Abstract
Background and Aim
While short and long attachment caps are available for colonoscopy, it is unclear which type is more appropriate for stigmata of recent hemorrhage (SRH) identification in acute hematochezia. This study aimed to compare the performance of short versus long caps in acute hematochezia diagnoses and outcomes.
Methods
We selected 6460 patients who underwent colonoscopy with attachment caps from 10 342 acute hematochezia cases in the CODE BLUE‐J study. We performed propensity score matching (PSM) to balance baseline characteristics between short and long cap users. Then, the proportion of definitive or presumptive bleeding etiologies found on the initial colonoscopy and SRH identification rates were compared. We also evaluated rates of blood transfusions, interventional radiology, or surgery, as well as the rate of rebleeding and mortality within 30 days after the initial colonoscopy.
Results
A total of 3098 patients with acute hematochezia (1549 short cap and 1549 long cap users) were selected for PSM. The rate of colonic diverticular bleeding (CDB) diagnosis was significantly higher in long cap users (P = 0.006). While the two groups had similar rates of the other bleeding etiologies, the frequency of unknown etiologies was significantly lower in long cap users (P < 0.001). The rate of SRH with active bleeding was significantly higher in long cap users (P < 0.001). Other clinical outcomes did not differ significantly.
Conclusion
Compared to that with short caps, long cap‐assisted colonoscopy is superior for the diagnosis of acute hematochezia, especially CDB, and the identification of active bleeding.
Keywords: acute hematochezia, attachment cap, cap‐assisted colonoscopy, colonic diverticular bleeding, stigmata of recent hemorrhage
While short and long attachment caps are available for colonoscopy, it is unclear which type is more appropriate for acute hematochezia. This study has shown that long cap‐assisted colonoscopy is superior to short cap colonoscopy for the diagnosis of acute hematochezia, particularly colonic diverticular bleeding, and the detection of active bleeding.
Introduction
An attachment cap, affixed to the tip of an endoscope, is a small, transparent plastic or resin cap that improves mucosal visualization by depressing and flattening colonic folds. 1 Previous studies have shown that cap‐assisted colonoscopy significantly reduces cecal intubation time 2 , 3 and improves adenoma detection rates. 3 , 4 , 5 Attachment caps are also reported to increase the rate identification of stigmata of recent hemorrhage (SRH) in acute lower gastrointestinal bleeding 6 , 7 and can be a predictor for SRH in colonic diverticular bleeding (CDB). 8 , 9 Although a retrospective study showed that the use of a transparent cap during single‐balloon enteroscopy was significantly associated with higher identification rates for arteriovenous malformations, 10 it remains unclear whether cap‐assisted colonoscopy is advantageous for diagnosing bleeding etiologies in acute hematochezia.
Diverse attachment cap types, categorized by their extension length, are commercially available for clinical use. 11 , 12 Generally, short and long caps extend 4 and 12 mm, respectively, from the scope tip. Length seems to be associated with efficacy, as previous studies on cap‐assisted colonoscopy have shown that long caps increase adenoma detection rates and decrease cecal intubation times. 12 , 13 , 14 , 15 Long caps can help depress haustral folds deeper than short caps, allowing decreases in blind mucosa. Unlike short caps, longer caps also enable suction and inversion of diverticular domes to aid in the identification of bleeding points inside diverticula. 16 Indeed, SRH identification rates were higher for long caps (60%) 17 versus short caps (17–51%) 6 , 8 , 9 in patients with CDB. These findings collectively support long caps as more useful for diagnostic purposes as well as SRH identification in acute hematochezia. However, to the best of our knowledge, no studies have been conducted to compare diagnostic value and clinical outcomes with regard to acute hematochezia in patients undergoing short cap‐assisted versus long cap‐assisted colonoscopy.
Recently, the CODE BLUE‐J Study, a nationwide, multicenter, retrospective, cohort study, was carried out in Japan to determine the value of colonoscopy in individuals with acute hematochezia. 18 , 19 The present study exploits the CODE BLUE‐J Study database to confirm the ideal cap length for diagnosis of bleeding etiologies and improvements in clinical outcomes in patients with acute hematochezia.
Methods
Study design and patients
This study used the CODE BLUE‐J Study, a retrospective, observational, multicenter study of 49 participating Japanese hospitals between January 2010 and December 2019, which examined patients hospitalized for acute hematochezia. Clinical patient characteristics and the methodology have been described previously. 18 , 19 The opt‐out method of consent was approved by the ethics committees and institutional review boards of all 49 participating institutions for this study (Table SS1). SRH was defined as the source of the bleeding, which included both active and non‐active bleeding. Non‐active bleeding meant a visible non‐bleeding vessel or an adherent clot. 20
Clinical data collection and outcomes
Gastroenterologists or dedicated researchers at each participating institution collected all variables from the electronic medical record and endoscopy databases. The following data were retrospectively collected: age at diagnosis of acute hematochezia, gender, body mass index, alcohol consumption, smoking status, vital signs, presenting symptoms, history of diverticular bleeding, comorbidities, medication use within 30 days of admission, computed tomography (CT)‐associated factors (contrast‐enhanced CT use prior to colonoscopy and the presence of extravasation in the colorectal region), colonoscopy‐associated factors (timing of colonoscopy, bowel preparation with polyethylene glycol [PEG], use of water‐jet devices, and type of endoscopic attachment cap), and diagnosis on initial endoscopy. The type of SRH (e.g. active bleeding or non‐active bleeding) and location (e.g. right colon or left colon) were assessed. The left colon included the descending, sigmoid colon, and rectum, whereas the right colon included the other parts of the colon.
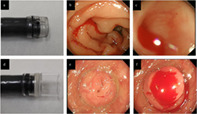
Attachment caps were divided into short and long groups. The representative short cap products included the Elastic Touch (slit and hole type [M or L or LL]) (Top, Tokyo, Japan, Fig. 1a) and the disposable distal attachment (Olympus, Tokyo, Japan). Long caps were primarily distal attachment MAJ‐663 (Olympus, Fig. 1d). Other types of attachment caps (e.g. ST hoods specialized for therapeutic endoscopy) were excluded. Based on the manufacturer's instructions, short and long caps were placed at approximately 4 or 12 mm from the tip of the endoscope to aid in flattening the folds of the colon and maintaining a fixed distance from the mucosa to improve mucosal imaging. While short caps are limited to suctioning a bleeding diverticulum from its dome (Fig. 1b,c), long caps enable visualization of the diverticular dome by inversion and identification of a bleeding source localized in the diverticular base (Fig. 1e,f). Endoscopic attachment cap types were selected at the discretion of each endoscopist and in accordance with the policies of each participating hospital.
Figure 1.

Representative images showing attachment caps and colonic diverticular bleeding. (a) A short cap (Elastic Touch [slit and hole type L], Top). (b and c) Identification of active bleeding from a diverticulum located in the ascending colon using the short cap. (d) A long cap (MAJ‐663 [Olympus]). (e) A colonic diverticulum located in the ascending colon. (f) The long cap inverts the diverticulum by suction and identifies active bleeding inside the diverticulum.
The main outcome included the proportion of bleeding etiologies on the initial colonoscopy and the rate of SRH identification in patients with acute hematochezia. The diagnosis of CDB had both definitive and presumptive CDB. Definitive CDB was defined as diverticulum with SRH identified by colonoscopy. 20 , 21 On the other hand, presumptive CDB included diverticulum without SRH and very little possibility of bleeding source except for colonic diverticulum determined by colonoscopy, which may be supported by negative findings of upper gastrointestinal or small bowel endoscopy, and CT visualization of contrast medium extravasation localized to the diverticulum. 20 , 21 SRH identification rates were evaluated according to location and type. We assessed the rate of patients who needed blood transfusions, interventional radiology (IVR), or surgery during admission as well as the length of hospital stay after the initial colonoscopy. We also evaluated the risk of rebleeding or death that occurred within 30 days after the initial colonoscopy. Rebleeding was defined as significant amounts of fresh bloody or wine‐colored stools after first hematochezia. 22 , 23 , 24 Patients were tracked from the time of admission until the occurrence of rebleeding or the conclusion of follow‐up (30 days after the initial colonoscopy or death). Using the last observation carried forward, missing data were examined.
Statistical analysis
Comparing categorical data was done using Fisher's exact test, and continuous data was compared with the Mann–Whitney U test. To compare outcomes between short and long caps, we conducted propensity score matching (PSM) to match baseline clinical data as well as CT‐ or colonoscopy‐associated factors between the two cohorts. PSM was applied to reduce the effect of selection bias and possible confounding factors. 25 For PSM, short and long cap users were matched one to one with nearest neighbors using a caliper width of 0.2 of the SD of the logit of the propensity score.
Statistical significance was defined as a P‐value <0.05. The statistical program IBM SPSS Statistics version 28.0 was used for all statistical analyses (IBM Corp., Armonk, New York, USA) and a free and open statistical software program R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria, URL https://www.R-project.org/).
Results
Patient characteristics
The CODE BLUE‐J study identified a total of 10 342 adult patients admitted for acute hematochezia. We excluded 1278 patients who did not undergo colonoscopy, 2527 patients who did not use attachment caps, and 77 patients who used specialized attachment caps. The remaining 6460 patients were ultimately included in this study (Fig. 2). Among them, short and long caps were used for 4638 (72%) and 1822 (28%) patients, respectively (Fig. 2).
Figure 2.

Flowchart of the assessment of patients with acute hematochezia in this study.
PSM in acute hematochezia (short vs long cap users)
To compare the rate of SRH identification between short and long cap use, we conducted PSM with 30 clinical factors (Table 1). While the proportions of some clinical factors were significantly higher in either short cap or long cap users before matching, all clinical factors were balanced between the two groups after matching (P‐values for all factors >0.10 and standardized mean differences for all factors <0.10) (Table 1). In the propensity‐matched cohort, a total of 3098 patients with acute hematochezia were selected (1549 short cap and 1549 long cap users).
Table 1.
Baseline characteristics between short and long cap users with acute hematochezia
| Unmatched cohort (n = 6460) | Propensity‐matched cohort (n = 3098) | ||||||
|---|---|---|---|---|---|---|---|
| Short cap (n = 4638) | Long cap (n = 1822) | P‐value | Short cap (n = 1549) | Long cap (n = 1549) | P‐value | SMD | |
| Age (years), median (range) | 74 (20–102) | 74 (20–99) | 0.14 | 73 (20–102) | 73 (20–99) | 0.88 | 0.005 |
| Sex (female/male) | 1583/3055 | 665/1157 | 0.077 | 576/973 | 549/1000 | 0.33 | 0.036 |
| Body mass index, median (range) | 22.8 (12.3–43.8) | 22.8 (12.2–49.8) | 0.57 | 22.7 (12.3–41.5) | 22.8 (12.2–49.8) | 0.81 | 0.014 |
| Current drinker | 1907 (49) | 802 (48) | 0.75 | 758 (49) | 746 (48) | 0.67 | 0.016 |
| Current smoker | 758 (19) | 265 (16) | 0.010 | 237 (15) | 238 (15) | 1.00 | 0.002 |
| Vital signs | |||||||
| Systolic blood pressure, mm Hg, median (range) | 128 (50–275) | 128 (40–221) | 0.60 | 127 (55–226) | 128 (40–221) | 0.83 | 0.002 |
| Heart rate/min, median (range) | 83 (30–149) | 84 (45–161) | <0.001 | 85 (42–149) | 84 (45–161) | 0.97 | 0.012 |
| Presenting symptoms | |||||||
| Loss of consciousness | 353 (7) | 104 (6) | 0.007 | 83 (5) | 87 (6) | 0.81 | 0.011 |
| Abdominal pain | 408 (9) | 138 (8) | 0.12 | 101 (7) | 105 (7) | 0.83 | 0.01 |
| Comorbidities | |||||||
| Dementia | 239 (5) | 59 (3) | <0.001 | 47 (3) | 52 (3) | 0.68 | 0.018 |
| Diabetes mellitus | 885 (19) | 384 (21) | 0.070 | 344 (22) | 335 (22) | 0.73 | 0.014 |
| Cerebrovascular disease | 705 (15) | 203 (11) | <0.001 | 155 (10) | 162 (11) | 0.72 | 0.015 |
| Chronic obstructive pulmonary disease | 164 (4) | 37 (2) | 0.001 | 32 (2) | 34 (2) | 0.90 | 0.009 |
| Connective tissue disease | 177 (4) | 77 (4) | 0.44 | 68 (4) | 69 (5) | 1.00 | 0.003 |
| Chronic heart failure | 370 (8) | 154 (9) | 0.54 | 113 (7) | 129 (8) | 0.32 | 0.038 |
| Chronic kidney disease | 707 (15) | 285 (16) | 0.70 | 260 (17) | 251 (16) | 0.70 | 0.016 |
| Liver disease | 172 (4) | 64 (4) | 0.77 | 46 (3) | 58 (4) | 0.27 | 0.043 |
| Hypertension | 2700 (58) | 1140 (63) | 0.001 | 968 (63) | 968 (63) | 1.00 | <0.001 |
| Hyperlipidemia | 1227 (27) | 594 (33) | <0.001 | 513 (33) | 517 (33) | 0.91 | 0.005 |
| Medication † | |||||||
| Low‐dose aspirin ‡ | 984 (21) | 384 (21) | 0.92 | 321 (21) | 335 (22) | 0.57 | 0.022 |
| Thienopyridines § | 508 (11) | 175 (10) | 0.12 | 145 (9) | 152 (10) | 0.71 | 0.015 |
| Cilostazol | 127 (3) | 36 (2) | 0.093 | 32 (2) | 30 (2) | 0.90 | 0.009 |
| Warfarin | 316 (7) | 143 (8) | 0.15 | 128 (8) | 124 (8) | 0.84 | 0.009 |
| DOAC ¶ | 307 (7) | 76 (4) | <0.001 | 59 (4) | 58 (4) | 1.00 | 0.003 |
| Corticosteroid | 262 (6) | 102 (6) | 1.00 | 87 (6) | 84 (5) | 0.88 | 0.008 |
| Colonoscopy‐associated factors | |||||||
| Timing of colonoscopy ≤24 h | 3171 (68) | 1374 (75) | <0.001 | 1162 (75) | 1165 (75) | 0.93 | 0.004 |
| Full preparation with polyethylene glycol | 3191 (69) | 1223 (67) | 0.20 | 1049 (68) | 1045 (68) | 0.91 | 0.006 |
| Use of water‐jet device | 3836 (83) | 1707 (94) | <0.001 | 1436 (93) | 1451 (94) | 0.32 | 0.038 |
| CT‐associated factors | |||||||
| Contrast‐enhanced CT before colonoscopy | 2296 (50) | 891 (49) | 0.68 | 772 (50) | 770 (50) | 0.97 | 0.003 |
| Extravasation in the colorectal region on CT | 630 (14) | 286 (16) | 0.029 | 238 (15) | 240 (16) | 0.96 | 0.004 |
Medication use was defined as intermittent or regular oral administration within 2 weeks before admission.
Low‐dose aspirin included enteric‐coated aspirin and buffered aspirin.
Thienopyridine included clopidogrel and ticlopidine.
DOAC included dabigatran etexilate, rivaroxaban, apixaban, and edoxaban.
Data are presented as n (%). Bold values indicate P < 0.05. Analyzed using Mann–Whitney U test and Fisher's exact test.
CT, computed tomography; DOAC, direct oral anticoagulant; SMD, standard mean difference.
In terms of definitive or presumptive bleeding etiologies on initial colonoscopy, the rate of CDB diagnosis was significantly higher in long cap users (83%) than short cap users (79%, odds ratio [OR] 1.29, 95% confidence interval [CI] 1.07–1.54, P = 0.006). While the two groups had similar rates of bleeding etiologies other than CDB, the frequency of unknown etiologies was significantly lower in long cap users (2.6%) than in short cap users (5.0%, OR 0.51, 95% CI 0.35–0.75, P < 0.001) (Table 2).
Table 2.
Rate of definitive or presumptive bleeding etiologies for the initial colonoscopy in acute hematochezia
| Propensity‐matched cohort (n = 3098) | |||||
|---|---|---|---|---|---|
| Diagnosis | Short cap (n = 1549) | Long cap (n = 1549) | OR | 95% CI | P‐value |
| CDB | 1224 (79) | 1284 (83) | 1.29 | 1.07–1.54 | 0.006 |
| Post‐procedure bleeding † | 63 (4.1) | 59 (3.8) | 0.93 | 0.65–1.34 | 0.71 |
| Ischemic colitis | 29 (1.9) | 30 (1.9) | 1.04 | 0.62–1.73 | 0.90 |
| Rectal ulcer | 25 (1.6) | 15 (1.0) | 0.60 | 0.31–1.14 | 0.12 |
| Colorectal angioectasia | 19 (1.2) | 17 (1.1) | 0.89 | 0.46–1.73 | 0.89 |
| Colorectal malignancy | 18 (1.2) | 8 (0.5) | 0.44 | 0.19–1.02 | 0.055 |
| Colorectal polyp | 5 (0.3) | 3 (0.2) | 0.60 | 0.14–2.51 | 0.48 |
| IBD | 15 (1.0) | 8 (0.5) | 0.53 | 0.22–1.26 | 0.15 |
| Infectious colitis | 2 (0.1) | 7 (0.5) | 3.51 | 0.73–16.9 | 0.12 |
| Radiation colitis | 5 (0.3) | 7 (0.5) | 1.40 | 0.44–4.43 | 0.57 |
| Other colitis ‡ | 9 (0.6) | 14 (0.9) | 1.56 | 0.67–3.62 | 0.30 |
| Hemorrhoids | 17 (1.1) | 12 (0.8) | 0.70 | 0.34–1.48 | 0.35 |
| Colorectal varix | 5 (0.3) | 3 (0.2) | 0.60 | 0.14–2.51 | 0.48 |
| Small bowel bleeding | 24 (1.5) | 31 (2.0) | 1.30 | 0.76–2.22 | 0.34 |
| Other diagnosis § | 11 (0.6) | 17 (1.0) | 1.55 | 0.72–3.32 | 0.26 |
| Unknown etiology | 78 (5.0) | 41 (2.6) | 0.51 | 0.35–0.75 | <0.001 |
Post‐procedure bleeding included post endoscopic submucosal dissection, post polypectomy, post endoscopic mucosal resection, and other procedures.
Other colitis included nonspecific colitis, drug‐induced ulcer, and nonspecific ulcer.
Other diagnosis included mucosal lymphoid hyperplasia, mucosal bleeding, dieulafoy ulcer, Cronkhite‐Canada syndrome, upper gastrointestinal bleeding, hematoma, gastrointestinal stromal tumor, postoperative ulcer, postoperative stenosis, anal bleeding, pseudoaneurysm, submucosal tumor of unknown origin, post‐biopsy, and bleeding from Meckel diverticulum.
Values are in number and %. Bold values indicate P < 0.05. Each OR was obtained by univariate logistic regression analysis.
CDB, colonic diverticular bleeding; CI, confidence interval; IBD, inflammatory bowel disease; OR, odds ratio.
The rate of overall SRH identification was not significantly different between short cap users (35%) and long cap users (38%, OR 1.13, 95% CI 0.98–1.31, P = 0.10) in the propensity‐matched cohort. Meanwhile, the identification rate of SRH with active bleeding was significantly higher in long cap users (23%) than short cap users (18%, OR 1.39, 95% CI 1.16–1.65, P < 0.001). The rate of SRH with active bleeding located in the right colon was significantly higher in long cap users (14%) than in short cap users (11%, OR 1.37, 95% CI 1.10–1.70, P = 0.005) (Table 3). As for SRH without active bleeding, there were no significant differences between short and long cap users (18 and 16% respectively, OR 0.88, 95% CI 0.73–1.07, P = 0.20). The identification rate of adherent clots was significantly lower in long cap users (9.0%) than in short cap users (11.5%, OR 0.77, 95% CI 0.61–0.97, P = 0.025), whereas the rate of visible vessels was not significantly different between the two groups (6.6% in short cap users and 7.2% in long cap users, OR 1.11, 95% CI 0.84–1.46, P = 0.48).
Table 3.
Comparative analysis regarding outcomes of acute hematochezia between short or long cap users
| Unmatched cohort (n = 6460) | Propensity‐matched cohort (n = 3098) | |||||||
|---|---|---|---|---|---|---|---|---|
| Short cap (n = 4638) | Long cap (n = 1822) | OR, (95% CI) | P‐value | Short cap (n = 1549) | Long cap (n = 1549) | OR, (95% CI) | P‐value | |
| All SRH | 1567 (34) | 720 (40) | 1.28 (1.15–1.43) | <0.001 | 544 (35) | 588 (38) | 1.13 (0.98–1.31) | 0.10 |
| SRH, active bleeding | 799 (17) | 435 (24) | 1.51 (1.32–1.72) | <0.001 | 273 (18) | 354 (23) | 1.39 (1.16–1.65) | <0.001 |
| SRH, non‐active bleeding † | 778 (17) | 301 (17) | 0.98 (0.85–1.14) | 0.81 | 277 (18) | 250 (16) | 0.88 (0.73–1.07) | 0.20 |
| SRH, visible vessels | 312 (6.7) | 134 (7.4) | 1.10 (0.89–1.36) | 0.37 | 102 (6.6) | 112 (7.2) | 1.11 (0.84–1.46) | 0.48 |
| SRH, adherent clot | 471 (10) | 169 (9.3) | 0.91 (0.75–1.09) | 0.29 | 178 (12) | 140 (9.0) | 0.77 (0.61–0.97) | 0.025 |
| Active bleeding in left‐sided colon ‡ | 293 (6.3) | 158 (8.7) | 1.41 (1.15–1.72) | <0.001 | 103 (6.6) | 128 (8.3) | 1.27 (0.97–1.66) | 0.088 |
| Active bleeding in right‐sided colon ‡ | 488 (11) | 266 (15) | 1.45 (1.24–1.71) | <0.001 | 164 (11) | 216 (14) | 1.37 (1.10–1.70) | 0.005 |
| Blood transfusion during admission | 1482 (32) | 585 (32) | 1.01 (0.90–1.13) | 0.91 | 494 (32) | 492 (32) | 0.99 (0.86–1.16) | 0.94 |
| Need for IVR during admission | 51 (1.1) | 20 (1.1) | 1.00 (0.59–1.68) | 1.00 | 13 (0.8) | 15 (1.0) | 1.16 (0.55–2.44) | 0.70 |
| Need for surgery during admission | 30 (0.6) | 12 (0.7) | 1.02 (0.52–1.99) | 0.96 | 5 (0.3) | 11 (0.7) | 2.21 (0.77–6.37) | 0.14 |
| Length of stay (days), median (range) | 6 (0–160) | 6 (0–118) | 0.99 (0.982–0.995) | <0.001 | 6 (0–100) | 6 (0–118) | 1.00 (0.99–1.01) | 0.89 |
| 30‐day rebleeding | 651 (14) | 269 (15) | 1.06 (0.91–1.24) | 0.45 | 238 (15) | 228 (15) | 0.95 (0.78–1.16) | 0.62 |
| 30‐day mortality | 53 (1.1) | 12 (0.7) | 0.57 (0.31–1.08) | 0.083 | 15 (1.0) | 11 (0.7) | 0.73 (0.34–1.60) | 0.43 |
Non‐active bleeding was defined as visible vessels and adherent clot.
The left‐sided colon was defined as descending and sigmoid colon and rectum; and the right‐sided colon was the other locations.
Values are in numbers and %. Bold values indicate P < 0.05. Each OR was obtained by univariate logistic regression analysis.
CI, confidence interval; IVR, interventional radiology; OR, odds ratio; SRH, stigmata of recent of hemorrhage.
The proportions of patients who needed blood transfusions, IVR, or surgery during admission were not significantly different between the two groups. Lengths of hospital stays after the initial colonoscopy were similar between short cap users (6 days [range: 0–100 days]) and long cap users (6 days [range: 0–118 days]). The risks of rebleeding and death that occurred within 30 days after the initial colonoscopy were not significantly different between the two cohorts.
Etiology and location of SRH with active bleeding among long cap users
Among the 354 long cap users with SRH with active bleeding, the most common etiology was CDB (81%), followed by post‐procedure bleeding (8.2%) and colorectal angioectasia (3.1%) (Table 4). As for the location, SRH with active bleeding was predominantly located in the right colon (61%) rather than the left colon (36%). Among patients with SRH with active bleeding in the right colon, CDB was more frequent than non‐CDB (91 vs 9%) (Table 4).
Table 4.
Etiology and location of stigmata of recent of hemorrhage (SRH) with active bleeding among long cap users (n = 354)
| Long cap (n = 354) | |
|---|---|
| Etiology of SRH with active bleeding | |
| CDB | 285 (81) |
| Postprocedure bleeding † | 29 (8.2) |
| Colorectal angioectasia | 11 (3.1) |
| Rectal ulcer | 9 (2.5) |
| Small bowel bleeding | 7 (2.0) |
| Radiation colitis | 4 (1.1) |
| Other colitis ‡ | 2 (0.6) |
| Colorectal varix | 2 (0.6) |
| Hemorrhoids | 1 (0.3) |
| Colorectal malignancy | 1 (0.3) |
| Colorectal polyp | 0 (0) |
| IBD | 0 (0) |
| Infectious colitis | 0 (0) |
| Ischemic colitis | 0 (0) |
| Other diagnosis § | 14 (1.2) |
| Location and etiology of SRH with active bleeding | |
| Left‐sided colon ¶ | 128 (36) |
| CDB | 87 (68) |
| Non‐CDB | 41 (32) |
| Right‐sided colon ¶ | 216 (61) |
| CDB | 197 (91) |
| Non‐CDB | 19 (9) |
Postprocedure bleeding was from post‐endoscopic submucosal dissection, post‐polypectomy, post‐endoscopic mucosal resection, and other procedure.
Other colitis included nonspecific colitis, drug‐induced ulcer, and nonspecific ulcer.
Other diagnoses included mucosal lymphoid hyperplasia, mucosal bleeding, dieulafoy ulcer, Cronkhite‐Canada syndrome, upper gastrointestinal bleeding, hematoma, gastrointestinal stromal tumor, postoperative ulcer, postoperative stenosis, anal bleeding, pseudoaneurysm, submucosal tumor of unknown origin, post‐biopsy, and bleeding from Meckel diverticulum.
The left‐sided colon was defined as descending and sigmoid colon and rectum, and the right‐sided colon was the other locations.
Values are in numbers and %.
CDB, colonic diverticular bleeding; IBD, inflammatory bowel disease; SRH, stigmata of recent of hemorrhage.
Discussion
This nationwide cohort study compared the proportion of bleeding etiologies and SRH identification rates in acute hematochezia patients who underwent short‐ and long cap‐assisted colonoscopy, to examine the impact of cap length on diagnostic and patient outcomes. We found that the proportion of CDB diagnosis was significantly greater in long cap users than short cap users. Since the rate of unknown etiologies was significantly lower in long cap users, our results suggest that using long caps during colonoscopy may positively contribute to more accurate diagnosis of bleeding etiologies, particularly CDB, in patients with acute hematochezia.
We also found that the identification rate of SRH with active bleeding was (i) significantly higher in long cap users than in short cap users and (ii) dominant on the right side. Our descriptive data of long cap users with SRH with active bleeding showed that 80% of diagnoses were CDB and 60% of SRH were located in the right colon. This suggests that cap length influences the detection of SRH with active bleeding in patients with acute hematochezia, especially for right‐sided CDB. Previous investigations have found that cap‐assisted colonoscopy significantly improved visualization of surfaces in the right colon 1 and was more effective than standard colonoscopy for the detection of right‐sided colorectal polyps. 26 In general, since these polyps tend to be overlooked because the haustral folds and anatomical flexures cause blind spots, 27 , 28 our findings show that long cap‐assisted colonoscopy may be more useful in the right colon where such blind spots are more likely to occur. Furthermore, long caps enable visualization of bleeding sources inside diverticula by suction and inversion of diverticular domes. 16 , 17 Given that diverticula in the right colon have wider necks and domes compared with the left colon, 29 diverticular inversion with long caps may result in higher identification rates for SRH with active bleeding.
Our analysis regarding SRH with inactive bleeding showed that the identification rate of adherent clots in long cap users was significantly lower than in short cap users. Meanwhile, there was no significant difference in the rate of visible vessels between the two groups, suggesting that this may be attributed to the diverticular inversion with long caps. For instance, when encountering diverticula with adherent clots using the long cap‐assisted colonoscopy, the inversion maneuver would be performed to identify active bleeding sources and might make diverticula with adherent clots into active bleeding. Indeed, long cap users showed higher identification rate of active bleeding compared with short cap users as described above. As for visible vessels, this finding may not necessarily require diverticular inversion, explaining the lack of difference in the rate of inactive SRH between the two groups.
On the other hand, our analysis showed no significant differences between short and long cap users in the percentages of patients who needed blood transfusions, IVR, or surgery and the rates of rebleeding and mortality, although long cap users had higher identification rates for SRH with active bleeding. When performing colonoscopy for CDB, identification of hemorrhagic stigmata is important, as patients with SRH, such as active bleeding, visible vessels, or adherent clots, have higher rates of rebleeding and hemorrhage interventions. 30 While previous studies found that SRH identification was associated with better CDB outcomes, 31 few studies were conducted to demonstrate any associations between SRH identification and clinical outcomes of acute hematochezia other than CDB. Further investigations are warranted to understand whether SRH identification with long caps can improve outcomes for acute hematochezia.
This study had a number of strengths and limitations. The inclusion of 6460 cap users with acute hematochezia is a significant strength of this large cohort study. Our PSM analysis also included >1500 cases of both long and short cap users. We were able to conduct this analysis to reduce bias and confounders in the baseline traits, significantly affecting SRH identification thanks to our large and comprehensive clinical dataset. To the best of our knowledge, this is the first analysis comparing the clinical usefulness of short and long caps in patients with acute hematochezia. These higher values may more accurately quantify the clinical utility of long caps for CDB. We do acknowledge, however, that the retrospective design of this multicenter study, which was conducted primarily in Japanese tertiary endoscopic centers, posed certain data limitations. Furthermore, given that cap types were selected in accordance with individual hospital policies and provider preferences, it is a possibility that endoscopists preferentially selected long caps when they expected CDB. Since long caps are not available in some of facilities included in this study, long cap‐assisted colonoscopy may be unevenly performed among hospitals. To reduce such bias, we conducted PSM, but unmeasured confounders associated with cap selection may exist. While the optimal type of cap for acute hematochezia remains to be fully elucidated, our results are useful for planning future studies.
In conclusion, we compared the usefulness of short versus long attachment caps in patients with acute hematochezia, including CDB, by using PSM analysis on data from a large, nationwide cohort study. Our result suggests that long cap‐assisted colonoscopy may be better for the diagnosis of bleeding etiologies, especially CDB, and the identification of SRH with active bleeding in patients with acute hematochezia.
Informed consent
The authors retrospectively analyzed data from January 2010 to December 2019. Thus, waiver of consent for this study was approved. All efforts were made to ensure confidentiality of the data.
Supporting information
Table S1. Affiliations, ethics committee approval numbers, and number of cases at the 49 participating hospitals.
Acknowledgments
The authors thank Yusuke Niisato, Yoshitaka Tange, Hirosumi Suzuki, Tsubasa Onoda, Yuya Hagiwara, Soma Fukuda, Satoshi Fukuda, Miki Tsuji, and Miho Nagafuchi for their assistance in data collection. They would also like to thank Dr. Bryan J. Mathis of the University of Tsukuba Hospital International Medical Center for English language revision.
Mariko Kobayashi and Shintaro Akiyama shared co‐first authorship.
Declaration of conflict of interest: All authors have no conflict of interest to disclose.
Author contribution: Naoyoshi Nagata was the principal investigator and designed the study. Mariko Kobayashi, Shintaro Akiyama, and Toshiaki Narasaka conducted the literature search and conducted the study. Mariko Kobayashi and Shintaro Akiyama performed all data analysis and created all figures and tables. Mariko Kobayashi, Shintaro Akiyama, Toshiaki Narasaka, Katsumasa Kobayashi, Atsushi Yamauchi, Atsuo Yamada, Jun Omori, Takashi Ikeya, Taiki Aoyama, Naoyuki Tominaga, Yoshinori Sato, Takaaki Kishino, Naoki Ishii, Tsunaki Sawada, Masaki Murata, Akinari Takao, Kazuhiro Mizukami, Ken Kinjo, Shunji Fujimori, Takahiro Uotani, Minoru Fujita, Hiroki Sato, Sho Suzuki, Junnosuke Hayasaka, Tomohiro Funabiki, Yuzuru Kinjo, Akira Mizuki, Shu Kiyotoki, Tatsuya Mikami, Ryosuke Gushima, Hiroyuki Fujii, Yuta Fuyuno, Naohiko Gunji, Yosuke Toya, Kazuyuki Narimatsu, Noriaki Manabe, Koji Nagaike, Tetsu Kinjo, Yorinobu Sumida, Sadahiro Funakoshi, Kiyonori Kobayashi, Tamotsu Matsuhashi, Yuga Komaki, and Mitsuru Kaise collected and interpreted the data. Mariko Kobayashi and Shintaro Akiyama drafted the article. Mariko Kobayashi, Shintaro Akiyama, Toshiaki Narasaka, Naoki Ishii, Kiichiro Tsuchiya, and Naoyoshi Nagata critically revised the manuscript. All authors read and approved the submitted version of the manuscript.
Financial support: This work was partially supported by grants from the Ministry of Health, Labour and Welfare, Japan (grant number: 19HB1003), Japan Society for the Promotion of Science KAKENHI (JP17K09365 and 20K08366), Smoking Research Foundation, Takeda Science Foundation, Tokyo Medical University Cancer Research Foundation, Tokyo Medical University Research Foundation, and Grants‐in‐Aid for Research from the National Center for Global Health and Medicine (29‐2001, 29‐2004, 19A1011, 19A1022, 19A‐2015, 29‐1025, and 30‐1020). The funders played no role in the study design, analysis, or decision to publish the manuscript.
Contributor Information
Toshiaki Narasaka, Email: tnarasaka@md.tsukuba.ac.jp.
Naoyoshi Nagata, Email: nnagata_ncgm@yahoo.co.jp.
Data availability statement
The datasets generated during and/or analyzed during the current study are not available.
References
- 1. Frieling T, Neuhaus F, Heise J et al. Cap‐assisted colonoscopy (CAC) significantly extends visualization in the right colon. Z. Gastroenterol. 2012; 50: 279–84. [DOI] [PubMed] [Google Scholar]
- 2. Morgan JL, Thomas K, Braungart S, Nelson RL. Transparent cap colonoscopy versus standard colonoscopy: a systematic review and meta‐analysis. Tech. Coloproctol. 2013; 17: 353–60. [DOI] [PubMed] [Google Scholar]
- 3. Rastogi A, Bansal A, Rao DS et al. Higher adenoma detection rates with cap‐assisted colonoscopy: a randomised controlled trial. Gut. 2012; 61: 402–8. [DOI] [PubMed] [Google Scholar]
- 4. Nutalapati V, Kanakadandi V, Desai M, Olyaee M, Rastogi A. Cap‐assisted colonoscopy: a meta‐analysis of high‐quality randomized controlled trials. Endosc Int Open. 2018; 6: E1214–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Desai M, Sanchez‐Yague A, Choudhary A et al. Impact of cap‐assisted colonoscopy on detection of proximal colon adenomas: systematic review and meta‐analysis. Gastrointest. Endosc. 2017; 86: 274–81.e3. [DOI] [PubMed] [Google Scholar]
- 6. Shibata S, Shigeno T, Fujimori K, Kanai K, Yoshizawa K. Colonic diverticular hemorrhage: the hood method for detecting responsible diverticula and endoscopic band ligation for hemostasis. Endoscopy. 2014; 46: 66–9. [DOI] [PubMed] [Google Scholar]
- 7. Aoki T, Yamada A, Kobayashi K et al. Development and validation of a novel model for predicting stigmata of recent hemorrhage in acute lower gastrointestinal bleeding: a multicenter nationwide study. Dig. Endosc. 2023. 10.1111/den.14533. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 8. Niikura R, Nagata N, Aoki T et al. Predictors for identification of stigmata of recent hemorrhage on colonic diverticula in lower gastrointestinal bleeding. J. Clin. Gastroenterol. 2015; 49: e24–30. [DOI] [PubMed] [Google Scholar]
- 9. Sato Y, Nakatsu‐Inaba S, Matsuo Y et al. Efficient Colonoscopic Identification of Colonic Bleeding Diverticulum Using Intradiverticular Water Injection with a Nontraumatic Tube. J Anus Rectum Colon. 2021; 5: 313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hasak S, Lang G, Early D et al. Use of a transparent cap increases the diagnostic yield in antegrade single‐balloon enteroscopy for obscure GI bleed. Dig. Dis. Sci. 2019; 64: 2256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanchez‐Yague A, Kaltenbach T, Yamamoto H, Anglemyer A, Inoue H, Soetikno R. The endoscopic cap that can (with videos). Gastrointest. Endosc. 2012; 76: 169–178.e1‐2. [DOI] [PubMed] [Google Scholar]
- 12. Ng SC, Tsoi KK, Hirai HW et al. The efficacy of cap‐assisted colonoscopy in polyp detection and cecal intubation: a meta‐analysis of randomized controlled trials. Am. J. Gastroenterol. 2012; 107: 1165–73. [DOI] [PubMed] [Google Scholar]
- 13. Tada M, Inoue H, Yabata E, Okabe S, Endo M. Feasibility of the transparent cap‐fitted colonoscope for screening and mucosal resection. Dis. Colon Rectum. 1997; 40: 618–21. [DOI] [PubMed] [Google Scholar]
- 14. Matsushita M, Hajiro K, Okazaki K, Takakuwa H, Tominaga M. Efficacy of total colonoscopy with a transparent cap in comparison with colonoscopy without the cap. Endoscopy. 1998; 30: 444–7. [DOI] [PubMed] [Google Scholar]
- 15. Horiuchi A, Nakayama Y. Improved colorectal adenoma detection with a transparent retractable extension device. Am. J. Gastroenterol. 2008; 103: 341–5. [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi K, Furumoto Y, Narasaka T. “Long‐hood method” for identification of the bleeding site in colonic diverticular hemorrhage. Dig. Endosc. 2020; 32: e28–e9. [DOI] [PubMed] [Google Scholar]
- 17. Akutsu D, Narasaka T, Kobayashi K et al. Newly developed endoscopic detachable snare ligation therapy for colonic diverticular hemorrhage: a multicenter phase II trial (with videos). Gastrointest. Endosc. 2018; 88: 370–7. [DOI] [PubMed] [Google Scholar]
- 18. Nagata N, Kobayashi K, Yamauchi A et al. Identifying bleeding etiologies by endoscopy affected outcomes in 10,342 cases with hematochezia: CODE BLUE‐J study. Am. J. Gastroenterol. 2021; 116: 2222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagata N, Kobayashi K, Yamauchi A et al. Nationwide large‐scale data of acute lower gastrointestinal bleeding in Japan uncover detailed etiologies and relevant outcomes: CODE BLUE J‐Study. medRxiv. 2021. 10.1101/2021.01.18.21250035. [DOI] [Google Scholar]
- 20. Nagata N, Ishii N, Manabe N et al. Guidelines for colonic diverticular bleeding and colonic diverticulitis: Japan Gastroenterological Association. Digestion. 2019; 99: 1–26. [DOI] [PubMed] [Google Scholar]
- 21. Nagata N, Niikura R, Aoki T et al. Colonic diverticular hemorrhage associated with the use of nonsteroidal anti‐inflammatory drugs, low‐dose aspirin, antiplatelet drugs, and dual therapy. J. Gastroenterol. Hepatol. 2014; 29: 1786–93. [DOI] [PubMed] [Google Scholar]
- 22. Nagata N, Sakurai T, Shimbo T et al. Acute severe gastrointestinal tract bleeding is associated with an increased risk of thromboembolism and death. Clin. Gastroenterol. Hepatol. 2017; 15: 1882–9.e1. [DOI] [PubMed] [Google Scholar]
- 23. Oakland K, Guy R, Uberoi R et al. Acute lower GI bleeding in the UK: patient characteristics, interventions and outcomes in the first nationwide audit. Gut. 2018; 67: 654–62. [DOI] [PubMed] [Google Scholar]
- 24. Strate LL, Orav EJ, Syngal S. Early predictors of severity in acute lower intestinal tract bleeding. Arch. Intern. Med. 2003; 163: 838–43. [DOI] [PubMed] [Google Scholar]
- 25. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat. Med. 1998; 17: 2265–81. [DOI] [PubMed] [Google Scholar]
- 26. Kim DJ, Kim HW, Park SB et al. Efficacy of cap‐assisted colonoscopy according to lesion location and endoscopist training level. World J. Gastroenterol. 2015; 21: 6261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miyamoto H, Naoe H, Oda Y et al. Impact of retroflexion in the right colon after repeated forward‐view examinations. JGH Open. 2018; 2: 282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kushnir VM, Oh YS, Hollander T et al. Impact of retroflexion vs. second forward view examination of the right colon on adenoma detection: a comparison study. Am. J. Gastroenterol. 2015; 110: 415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meyers MA, Alonso DR, Gray GF, Baer JW. Pathogenesis of bleeding colonic diverticulosis. Gastroenterology. 1976; 71: 577–83. [PubMed] [Google Scholar]
- 30. Jensen DM, Ohning GV, Kovacs TO et al. Natural history of definitive diverticular hemorrhage based on stigmata of recent hemorrhage and colonoscopic Doppler blood flow monitoring for risk stratification and definitive hemostasis. Gastrointest. Endosc. 2016; 83: 416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gobinet‐Suguro M, Nagata N, Kobayashi K et al. Treatment strategies for reducing early and late recurrence of colonic diverticular bleeding based on stigmata of recent hemorrhage: a large multicenter study. Gastrointest. Endosc. 2022; 95: 1210–22.e12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Affiliations, ethics committee approval numbers, and number of cases at the 49 participating hospitals.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not available.


