in the bile. 30 , 31 Moreover, Arima et al. reported that bacterial cholangitis caused by impacted bile duct stones is a serious condition despite emergency biliary decompression. 32 This is related to an abrupt increase of intrabiliary pressure, which leads to septicemia and endotoxemia by adverse effects on the defensive mechanisms. 33 Type‐2 diabetes mellitus (DM) patients had higher infection rates with poorer prognosis. As a complication of choledocholithiasis, they have more severe forms of bacterial cholangitis as compared to nondiabetic individuals. In this study, the coexistence of diabetes mellitus was found to be a risk factor for positive bile culture in 32% of cases. This agrees with the literature that diabetes mellitus is significantly associated with hyperglycemia in bile metabolism and is more susceptible to high bacterial growth. 34 This is relevant to patients with type‐2 DM who have poor glycemic control and diabetic neuropathy. Also, type‐2 DM is associated with reduced response of T cells and neutrophils, impaired phagocytosis, intracellular killing, and leukocyte chemotaxis. 11 Our study showed that the positive results of bile culture is significantly associated with a confirmed history of ascending cholangitis in 34% of patients. These observations were in agreement with the study of Sharma et al. who reported bacteriobilia was found more often in patients with a pervious history of cholangitis (47%) than those without (19%). 31 In this study, bacteriobilia was identified through ERCP/stent or PTBD in 36% of cases. This is in agreement with the results of Negm et al., who showed that risk factors of bacteriobilia include biliary stenting and repeated biliary interventions resulting in contamination of bile ducts by intestinal bacteria. 20 Microbiological analysis of the bile culture is a valuable diagnostic tool that helps to establish antibiotic guidelines before biliary surgical procedures associated with bacteriobilia. In this study, E. coli was the predominant pathogen isolated from bile cultures (36%) followed by Klebsiella species (14%), P. aeruginosa (12%), S. aureus (2%), and C. freundii (2%). These results are consistent with international studies that reported E. coli and Klebsiella species are the frequently isolated bacteria in bile cultures. 20 , 21 , 27 Sharma et al. found, in the bile cultures, E. coli (50%), Pseudomonas (25%), Klebsiella (7%), Citrobacter (7%), and Proteus (6%). 31 Also, Bajaj et al. found that the commonest bacteria that were isolated from bile culture were E. coli (47.94%), K. pneumoniae (17.53%), P. aeruginosa (16.49%), and Enterococcus spp. (11.34%). 35 Moreover, other studies in the literature have detected pathogens in bile specimens most frequently as follows: E. coli (40.5%), Enterococcus spp. (faecalis, faecium) (38.1%), and Klebsiella (23.8%). 12 , 29 Another study found that the gram‐negative enteric organisms formed 84.3% of the bile isolates in which P. aeruginosa (35.7%), E. coli (29.4%), Klebsiella spp. (21.5%), and Citrobacter spp. (5%) were the most common organisms. 8 These findings are consistent with our results. This is due to the careful setup in our microbiology laboratory, which led to better pathogen isolation without any delay due to transportation. Our results are consistent with other international studies that reported E. coli as the most common bacterial pathogen found in the bile of malignant biliary obstruction, diabetic patients, and after ERCP intervention. 17 , 18 , 19 , 20 , 21 Also, E. coli was found to have a strong association with ascending cholangitis, which leads to poor clinical outcome. We carried out extensive search of published studies in the literature but could not find any specific findings describing poor clinical outcome or poor sepsis control of E. coli. Based on antibiotic sensitivity tests, we found that biliary bacterial microorganisms were sensitive to meropenem in 54% of cases and ciprofloxacin in 46% of cases. There have been reports in the literature on antibiotic sensitivity toward meropenem, amikacin, imipenem, and piperacillin‐tazobactam. 31 , 35 Also, Bajaj et al. found sensitivity for colistin and aminoglycosides (gentamicin and amikacin) as 26–38%. 35 Furthermore, Suna et al. found that carbapenems are extremely effective against gram‐negative bacteria in bile. 36 Eventually, in all patients of this study, biliary bacterial pathogens were found to be resistant to a broad spectrum of antibiotics including cefixime (38%), ceftriaxone (26%), amoxyclav (14%), cefotaxime (14%), and ceftazidime (8%). Bajaj et al. found that biliary gram‐negative bacteria showed antibiotic resistance for ampicillin (95–97%), cephalosporins (ceftriaxone and cefotaxime) (79–91%), and quinolones (ciprofloxacin and ofloxacin) (55–90%). 35 In addition, Reiter et al. found that gram‐negative bacteria in bile showed high resistance for the following antibiotics: meropenem (94.8%), gentamicin (93.2%), cefepime (80.4%), ciprofloxacin (79.4%), piperacillin‐tazobactam (72.9%), and third‐generation cephalosporins (cefotaxime/ceftriaxone) (71.7%). 12 Furthermore, Kruger et al. reported that the prevalence of antibiotic resistance in bacteriobilia was 50% for cefotaxime versus 26% for piperacillin‐tazobactam. 37 This high level of resistance is related to the widespread and indiscriminate use of antibiotic therapy. The main strength of this study is the applicability of its updated results to improve the global treatment of patients and reach an excellent practice at Sudanese national surgical centers. Also, this is the first study describing bacteriobilia and antibiotic sensitivity test patterns in Sudan, where no prevalence data of bile infection was available prior to this study. The small number of patients is a limitation of our study that needs to be addressed. Future studies are recommended to compare the findings of this study with larger sample sizes and longer periods. In conclusion, careful selection of empirical antibiotic therapy based on surveillance of routine bile cultures during biliary tree procedures in patients with high risk of bacteriobilia will potentially help in improving the surgical outcomes and optimize treatment of acute cholangitis, which is associated with high mortality.
Acknowledgment
The authors would like to thank their colleagues in the surgical gastroenterology department and operating room staff at Ibn Sina Specialized Hospital for their unlimited support and kind suggestions.
Declaration of conflict of interest: The authors have no conflict of interest to declare.
Author contribution: Wael Mohialddin Ahmed Doush was responsible for original manuscript writing, editing, supervision, and critical revision of the content. Wael Mohialddin Ahmed Doush, Elfatih Yousif Abdelrahim, and Mohammed Salah Babekir were responsible for data collection, data analysis, and manuscript design. Wael Mohialddin Ahmed Doush and Muataz S. Abdelaziz were responsible for manuscript drafting and revision. All authors read and gave the final approval of the manuscript to be published.
Data availability statement
Datasets are not to be made available publicly by the local Research Ethics Committee because of ethical reasons. However, the data might be available from the corresponding author upon reasonable request.
References
- 1. Zimmer V, Lammert F. Acute bacterial cholangitis. Viszeralmedizin. 2015; 31: 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rupp C, Bode K, Weiss KH et al. Microbiological assessment of bile and corresponding antibiotic treatment: a strobe‐compliant observational study of 1401 endoscopic retrograde cholangiographies. Medicine. 2016; 95: e2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agarwal N, Sharma BC, Sarin SK. Endoscopic management of acute cholangitis in elderly patients. World J. Gastroenterol. 2006; 12: 6551–6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee JG. Diagnosis and management of acute cholangitis. Nat. Rev. Gastroenterol. Hepatol. J. 2009; 6: 533–541. [DOI] [PubMed] [Google Scholar]
- 5. Morris‐Stiff GJ, O'Donohue P, Ogunbiyi S, Sheridan WG. Microbiological assessment of bile during cholecystectomy: is all bile infected? HPB. 2007; 9: 225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bang CS, Yoon JH, Kim YJ et al. Clinical impact of body mass index on bactibilia and bacteremia. BMC Gastroenterol. 2014; 14: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beger HG, Schwarz A. Spectrum of biliary infections in the west and in the east. HPB Surg. 1995; 8: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shenoy SM, Shenoy S, Gopal S, Tantry BV, Baliga S, Jain A. Clinicomicrobiological analysis of patients with cholangitis. Indian J. Med. Microbiol. 2014; 32: 157–160. [DOI] [PubMed] [Google Scholar]
- 9. Thabit AK. Antibiotics in the biliary tract: a review of the pharmacokinetics and clinical outcomes of antibiotics penetrating the bile and gallbladder wall. Pharmacotherapy. 2020; 40: 672–691. [DOI] [PubMed] [Google Scholar]
- 10. Wada K, Takada T, Kawarada Y et al. Diagnostic criteria and severity assessment of acute cholangitis: Tokyo guidelines. J. Hepatobiliary Pancreat. Surg. 2007; 14: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cozma MA, Dobrica EC, Shah P, Shellah D, Gaman MA, Diaconu CC. Implications of type 2 diabetes mellitus in patients with acute cholangitis: a systematic review of current literature. Healthcare. 2022; 10: 2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reiter FP, Obermeier W, Jung J et al. Prevalence, resistance rates, and risk factors of pathogens in routine bile cultures obtained during endoscopic retrograde cholangiography. Dig. Dis. 2021; 39: 42–51. [DOI] [PubMed] [Google Scholar]
- 13. Lv J, Deng S, Zhang L. A review of artificial intelligence applications for antimicrobial resistance. Biosaf. Health. 2021; 3: 22–31. [Google Scholar]
- 14. Park JW, Lee JK, Lee KT, Lee KH, Sung YK, Kang CI. How to interpret the bile culture results of patients with biliary tract infections. Clin. Res. Hepatol. Gastroenterol. 2014; 38: 300–309. [DOI] [PubMed] [Google Scholar]
- 15. Stewart L, Grifiss JM, Jarvis GA, Way LW. Elderly patients have more severe biliary infections: influence of complement‐killing and induction of TNF‐α production. Surgery. 2008; 143: 103–112. [DOI] [PubMed] [Google Scholar]
- 16. Salvador VB, Lozada MC, Consunji RJ. Microbiology and antibiotic susceptibility of organisms in bile cultures from patients with and without cholangitis at an Asian academic medical center. Surg. Infect. 2011; 12: 105–111. [DOI] [PubMed] [Google Scholar]
- 17. Howard TJ, Yu J, Greene RB et al. Influence of bactibilia after preoperative biliary stenting on postoperative infectious complications. J. Gastrointest. Surg. 2006; 10: 523–531. [DOI] [PubMed] [Google Scholar]
- 18. Namias N, Demoya M, Sleeman D et al. Risk of postoperative infection in patients with bactibilia undergoing surgery for obstructive jaundice. Surg. Infect. 2005; 6: 323–328. [DOI] [PubMed] [Google Scholar]
- 19. Ballal M, Shenoy PA, Rodrigues GS et al. Biliary tract infections and their microbiological spectrum – a study from coastal region of southern India. Infectio. 2019; 23: 253–258. [Google Scholar]
- 20. Negm AA, Schott A, Vonberg RP et al. Routine bile collection for microbiological analysis during cholangiography and its impact on the management of cholangitis. Gastrointest. Endosc. 2010; 72: 284–291. [DOI] [PubMed] [Google Scholar]
- 21. Csendes A, Burdiles P, Maluenda F, Diaz JC, Csendes P, Mitru N. Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch. Surg. 1996; 131: 389–394. [DOI] [PubMed] [Google Scholar]
- 22. Wu ZY, Wu XS, Yao WY, Wang XF, Quan ZW, Gong W. Pathogens' distribution and changes of antimicrobial resistance in the bile of acute biliary tract infection patients. Chin. J. Surg. 2021; 59: 24–31. [DOI] [PubMed] [Google Scholar]
- 23. Jo IH, Kim YJ, Chung WC et al. Microbiology and risk factors for gram‐positive Cocci bacteremia in biliary infections. Hepatobiliary Pancreat. Dis. Int. 2020; 19: 461–666. [DOI] [PubMed] [Google Scholar]
- 24. Kabar I, Husing A, Cicinnati VR et al. Analysis of bile colonization and intestinal flora may improve management in liver transplant recipients undergoing ERCP. Ann. Transplant. 2015; 20: 249–255. [DOI] [PubMed] [Google Scholar]
- 25. Mohammed S, Evans C, VanBuren G et al. Treatment of bacteriobilia decreases wound infection rates after pancreaticoduodenectomy. HPB. 2014; 16: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herzog T, Belyaev O, Muller CA et al. Bacteribilia after preoperative bile duct stenting: a prospective study. J. Clin. Gastroenterol. 2009; 43: 457–462. [DOI] [PubMed] [Google Scholar]
- 27. Kaya M, Beştaş R, Bacalan F, Bacaksız F, Arslan EG, Kaplan MA. Microbial profile and antibiotic sensitivity pattern in bile cultures from endoscopic retrograde cholangiography patients. World J. Gastroenterol. 2012; 18: 3585–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehta V, Grebriyal V, Loomba PS, Mishra B, Sharma A. Microbiological spectrum, antibiogram and bacteraemia in biliary tract infections – a study from tertiary centre of north India. J. Adv. Med. Med. Res. 2022; 34: 28–40. [Google Scholar]
- 29. Englesbe MJ, Dawes LG. Resistant pathogens in biliary obstruction: importance of cultures to guide antibiotic therapy. HPB. 2005; 7: 144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kiriyama S, Kozaka K, Takada T et al. Tokyo guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J. Hepatobiliary Pancreat. Sci. 2018; 25: 17–30. [DOI] [PubMed] [Google Scholar]
- 31. Sharma V, Ghoshal U, Baijjal SS, Sharma R, Choudhuri G. Frequency of Biliary infection and antimicrobial susceptibility pattern in patients with extra‐hepatic biliary obstruction undergoing non‐surgical interventions with reused accessories. J. Liver Res. Disord. Ther. 2016; 2: 91–96. [Google Scholar]
- 32. Arima N, Uchiya T, Hishikawa R et al. Clinical characteristics of impacted bile duct stone in the elderly. Nihon Ronen Igakkai Zasshi. 1993; 30: 964–968. [DOI] [PubMed] [Google Scholar]
- 33. Sung JY, Costerton JW, Shaffer EA. Defense system in the biliary tract against bacterial infection. Dig. Dis. Sci. 1992; 37: 689–696. [DOI] [PubMed] [Google Scholar]
- 34. Al‐Jameel SS. Association of diabetes and microbiota: an update. Saudi J. Biol. Sci. 2021; 28: 4446–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bajaj A, Thakur A, Mishra B et al. Multidrug resistant bacteria causing biliary tract infection: a tertiary care centre study from north India. Saudi J. Pathol. Microbiol. 2019; 4: 245–249. [Google Scholar]
- 36. Suna N, Yıldız H, Yuksel M et al. The change in microorganisms reproducing in bile and blood culture and antibiotic susceptibility over the years. Turk. J. Gastroenterol. 2014; 25: 284–290. [DOI] [PubMed] [Google Scholar]
- 37. Kruger CM, Adam U, Adam T et al. Bacterobilia in pancreatic surgery‐conclusions for perioperative antibiotic prophylaxis. World J. Gastroenterol. 2019; 25: 6238–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets are not to be made available publicly by the local Research Ethics Committee because of ethical reasons. However, the data might be available from the corresponding author upon reasonable request.


