Abstract
The indication for transcatheter aortic valve replacement to younger patients remains controversial. Here, we report a successful implantation of the first polymeric transcatheter aortic valve replacement device in a patient with severe calcific aortic stenosis. Compared with conventional valves, the novel valve has better durability, larger orifice area, and better morphological adaptability. (Level of Difficulty: Intermediate.)
Key Words: bioprosthetic durability, polymeric device, transcatheter aortic valve replacement
Graphical abstract
Patient Presentation
An 80-year-old man with severe calcific aortic stenosis was admitted to the hospital with worsening shortness of breath on exertion and paroxysmal nocturnal dyspnea. He reported initial symptoms of palpitations and chest distress 6 months previously. On examination, a severe systolic ejection murmur (grade 4) was observed at the aortic valve area.
Learning Objectives
-
•
To recognize the importance of material development in TAVR.
-
•
To understand the management of patients with aortic stenosis.
-
•
To understand the characteristics of the first polymeric TAVR device.
Past Medical History
He had a history of hypertension, chronic atrial fibrillation, and severe chronic obstructive pulmonary disease.
Differential Diagnosis
Differential diagnoses included coronary artery disease, rheumatic heart disease, hypertrophic myocardiopathy, and coarctation of the aorta.
Investigations
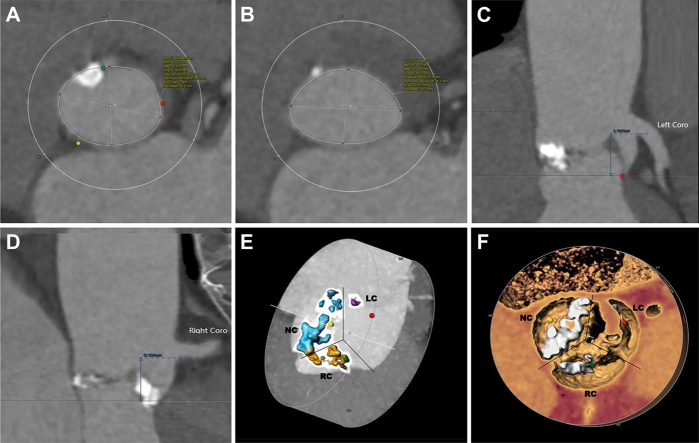
This patient had severe eccentric calcific stenosis of the aortic valve combined with moderate-to-severe regurgitation (Videos 1 and 2). The preprocedure left ventricular end-diastolic and systolic diameters were 54 and 36 mm, respectively. The peak transvalvular flow velocity and mean pressure gradient were 4.5 m/s and 81 mm Hg, respectively (Supplemental Figures 1 and 2). Preoperative computed tomography (CT) demonstrated a significant stenotic type 1 bicuspid valve (non–right coronary cusp fused) with bulky asymmetric leaflet calcification (Figure 1).
Figure 1.
Preprocedure Computed Tomography
(A) The annular diameter (area) was 26.3 mm. (B) The outflow tract diameter (area) was 25.6 mm. (C) The left coronary opening height was 16 mm. (D) The right coronary opening height was 17 mm. (E, F) A significant stenotic type 1 bicuspid valve (non–right coronary cusp fused) with bulky, asymmetric leaflet calcification.
Our heart team determined that elderly patient with multiple complications was at high risk for surgery (Society of Thoracic Surgeons Score 9.812%). Transcatheter aortic valve replacement (TAVR) for the bicuspid valve with severe calcification was somewhat challenging but feasible. Considering the effects, risks, and patient willingness, TAVR with a SIKELIA valve (MitrAssist Lifesciences Limited) (Figure 2) was finally indicated. The leaflets and sealing cuff are made of newly developed BioDura polyurethane material. More parameters are provided in Supplemental Figure 3 and Supplemental Table 1.
Figure 2.
The Polymeric Transcatheter Aortic Valve Replacement Device
It was formed by a self-expandable nitinol wire frame and a one-piece membrane constituting the moulded nanocomposite leaflets and a sealing off. Reprinted with permission from MitrAssist Lifesciences Limited.
For the first experimental implantation, we chose an elderly patient with an acceptable indication for TAVR instead of a younger patient to highlight its bioprosthetic durability. Anatomical structures such as leaflet calcification and irregular stenotic aortic orifice increase the risk of perivalvular leak (PVL). This valve may have a better sealing effect, owing to its flexible frame, which is composed of sparse nitinol wires and an adaptable sealing cuff. In addition, it is a fully retrievable and repositionable valve after 100% expansion, significantly improving release accuracy and safety, which is important for this patient with aortic regurgitation. For this reason, the delivery system is relatively large (22-F); the smallest diameter of the right femoral artery of the patient was 8.2 mm, which was sufficient to accommodate the delivery sheath. The polymeric leaflets have also better durability than pericardium leaflets.1 Finally, the novel valve was implanted according to patient's wishes.
Management
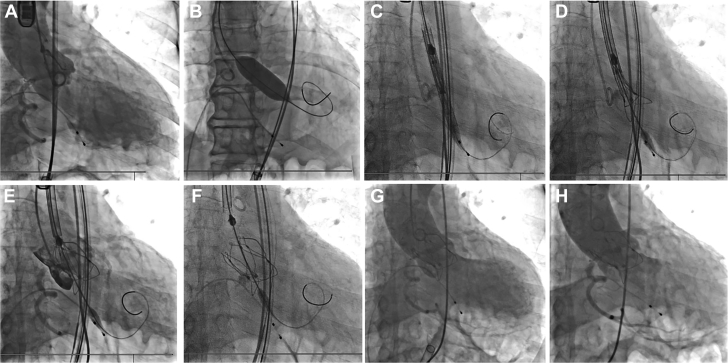
TAVR was performed under general anesthesia. The right femoral artery of the patient was chosen, as the primary vascular access with a 22-F-long introducer. The stenotic aortic valve was dilated using a balloon catheter with a diameter of 20 mm and a length of 40 mm. A 26-mm polymeric valve was advanced through a 22-F sheath. After passing through the aortic arch, the cusp overlap and commissural alignment were achieved. The delivery system was then moved to a predetermined height; the annulus marker was located 2 mm above the sinus plane to yield the largest radial support force based on preoperative CT analysis and annulus simulation. The valve was released during pacing at 180 beats/min (Video 3). Transesophageal echocardiography and digital subtraction angiography showed the valve in the correct position but moderate PVL (Figure 3, Video 4). After 15 minutes, the PVL improved to mild severity (Figure 3, Video 5). The average transvalvular pressure was 4 mm Hg and the effective orifice area (EOA) was 2.3 cm2. No new conduction blocks were observed. His symptoms gradually improved within 48 hours. The PVL remained mild and the EOA increased to 2.8 cm2. The patient was discharged on the sixth day postoperation.
Figure 3.
Procedure
(A) Live fluoroscopy illustrating the root of aorta. (B) The balloon inflation at the calcified aortic valve. (C) Passing the delivery system. (D) Gradually deploying the valve after aligning the reference points with orthotopic annulus. (E) Confirming valve position and coronary blood flow. (F) Completing valve deployment. (G) Immediate angiography showed moderate perivalvular leakage. (H) The perivalvular leakage decreased to mild after 15 minutes.
Discussion
TAVR is an effective alternative to surgery for severe aortic stenosis; however, its indication for expansion in younger patients remains controversial due to limited device durability. Hence, the exploration of prosthetic degeneration is of utmost clinical importance. A recently developed surgical valve with siloxane-based polyurethane urea has shown good safety and efficacy.2,3 However, transcatheter valves with polymeric leaflets have not yet been investigated.
The valve used in this case is a self-expandable frame composed of shape memory nitinol wires and proprietary polymeric leaflets. The highly elastic nitinol wire frame allows the valve to be fully retrieved and repositioned, even after being fully deployed. Therefore, the delivery system has a larger sheath than contemporary systems. This limits their application to patients with small femoral arteries. In addition, this valve has a sparser and shorter metal frame, making future coronary interventional therapies easier after TAVR. In this heavily calcified patient, the valve was proven to be well anchored and adaptive to the irregular bicuspid orifice.
Thus, the demand for artificial valves has increased. Mechanical valves, which have better durability and larger orifice areas, are the first choice of treatment for young patients. For the elderly who cannot tolerate anticoagulation therapy, a biological valve is a better choice, although its orifice area and durability are limited. Therefore, a valve with both these advantages is required. The novel valve has thinner polymer leaflets that are approximately one-third the thickness of the biological leaflets made from the pericardium. This can improve the hemodynamics of the valve and achieve a larger orifice area. This 26-mm valve can achieve an EOA of up to 2.8 cm2 in a heavily calcified bicuspid aortic valve. The leaflets and sealing cuff show good hemocompatibility, biostability, and resistance to calcification.2 Attempts have been made to improve the durability of bioprosthetic valves.4 Recently, the use of polymers instead of tissue to produce valve leaflets has gain more attention. As the first polymeric TAVR device implanted in humans, the valve has already passed up to 400 million cycles without damage in the accelerated wearing test, according to the ISO 5840-3 standard. The long-term safety and effectiveness need to be verified using more clinical data.
This 80-year-old patient with a poor primary condition was regarded as a high-risk patient for surgery. TAVR is the preferred choice. The patient had moderate PVL immediately after valve release. Subsequently, PVL gradually decreased to a mild level. This may be because the novel valve is self-expandable with a sparser metal frame, which may gradually conform to the calcified native aortic anatomy after implantation. This patient with aortic stenosis and regurgitation may also benefit from the perioperative management for heart failure. In the long term, endothelialization also reduces PVL.
Follow-Up
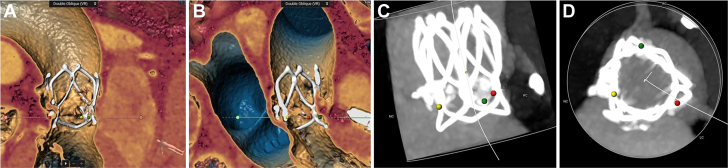
Postprocedural treatments included warfarin, diuretics, antihypertensives, and beta-blockers. Anticoagulation therapy with excellent biocompatibility is not essential for this novel valve. However, the patient had indications for anticoagulation therapy because of atrial fibrillation. After discharge, the patient was administered rivaroxaban. Follow-up transesophageal echocardiography indicated that the valve worked well (Table 1, Video 6). At 6-month follow-up, CT showed that the morphology of the prosthesis was normal (Figure 4). NYHA functional classification improved from IV to II. Six-minute walking distance increased to 430 m. No nocturnal paroxysmal dyspnea, shortness of breath, or syncope occurred.
Table 1.
The Echocardiography Follow-Up
| Baseline | Immediately | 7 d | 30 d | 6 mo | |
|---|---|---|---|---|---|
| EOA, cm2 | 1.00 | 2.30 | 2.80 | 2.77 | 2.78 |
| MPG, mm Hg | 47 | 4 | 4 | 5 | 3 |
| PVR | — | Moderate | Mild | Mild | Mild |
| LVEDD, mm | 54 | 52 | 45 | 44 | 44 |
| LVESD, mm | 36 | 37 | 30 | 32 | 29 |
| PASP, mm Hg | 56 | 45 | 35 | 34 | 28 |
| LVEF, % | 52 | 54 | 62 | 60 | 63 |
EOA = effective orifice area; LVEDD = left ventricular end diastolic diameter; LVEF = left ventricular ejection fraction; LVESD = left ventricular end systolic diameter; MPG = mean pressure gradient; PASP = pulmonary artery systolic pressure; PVR = perivalvular regurgitation.
Figure 4.
Computed Tomography Scan at 6-Month Follow-Up
(A to D) Morphology of the prosthesis and the relationship with surrounding tissues.
Conclusions
The successful implantation of the first polymeric TAVR device in humans is a significant leap in the history of TAVR technology. Compared with conventional transcatheter valves, this novel valve has better durability and a larger orifice area; it is fully retrievable and repositionable and has better adaptability to irregular valve orifices. Safety and efficacy still need to be verified using more clinical data on a larger scale and for a longer duration.
Funding Support and Author Disclosures
This work was supported by the Shanghai Clinical Research Center for Interventional Medicine (funding number: 19MC1910300). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors express gratitude to Professor Gaetano Burriesci for his contribution to the design of the novel valve and Dr Wei Zhong for his contribution to the synthesis of polymeric material.
The authors attest that they are in compliance with human studies committees of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures, a table, and videos, please see the online version of this paper.
Appendix
Preoperative biplane echocardiography.
Preoperative Doppler echocardiography.
The process of valve release.
Immediate echocardiography after valve release.
Echocardiography 15 minutes after valve release.
Echocardiography at 6-month follow-up.
References
- 1.Rezvova M.A., Klyshnikov K.Y., Gritskevich A.A., Ovcharenko E.A. Polymeric heart valves will displace mechanical and tissue heart valves: a new era for the medical devices. Int J Mol Sci. 2023;24:3963. doi: 10.3390/ijms24043963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenney C., Millson P., Grainger D.W., et al. Assessment of a siloxane poly(urethane-urea) elastomer designed for implantable heart valve leaflets. Adv Nanobiomed Res. 2021;1 [Google Scholar]
- 3.Kereiakes D.J., Answini G.A., Yakubov S.J., et al. Preliminary evaluation of a novel polymeric valve following surgical implantation for symptomatic aortic valve disease. J Am Coll Cardiol Intv. 2021;14:2754–2756. doi: 10.1016/j.jcin.2021.08.071. [DOI] [PubMed] [Google Scholar]
- 4.Neethling W., Rea A., Forster G., Bhirangi K. Performance of the ADAPT-Treated CardioCel® scaffold in pediatric patients with congenital cardiac anomalies: medium to long-term outcomes. Front Pediatr. 2020;8:198. doi: 10.3389/fped.2020.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preoperative biplane echocardiography.
Preoperative Doppler echocardiography.
The process of valve release.
Immediate echocardiography after valve release.
Echocardiography 15 minutes after valve release.
Echocardiography at 6-month follow-up.