Abstract
Human herpesvirus-6 (HHV-6) is an increasingly recognized cause of myocarditis. We present the case of a 46-year-old woman who presented with fulminant HHV-6 myocarditis requiring heart transplantation. (Level of Difficulty: Advanced.)
Key Words: acute heart failure, cardiac transplant, cardiomyopathy
Graphical abstract
History of Presentation
A 46-year-old woman presented with chest pain and fevers. The week before presentation, she attended a family event with 40 people, and multiple attendees developed a viral illness. Several days before presentation, she started to experience generalized fatigue and myalgias. She was febrile to 102 °F at home, and because of chest pain and shortness of breath, she presented to the hospital. On presentation, she was febrile to 39.2°C and tachycardic, with a heart rate of 125 beats/min. Her blood pressure was 135/83 mm Hg. She was tachypneic to 18 breaths/min but had oxygen saturation of 97% on room air. Cardiac examination displayed tachycardia. No murmurs or extra heart sounds were appreciated. Her jugular venous pressure was estimated at 14 cm H2O. Her lungs were clear to auscultation.
Learning Objectives
-
•
To recognize and create a differential diagnosis for fulminant myocarditis.
-
•
To review treatment options for viral myocarditis as well as myocarditis complicated by worsening cardiogenic shock.
Past Medical History
This patient had a past medical history of essential hypertension independent of pregnancy, pre-eclampsia, and well-controlled ulcerative colitis treated with mercaptopurine. Her past surgical history included cholecystectomy and cesarean delivery.
Investigations
The initial electrocardiogram (ECG) showed ST-segment elevations in leads V1 to V2, and aVR, as well as ST-segment depressions in leads V3 to V6, II, III, and aVF (Figure 1). Presentation laboratory test results were notable for a transaminitis (aspartate transferase, 115 U/L; alanine transaminase, 74 U/L), a high-sensitivity troponin level of 17,302 ng/L that peaked at 25,677 ng/L, and a C-reactive protein level of 132.5 mg/L. Results of the respiratory viral panel and COVID-19 testing were both negative. Left-sided heart catheterization displayed angiographically normal coronary arteries, and right-sided heart catheterization showed a central venous pressure of 15 mm Hg, a pulmonary artery pressure of 48/30 (mean, 36) mm Hg, a pulmonary capillary wedge pressure of 26 mm Hg, and a Fick cardiac index of 2.1. The initial transthoracic echocardiogram (TTE) displayed a left ventricular ejection fraction (LVEF) of 50% and increased left ventricular wall thickness. The patient’s hemodynamic values were monitored with a Swan-Ganz catheter for the duration of the presentation. Because of a drop in cardiac index to 1.7 and lactic acidosis despite treatment of heart failure with diuresis and milrinone, an intra-aortic balloon pump was placed on day 3, with subsequent improvement in cardiac index to 2 on day 4. A repeat TTE on day 4 displayed a newly reduced LVEF of 25% and new left ventricular dilation. A repeat ECG on day 4 showed resolution of ST-segment elevation but with new Q waves in leads III, aVF, and V1, and low voltage (Figure 2). On day 5, in response to a subsequent drop in cardiac index to 1.7 with an associated rise in arterial lactate, an Impella 5.5 LVAD (Abiomed) was placed, and the patient was intubated. Despite this treatment, her cardiac index remained low, and the lactate failed to clear. On day 6, the patient was cannulated to venoarterial extracorporeal membrane oxygenation (VA-ECMO) with a right axillary intravascular microaxial left ventricular assist device (LVAD) for left ventricular decompression. On day 7, in hopes of transitioning to a more durable form of biventricular support, the patient was decannulated from VA-ECMO and underwent placement of a percutaneous oxygenated right ventricular assist device (RVAD) with increased intravascular microaxial LVAD flows. A repeat ECG on day 11 displayed virtually no ventricular electrical activity (Figure 3). A subsequent TTE on day 11 displayed an LVEF of <5% bordering on ventricular standstill (Videos 1 and 2). The arterial lactate level at this point was 4 mmol/L despite biventricular support with an RVAD and the previously mentioned intravascular microaxial LVAD.
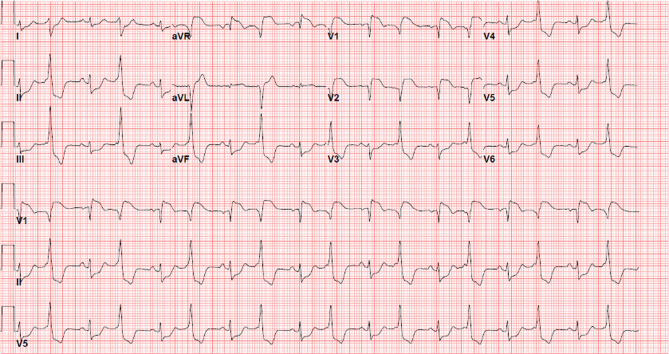
Figure 1.
Presentation Electrocardiogram, Hospital Day 1
Diffuse ST-segment elevations are noted.
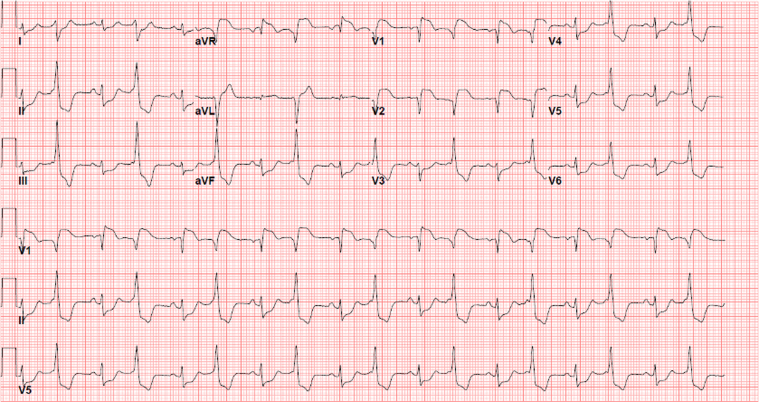
Figure 2.
Electrocardiogram, Hospital Day 4
Resolution of ST-segment elevation is seen, but with new Q waves in leads III, aVF, and V1 and low voltage.
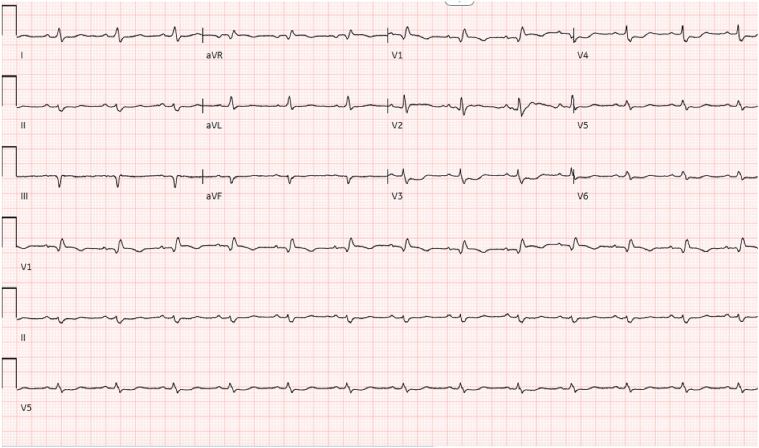
Figure 3.
Electrocardiogram, Hospital Day 11
Minimal electrical activity is noted.
Differential Diagnosis
The constellation of a young woman presenting with fulminant heart failure, diffuse ST-segment changes on the ECG, clean coronary arteries, and elevated troponin and C-reactive protein levels raised concern for myocarditis. There was concern for viral myocarditis given the viral prodrome despite the negative results of a respiratory viral pathogen panel. The differential diagnosis included giant cell myocarditis given her fulminant presentation (although notably the patient lacked ventricular arrhythmias), as well as autoimmune myocarditis in the setting of a previous history of autoimmune disease.
Management
The day of presentation, the patient was started on intravenous diuresis with furosemide for treatment of heart failure. The next day, she was started on steroids and intravenous immunoglobulin (IVIG) for empiric treatment of fulminant viral myocarditis. On day 3, in response to worsening shock, she was started on milrinone (in the setting of a national dobutamine shortage), and an intra-aortic balloon pump was placed. On day 5, as a result of worsening hemodynamics, the balloon pump was upgraded to an intravascular microaxial LVAD, and on day 6 she was cannulated to VA-ECMO for biventricular support (Table 1). She was subsequently transferred to a transplant-capable center for consideration for heart transplantation. At the transplant-capable center, to unload the heart and provide a more durable method of biventricular support with fewer complications, the patient was decannulated from VA-ECMO and underwent placement of a percutaneous oxygenated RVAD with increased intravascular microaxial LVAD flows. Despite these interventions, the patient continued to deteriorate, with suboptimal intravascular microaxial LVAD flows. She developed a worsening mental status and an increasing lactate level, so she was listed for heart transplantation as status 1 on hospital day 11. On hospital day 14, she received a heart transplant.
Table 1.
Trend in Cardiac Output, Cardiac Index, and Arterial Lactate With Associated Levels of Inotropic or Mechanical Support Throughout Admission
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 11 |
|---|---|---|---|---|---|---|---|---|
| Fick cardiac output, L/min | 3.5 | 3.1 | 2.9 | 3.4 | 3.1 | 5 | 5.2 | 3.9 |
| Fick cardiac index, L/min/m2 | 2.1 | 1.8 | 1.7 | 2 | 1.8 | 2.9 | 3.1 | 2.3 |
| Arterial lactate, mmol/L | 2.6 | 2.7 | 4 | 3 | 4.2 | 3 | 3 | 4.9 |
| Level of support | None | Milrinone | IABP | IABP | Intravascular microaxial LVAD | VA-ECMO + intravascular microaxial LVAD | RVAD + Intravascular microaxial LVAD | RVAD + intravascular microaxial LVAD |
Discussion
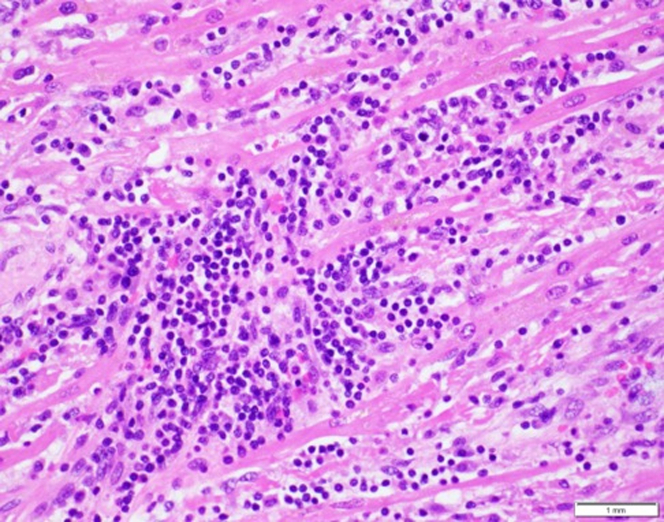
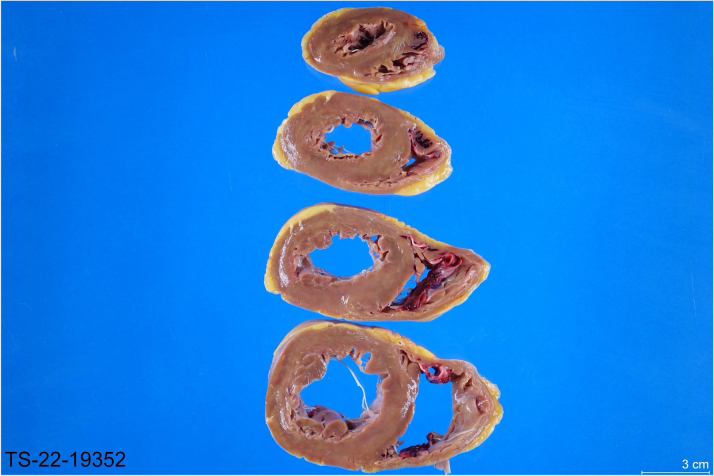
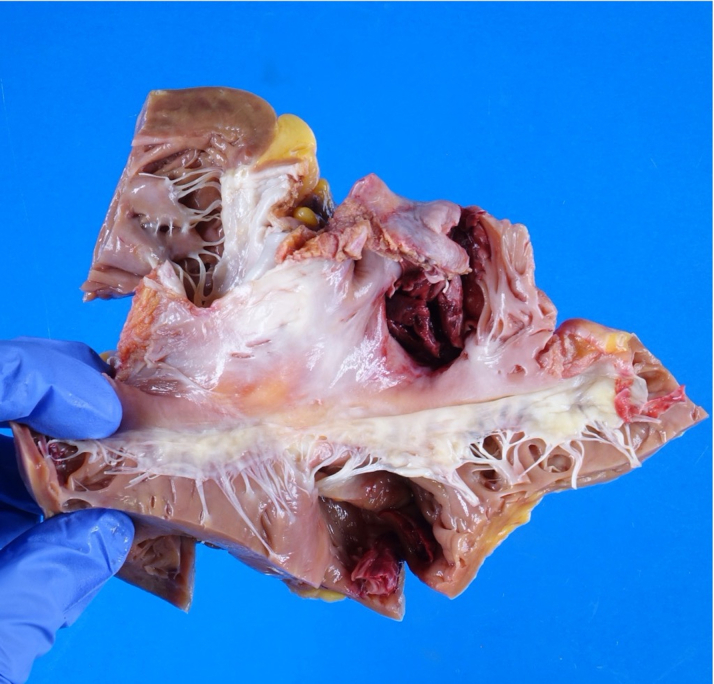
The explanted heart displayed lymphocytic-predominant myocarditis and was polymerase chain reaction–positive for human herpesvirus-6 (HHV-6) (Figure 4). The explanted heart displayed ventricular dilatation as well as thrombus in the right atrial appendage (Figures 5 and 6). Myocarditis, an inflammatory disease of the myocardium, is a frequent cause of cardiomyopathy and sudden cardiac death. Myocarditis can be caused by a variety of conditions but is commonly caused by viral agents, with a cardiotropic viral infection followed by active inflammatory destruction of the myocardium. Given the varying tropisms of viruses, their mechanism of myocardial injury can differ. Coxsackievirus (an enterovirus that is the classic viral cause of myocarditis) causes injury through viral entry into myocardial cells through the coxsackie and adenovirus receptor (CAR) and activation of innate immunity. In contrast, parvovirus has a tropism for endothelial cells, and Herpesviridae have tropisms for T lymphocytes.1
Figure 4.
Histologic Features of the Explanted Heart
Pink cells are myocytes. Blue cells are inflammatory cells. The round nucleus and scant cytoplasm of blue cells suggest lymphocytes consistent with lymphocytic-predominant myocarditis. Extensive myocyte injury is also present.
Figure 5.
Cross Sections of the Explanted Heart
Ventricular dilatation and the presence of thrombus are noted.
Figure 6.
Pathologic Features of the Explanted Heart Through the Tricuspid Valve
HHV-6 is an increasingly recognized cause of viral myocarditis.2 Acute myocarditis can progress to more chronic ventricular dysfunction and dilated cardiomyopathy. Studies have shown that two-thirds of hearts with “idiopathic” left ventricular dysfunction are positive for viral genomes on polymerase chain reaction and that viral persistence in the myocardium is associated with progressive cardiac dysfunction.3,4
Although traditionally fulminant myocarditis is thought to have a better prognosis and chance of recovery than acute myocarditis, previous studies have not included patients with left ventricular function as severely diminished as in our patient. Steroids and azathioprine have previously been used to some effect in the treatment of HHV-6 myocarditis.5 IVIG has also been trialed with varying success in myocarditis.6 Despite treatment with steroids and IVIG, our patient progressed to ventricular standstill requiring heart transplantation. In immunosuppressed patients who present with severe myocarditis, advanced heart failure and transplantation teams should be engaged early because of the risk of progression to severe decompensated heart failure.
Follow-Up
Our patient received a heart transplant on day 14 of hospitalization. She was ultimately discharged to a rehabilitation facility and then home.
Conclusions
Although fulminant myocarditis is thought to have an overall better prognosis, a precipitous decline in left ventricular function and worsening shock despite escalation of support portend a poor outcome, and heart transplantation should be considered early.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Transthoracic Echocardiogram, Parasternal Long-Axis View, Hospital Day 11
Imaging revealed a severely reduced EF, thickening of the anteroseptal and inferolateral walls, and an intravascular microaxial LVAD in place.
Transthoracic Echocardiogram, Apical 4-Chamber View, Hospital Day 11
Imaging revealed biventricular dysfunction and an LVEF estimated at 5%.
References
- 1.Pollack A., Kontorovich A.R., Fuster V., Dec G.W. Viral myocarditis—diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12(11):670–680. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 2.Tschöpe C., Ammirati E., Bozkurt B., et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18(3):169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kühl U., Pauschinger M., Noutsias M., et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111(7):887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 4.Kühl U., Pauschinger M., Seeberg B., et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112(13):1965–1970. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy R.E., Boehmer J.P., Hruban R.H., et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342(10):690–695. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 6.Elsanhoury A., Kühl U., Stautner B., et al. The spontaneous course of human herpesvirus 6 DNA-associated myocarditis and the effect of immunosuppressive intervention. Viruses. 2022;14(2):299. doi: 10.3390/v14020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic Echocardiogram, Parasternal Long-Axis View, Hospital Day 11
Imaging revealed a severely reduced EF, thickening of the anteroseptal and inferolateral walls, and an intravascular microaxial LVAD in place.
Transthoracic Echocardiogram, Apical 4-Chamber View, Hospital Day 11
Imaging revealed biventricular dysfunction and an LVEF estimated at 5%.