Abstract
An increasing proportion of patients with chronic limb-threatening ischemia are older and have multiple comorbidities, including diabetes and renal failure. For those who are not candidates for a surgical bypass, this set of patients presents a challenge to vascular surgeons and interventionalists owing to the complex below-the-knee and increasingly below-the-ankle disease pattern that can fail traditional approaches for endovascular intervention. Two techniques, the retrograde pedal access and the pedal-plantar loop technique, can be useful in these settings and in skilled hands can be used safely, with a high technical success rate. In patients with chronic limb-threatening ischemia who are not candidates for a single-segment saphenous vein bypass, the retrograde pedal access technique can be used not only in the setting of failed antegrade treatment, but also primarily when faced with a difficult groin or as an adjunct during a planned antegrade-retrograde intervention. The pedal plantar loop technique allows for retrograde access to tibial vessels without retrograde vessel puncture and additionally offers the ability to treat the pedal-plantar arch, which may have added benefit in wound healing. We describe the tips and tricks for these two techniques used in our limb salvage practice.
Keywords: Pedal arch revascularization, Pedal loop revascularization, Pedal access, Retrograde access, Pedal-plantar arch, Pedal-plantar loop, CLTI, Limb salvage
Peripheral artery disease (PAD) affects 230 million adults aged ≥25 years (5.6%) worldwide, and approximately 8.5 million adults (7%) in the United States.1 In addition to the increased risk of cardiovascular morbidity and mortality, patients with PAD have decreased physical function and are at an increased risk for limb loss.2, 3, 4 More than 80% of major limb amputations occur in patients with chronic limb-threatening ischemia (CLTI),5 which accounts for approximately 11% of the population with PAD.3 Patients with CLTI are more likely to be older, with multiple comorbidities, including renal failure and diabetes,5 and have a disease pattern that is more diffuse with a greater incidence of below-the-knee (BTK) vessel involvement and chronic total occlusion (CTO).6, 7, 8 Below-the-ankle (BTA) vessel disease is also frequently encountered in the setting of CLTI, especially in patients with diabetes, where the prevalence of BTA vessel involvement has been reported in ≤44% of patients presenting with CLTI.9,10
The mainstay of CLTI treatment is optimal medical therapy and to establish inline flow to the foot to optimize wound healing and decrease the risk of amputation. The benefits of open surgical bypass are well-established11,12; however, there are an increasing number of patients with CLTI with advanced comorbidities, lack of am adequate autologous conduit, and extensive multilevel arterial occlusive disease in whom traditional open approaches are challenging, if not impossible. Fortunately, there has been a significant improvement in our endovascular armamentarium of tools and techniques to help with these complex patients with CLTI.4 Two such techniques that we use regularly in our limb salvage practice include retrograde pedal access and the pedal-plantar loop technique. Tips and tricks on the use of these techniques are described elsewhere in this article.
Retrograde pedal access
Retrograde pedal access was first described almost 30 years ago by Iyer et al 13 using an open cutdown of the posterior tibial artery. The percutaneous approach to retrograde pedal access was subsequently described in 2003 by Botti et al14 and Spinosa et al15 and has since gained popularity as endovascular tools and techniques improved.
Why use this technique?
Published data for BTK percutaneous angioplasties performed for CLTI suggest that 11%16 and, more recently, ≤39%17,18 of lesions cannot be crossed with traditional antegrade approaches. Technical failure rates are higher in the setting of occlusive lesions compared with stenosis.19 Several factors are thought to play a favorable role in the retrograde crossing of a CTO. These factors include a convex distal cap morphology that favors tracking of the wire to the center of the lumen,20 less engagement of collaterals during retrograde travel of a wire, and increased pushability and control of the wire through an access point that is smaller, and closer to the occlusive lesion.
The retrograde pedal access is not only useful in the setting of a failed antegrade revascularization, but also when used in isolation during a tibiopedal arterial minimally invasive revascularization21 when faced with a difficult groin or as an adjunct when a sturdy wire platform is needed to facilitate the treatment of particularly difficult lesions, which can be achieved with a through and through access like that achieved during subintimal arterial flossing with antegrade-retrograde intervention.22
Technique tips and tricks
Planning
Preoperative assessment of imaging such as duplex examination, computed tomography angiography or magnetic resonance angiography can assist in preoperative planning. Several angiographic scoring systems exist for predicting successful antegrade crossing of BTK lesions including the CTO plaque cap morphology classification,20 the infrapopliteal CTO score,18 and the Japanese-BTK CTO score.17 Several lesion characteristics associated with unsuccessful antegrade crossing can be assessed using noninvasive preoperative imaging, including lesion length >100 mm20 or >200 mm,17,18 severe calcification,18,20 vessel diameter of <2 mm,17 and distal reconstitution of target vessel.17 However, we find it best to expect the possibility of retrograde access for every patient with CLTI and thus prepare every patient with CLTI appropriately. Prepping of the foot for every patient will significantly increase the chances of using the technique more expeditiously, and successfully.
Positioning
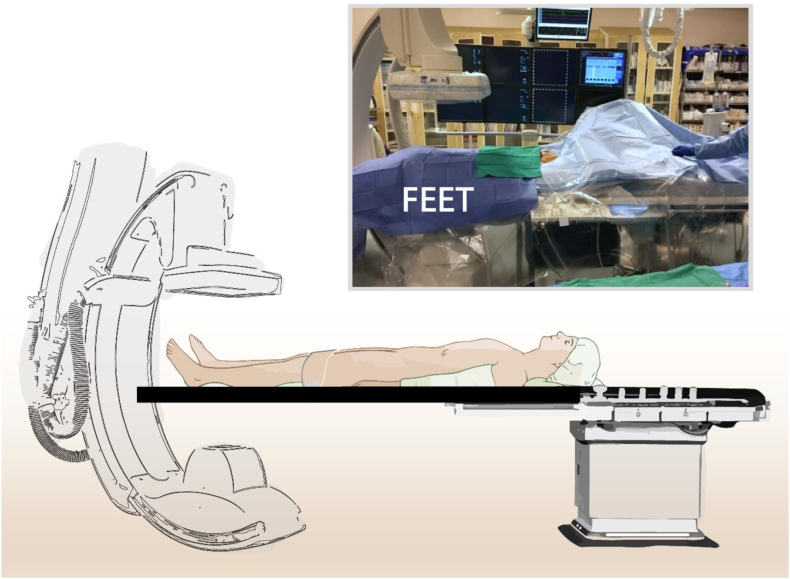
We ensure that the patient is positioned on the angiographic table so that a complete angiogram of the whole leg and foot can be obtained, ideally without flexing the leg. Flipping the patient on the bed so that the feet are where the head normally lies can help ensure such imaging (Fig 1). This strategy also facilitates antegrade femoral access, which we use almost exclusively when treating BTK lesions.
Fig 1.
Patient positioning to facilitate access to the pedal vessels and imaging of the foot. The patient can be positioned on the table such that the foot is at the head of the angiographic table.
Access
Retrograde access can be obtained with ultrasound and or angiography guidance depending on how calcified the target vessel is. We prefer ultrasound-guided access of the dorsalis pedis, distal anterior tibial artery, or posterior tibial artery when possible. Accessing more distal locations in the foot can be difficult owing to the smaller size of the vessel, but can also be easier because there is less movement of the vessels as they are held in tighter by the tendons of the foot. If possible, try to avoid distal access of the single patent vessel to the foot in case there is a need for pedal bypass in the future.
For ultrasound-guided access, we recommend using a small footprint linear array transducer such as the hockey stick probe, which is ideal for visualization of the very superficial pedal vessels. We evaluate the vessel in both the long and short axes and identify a relatively straight portion of the vessel at their least diseased or calcified portions. It is crucial to keep the tip of the needle in view at all times, and confirm access by ultrasound visualization of the tip of the needle in the lumen of the vessel because pulsatile flow is often absent to confirm arterial access.
Fluoroscopic or angiographic guidance can be particularly useful for heavily calcified lesions where ultrasound images are difficult to interpret or for retrograde access of more proximal vessels such as peroneal and proximal anterior tibial artery access, which is less commonly used in our practice. Fluoroscopic-guided access can be performed with or without road mapping or image overlay techniques. We often find it unnecessary, given that vessels are often heavily calcified and readily visible. Advancing the needle in one direction, then changing the imaging to an orthogonal view can help to provide an understanding of the vessel location and depth to aid vessel puncture.
Once access to the vessel is obtained, drop the needle angle to ensure the needle entry is at a soft angle to facilitate passage of the wire into the true lumen. We recommend using a longer workhorse 0.014″ or 0.018″ guidewire immediately after needle access instead of the enclosed initial access wire to minimize the need for wire exchange.
Treatment considerations
Placing a sheath can be helpful if one needs to treat or use more tools for crossing the target lesion from a retrograde approach. However, starting sheathless with only a wire with or without a 0.014″ or 0.018″ support or crossing catheter is often adequate for retrograde crossing of the target lesion. After crossing the lesion, we recommend reconnection with the antegrade approach (often by advancing the wire into an angled 0.035″ antegrade catheter, or snaring) as soon as possible. This practice allows the retrograde wire to be externalized and the procedure to continue from an antegrade approach. If the wire remains in the subintimal plane after crossing the lesion from the retrograde approach, multiple re-entry techniques such as controlled antegrade and retrograde tracking (CART), reverse CART, confluent or parallel balloon, and facilitated re-entry techniques can be used for re-entry into the true lumen.23,24 With the CART, reverse CART, and confluent or parallel balloon techniques, connection between the antegrade and retrograde subintimal plane is achieved by balloon dilation of the subintimal space either from the retrograde (CART) or the antegrade (reverse CART) wire, or both (confluent or parallel balloon technique).
Hemostasis
Hemostasis at the pedal access site can usually be achieved using light manual compression for 10 to 20 minutes. Radial access hemostatic compression devices such as the TR BAND radial compression device (Terumo Medical Corporation, Somerset, NJ) can be used at the level of the ankle, but we do not commonly use them in our practice. If hemostasis cannot be achieved by external compression, a low-profile balloon from the antegrade wire can be insufflated for 2 to 5 minutes.
Tools and additional tips
-
•
We use a short, 4F micropuncture kit such as the Micropuncture Pedal Introducer Access Set (Cook Medical, Bloomington, IN), which contains a 4-cm access needle and a 2.9F ID introducer that can be converted to an interventional retrograde sheath using the enclosed check-Flo valve.
-
•
Either 0.014″ or 0.018″ guidewires can be used, but we often start with and prefer using an 0.018″ wire to enhance the support and pushability to cross the lesion. Wires we commonly use include the V18 ControlWire Guidewire (Boston Scientific Corporation, Marlborough, MA), Hi-Torque Command 18 ST (Abbott Cardiovascular, Plymouth, MD), and Nitrex guidewire (Medtronic, Minneapolis, MN).
-
•
For the support catheter, we use a straight or angled 0.014″ or 0.018″ CXI Support Catheter (Cook Medical) for most of our work.
Pedal-plantar loop technique
The initial description of the pedal-plantar loop technique originated from case reports in Italy. It was described by Fusaro et al25 as an alternative technique for retrograde access to either the anterior tibial or posterior tibial artery in patients undergoing BTK revascularization where retrograde pedal access was not feasible. Graziani,26 in contrast, described the technique as a means of recanalizing an occluded pedal arch. Manzi et al27 subsequently published a consecutive case series where it established the pedal-plantar loop technique as a means for the pedal/plantar artery and pedal arch revascularization.
Why use this technique?
Patients with CLTI are more likely to present with concurrent BTA vessel disease, especially in the setting of diabetes.9,10 In addition to successful BTK revascularization, recanalization of BTA vessels has been shown to decrease the risk of amputation,9,28 likely by increasing the rate of wound healing,29 which is an independent predictor of major adverse limb events in patients with CLTI.30 Moreover, the patency of the pedal arch has been shown to be an important predictor of the rate of wound healing in CLTI and is especially important in the setting of a single vessel runoff to the foot, where a patent pedal arch is an important predictor of an improved limb salvage rate.31
The pedal-plantar loop technique is primarily used in our practice for the revascularization of pedal vessels and the pedal arch. However, we also use the technique as an alternative for retrograde access to the tibial artery. Although we primarily use the technique through an antegrade femoral puncture, a variation of the technique where the pedal arch is accessed from an antegrade pedal access has also been described.32
Technique tips and tricks
Planning
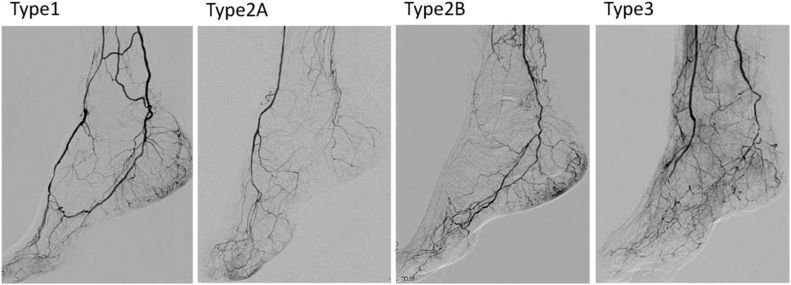
A deep understanding of pedal arch anatomy along with variants is imperative for successful use of this technique. The pedal arch involves the connection between the anterior and posterior circulation and typically involves a connection between the dorsalis pedis artery and the lateral plantar artery with the deep perforator at the first metatarsal interspace. The lateral plantar artery then joins the medial plantar artery to become the posterior tibial artery. In approximately 10% of patients, the anterior and posterior circulations of the foot are completely separate, precluding the use of the pedal-plantar loop technique. In 7% to 9% of patients, the dorsalis pedis artery arise from the peroneal artery.33 Knowing these variations a priori may be difficult without prior angiographic images. The pedal arch anatomy should be assessed closely at the time of intervention. In addition to anatomic variations, findings can be classified by the Kawarada classification system.34 A type 1 arch describes a patent arch with patent dorsal and lateral plantar arteries. In a type 2 arch, either the dorsalis pedis (2A) or lateral plantar artery (2B) is patent. In a type 3 arch, both arteries are occluded (Fig 2).
Fig 2.
Kawarada pedal arch classification of the pedal arch based on the Kawarada classification. The pedal arch is patent in type 1. The pedal arch is not visualized in types 2 and 3. In a type 2 arch, the dorsalis pedis artery (2A) or lateral plantar artery (2B) is patent. In a type 3 arch, both the dorsalis pedis and lateral plantar arteries are occluded. Reproduced with permission from Kawarada et al. Cathet Cardiovasc Intervent 2012;80:861-871.34
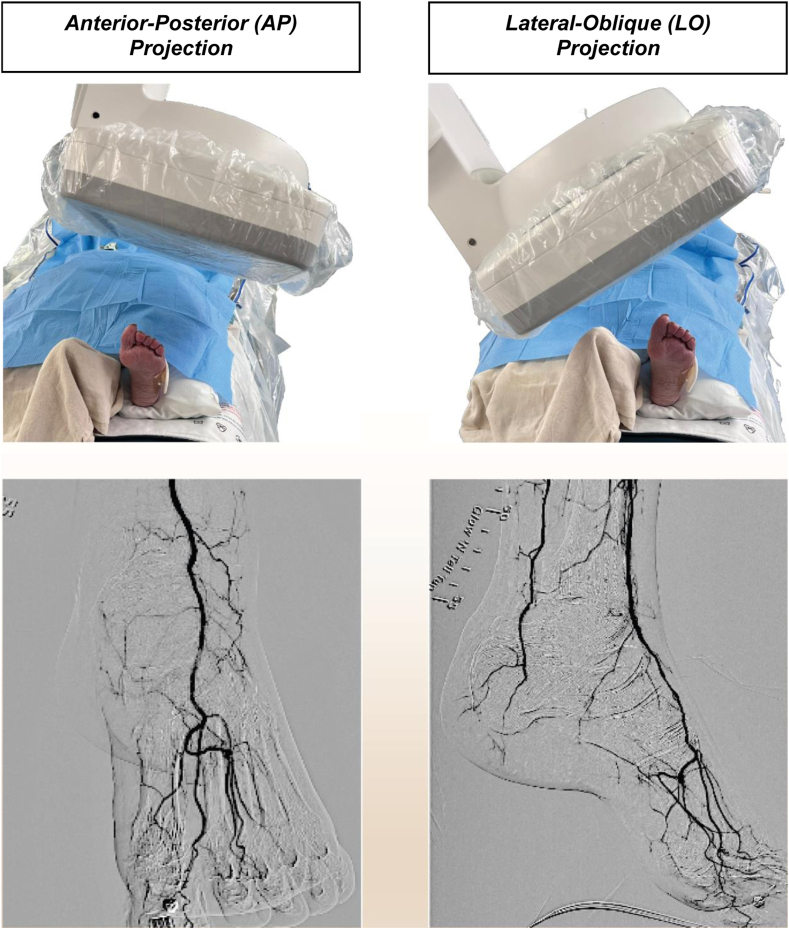
Ensuring a high-quality image of the entire foot in two dimensions is particularly important. Position the foot such that one can obtain an anterior-posterior (AP) projection and a lateral-oblique (LO) projection of the foot. The AP projection allows for visualization of the dorsal anatomy, and involves cranial positioning of the Digital Detector, inline with the tilt/angle of the foot. The LO projection allows for optimal visualization of the plantar anatomy (Fig 3).
Fig 3.
Angiographic views of the foot. Angiogram of the foot should be obtained in two views to allow for the full visualization and assessment of the pedal vessels and the pedal arch. The standard views included the anterior-posterior (AP) projection (with cranial positioning of the imager), and the lateral-oblique (LO) projection.
Positioning and access
The same principles for positioning the patient for retrograde pedal access applies for the pedal-plantar loop technique (Fig 1). An additional consideration is that a moving patient makes this technique near impossible to perform. Therefore, if necessary, general anesthesia should be used and the necessary equipment moved to accommodate the patient flipped on the angiographic table. Antegrade femoral access is essential for this approach. Access from the contralateral femoral artery increases the lengths needed for treatment and decreases substantially the pushability of the catheters and wires used for treatment.
Treatment considerations
The longest sheath possible should be advanced from the antegrade access site to optimize the pushability of the wires and catheters, decrease the contrast dose, and improve image quality. We prefer the use of 0.014″ guidewires supported by microcatheters, crossing catheters, or even low-profile balloons for navigating the pedal arch. Creating the pedal-plantar loop can be done by accessing the anterior tibial artery, navigating around the arch, and obtaining retrograde access to the posterior tibial artery, or vice versa.
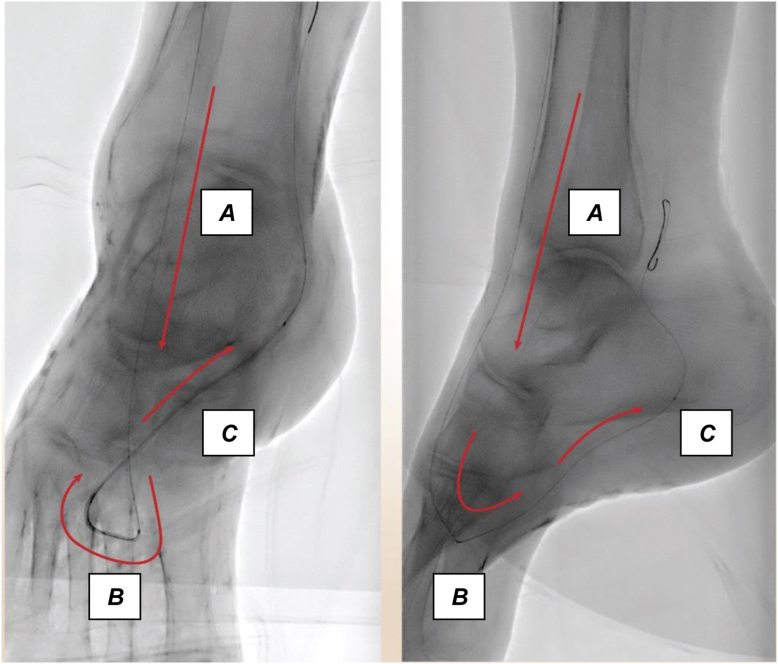
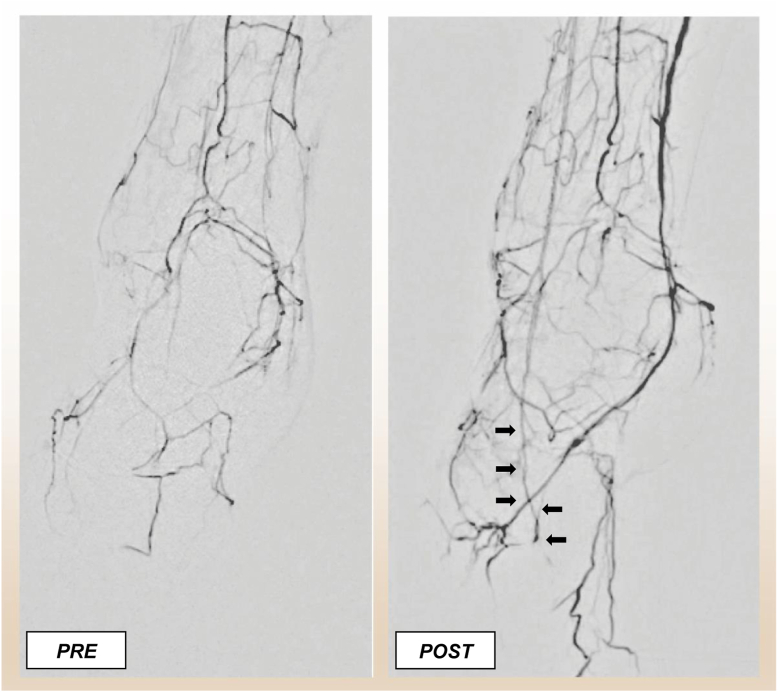
The technique can be used even in the settings of an occluded pedal arch. Navigating the arch requires imagining the path of the loop and targeting the turn in the first metatarsal space. Evaluating the wire passage in both the AP and LO projection is key while navigating the arch (Fig 4). Additionally, vasodilators should be used liberally while the wires are in the pedal vessels. Once the wire has navigated the pedal arch, 2- to 3-mm balloons can be used to dilate or recanalize the arch. An example of preintervention and postintervention angiograms using the pedal-plantar loop technique is shown in Fig 5.
Fig 4.
Expected wire trajectory during the pedal-plantar loop technique. The expected wire trajectory during a pedal plantar loop technique in a normal pedal arch configuration. Here, the wire was advanced from the anterior tibial artery (A) to the dorsalis pedis and through the deep perforator (B) in the first metatarsal interspace of the foot, and into the lateral plantar artery (C) to gain retrograde access to the posterior tibial artery.
Fig 5.
Angiogram of the foot before and after pedal angioplasty performed as part of the pedal-plantar loop technique. Pre- and postintervention angiogram after tibial and pedal artery intervention using the pedal-plantar loop technique. Note that the pedal arch is reconstituted after the intervention (black arrows).
Tools and additional tips
-
•
Our preferred 0.014″ guidewires for the pedal-plantar loop technique include Terumo GLIDEWIRE ADVANTAGE Guidewire (Terumo Medical Corporation), and the Approach Hydro ST Microwire (Cook Medical). These wires have an excellent combination of maneuverability and durability.
-
•
We often use the ASAHI Corsair Pro catheter (ASAHI INTECC, Irvine, CA) and the Armada 14 XT balloon (Abbott Cardiovascular) as the support or crossing catheter owing to their low profile.
-
•
Nitroglycerin can be administered at 200-μg dose increments every 30 minutes through the antegrade catheter with the use of a Tuohy Borst Y connector.
-
•
Balloon angioplasty of the pedal vessels, if performed, is accomplished using prolonged dilation of balloons (ideally ≥3 minutes). Typical balloon sizes range from 2.0 to 3.5 mm. Shorter balloons are generally used, but long tapered balloons such as the NanoCross Elite balloon catheter (Medtronic) can be used as well.
Discussion
A recent systematic review and meta-analysis by Giannopoulos et al35 showed that retrograde pedal access used during the treatment of complex infrainguinal lesions mostly composed of CTOs in the setting of CTLI is safe, with a technical success rate estimated at approximately 91%. In this analysis of 31 retrospective and prospective studies, the immediate procedural complication rates ranged from 0.1% to 2.1%, with the most common immediate procedural complication rate being iatrogenic perforation of the distal accessed artery. The rate of hematoma at the retrograde access site was approximately 1.3%, with a 30-day access site stenosis or occlusion rate of 1.3%.
Other potential complications that can arise from pedal access include arteriovenous fistula formation and compartment syndrome from persistent bleeding from the access site. Although we have not seen compartment syndrome related to retrograde pedal access, and unlikely when the dorsalis pedis and posterior tibial arteries are used, it could potentially be of greater concern when the peroneal artery is accessed owing to its location in the deep posterior compartment and difficulty with postprocedural hemostasis. With regard to operator safety, an important consideration is the potential for higher radiation exposure owing to the surgeon's proximity to the limb during the procedure. Radiation exposure can be minimized by decreasing the overall fluoroscopy time through early recognition for the need of pedal access, use of shutters, and use of intravascular ultrasound examination.
Given the possibility of occlusion of the distal vessel run-off, the technique must be applied with caution, especially in the setting of a tenuous distal vessel run-off, and especially in the setting of a single vessel run-off. An access site complication in these cases may render the limb unsalvageable. Expanding the spectrum of lesions that can be intervened by using retrograde pedal access also raises concern regarding the outcomes in patients who may be potential candidates for a bypass. Prior studies36,37 and a recent meta-analysis by Hossain et al38 have shown that infrainguinal bypass after failed endovascular interventions have worse outcomes compared with primary bypass. Data specific to infrapopliteal bypass after failed tibial intervention are scarce, but a study by Enzmann et al39 showed that patients undergoing primary tibiodistal vein bypass after failed tibial angioplasty had no impact on outcome compared with a primary tibiodistal vein bypass. In light of the outcomes of the Best Surgical Therapy in Patients With Critical Limb Ischemia (BEST-CLI) study,12 and until additional data become available, the use of retrograde tibial access should be reserved for a specific cohort of patients with CLTI in which single-segment saphenous vein bypass is not an option.
Compared with retrograde pedal access, published outcomes related to the pedal-plantar loop technique are limited, likely owing to the relatively low frequency at which the technique is used, which we estimate at approximately 10% to 18% of patients with CLTI undergoing BTK interventions.27,28 In experienced hands, the procedural success rate for the pedal-plantar loop technique is approximately 85%.27 The procedural complication rate specific to the pedal-plantar loop technique is not well-reported, but is attributable mostly to access-related complications from antegrade femoral access used for the technique, which has been reported at 3.7%.40 Outcomes for the technique when used for pedal artery and pedal arch revascularization are limited and have been recently summarized in a systematic review by Machin et al.41
With the changing landscape of baseline patient characteristics, including a greater proportion of patients with diabetes and renal failure offered treatment for PAD,42 the proportion of patients with complex BTK and BTA disease in a limb salvage practice will continue to increase. Retrograde pedal access and the pedal-plantar loop technique are safe techniques that can be used in the treatment of complex CLTI, not only as a bailout procedure, but as a part of a planned treatment strategy in select clinical scenarios. In an era of increasingly complex patients, these techniques are here to stay, and more physicians involved in the treatment of CLTI should become familiar and comfortable with these techniques.
Footnotes
Author conflict of interest: V.C. serves as consultant for Cook Medical, Shockwave Medical, Medtronic, W. L. Gore & Associates, Penumbra, and Abbott.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Song P., Rudan D., Zhu Y., et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–e1030. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 2.Eid M.A., Mehta K.S., Goodney P.P. Epidemiology of peripheral artery disease. Semin Vasc Surg. 2021;34:38–46. doi: 10.1053/j.semvascsurg.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Aday A.W., Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ Res. 2021;128:1818–1832. doi: 10.1161/CIRCRESAHA.121.318535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman J.A., Schneider P.A., Conte M.S. Advances in revascularization for peripheral artery disease: revascularization in PAD. Circ Res. 2021;128:1885–1912. doi: 10.1161/CIRCRESAHA.121.318261. [DOI] [PubMed] [Google Scholar]
- 5.Conte M.S., Bradbury A.W., Kolh P., et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69:3S–125S.e40. doi: 10.1016/j.jvs.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel K., Liu Y., Etaee F., et al. Differences between patients with intermittent claudication and critical limb ischemia undergoing endovascular intervention: insights from the excellence in peripheral artery disease registry. Circ Cardiovasc Interv. 2021;14 doi: 10.1161/CIRCINTERVENTIONS.121.010635. [DOI] [PubMed] [Google Scholar]
- 7.Graziani L., Silvestro A., Bertone V., et al. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg. 2007;33:453–460. doi: 10.1016/j.ejvs.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Rueda C.A., Nehler M.R., Perry D.J., et al. Patterns of artery disease in 450 patients undergoing revascularization for critical limb ischemia: implications for clinical trial design. J Vasc Surg. 2008;47:995–999. doi: 10.1016/j.jvs.2007.11.055. discussion: 9-1000. [DOI] [PubMed] [Google Scholar]
- 9.Meloni M., Izzo V., Giurato L., Gandini R., Uccioli L. Below-the-ankle arterial disease severely impairs the outcomes of diabetic patients with ischemic foot ulcers. Diabetes Res Clin Pract. 2019;152:9–15. doi: 10.1016/j.diabres.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 10.Ferraresi R., Mauri G., Losurdo F., et al. BAD transmission and SAD distribution: a new scenario for critical limb ischemia. J Cardiovasc Surg (Torino) 2018;59:655–664. doi: 10.23736/S0021-9509.18.10572-6. [DOI] [PubMed] [Google Scholar]
- 11.Bradbury A.W., Adam D.J., Bell J., et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg. 2010;51:5S–17S. doi: 10.1016/j.jvs.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 12.Farber A., Menard M.T., Conte M.S., et al. Surgery or endovascular therapy for chronic limb-threatening ischemia. N Engl J Med. 2022;387:2305–2316. doi: 10.1056/NEJMoa2207899. [DOI] [PubMed] [Google Scholar]
- 13.Iyer S.S., Dorros G., Zaitoun R., Lewin R.F. Retrograde recanalization of an occluded posterior tibial artery by using a posterior tibial cutdown: two case reports. Cathet Cardiovasc Diagn. 1990;20:251–253. doi: 10.1002/ccd.1810200408. [DOI] [PubMed] [Google Scholar]
- 14.Botti C.F., Jr., Ansel G.M., Silver M.J., Barker B.J., South S. Percutaneous retrograde tibial access in limb salvage. J Endovasc Ther. 2003;10:614–618. doi: 10.1177/152660280301000330. [DOI] [PubMed] [Google Scholar]
- 15.Spinosa D.J., Leung D.A., Harthun N.L., et al. Simultaneous antegrade and retrograde access for subintimal recanalization of peripheral arterial occlusion. J Vasc Interv Radiol. 2003;14:1449–1454. doi: 10.1097/01.rvi.0000096764.74047.21. [DOI] [PubMed] [Google Scholar]
- 16.Romiti M., Albers M., Brochado-Neto F.C., Durazzo A.E., Pereira C.A., De Luccia N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47:975–981. doi: 10.1016/j.jvs.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Tan M., Ueshima D., Urasawa K., et al. Prediction of successful guidewire crossing of below-the-knee chronic total occlusions using a Japanese scoring system. J Vasc Surg. 2021;74:506–513.e2. doi: 10.1016/j.jvs.2021.01.059. [DOI] [PubMed] [Google Scholar]
- 18.Kokkinidis D.G., Strobel A., Jawaid O., et al. Development and validation of a predictive score for anterograde crossing of infrapopliteal chronic total occlusions: (The Infrapop-CTO Score) Catheter Cardiovasc Interv. 2020;95:748–755. doi: 10.1002/ccd.28693. [DOI] [PubMed] [Google Scholar]
- 19.Soder H.K., Manninen H.I., Jaakkola P., et al. Prospective trial of infrapopliteal artery balloon angioplasty for critical limb ischemia: angiographic and clinical results. J Vasc Interv Radiol. 2000;11:1021–1031. doi: 10.1016/s1051-0443(07)61332-3. [DOI] [PubMed] [Google Scholar]
- 20.Saab F., Jaff M.R., Diaz-Sandoval L.J., et al. Chronic total occlusion crossing approach based on plaque cap morphology: the CTOP classification. J Endovasc Ther. 2018;25:284–291. doi: 10.1177/1526602818759333. [DOI] [PubMed] [Google Scholar]
- 21.Mustapha J.A., Saab F., McGoff T., et al. Tibio-pedal arterial minimally invasive retrograde revascularization in patients with advanced peripheral vascular disease: the TAMI technique, original case series. Catheter Cardiovasc Interv. 2014;83:987–994. doi: 10.1002/ccd.25227. [DOI] [PubMed] [Google Scholar]
- 22.Spinosa D.J., Harthun N.L., Bissonette E.A., et al. Subintimal arterial flossing with antegrade-retrograde intervention (SAFARI) for subintimal recanalization to treat chronic critical limb ischemia. J Vasc Interv Radiol. 2005;16:37–44. doi: 10.1097/01.RVI.0000141336.53745.4A. [DOI] [PubMed] [Google Scholar]
- 23.Shishehbor M.H., Jaff M.R. Percutaneous therapies for peripheral artery disease. Circulation. 2016;134:2008–2027. doi: 10.1161/CIRCULATIONAHA.116.022546. [DOI] [PubMed] [Google Scholar]
- 24.Kawarada O., Sakamoto S., Harada K., Ishihara M., Yasuda S., Ogawa H. Contemporary crossing techniques for infrapopliteal chronic total occlusions. J Endovasc Ther. 2014;21:266–280. doi: 10.1583/13-4460MR.1. [DOI] [PubMed] [Google Scholar]
- 25.Fusaro M., Dalla Paola L., Biondi-Zoccai G. Pedal-plantar loop technique for a challenging below-the-knee chronic total occlusion: a novel approach to percutaneous revascularization in critical lower limb ischemia. J Invasive Cardiol. 2007;19:E34–E37. [PubMed] [Google Scholar]
- 26.Graziani L. Crossing the rubicon: a closer look at the pedal loop technique. Ann Vasc Surg. 2017;45:315–323. doi: 10.1016/j.avsg.2017.06.135. [DOI] [PubMed] [Google Scholar]
- 27.Manzi M., Fusaro M., Ceccacci T., Erente G., Dalla Paola L., Brocco E. Clinical results of below-the knee intervention using pedal-plantar loop technique for the revascularization of foot arteries. J Cardiovasc Surg (Torino) 2009;50:331–337. [PubMed] [Google Scholar]
- 28.Jung H.W., Ko Y.G., Hong S.J., et al. Editor's choice - impact of endovascular pedal artery revascularisation on wound healing in patients with critical limb ischaemia. Eur J Vasc Endovasc Surg. 2019;58:854–863. doi: 10.1016/j.ejvs.2019.07.034. [DOI] [PubMed] [Google Scholar]
- 29.Nakama T., Watanabe N., Haraguchi T., et al. Clinical outcomes of pedal artery angioplasty for patients with ischemic wounds: results from the multicenter RENDEZVOUS registry. JACC Cardiovasc Interv. 2017;10:79–90. doi: 10.1016/j.jcin.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Reed G.W., Salehi N., Giglou P.R., et al. Time to wound healing and major adverse limb events in patients with critical limb ischemia treated with endovascular revascularization. Ann Vasc Surg. 2016;36:190–198. doi: 10.1016/j.avsg.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 31.Higashimori A., Iida O., Yamauchi Y., et al. Outcomes of One straight-line flow with and without pedal arch in patients with critical limb ischemia. Catheter Cardiovasc Interv. 2016;87:129–133. doi: 10.1002/ccd.26164. [DOI] [PubMed] [Google Scholar]
- 32.Palena L.M., Manzi M. Antegrade pedal approach for recanalizing occlusions in the opposing circulatory pathway of the foot when a retrograde puncture is not possible. J Endovasc Ther. 2014;21:775–778. doi: 10.1583/14-4801R.1. [DOI] [PubMed] [Google Scholar]
- 33.Ferraresi R., Palena L.M., Mauri G., Manzi M. 2014. Interventional treatment of the below-the-ankle Peripheral artery disease. PanVascular medicine: Springer Berlin Heidelberg; pp. 1–23. [Google Scholar]
- 34.Kawarada O., Fujihara M., Higashimori A., Yokoi Y., Honda Y., Fitzgerald P.J. Predictors of adverse clinical outcomes after successful infrapopliteal intervention. Catheter Cardiovasc Interv. 2012;80:861–871. doi: 10.1002/ccd.24370. [DOI] [PubMed] [Google Scholar]
- 35.Giannopoulos S., Palena L.M., Armstrong E.J. Technical success and complication rates of retrograde arterial access for endovascular therapy for critical limb ischaemia: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2021;61:270–279. doi: 10.1016/j.ejvs.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Nolan B.W., De Martino R.R., Stone D.H., et al. Prior failed ipsilateral percutaneous endovascular intervention in patients with critical limb ischemia predicts poor outcome after lower extremity bypass. J Vasc Surg. 2011;54:730–735. doi: 10.1016/j.jvs.2011.03.236. discussion: 5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradbury A.W., Adam D.J., Bell J., et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: analysis of amputation free and overall survival by treatment received. J Vasc Surg. 2010;51(5 Suppl):18S–31S. doi: 10.1016/j.jvs.2010.01.074. [DOI] [PubMed] [Google Scholar]
- 38.Hossain S., Leblanc D., Farber A., et al. Editor's choice - infrainguinal bypass following failed endovascular intervention compared with primary bypass: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2019;57:382–391. doi: 10.1016/j.ejvs.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 39.Enzmann F.K., Eder S.K., Aschacher T., et al. Tibiodistal vein bypass in critical limb ischemia and its role after unsuccessful tibial angioplasty. J Vasc Surg. 2018;67:1191–1198. doi: 10.1016/j.jvs.2017.07.127. [DOI] [PubMed] [Google Scholar]
- 40.Cragg J., Lowry D., Hopkins J., et al. Safety and outcomes of ipsilateral antegrade angioplasty for Femoropopliteal disease. Vasc Endovascular Surg. 2018;52:93–97. doi: 10.1177/1538574417739762. [DOI] [PubMed] [Google Scholar]
- 41.Machin M., Younan H.C., Gueroult A.M., Onida S., Shalhoub J., Davies A.H. Systematic review of inframalleolar endovascular interventions and rates of limb salvage, wound healing, restenosis, rest pain, reintervention and complications. Vascular. 2022;30:105–114. doi: 10.1177/17085381211004246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mustapha J.A., Finton S.M., Diaz-Sandoval L.J., Saab F.A., Miller L.E. Percutaneous transluminal angioplasty in patients with infrapopliteal arterial disease: systematic review and meta-analysis. Circ Cardiovasc Interv. 2016;9 doi: 10.1161/CIRCINTERVENTIONS.115.003468. [DOI] [PubMed] [Google Scholar]