Abstract
Objective
The optimal degree of proximal thoracic endograft oversizing when aiming for durable sealing in prosthetic grafts is unknown. The aim of the present study was to create an in vitro model for testing different oversized thoracic endografts in a reproducible and standardized manner and, subsequently, determine the optimal oversizing range when planning procedures with a proximal landing in prosthetic zones in the descending thoracic aorta or aortic arch.
Methods
An in vitro model consisting of a fixed 24-mm polyethylene terephthalate (Dacron; DuPont) graft sutured proximally and distally to two specifically designed 40-mm rings, with four force sensing resistors attached at four equally distant positions and a USB camera attached proximally for photographic and video documentation was used for deployment of Zenith TX2 (Cook Medical Inc) dissection platform endografts with diameters between 24 and 36 mm. After deployment, ballooning with a 32-mm compliant balloon was performed to simulate real-life conditions. The assessment of oversizing included visual inspection, calculation of the valley areas created between the prosthetic wall and the stent graft fabric, distance between the stent graft peaks, the radial force exerted by the proximal sealing stent, and the pull-out force necessary for endograft extraction.
Results
A total of 70 endografts were deployed with the oversizing ranging from 0% to 50%: 10 × 24 mm, 10 × 26 mm, 10 × 28 mm, 10 × 30 mm, 10 × 32 mm, 10 × 34 mm, and 10 × 36 mm. Two cases of infolding occurred with 50% oversizing. The valley areas increased from 8.79 ± 0.23 mm2 with 16.7% oversizing to 14.26 ± 0.45 mm2 with 50% oversizing (P < .001). A significant difference was found in the pull-out force required for endografts with <10% oversizing vs ≥10% oversizing (P < .001). The difference reached a plateau at ∼4 N with oversizing of >15%. The mean radial force of the proximal sealing stent was greater after remodeling with a compliant balloon (0.55 ± 0.02 N vs 0.60 ± 0.02 N after ballooning; P < .001). However, greater oversizing did not lead to an increase in the radial force exerted by the proximal sealing stent.
Conclusions
The findings from the present study offer additional insight into the mechanics of oversized stent grafts in surgical grafts. In endografts with the Zenith stent design (TX2), oversizing of <16.7% resulted in reduced resistance to displacement forces, and oversizing of >50% was associated with major infolding in 20% of cases. Long-term in vitro and in vivo testing is required to understand how these mechanical properties affect the clinical outcomes of oversizing.
Keywords: Aortic case planning, Aortic endografts, Aortic endovascular repair, In vitro models, Oversizing, Proximal landing zone
Clinical Relevance
A paucity of data is available regarding the optimal thoracic endograft oversizing that should be used when landing proximally in prosthetic grafts located in the descending thoracic aortic or aortic arch. The present study is one of the first to attempt to establish a definition of optimal oversizing for in vitro studies. It is also one of the few studies to specifically consider this subcohort of patients with complex aortic disease. Evidence from the present study suggests using oversizing of 20% to 30% for proximal sealing in prosthetic grafts. The results showed low pull-out force requirements for endografts with <16.7% oversizing but worse wall apposition for endografts with ≥30% oversizing.
Thoracic endovascular aortic repair and endovascular aortic repair have become the leading therapeutic options for most pathologies involving the descending or infrarenal abdominal aorta, mainly owing to the improved perioperative and short-term outcomes.1, 2, 3 One of the most important aspects of endovascular repair is preoperative anatomic and morphologic case planning, a crucial step for determining the most suitable stent graft design and size. This allows for correct anchorage and apposition of the device, maximizing the chances of obtaining a durable repair. To ensure correct sealing and exclusion of the diseased aortic segment, a device with a larger diameter than that of the proximal and distal landing zones should be implanted (oversizing).4 The optimal percentage of oversizing, however, has not been standardized and ranges from 0% to 30%, depending on the type of pathology, device-specific instructions for use (IFU), material availability, and physician preference. Type B aortic dissection often requires a lower degree of oversizing (0%-10%) to avoid the risk of retrograde type A dissection developing. In contrast, for aneurysmal pathology, 15% to 30% oversizing can avoid treatment failures and long-term migration.2,3,5, 6, 7 Finally, excessive oversizing should be avoided because of the risk endograft collapse and infolding.5, 6, 7
However, not all endografts are deployed in native aortic segments. Extensive aortic pathologies, especially those involving the ascending aorta and arch are frequently treated with single-stage or multistage hybrid procedures,8,9 and prosthetic segments are often used as proximal landing zones for endografts.10 The physical and mechanical in vivo properties of surgical prostheses, however, differ from those of native vessels, especially regarding their elasticity. Bustos et al11 have reported polyethylene terephthalate graft (Dacron; DuPont) prostheses to have ≤24 times more rigidity than that of the native aortic wall. Thus, it is probable that general oversizing recommendations intended for native aortic segments should not be directly applied to surgical grafts. Finally, both clinical and in vitro data regarding endograft oversizing in surgical grafts are scarce, leaving a high degree of uncertainty for clinicians when sizing and planning these procedures.
Considering the importance of durability and aneurysm sealing and the lack of knowledge regarding the optimal oversizing degree for endografts with a proximal seal zone inside surgical grafts, a study was designed to perform an in vitro evaluation of the mechanical outcomes of differently oversized thoracic endografts deployed inside surgical grafts in the descending aorta and aortic arch. Subsequently, we sought to determine the degree of oversizing that would result in optimal mechanical performance.
Methods
The chosen endograft for deployment in the present pilot study was the Zenith TX2 Dissection Endograft with Pro-Form (Cook Medical Inc). The Zenith TX2 stent graft is constructed of full-thickness woven polyester fabric sewn to self-expanding stainless steel Z-stents with braided polyester and monofilament polypropylene suture. The graft is fully stented to provide stability and the expansive force necessary to open the lumen of the graft during deployment. However, sealing with the TX2 stent graft is achieved through the first and last endograft stents, in which the stainless steel stents are sutured to the inside of the fabric, generating an outward radial force. Thus, the Cook Zenith stents provide the necessary attachment and seal of the graft to the vessel wall.12 Endografts between 24 and 26 mm in diameter have a total of 10 sealing stent peaks, and endografts with a diameter between 28 and 36 mm have a total of 12 stent peaks (Fig 1). The Zenith graft was chosen because of the absence of a proximal bare metal stent, hooks, barbs, or other additional fixation devices.
Fig 1.
Photograph of a Zenith TX2 Dissection Endograft with Pro-Form (Cook Medical Inc) with a 32-mm proximal diameter and 82-mm length. This endograft, irrespective of its total length, has one proximal and one distal sealing stent, in which stainless steel stents are sewn onto the fabric interior, exerting radial force outward into the aortic wall. The remainder of the endograft has stents on the outside of the fabric, with no adjunct proximal fixation mechanisms (no barbs, hooks, or other additional fixation devices). The TX2 endografts with a diameter between 24 and 26 mm have a proximal stent with 10 stent peaks, and endografts between 28 and 36 mm have a proximal stent with 12 stent peaks.
Creation of an in vitro model
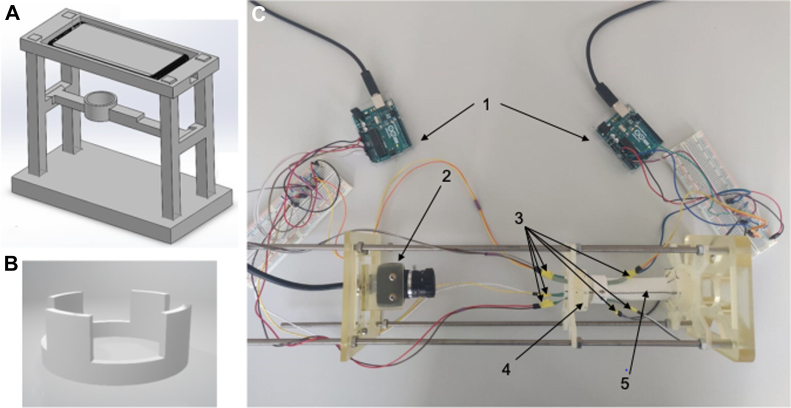
A bench model for endograft deployment was designed using commercial computer-aided design software (Solidworks 2020; Dassault Systèmes; Fig 2). Most of the necessary parts were printed using a commercial polyjet three-dimensional printer (Agilista 3200W; Keyence Co; Fig 2). An external scaffolding was designed to allow for placement of a 24-mm Dacron graft, with a camera for documentation of the process and sufficient space for endograft deployment, ballooning, and endograft removal.
Fig 2.
Model simulations and final construction, including three-dimensional rendering of a preliminary model (A) with pressure sensor rings (B). C, Final constructed model showing the Arduino microcomputers (1, arrows) hooked up to the pressure sensors (3, arrows), distributed homogenously at the 12-, 3-, 6-, and 9-o'clock positions around the first two proximal stents of the endograft with the high-resolution camera (2, arrow) positioned proximally for image and video documentation of the consecutive endograft deployments. Finally, the polyethylene terephthalate graft (5, arrow) was sutured proximally and distally to two specifically designed 40-mm rings (4, arrow), each with 10 small holes, allowing for equidistant placement of the suture stitches.
To avoid constriction of the Dacron graft (24-mm Gelweave straight; Terumo Aortic) by the external structures that could potential affect its distensibility, the graft was held in place by 10 equidistant suture stitches to two specifically designed 40-mm rings, one placed proximally and one distally. Two 25-mm concentric rings were attached 10 mm and 40 mm from the proximal edge of the graft, corresponding to the middle of the first and second row of endografts stents. On the inside of both concentric rings, four force sensing resistors (FSR400; Interlink Electronics Inc) were attached at the 3-, 6-, 9-, and 12-o'clock positions and connected to an Arduino Uno Rev3 microcontroller (Arduino AG). Pressure reading was conducted according to the manufacturer's specifications. At the proximal end of the model, a USB camera (UI3580LE; IDS Imaging Development Systems GmbH) was fixed to allow for photographic and video documentation of the deployment sequence.
Endograft deployment
The endografts were advanced into the Dacron prosthesis with the aid of a super-stiff Amplatz guidewire (Boston Scientific) from the bottom of the model proximally in the direction of the camera. The nose of the delivery system was advanced on the Amplatz guidewire until it had reached the outside of the graft. Next, the proximal edge of the endograft fabric was aligned with the proximal edge of the Dacron prosthesis. The endograft was slowly deployed under visual guidance, and, if necessary, small manual adjustments were made to achieve precise proximal alignment. After complete deployment, the proximal stent was ballooned with a compliant 32-mm Coda balloon (Cook Medical Inc) inflated with 30 mL of saline solution in a 50-mL syringe to reproduce standard clinical practice and ensure that the balloon was inflated to its maximum diameter. Video documentation was performed of the deployment sequent, and two images were taken for each deployed endograft, one immediately after endograft deployment and one after balloon remodeling.
Assessment of oversizing
Visual assessment of the two images taken for each deployed endograft was performed. The items evaluated included (1) apposition of the endograft with the Dacron prosthesis; (2) the valley size, which was estimated by analyzing the area created between the curvature of the Dacron prosthesis and endograft fabric; (3) the occurrence of infolding; and (4) the distance between the stent graft peaks. The distance between the stent peaks was measured using ImageJ (National Institutes of Health). The theoretical ideal deployment was considered to be equally distant stent peaks to achieve a homogeneous radial pressure.13
The radial force was evaluated with the implanted pressure sensors, four on the proximal sealing stent and four on the second endograft stent. The eight pressure sensors were leveled before endograft deployment, ensuring that all the sensors were set at zero. Continuous pressure measurements were obtained throughout the deployment, ballooning, and postballooning sequences. The sensors were then averaged to obtain one final value for each phase of the sequences.
After the visual evaluation and radial force measurements, the endografts were extracted and resheathed into the delivery system in standard fashion.14 The Zenith TX2 Dissection with Pro-Form endografts were then deployed in a new 24-mm Dacron prosthesis with distal fixation to the lower mounting clamp of a uniaxial tensile testing machine (Zwick Line Z2.5 TN; Zwick Roell GmbH). Deployment was performed under visual guidance, and the necessary manual adjustments were made to obtain an exact overlap of two stents. Again, after deployment, remodeling of the proximal stent was performed with a 32-mm Coda compliant balloon and 30 mL of saline solution. Once satisfactorily deployed, the distal end of the endograft was attached with 2-0 Prolene suture to the load cell (Xforce HP 0.2 kN; Zwick Roell GmbH) of the tensile testing machine. After attachment, the load cell was moved upward along the traverse with a testing speed of 20 mm/s until the endograft was successfully pulled from the Dacron prosthesis.
Statistical analysis
Statistical analysis was performed using SPSS, version 26 (IBM Corp). Normality was evaluated using the Kolmogorov-Smirnov test. Normally distributed variables are expressed as the mean ± standard deviation and non-normally distributed variables as the median and interquartile range. The radial force measurements presented were collected by the pressure sensors positioned at the level of the proximal sealing stent. A related-samples Friedman two-way analysis of variance by ranks was used to compare the differences observed before and after remodeling, and the differences between groups were compared using the Kruskal-Wallis test. Significant values were adjusted using the Bonferroni correction for multiple tests. P < .05 was considered statistically significant. Ethical approval for the present study was waived owing to the lack of patient data.
Results
Visual results: apposition, infolding, stent peak distribution, and valley size
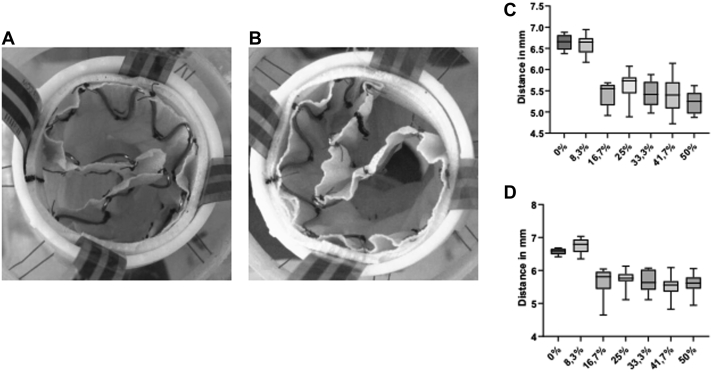
A total of 70 Zenith TX2 endografts were deployed inside the bench model (Fig 3), including 10 × 24 mm (0% oversizing), 10 × 26 mm (8.3% oversizing), 10 × 28 mm (16.7% oversizing), 10 × 30 mm (25% oversizing), 10 × 32 mm (33.3% oversizing), 10 × 34 mm (41.7% oversizing) and 10 × 36 mm (50% oversizing), for an oversizing range of 0% to 50%. Of the 10 endografts deployed with 50% oversizing, two (the second and eighth deployed endografts) developed infolding, with unsuccessful correction after balloon remodeling (Fig 4, A and B). Visual inspection showed that the best “endograft–prosthesis apposition” with the 24-mm and 26-mm endografts (<10%). In contrast, the 32-, 34-, and 36-mm endografts (>30% oversizing) all showed significant fabric excess between the stent peaks, leading to the creation of valleys and infolding in 20% of the endografts with extreme oversizing.
Fig 3.
Photographs of the proximal landing zone after remodeling with a 32-mm compliant balloon: 24 mm, 0% oversizing (A); 26 mm, 8.3% oversizing (B); 28 mm, 16.7% oversizing (C); 30 mm, 25% oversizing (D); 32 mm, 33.3% oversizing (E); 34 mm, 41.7% oversizing (F); and 36 mm, 50% oversizing (G). Note the increase in the size of the valleys created between the stent peaks.
Fig 4.
A, Photograph of the first case of infolding, corresponding to the second 36-mm endograft deployed. B, Photograph of the second infolding case, corresponding to the eighth 36-mm endograft deployed. C, Graph showing the distribution of the distance between stent peaks in the endografts. D, Graph showing the distribution of the distance between stent peaks in the endografts.
No differences were observed in the peak distribution per oversizing category. The 24-mm and 26-mm endografts, both with 10 stent peaks, showed a comparable distribution. The endografts with a diameter from 28 to 36 mm also presented with comparable distributions. These similarities between groups remained constant before and after ballooning. However, although the distance between the stent peaks in the 24- and 26-mm endografts (10 stent peaks; <10%) remained stable before and after ballooning (P = .66), it increased from a mean of 5.22 ± 0.26 mm to 5.61 ± 0.31 mm after ballooning for the 36-mm stent grafts (P = .004; Fig 4, C and D).
A significant difference between all oversizing groups was observed in the estimated valley area, with the valley areas increasing from 8.79 ± 0.23 mm2 with 16.7% oversizing to 14.26 ± 0.45 mm2 with 50% oversizing. The size and morphology of these valleys in the >30% oversizing group did not change significantly after the use of the compliant balloon in the dry laboratory (Fig 5).
Fig 5.
Graph depicting the valley area (A) before remodelling, (B) after remodelling and (C) comparing before and after remodelling.
Radial force
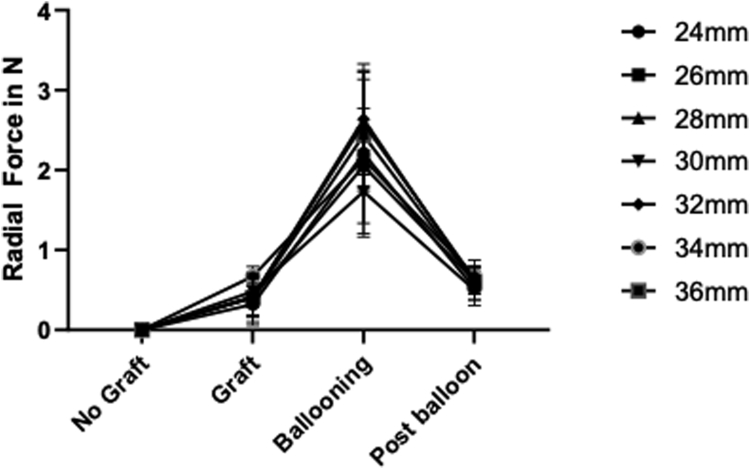
The radial force exerted on the prosthesis was significantly greater after remodeling than before remodeling (P < .001), with a mean radial force immediately after endograft deployment of 0.55 ± 0.02 N vs 0.60 ± 0.02 N after ballooning (Fig 6). Although a statistically significant difference was observed in the radial force exerted before remodeling (P = .041) between the oversizing groups, this significance was lost after correction for multiple testing (P = .082). No difference in the radial force was observed between groups after remodeling.
Fig 6.
Graph depicting the mean radial force exerted by each endograft size, before, during, and after remodeling. The mean was calculated by averaging the values from the four pressure sensors placed at the proximal sealing stent and obtaining a mean measurement of the 10 endograft deployments.
Pull-out force
The mean force required to extract a 0% oversized endograft was 1.35 ± 0.05 N, which increased to 2.65 ± 0.06 N for 8.3% oversizing and 4.02 ± 0.05 N for 16.7% oversizing. At oversizing between 25% and 50%, the mean force necessary for extraction was ∼4 N (4.14 ± 0.15 N for 25%; 3.71 ± 0.07 N for 33.3%; 4.22 ± 0.1 N for 41.7%, and 4.77 ± 0.05 N for 50% oversizing; Fig 7). When evaluating the differences between groups, oversizing percentages from 16.7% to 50% were all significantly different from 0% and 8.7% oversizing (P < .001). Between 16.7% and 50%, the only observed difference was between 33.3% and 50% oversizing (P = .005).
Fig 7.
A, Setup of the pull-out test experiment, with the 24-mm polyethylene terephthalate graft attached distally to the base of the machine and the endograft attached by its distal sealing stent to the pulling device. B, Graph depicting box plots of the force required to pull each size endograft from the 24-mm prosthetic graft. A significant difference was found in the endografts oversized with <10% and >10%.
Discussion
Oversizing is one of the fundamental aspects of aortic case planning.15 Optimal oversizing should be able to guarantee a durable treatment, with adequate proximal and distal sealing and the absence of migration, without stent-induced complications. However, a consensus has not yet been reached regarding the optimal degree of oversizing.1, 2, 3 Charlton-Ouw et al16 showed that of the 3607 patients included in the GREAT Registry (W.L. Gore & Associates global registry for endovascular aortic treatment), only 53% had been treated in accordance with the oversizing IFU recommendations, 22% were oversized, 15% were undersized, and 10% had both over- and undersized components. These variations had an effect on the treatment outcomes, with undersizing related to increased aortic and all-cause mortality (hazard ratio, 60.5 and 18.0, respectively).16 Similarly, Tanious et al17 evaluated 219 patients undergoing thoracic endovascular aortic repair for aneurysmatic pathology and found an increased risk of mortality for patients with >30% oversizing (hazard ratio, >10; P = .049). Excessive oversizing in the infrarenal/visceral segment has also been associated with neck dilation and an increased incidence of late treatment failure.18,19 Finally, a recent systematic review found a significant increase in the combined incidence of adverse clinical events (endoleak type I, migration, and reintervention during follow-up) for patients with neck dilatation, although the extent to which this was an effect of stent oversizing or disease progression remains unclear.7
Optimal oversizing is even more uncertain when considering landing endografts inside surgical grafts. Manufacturers' IFU regarding oversizing usually apply to native aortic segments. However, because surgical grafts have significantly different structural properties,10 it is probable that these standard oversizing recommendations will not apply. Moreover, Dacron prosthetic conduits have a tendency toward expansion over time, with postoperative prosthetic diameters larger than those of the original grafts.20 Kotha et al20 specifically studied proximal landing zone complications of stent grafts deployed in surgical prostheses (a 37-mm endograft in a 28-mm Dacron prosthesis; 32% oversizing). They reported a 20% proximal landing zone complication rate, including two cases of a type I endoleak, one case of infolding, and one case of migration.20
This significant risk of proximal fixation complications coupled with the lack of scientific knowledge was the main reason we sought to create an in vitro model that would allow us to understand the mechanical and physical differences associated with various degrees of oversizing and, subsequently, define an optimal range of oversizing for prosthetic landing zones. We designed a study plan with four different stages:
Stage I: creation of a dry bench model with straight anatomic features (pilot study phase)
Stage II: testing of different grafts (Dacron, polytetrafluoroethylene, and animal aortas) in the dry bench model
Stage III: evaluation of the different grafts in a model with pulsatile flow
Stage IV: evaluation of the effects of oversizing in different curved anatomies and three-dimensional printed models with pulsatile flow
In the present report, we describe the first stage of our study plan. First, establishing a definition of optimal oversizing in an in vitro model was challenging given the lack of prior studies. To overcome this, we created a composite end point, including visual appreciation, percentage of infolding, homogeneity of the peak distribution and estimated valley area, and the exerted pull-out force required for endograft extraction. Additionally, to replicate clinical conditions, endograft deployment was followed by remodeling with a compliant balloon. In addition, differences in the graft configurations before and after remodeling were analyzed. Visual inspection showed the >30% oversized endografts to be associated with worse endograft apposition, with greater infolding and valley formation, which did not improve after ballooning. This was especially significant for the 50% oversized grafts. Probably the most significant findings from our study were the differences in pull-out-forces. These forces increased significantly in the first oversizing groups (0%-16%). Similar pull-out-forces were observed after 16% oversizing, probably indicating that little benefit results from endograft fixation with higher oversizing. Other studies have also assessed the endograft pull-out forces. Chiang et al21 created an in vitro model with the objective of providing a testing setup that would allow for a comparison of the pull-out forces for various thoracic stent grafts at various neck angulations and oversizing. They tested three types of commercial endovascular thoracic stent grafts (Valiant [Medtronic]; Zenith TX2 Pro-Form [Cook Medical Inc]; and TAG (W.L. Gore & Associates]) with 6.25% to 25% oversizing and at four different angulations (0°, 45°, 90°, and 135°) deployed in a silicone tube.21 The Valiant device was associated with the highest dislodgment force, and the Zenith TX2 and TAG devices had comparable forces. However, the Zenith TX2 required higher dislodgment forces in more angulated necks (>90%). Similar to our findings, they also reported that increasing the oversizing to >20% for all three devices did not significantly change the dislodgment force.21 Finally, a surprising finding in our study was the absence of differences observed in the radial force exerted on the Dacron material between the oversizing groups. This could be secondary to the high rigidity of this material, constricting the endograft and limiting the radial force exerted on the prosthesis. We believe this situation is substantially different from that with native aortic tissue, given its higher distensibility and elasticity, and one of the important mechanical characteristics resulting in different sealing behavior of stent grafts in native aortas and prosthetic landing zones. These findings, combined with the documented tendency of Dacron endografts to dilate and expand over time and the high rate of proximal complications associated with >30% oversizing, might indicate that the best oversizing rate for endografts deployed within Dacron prosthetic segments is 20% to 30%. However, more complex models, including evaluations with different curvatures and different size Dacron endografts, and a long-term durability assessment in a model with pulsatile flow to allow for dynamic analysis and fatigue testing are needed for any definite conclusions. A model with pulsatile flow has been designed and is currently being assembled, allowing for testing of different anatomic features under more real-life conditions (phases 3 and 4 of our study plan). Furthermore, we are attempting to obtain 24-mm bovine aortas to enable a comparison group for our dry and straight bench model (phase 2).
The present study has some limitations. The percentage of oversizing tested was inside a 24-mm Dacron prosthetic segment. Thus, it is possible that the same degree of oversizing would behave differently in prosthetic segments with different diameters. The endograft used, the Zenith TX2, does not have any adjunct proximal fixation systems other than the radial force, enabling a larger number for analysis and maximizing the internal validity. However, it is possible that the first endograft choice for these repairs would be a device with adjunct fixation devices. Therefore, for a more in-depth assessment, different endograft models must be tested, including nitinol scaffolding, polytetrafluoroethylene fabric, and proximal fixation adjuvants. As stated, the present version of the model is a dry straight bench model, which did not allow for simulation of the real-life conditions the aorta is subjected to, including pulsatility, mobility, and shear stress, limiting the long-term validity of our results. Finally, we also could not properly evaluate the documented dilation of Dacron endografts over time in the current model.
Despite these limitations, our findings offers further insight into the characteristics of the proximal sealing zone of thoracic endografts deployed in surgical grafts. Although these results are from the pilot phase, we have already began work on phases 2 to 4, which we hope will lead to evidence-driven recommendations for oversizing when landing proximally in surgical prostheses.
Conclusions
The findings from the present study offer additional insight into the mechanics of oversized stent grafts in surgical grafts. In endografts with the Zenith stent design (TX2), oversizing of <16.7% resulted in reduced resistance to displacement forces. In contrast, oversizing of >50% was associated with major infolding in 20% of the cases. Long-term in vitro and in vivo testing is required to understand how these mechanical properties affect the clinical outcomes of oversizing.
Acknowledgments
The authors thank the Fundacion Alfonso Martin Escudero for their contribution and financial support in the realization of this work.
Footnotes
The present study was supported in part by the Fundacion Alfonso Martin Escudero.
Author conflict of interest: none.
CP and MG contributed equally to this article and share co-first authorship.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Erbel R., Aboyans V., Boileau C., et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 2.Wanhainen A., Verzini F., Van Herzeele I., et al. European Society for Vascular surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal Aorto-iliac Artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57:8–93. doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Riambau V., Böckler D., Brunkwall J., et al. Management of descending thoracic aorta diseases. Eur J Vasc Endovasc Surg. 2017;53:4–52. doi: 10.1016/j.ejvs.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Nation D., Wang G. TEVAR: endovascular repair of the thoracic aorta. Semin Intervent Radiol. 2015;32:265–271. doi: 10.1055/s-0035-1558824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spanos K., Tsilimparis N. The Pac-Man Sign. Eur J Vasc Endovasc Surg. 2018;56:56. doi: 10.1016/j.ejvs.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Canaud L., Alric P., Desgranges P., Marzelle J., Marty-Ané C., Becquemin J.P. Factors favoring stent-graft collapse after thoracic endovascular aortic repair. J Thorac Cardiovasc Surg. 2010;139:1153–1157. doi: 10.1016/j.jtcvs.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Kouvelos G.N., Oikonomou K., Antoniou G.A., Verhoeven E.L.G., Katsargyris A. A systematic review of proximal neck dilatation after endovascular repair for abdominal aortic aneurysm. J Endovasc Ther. 2017;24:59–67. doi: 10.1177/1526602816673325. [DOI] [PubMed] [Google Scholar]
- 8.Ranney D., Yerokun B., Benrashid E., et al. Outcomes of planned two-stage hybrid aortic repair with Dacron-replaced proximal landing zone. Ann Thorac Surg. 2018;106:1136–1142. doi: 10.1016/j.athoracsur.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 9.Gargiulo M., Gallitto E., Freyrie A., Stella A. Fenestrated endograft for recurrent paravisceral aortic pseudoaneurysm after thoracoabdominal aortic aneurysm open repair. J Vasc Surg. 2013;58:790–793. doi: 10.1016/j.jvs.2012.11.082. [DOI] [PubMed] [Google Scholar]
- 10.Czerny M., Schmidli J., Adler S., et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society for Vascular Surgery (ESVS) Eur J Cardiothorac Surg. 2019;55:133–162. doi: 10.1093/ejcts/ezy313. [DOI] [PubMed] [Google Scholar]
- 11.Bustos C., García-Herrera C., Celentano D. Mechanical characterisation of dacron graft: experiments and numerical simulation. J Biomech. 2016;49:13–18. doi: 10.1016/j.jbiomech.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Zenith TX2 dissection endovascular graft with Pro-Form and the Z-Track plus introduction system. https://ifu.cookmedical.com/ifuPub/ReadFile?fileName=I-ZDEG-EU-1105-394-02.PDF
- 13.Rasband W.S. ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA. https://imagej.nih.gov/ij/ 1997-2018.
- 14.Oderich G.S., Ricotta J.J. Modified fenestrated stent grafts: device design, modifications, implantation, and current applications. Perspect Vasc Surg Endovasc Ther. 2009;21:157–167. doi: 10.1177/1531003509351594. [DOI] [PubMed] [Google Scholar]
- 15.Terara M., Hazenberg C.E., Houben I.B., Trimarchi S., van Herwaarden J.A. Important issues regarding planning and sizing for emergent TEVAR. J Cardiovasc Surg (Torino) 2020;61:708–712. doi: 10.23736/S0021-9509.20.11571-4. [DOI] [PubMed] [Google Scholar]
- 16.Charlton-Ouw K.M., Ikeno Y., Bokamper M., Zakhary E., Smeds M.R., GREAT participants Aortic endograft sizing and endoleak, reintervention and mortality following endovascular aneurysm repair. J Vasc Surg. 2021;74:1519–1526.e2. doi: 10.1016/j.jvs.2021.04.045. [DOI] [PubMed] [Google Scholar]
- 17.Tanious A., Boitano L., Canha L., et al. Thoracic aortic remodeling with endografting after a decade of thoracic endovascular aortic repair experience. J Vasc Surg. 2021;73:844–849. doi: 10.1016/j.jvs.2020.06.120. [DOI] [PubMed] [Google Scholar]
- 18.Tran K., Deslarzes-Dubuis C., Lee J.T. Quantification of suprarenal aortic neck dilation after fenetrated endovascular aneurysm repair. J Vasc Surg. 2021;73:31–38. doi: 10.1016/j.jvs.2020.04.522. [DOI] [PubMed] [Google Scholar]
- 19.Tsilimparis N., Dayama A., Ricotta J.J., 2nd Remodeling of aortic aneurysm and aortic neck on follow-up after endovascular repair with suprarenal fixation. J Vasc Surg. 2015;61:28–34. doi: 10.1016/j.jvs.2014.06.104. [DOI] [PubMed] [Google Scholar]
- 20.Kotha V., Herget E., Appoo J. Complications at the proximal landing zone of endovascular stent grafts deployed in surgically replaced ascending aorta. Ann Thorac Surg. 2016;102:1490–1497. doi: 10.1016/j.athoracsur.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 21.Chiang C.H., Yeh M.L., Chen W.L., Kan C.D. Apparatus for comparison of pullout forces for various thoracic stent grafts at varying neck angulations and oversizes. Ann Vasc Surg. 2016;31:196–204. doi: 10.1016/j.avsg.2015.10.007. [DOI] [PubMed] [Google Scholar]