Summary
Active components with suitable supports are the common paradigm for industrial catalysis, and the catalytic activity usually increases with minimizing the active component size, generating a new frontier in catalysis, single-atom catalysts (SACs). However, further improvement of SACs activity is limited by the relatively low loading of single atoms (SAs, which are heteroatoms for most SACs, i.e., external active sites) because of the highly favorable aggregation of single heteroatoms during preparation. Research interest should be shifted to investigate SACs with intrinsic SAs, which could circumvent the aggregation of external SAs and consequently increase the SAs loading while maintaining them individual to further improve the activity. In this review, SACs with external or intrinsic SAs are discussed and, at last, the perspectives and challenges for obtaining high-loading SACs with intrinsic SAs are outlined.
Subject areas: Chemistry, Catalysis, Materials science
Graphical abstract
Chemistry; Catalysis; Materials science.
Introduction
Heterogeneous catalysis plays a vital role in energy storage and conversion technologies, such as hydrogen evolution reaction (HER) for hydrogen economy, oxygen reduction reaction (ORR) for metal-air batteries, and nitrogen reduction reaction (NRR) for green NH3 synthesis, holding great promise to accelerate a sustainable future.1,2,3,4,5 Developing high-performance catalysts is the prerequisite for industrializing a specific catalytic process, and currently many industrial catalysts are metal particles supported on solid surfaces.6,7,8 The catalytic efficiency of heterogeneous catalysts is highly related to the surface/volume ratio of active components, and the specific activity per atom usually increases with decreasing the particle size. Much effort has been devoted to downsizing metal particles to achieve better catalytic performance.9,10,11,12,13 The ultimate limit for downsizing metal particles is single-atom catalysts (SACs), where highly dispersed metal single atoms (SAs) are anchored on the surface of solid supports (Figure 1A). The first practical SAC with Pt SAs supported on iron oxide (Pt1/FeOx) was reported in 2011,14 and it shows higher activity toward CO oxidation than the cluster/particle counterpart. Another advantage of SACs is the precisely identified and uniform active sites, which is conducive to revealing the catalytic mechanism. Such an advantage used to be only owned by homogeneous catalysts, of which the application is limited by the poor stability and recyclability. SACs bridge the gap between homogeneous and heterogeneous catalysis. Since then, SACs have attracted extensive research attention because of the excellent catalytic performances.15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39
Figure 1.
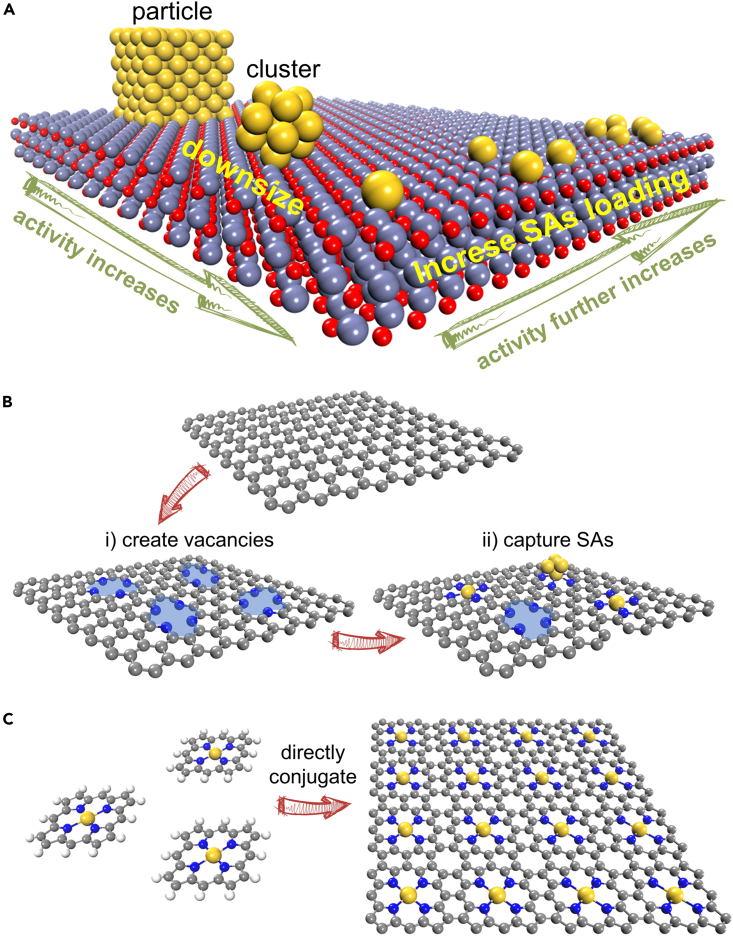
External and intrinsic SAs in carbon-based SACs
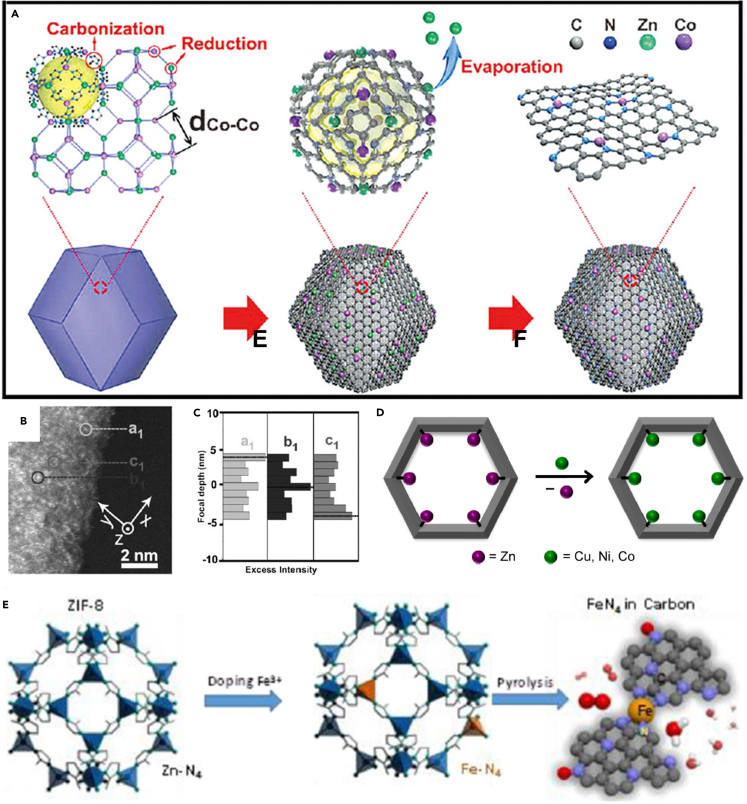
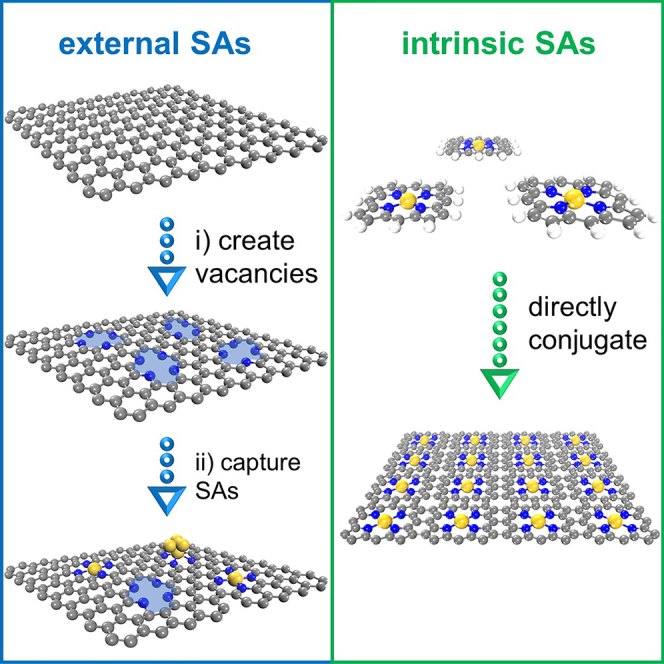
(A–C) Schematic illustrations of the increase in catalytic activity by downsizing the active component and the further increase by increasing the SAs loading (A), the common approach to synthesize carbon-based SACs with the active center of M1N4: (i) create vacancies and (ii) capture single heteroatoms (i.e., external SAs) (B), and the conjugation of MTP molecules having natural M1N4 centers to SACs with high-loading intrinsic SAs (C).
Over the last decade, large amounts of SACs have been synthesized and utilized toward catalytic reactions including HER, ORR, and NRR, and many good reviews have summarized the design concept, synthesis, and application of SACs from both the experimental and theoretical sides.12,40,41,42,43,44,45,46,47,48 For example, Zhuo et al. focused on the stability of metal SAs anchored on different sites of graphene support and the intrinsic connection between theoretical understanding on electronic structures and catalytic properties40; Wang et al. summarized the concept of SACs with two-dimensional (2D) supports for catalysis and pointed out the interplay between the unique geometric and electronic structures of 2D supports and the catalytic performance of confined single active sites42; Li et al. discussed the computational simulations on a couple of important applications of SACs and provided good perspectives on future computational simulations for SACs.43 Increasing the SAs loading while maintaining SAs as individual sites is the grail to achieve better catalytic performances (Figure 1A). However, SAs are heteroatoms for most SACs,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 even for most metal-organic frameworks (MOFs)-derived carbon-based SACs,34,35,36,37,38 where vacancies are first created on support surface and then used to capture single heteroatoms (Figure 1B). In this case, SAs are regarded as external active sites herein. Owing to the high surface energy of single heteroatoms compared to cluster/particle counterparts, the self-nucleation process of external SAs occurs very easily during preparation. Aiming at circumventing the self-nucleation of external SAs, the precursors of SAs should be controlled at a low concentration, resulting in the relatively low loading of external SAs (typically <2 wt % on non-carbon supports).14,16,49,50,51 Hence, it is worthy to shift research interest to SACs with intrinsic SAs. Figure 1C gives an example of intrinsic SAs with the same active centers in Figure 1B (M1N4, M: transition metal) to illustrate the difference between external and intrinsic SAs. Metalloporphyrin (MTP) molecules have natural M1N4 centers (Figure 1C, left), rather than capture single heteroatoms to construct M1N4 centers. After the direct conjugation of MTP molecules to 2D sheets (Figure 1C, right), SACs with high-loading intrinsic SAs can be synthesized.
In this contribution, we discuss the recent advances in SACs with external and intrinsic SAs. First, SACs with external SAs, including noble and non-noble metals as well as non-metal atoms, are summarized, and the corresponding loading/density of external active sites is discussed. Then, we discuss some studies on SACs with intrinsic SAs, which is a promising field because of the great advantages in achieving high-loading SAs and high catalytic performance but rarely summarized. In the discussion sections, the applications of SACs in thermocatalysis, electrocatalysis, and photocatalysis are involved. Finally, the perspectives and challenges on SACs with high-loading intrinsic SAs are outlined.
SACs with external SAs
Catalytic performances of SACs are determined by both SAs and supports. The interactions between SAs and supports have great impact on the electronic structure of SAs, and consequently regulating the coordination environment of SAs is highly efficient in tailoring the activity and selectivity. By sufficient and fine regulations, SAs could be totally different from the elemental bulk counterpart, showing excellent activity/selectivity. The application of SACs determines what kind of supports should be utilized. In detail, high-conductivity supports, such as carbon-based materials, are desirable for electrocatalysis, whereas high stability and suitable bandgap are required for thermocatalysis and photocatalysis, respectively.
Noble metal SAs
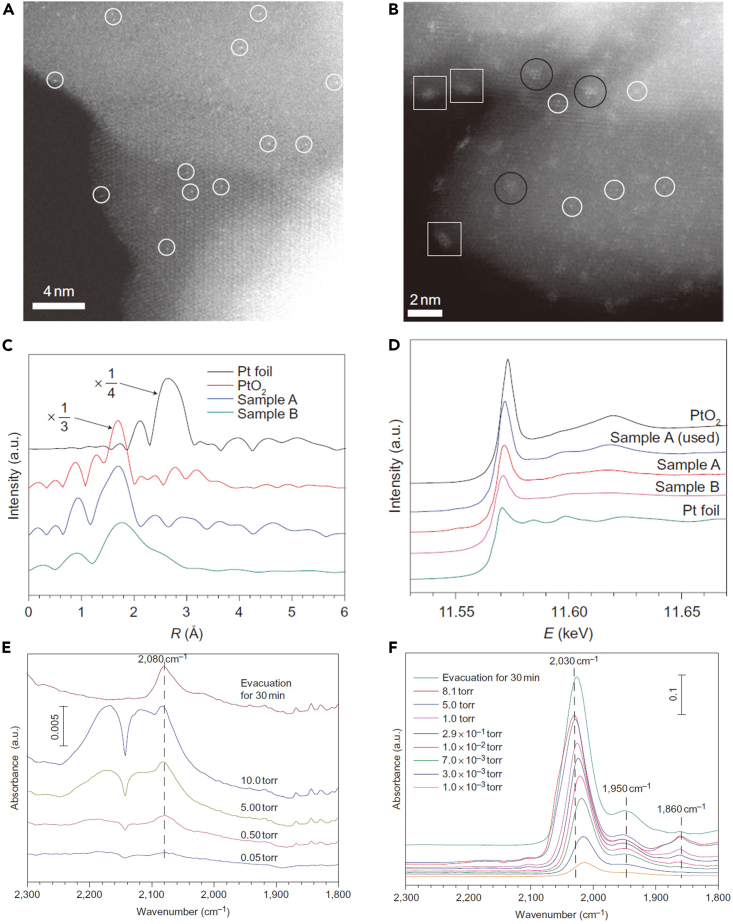
State-of-the-art catalysts for HER, oxygen evolution reaction (OER), etc., mainly rely on noble metals, such as Pt, Pd, Ru, Ir.52,53,54,55 Since noble metals are scarce and expensive, making the best of the noble resources is a big but challenging task for industrial catalysis. Increasing the efficiency of Pt is a significant innovation for the first practical SACs, Pt1/FeOx.14 Using the co-precipitation method, a catalyst with Pt SAs anchored onto the defects of FeOx surfaces (sample A, the Pt loading of 0.17 wt %), as well as a similar catalyst with the Pt loading of 2.5 wt % (sample B), was synthesized. The aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HADDF-STEM) images (Figures 2A and 2B) suggested that only Pt SAs can be seen in sample A, whereas Pt SAs, 2D Pt rafts, and three-dimensional Pt clusters coexist in sample B. In the extended X-ray absorption fine structure (EXAFS) spectra (Figure 2C), sample A has a strong peak at ∼ 1.7 Å from the Pt-O contribution and a weak peak at ∼ 2.5 Å from the Pt-Pt or Pt-Fe contribution, further suggesting that sample A contains only Pt SAs. Moreover, Pt SAs in sample A carry positive charges, whereas Pt0 clusters are dominant in sample B according to the normalized X-ray absorption near-edge structure (XANES) spectra (Figure 2D). In situ Fourier-transform infrared (FTIR) spectroscopy was used to explore the dispersion and oxidation state of Pt. The band related to CO adsorbed on Ptδ+ (2080 cm−1, Figure 2E) indicated the isolation of Pt SAs in sample A, whereas the bands for linearly bonded CO on Pt0 sites (1860, 1950, and 2030 cm−1, Figure 2F) verified the existence of Pt dimers or clusters in sample B. Owing to the highly dispersed Pt SAs, sample A exhibited higher catalytic performances toward CO oxidation and preferential oxidation than sample B and the standard Au/Fe2O3 catalyst. The same group also synthesized a series of catalysts with different Ir loadings (0.01, 0.22, 0.32, and 2.40 wt %) on FeOx supports.56 Similar to Pt1/FeOx, Ir clusters and particles were formed with increasing Ir loading (Figure 3A). The as-prepared Ir1/FeOx SAC with the Ir loading of 0.01 wt % exhibited high activity for water gas shift reaction (CO + H2O → CO2 + H2), and the theoretical contribution from Ir SAs to the total CO conversion was claimed to be around 70% based on the weight percentage of Ir SAs (Figure 3B). These two pioneering works demonstrate the successful synthesis of promising catalysts with minor usage of noble metals and the importance of SAs compared with the cluster and nanoparticle counterparts. Later, Pd1/FeOx,57,58 Rh1/FeOx,57 Au1/FeOx,57,59 and Ru1/FeOx60 were synthesized as well; Pt1/FeOx and Ir1/FeOx were also used in NO reduction,61 nitroarenes hydronation,62 oxygen activation,63 etc.
Figure 2.
Characterizations of the first practical SAC
(A and B) HAADF-STEM images of sample A (A) and sample B (B).
(C–D) The Fourier transform spectra from EXAFS (C) and the normalized XANES spectra (D) of sample A, sample B, PtO2, and Pt foil.
(E–F) In situ FTIR spectra of CO adsorption for sample A (E) and sample B (F).
(A–F) Reproduced with permission.14 Copyright 2011, Nature Publishing Group.
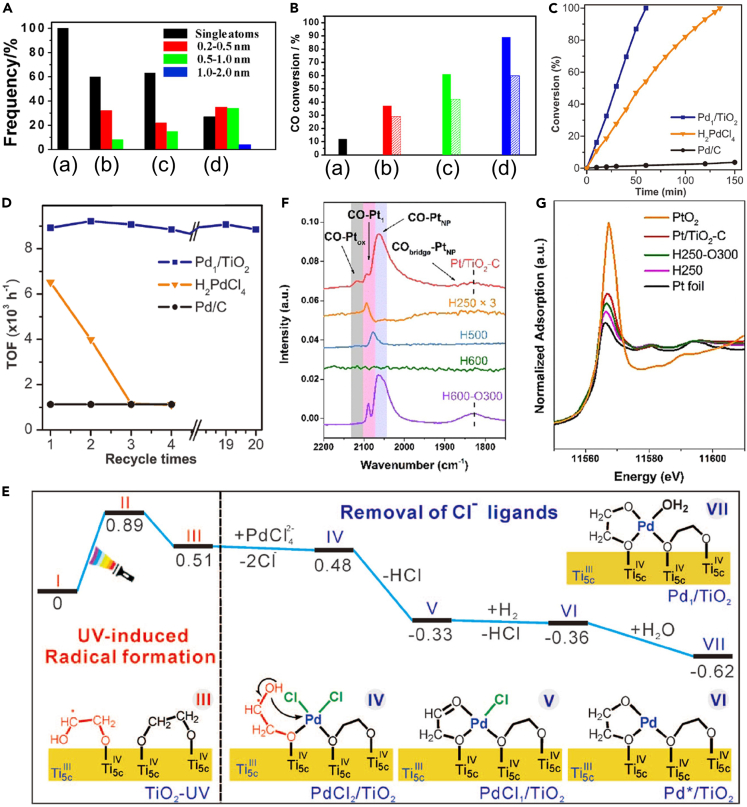
Figure 3.
Noble metal SACs with external Ir, Pd, and Pt SAs
(A and B) The frequencies of the observed size scope (A) and the differences between the experimental CO conversions at the steady state (solid) and the estimated ones based on the contributions from Ir SAs (stripe) (B) of Ir/FeOx catalysts with the Ir loadings of 0.01 (a), 0.22 (b), 0.32 (c), and 2.40 wt % (d). (A and B) Reproduced with permission.56 Copyright 2013, American Chemical Society.
(C and D) Catalytic performances for the first run (C) and turnover frequency (TOF) (D) of several recycles for Pd1/TiO2, H2PdCl4, and commercial Pd/C.
(E) Energies and models of intermediates and transition states in the stepwise preparation mechanism of Pd1/TiO2.
(C–E) Reproduced with permission.69 Copyright 2016, American Association for the Advancement of Science.
(F and G) In situ DRIFT (F) and XANES spectra (G) of different Pt/TiO2 samples.
(F–G) Reproduced with permission.70 Copyright 2020, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Inspired by the high performances of Pt1/FeOx and Ir1/FeOx,14,56 SACs with atomically dispersed earth-scarce noble metals have attracted increasing research attention to create more cost-effective catalysts.64,65,66,67,68 These SACs indeed exhibited high catalytic performances, but the loading of SAs is relatively low, e.g., 0.17 and 0.01 wt % for Pt1/FeOx and Ir1/FeOx,14,56 respectively. To increase the loading of noble metal SAs, Liu et al. reported a room-temperature photochemical strategy to fabricate atomically dispersed Pd catalyst on TiO2 nanosheets (Pd1/TiO2) with the Pd loading up to 1.5 wt %.69 They first synthesized two-atom–thick TiO2 nanosheets stabilized by ethylene glycolate (EG) and then dispersed them into water with H2PdCl4 solution under ultraviolet (UV) treatment. The TEM and HAADF-STEM images and the results of energy-dispersive X-ray spectroscopy (EDS) mapping, XANES and EXAFS spectrometry, FTIR spectra of CO adsorption suggested that Pd SAs are highly dispersed in the as-prepared Pd1/TiO2 catalyst even when the Pd loading increases to 1.5 wt %. The Pd1/TiO2 catalyst exhibited extremely high catalytic activity and stability toward styrene hydrogenation compared with the commercial Pd/C catalyst and the unsupported homogeneous H2PdCl4 catalyst (Figures 3C and 3D). During preparation, the UV-induced formation of EG radicals on TiO2 promoted the Cl− removal (Figure 3E), which is beneficial to the high performance of Pd1/TiO2. The PdCl2/TiO2 catalyst synthesized by the same method as for Pd1/TiO2 but without the UV treatment showed poorer catalytic performance than Pd1/TiO2. Han et al. modified such a photochemical method and synthesized a Pt1/TiO2 catalyst with the Pt loading of 1 wt %.70 Using in situ diffuse reflection infrared Fourier transform spectroscopy (DRIFTs) and in situ XANES spectroscopy, they found that the strong metal-support interaction (SMSI) can also occur between Pt SAs and TiO2 at the reduction temperature of 600°C, whereas the SMSI between Pt nanoparticles and TiO2 occurred at 250°C (Figures 3F and 3G). Moreover, Pt SAs were confirmed to be the true active sites toward the hydrogenation of 3-nitrostyrene. SACs with increasing SAs loading are promising catalysts, which have not only high activity but also high catalytic capability that conventional catalysts do not possess. With TiO2 as the support, Guo et al. used the ball milling method to synthesize the Pd1/TiO2 catalyst and utilized it to catalyze the photo-thermo semi-hydrogenation of acetylene, where the photo-induced electrons transferred from TiO2 support to the adjacent Pd SAs facilitated the activation of acetylene71; Tang et al. synthesized a Rh1/TiO2 catalyst and demonstrated that Rh SAs can change their structures and adapt their catalytic sites under reaction conditions72; Thang et al. explained that the high thermal stability of the Pt1/TiO2 catalyst through oxidative and reductive treatments is because every Pt SA binds to two excess O atoms, forming PtO2 on surface.73
Non-noble metal SAs
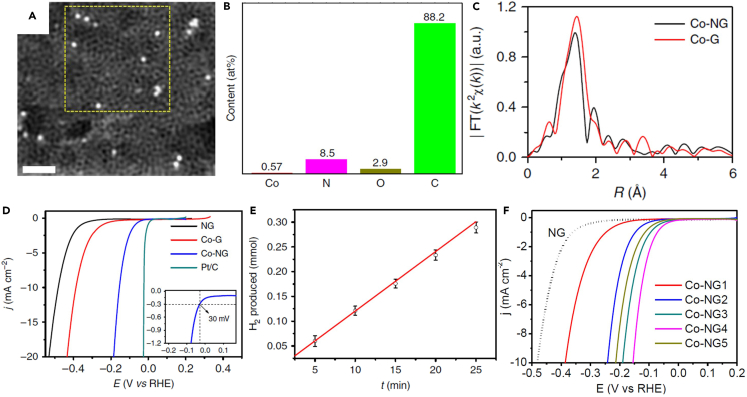
Although noble metals usually act well in catalyzing a specific reaction, non-noble metals also play a vital role in catalysis, such as Fe-based catalysts for the Haber-Bosch process, a method for industrial NH3 synthesis.74,75,76,77 For metal SAs, their properties change significantly and are even no longer similar to the elementary bulk counterparts, further indicating the great potential of SACs with non-noble metal SAs. Using the machine learning (ML) techniques, Wu et al. predicted the catalytic performances of carbon-based SACs toward OER and ORR and found that SACs with non-noble metal SAs were superior,78,79 which also occurs for double-atom catalysts (DACs).80 To circumvent the limitation of high cost of Pt catalysts toward HER, Fei et al. synthesized a Co SAC (Co-NG) with Co SAs on N-doped graphene oxide (GO), which exhibited extraordinarily high catalytic performance toward HER in both acidic and basic media.81 GO and cobalt salts were sonicated with the weight ratio of GO:Co = 135:1, and then the well-mixed precursor solution was freeze-dried and heated, which indicates an inexpensive, concise, and scalable method to synthesize non-noble metal SACs. The HAADF-STEM image (Figure 4A) clearly showed Co SAs in the carbon matrix, and the contents of Co, N, O, and C in Co-NG were determined to be 0.57, 8.5, 2.9, and 88.2 at%, respectively, by the inductively coupled plasma optical emission spectrometry (ICP-OES, Figure 4B). The EXAFS analysis was performed at the Co K-edge using the Fourier transform (FT) (Figure 4C) to further confirm the individual Co SAs in Co-NG. The linear sweep voltammograms (LSVs) results in acidic 0.5 M H2SO4 media (Figure 4D) suggested that Co-NG is superior HER catalyst though slightly poor as compared to the commercial Pt/C catalyst, and the Faradaic efficiency of the Co-NG catalyst was determined to be ∼100% (Figure 4E). The Co-NG catalyst also exhibited high HER activity when tested in alkaline 1 M NaOH media. Moreover, Co-NG catalysts with different Co loadings (from 0.03 to 1.23 at%) were synthesized, and the HER activity did not always increase with the increasing Co loading (Figure 4F). This can be ascribed to that not all Co atoms can be incorporated into the N-doped graphene matrix and consequently Co-containing clusters or particles occur. This Co-NG catalyst is the first inorganic solid-state SAC exhibiting excellent catalytic performance toward HER, proving that non-noble metal SACs hold great promise to replace noble metal-based catalysts.
Figure 4.
Non-noble metal SACs with external Co SAs
(A) HAADF-STEM image of the Co-NG catalyst with the scale bar of 1 nm.
(B) Percentages of Co, N, O, and C in the Co-NG catalyst measured by XPS and ICP-OES.
(C) The k2-weighted EXAFS in k-space for Co-NG and Co-G.
(D) LSVs of NG, Co-G, Co-NG, and Pt/C in 0.5 M H2SO4 at the scan rate of 2 mV s−1.
(E) The molar number of produced H2 as a function of time, where the red line denotes the theoretically calculated amounts of H2 with 100% Faradaic efficiency.
(F) LSV polarization curves for catalysts with the Co loading of 0.03 (Co-NG1), 0.09 (Co-NG2), 0.29 (Co-NG3), 0.57 (Co-NG4), and 1.23 (Co-NG5).
(A–F) Reproduced with permission.81 Copyright 2015, Nature Publishing Group.
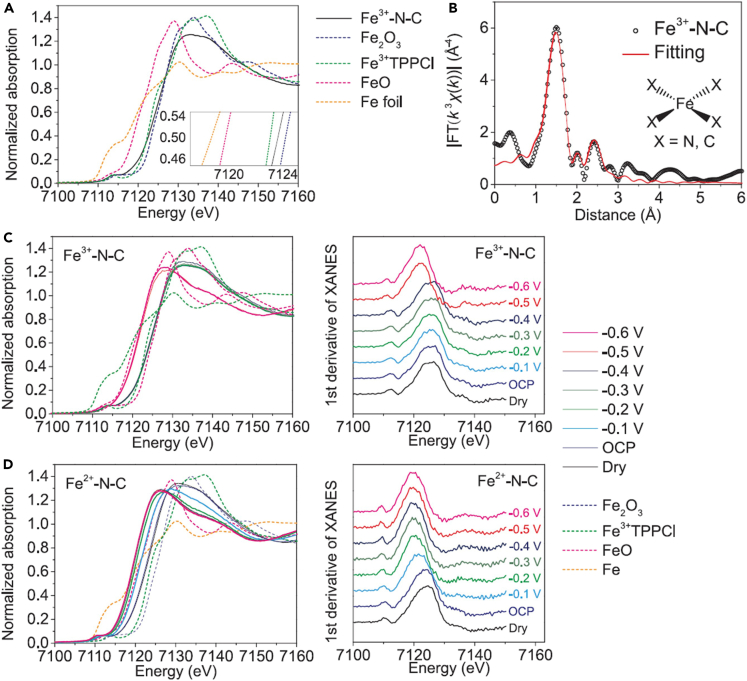
MOFs are a class of porous crystalline materials consisting of metal-containing nodes and organic linkers. Owing to the natural single-atom metal sites separated by linkers, MOFs have been widely studied as templates/precursors for noble metal and non-noble metal SACs.33,34,35,36,37,38 In MOFs and derivates, SAs can be introduced into the metal nodes, organic linkers, or pores, and SACs (mainly M1Nx-C catalysts) can be synthesized after the high-temperature pyrolysis. However, for most MOFs and derivates with SAs stabilized by linkers or pores, vacancies in (N-doped) carbon matrix generated by the evaporation of low-boiling point metal sites are still required to capture SAs. In this case, SAs are still external. Hence, only SAs introduced into the metal nodes of MOFs and derivates, i.e., mixed metal nodes, can be regarded as intrinsic, of which the coordination environment (at least the first-shell coordination) is unchanged before and after pyrolysis. Gu et al. synthesized Fe3+-N-C catalyst with the Fe loading of 2.8 wt % by the pyrolysis of Fe-doped ZIF-8, where SAs were stabilized in pores, and investigated its properties and performance by operando techniques.38 The Fe K-edge XANES spectrum (Figure 5A) suggested that Fe ions in the as-prepared Fe3+-N-C catalyst are in the +3 oxidation state, and the Fe K-edge EXAFS spectrum (Figure 5B) indicated that Fe3+ sites adopt a Fe1X4 (X = N or C) structure. The operando X-ray absorption spectroscopy measurements were performed to explore the change of the Fe3+-N-C catalyst during the CO2 reduction reaction (CO2RR). The Fe3+ sites were reduced to Fe2+ sites with the decreasing applied potential (Figure 5C), along with the first-shell coordination number of Fe ions decreased from 4 to 3. The Fe2+-N-C catalyst was also prepared, where Fe2+ sites coordinated with four pyridinic N atoms (pyrrolic N atoms for Fe3+ sites in Fe3+-N-C), and there also existed important number of Fe3+ sites in Fe2+-N-C. The in situ XANES analysis suggested that Fe3+ sites in the Fe2+-N-C catalyst are reduced to Fe2+ at −0.1 to −0.2 V versus RHE (Figure 5D). The activity and stability of Fe2+-N-C catalyst were both poorer than the Fe3+-N-C catalyst. This work proves the significant impact of the coordination environment of SAs in catalytic performances.
Figure 5.
MOF-derived SACs with external Fe SAs
(A) Fe K-edge XANES spectra of Fe3+-N-C, Fe2O3, Fe3+TPPCl, FeO, and Fe foil.
(B) R-space Fe K-edge EXAFS spectra.
(C and D) Fe K-edge XANES spectra (left) and the first derivative of the spectra (right) of Fe3+-N-C (C) and Fe2+-N-C (D) as dry powder (black) and loaded on glassy carbon electrodes at open circuit potential (OCP) (blue), −0.1 to −0.6 V versus RHE, with the spectra of Fe2O3, Fe3+TPPCl, FeO, and Fe foil as references.
(A–D) Reproduced with permission.38 Copyright 2019, American Association for the Advancement of Science.
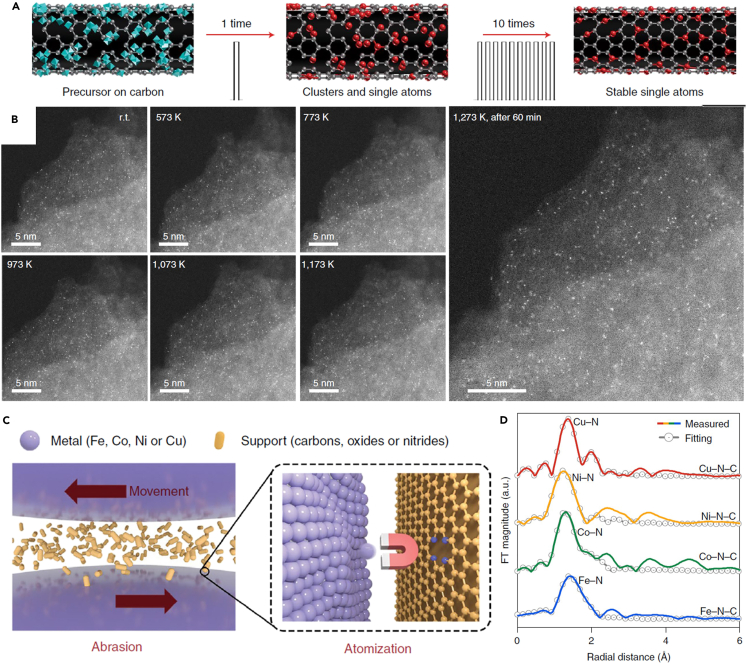
Co and other non-noble metal (Fe, Ni, Cu) SACs can be also synthesized by the high-temperature shockwave method and the mechanochemical abrasion method.16,32 In the high-temperature shockwave method, defects in supports can capture SAs generated by the on-state shockwave heating, and the off-state ensures the stability of whole system (Figure 6A). In situ STEM images (Figure 6B) confirmed that SAs have a superior structural stability and remain single from room temperature up to 1273 K. Using this method, noble metal SACs, e.g., Pt SACs with the Pt loading of ∼0.24 wt %, can be synthesized as well, and the support can be carbon materials and TiO2. As regards the mechanochemical abrasion method, SAs abraded from the corresponding elementary bulk metals were also stabilized by defects in supports (Figure 6C). Taking the as-prepared carbon-based SACs as the example, the EXAFS results suggested that Cu, Co, Ni, and Fe exist in the single-atom state (Figure 6D). The Fe loading in the Fe-N-C SAC reached ∼1.10 ± 0.3 wt %, and the SAs loading can be tuned by changing the abrasion rate. Similar to the universality of the high-temperature shockwave method, this method was also useful to synthesize SACs with the support of C3N4, MgO, SiO2, CeO2, etc. These newly developed SACs preparation methods suggest the important role of support defects in capturing single heteroatoms.
Figure 6.
Strategies to synthesize SACs with external metal SAs
(A) Schematic diagram of the synthesis and dispersion process of SACs by the high-temperature shockwave.
(B) In situ STEM images of the representative Pt SACs from room temperature up to 1273 K.
(A and B) Reproduced with permission.32 Copyright 2019, Nature Publishing Group.
(C) Schematic diagram of SACs formation by the mechanochemical abrasion.
(D) The radial distribution functions of the as-prepared Fe-N-C, Co-N-C, Ni-N-C, and Cu-N-C catalysts.
(C and D) Reproduced with permission.16 Copyright 2022, Nature Publishing Group.
Non-metal SAs
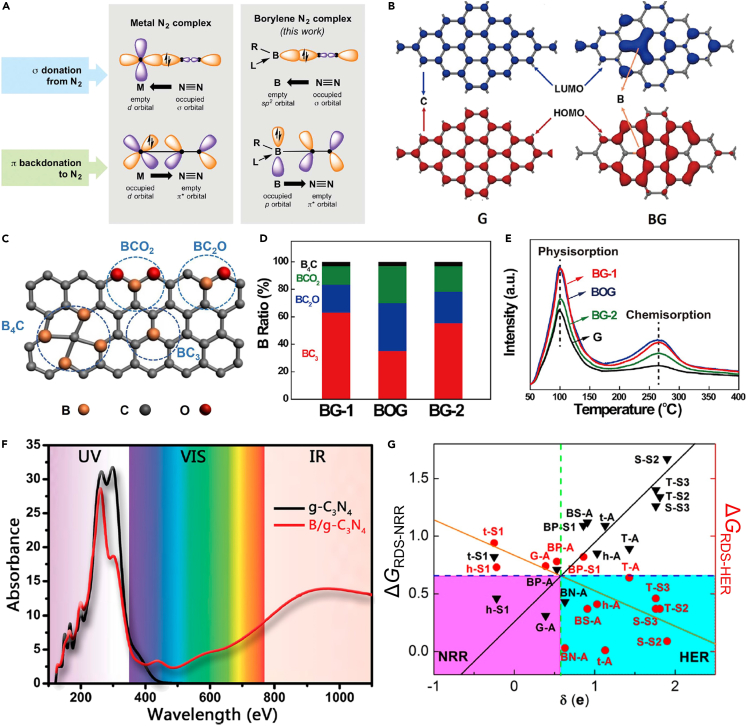
Industrial catalysis heavily relies on metal-based catalysts, such as Fe-based catalysts for industrial NH3 synthesis.74,75,76,77 Moreover, natural N2 fixation is achieved by nitrogenases, where the active sites are also transition metals, mainly Mo and V.82,83,84 Compared to metal compounds, metal-free materials are recently emerging as highly promising catalytic candidates because of their abundant natural resources, stability in acidic and/or alkaline environment, and environmental friendliness.85,86,87,88,89,90 Taking N2 fixation as the example, the underlying reason why metal compounds are efficient in binding and activating N2 molecule is the advantageous combination of unoccupied and occupied d orbitals, which can synergically accept electrons from and back-donate to N2 molecules, i.e., the “accept and back-donate” mechanism (Figure 7A). Recently, Légaré et al. found that some boron-contained compounds are also active for N2 fixation, where boron sites can be active centers because of the combination of filled and empty p orbitals (Figure 7A).91 Yu et al. extended this concept to heterogeneous catalysis and synthesized boron-doped graphene (BG) with boron SAs on graphene (G) by the thermal reduction of H3BO3 with GO in a mixed H2/Ar gas.92 The highest occupied molecular orbital (HOMO) and the unoccupied molecular orbital (LUMO) of G were non-localized and symmetric, but both HOMO and LUMO of BG were redistributed (Figure 7B). The redistribution of electron densities resulted in positively charged boron SAs in BG samples, which is in favor of N2 adsorption. There were four structures of boron SAs, including BC4, BC3, BC2O, and BCO2 (Figure 7C), and their proportions were different in BG samples (Figure 7D). Only physical N2 adsorption was observed for G, and chemical N2 adsorption was present for BG samples (Figure 7E). Moreover, the chemical N2 adsorption on BG samples increased as the boron loading increases, suggesting that chemical N2 adsorption sites are boron SAs. The BG-1 sample with the highest boron loading of 6.2 at% exhibited higher performance toward NRR than other BG samples and other reported NRR catalysts in aqueous solutions at ambient conditions.93,94,95,96,97 This work provides strong evidence of the broad applications of SACs with the active sites of non-metal SAs.
Figure 7.
Non-metal SACs with external boron SAs
(A) Schematic illustration of the bonding in well-known end-on-bound transition metal N2 complexes and prospective application to monovalent boron species. (A) Reproduced with permission.91 Copyright 2018, American Association for the Advancement of Science.
(B) LUMO (blue) and HOMO (red) of BG and undoped G.
(C) Schematic illustrations of the B4C, BC3, BC2O, and BCO2 structures.
(D) Percentages of different boron-doped types in BG samples.
(E) N2 temperature-programmed desorption curves of BG samples and G. (B-E) Reproduced with permission.92 Copyright 2018, Cell press.
(F) Optical adsorption spectra of g-C3N4 (black) and B/g-C3N4 (red). (F) Reproduced with permission.98 Copyright 2018, American Chemical Society.
(G) Computational screening of fourteen catalyst combinations in terms of ΔGRDS-NRR and ΔGRDS-HER vs. δ.
(G) Reproduced with permission.103 Copyright 2019, American Chemical Society.
Inspired by the high performance toward NRR of the experimentally synthesized boron SACs,92 the theoretical design of boron SACs toward NRR became active. The boron sites in homogeneous catalysts with sp2 hybridization have been proved to be efficient in N2 fixation (Figure 7A).91 Ling et al. analyzed that boron sites with sp3 hybridization could also act as active centers for N2 fixation because of the concurrence of occupied and empty p orbitals and N2 molecules could be adsorbed with the side-on configuration on boron sites.98 They designed a metal-free SAC, boron-doped graphitic-carbon nitride (B/g-C3N4), toward photocatalytic NRR, where g-C3N4 was chosen because of its broad applications in photocatalysis with the suitable bandgap.99,100,101 The as-designed B/g-C3N4 catalyst had not only high activity (the onset potential of −0.20 V) but also high photoconversion efficiency. In detail, the bandgap of g-C3N4 decreased from 2.98 eV to 1.12 eV because of the introduction of boron SAs, leading to the improved absorbance of the visible (VIS) light and infrared (IR) light (Figure 7F). Besides B/g-C3N4, the introduction of boron SAs is also efficient to boost the photocatalytic performance of g-CN (B/g-CN), which could activate N2 and reduce it to NH3 with a low overpotential of 0.15 V102 g-C2N is another import carbon nitride material, and the boron-doped g-C2N (B/g-C2N) is also a promising NRR SAC with the theoretical limiting potential of −0.15 V.87 High catalytic performance has been universally predicted for SACs with boron SAs supported on 2D carbon nitride materials. In addition, extensive density functional theory (DFT) calculations were carried out to investigate the design principles of boron SACs and reveal how the NRR activity and selectivity are affected by the local bonding structure of boron SAs and the boron-substrate charge transfer.103 By incorporating boron SAs into eight 2D materials, twenty-one models were constructed. Figure 7G shows their free energy changes of the rate-determining step (RDS) of NRR (ΔGRDS-NRR) and HER (ΔGRDS-HER), and smaller ΔGRDS-NRR occurs when δ gets smaller, whereas ΔGRDS-HER is relatively positive. This is because the electron transfer from boron SAs to adsorbed N2 is important for N2 activation, where more electrons in boron SAs could increase the possibility for such an electron injection, and negatively charged boron SAs could bind the H+ adatom strongly, leading to high selectivity. The relationship between the catalytic performance toward NRR and the locally chemical environment of boron SAs has been clarified in boron SACs. Beside electrocatalytic and photocatalytic NRR, SACs with non-metal SAs were also promising in other reactions. For example, Jiao et al. established the HER activity of graphene-based materials with boron, N, O, P, or S SAs and explored their reactivity origin by combining spectroscopic characterization, electrochemical measurements, and DFT calculations.86
SACs with intrinsic SAs
Beside transition metal atoms, some main-group elements, such as boron and Al, have also been proved to be efficient active sites in SACs. After another decade, at least half of the non-radioactive elements in the periodic table might be fabricated in SACs. However, as aforementioned, the favorable aggregation of external SAs and the limited concentrations of surface vacancy defects make the loading of external SAs relatively low, restricting the further improvement in the catalytic performances of SACs. If the single-atom structure is naturally possessed, high-loading SAs will be expectable.
Noble metal SAs
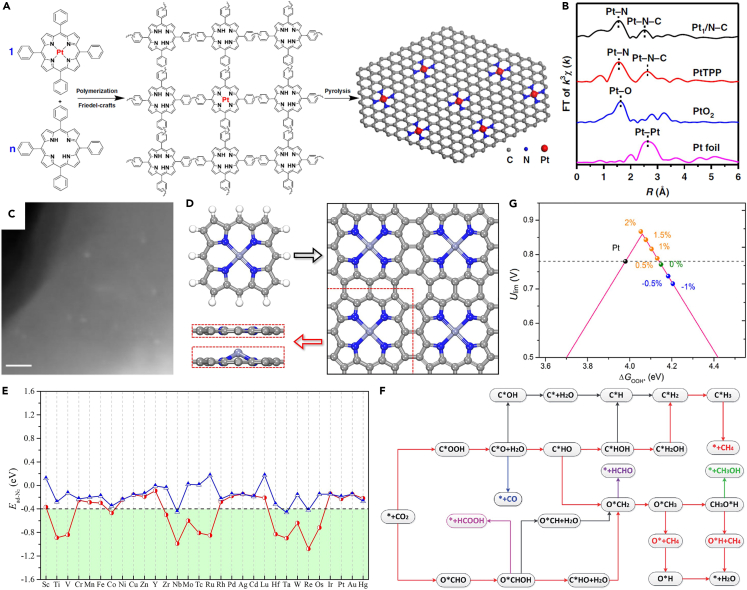
M1Nx centers (x = 3 or 4) have been widely confirmed as promising active sites for numbers of thermocatalytic, electrocatalytic, and photocatalytic reactions.26,104,105,106,107,108,109,110,111,112,113 As a class of molecules having natural M1Nx centers, MTPs are attractive as SACs or SACs precursors. He et al. developed a universal precursor-dilution strategy to synthesize a series of SACs and even bimetallic SACs.114 Metals tetraphenylporphyrins (M-TPPs), analogous materials of MTP with natural M1N4 centers, were co-polymerized with free TPPs, where free TPPs can extend the mean distance between metal atoms dispersed on the as-prepared polymer matrix and thus prevent the aggregation of metal atoms during the subsequent high-temperature pyrolysis. Figure 8A shows the synthesis of the Pt1N4-C catalyst using the precursor-dilution strategy with Pt-TPP:TPP = 1:40. Because the first-shell coordination of Pt SAs was unchanged before and after pyrolysis (Figure 8B), the active sites in the Pt1N4-C catalyst can be regarded as intrinsic SAs. Several characterization techniques confirmed that Pt is atomically dispersed in carbon matrix with the loading of 0.43 wt %. According to the molar ratio of Pt-TPP:TPP (1:40), the Pt loading should be 0.73 wt %, which suggested the metal loss during preparation. However, without the dilution of free TPPs, Pt atoms aggregated to nanoparticles (Figure 8C). By adjusting the molar ratio of Pt-TPP:TPP, different Pt SAs loadings (0.06, 0.21, 0.43, and 0.73 wt %) can be achieved in Pt1N4-C samples. More importantly, with suitable ratios of M-TPP:TPP, Ru1N4-C, Rh1N4-C, Pd1N4-C, In1N4-C, Au1N4-C, and Ag1N4-C can be synthesized; Cd1N4-C, Bi1N4-C, and Er1N4-C that have never been reported can be synthesized; this strategy was effective to synthesis bimetallic SACs with two different precursors, such as Pt-TPP and Sn-TPP to synthesize the Pt1-Sn1/N-C catalyst.
Figure 8.
Porphyrin-based SACs with intrinsic SAs
(A) Schematic illustration of the preparation of Pt1N4-C catalyst.
(B) The k3-weighted R-space FT spectra of EXAFS for Pt1N4-C, Pt-TPP, PtO2, and Pt foil.
(C) STEM image of Pt nanoparticles on the polymer matrix with Pt-TPP:TPP = 1:0.
(A–C) Reproduced with permission.114 Copyright 2019, Nature Publishing Group.
(D) Top views of the optimized MTP molecules and 2D MTP sheets in a 2 × 2 computational supercell.
(E) Adsorption energies of side-on and end-on adsorbed N2 (Ead-N2) on thirty 2D MTP sheets, where the green area denotes that Ead-N2 < −0.40 eV.
(D and E) Reproduced with permission.117 Copyright 2020, Elsevier Inc.
(F) Investigated pathways for CO2RR on 2D MTP sheets. (F) Reproduced with permission.107 Copyright 2019, The Royal Society of Chemistry.
(G) Theoretical limiting potential (Ulim) as a function of ΔGOOH∗ with an activity volcano plot on 2D MTP sheet, where the black line denotes Pt (111). (G) Reproduced with permission.118 Copyright 2017, Elsevier Inc.
As regards MTPs, since the planar tetramer and one-dimensional linear oligomer assembled by individual MTPs molecules were already synthesized,115,116 2D MTP sheets are highly expectable (Figure 8D). After forming 2D MTP sheets, the SAs loading can be significantly improved, such as 25.44 wt % for Ru in 2D RuTP sheet. Using DFT calculations, Yao et al. investigated the catalytic performances of thirty 2D MTP sheets toward NRR.117 Starting with exploring N2 adsorption (Figure 8E), they found that the end-on adsorption is stronger than the side-on one when metal atoms are in d1 ∼ d6 series but little difference for d7 ∼ d10 series. This is because N2 is always physically adsorbed on these 2D MTP sheets caused by the absence of empty d orbitals for d7 ∼ d10 metal atoms. N2 adsorption on 2D MTP sheets with the metal atoms in d2 ∼ d6 series was relatively strong (Ead-N2 < −0.4 eV) because of the coexistence of empty and occupied d orbitals. For 2D MTP sheets with d1 metal atoms, the coordinated N atoms might take away d electrons of metal atoms, and few electrons thus can be back-donated to adsorbed N2. After computing the free energy profiles of NRR, metal atoms in the three most active 2D MTP sheets were all in 5d series, which was attributed to the fact that MN4 centers with 5d metal atoms can transfer electron easier. Besides NRR, 2D MTP sheets have also been explored as SACs toward CO2RR (Figure 8F),107 ORR (Figure 8G),118 etc.119,120 Utilizing intrinsic SAs achieves not only higher SAs loading but also better activity per specific active site.
Non-noble metal SAs
MOF-based
Currently, pyrolyzing MOFs are widely used to synthesize carbon-based SACs, and this method is first reported by Yin et al.33 They prepared a Zn/Co bimetallic MOF with homogeneous distributions of Co2+ and Zn2+, forming mixed metal nodes, where Zn2+ sites replace Co2+ sites and serve as “fences” to expand the adjacent distance between Co2+ sites. During the subsequent high-temperature pyrolysis, Zn2+ sites were evaporated away because of low boiling point (907°C) and Co2+ sites were it situ reduced (Figure 9A), forming Co SAC, Co SAs/N-C(800), with Co SAs dispersed on N-doped porous carbon and the Co loading of 1.5 wt %. By increasing the pyrolysis temperature, a higher Co loading (4.3 wt %) was achieved in Co SAs/N-C(900). Co clusters or nanoparticles cannot be observed after careful examination of most areas of the SACs samples. Note that without the introduction of Zn2+ sites, Co2+ sites preferred to aggregate during pyrolysis, forming Co nanoparticle supported on N-doped carbon. The spatial distribution of Co SAs in the as-prepared Co SAs/N-C catalysts was investigated by analyzing a series of HAADF-STEM images with different focal depths. At specific focal depths, three selected atoms (a1, b1, and c1, Figure 9B) should have different intensities, and the intensity versus defocus function plot indeed indicated that the intensities of three atoms are different (Figure 9C) and c1 atom located between a1 and b1 along the x-axis has the lowest intensity at a focal position along the z axis. Hence, Co SAs were highly dispersed in N-doped carbon, rather than only on the surface. The as-prepared Co SAs/N-C catalysts were more active than the commercial Pt/C catalyst toward ORR in O2-saturated 0.1 M KOH media, and no obvious decay in activity can be observed even after 5000 continuous cycles. Owing to the high structural adjustability of MOFs, a general strategy to synthesize high-loading and high-performance SACs is provided.
Figure 9.
Strategies to synthesize SACs with intrinsic SAs
(A) Schematic illustration of the preparation of Co SAs/N-C catalyst.
(B) Sum of nine aligned HAADF-STEM images of Co SAs/N-C(800).
(C) Excess intensity of a1, b1, and c1 atoms shown in (B).
(A–C) Reproduced with permission.33 Copyright 2016, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
(D) Schematic illustration of AIM-installed metal atoms undergoing metal exchange. (D) Reproduced with permission.121 Copyright 2016, American Chemical Society.
(E) Synthesis principles of Fe-doped ZIF-derived catalysts. (E) Reproduced with permission.122 Copyright 2017, American Chemical Society.
Metal exchange is another efficient approach to fabricate MOFs with mixed metal nodes. Klet et al. successfully installed Cu, Co, and Ni SAs into Zn-MOF by combining the atomic layer deposition in MOFs and metal exchange technique (Figure 9D).121 After successful metal exchange, the color of Zn-AIM changed from yellow to pale green. The as-prepared precursors held great potential to synthesize SACs with intrinsic Cu, Co, and Ni SAs. Moreover, Zhang et al. simultaneously added Fe and Zn ions with 2-methylimidazole to prepared Fe-doped ZIF-8 containing Zn1-N4 and Fe1-N4 complexes, and Fe1N4 centers were formed by one-step thermal activation with the Fe loading of ∼0.5 at% (Figure 9E).122 The as-prepared Fe SAC achieved respectable ORR activity in challenging acidic media (0.5 M H2SO4).
Intrinsic defect-based
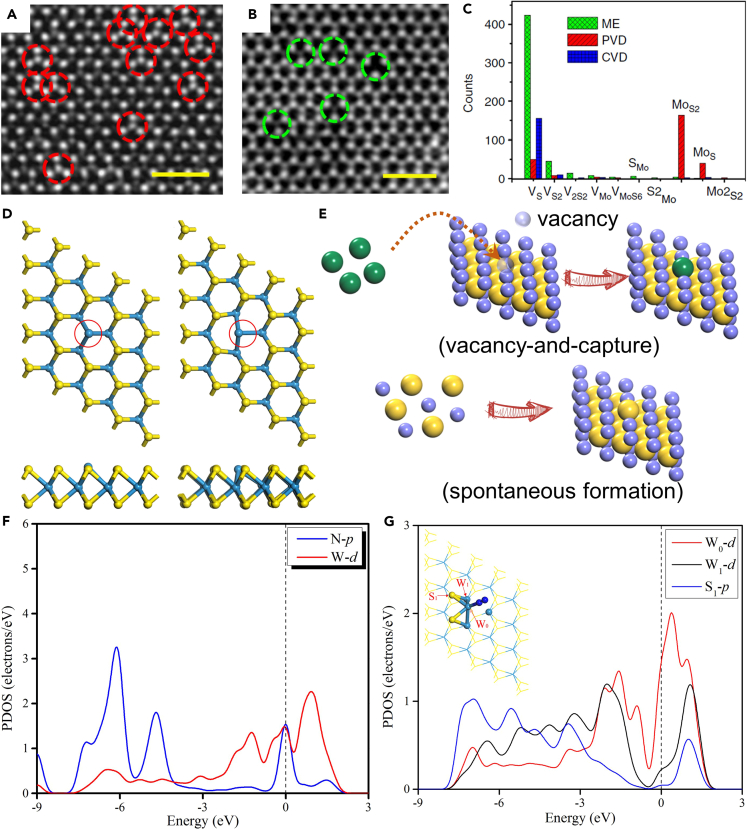
Though MTPs/M-TPPs or MOFs are high useful to synthesize SACs with intrinsic SAs, only carbon-based SACs can be prepared. Aiming at broad applications, metal compound-based SACs with intrinsic SAs should be explored. In this regard, intrinsic structural defects with the single-atom structure are attractive, because the concentration of intrinsic defects could be higher than that of single heteroatoms. Regarding high-loading intrinsic defects, 2D materials are promising candidates, because the ultrahigh surface/volume ratio can ensure the exposure of all intrinsic defects to reactants. Beyond graphene, transition metal dichalcogenides (MX2, X: chalcogen) are another representative family of 2D materials and have been widely used in electronics, electrocatalysis, energy storage and conversion, etc.123,124,125,126,127,128,129 Because the practical physiochemical properties of MX2 are far behind the theoretical predictions,130,131 Hong et al. comprehensively investigated the point defects in monolayer MoS2 samples synthesized by mechanical exfoliation (ME), physical vapor deposition (PVD), and chemical vapor deposition (CVD).132 Figures 10A–10C summarize the analysis of defects and their concentrations for ten optimally fabricated samples by each method. For PVD samples, antisite defects with one Mo atom replacing one or two S atoms (MoS or MoS2) were dominant defects (Figures 10A and 10C), and vacancies with one or two missing S atoms (VS or VS2) were frequently observed in ME and CVD samples (Figures 10B and 10C). The concentrations of MoS and MoS2 in PVD samples reached 7.0 × 1012 and 2.8 × 1013 cm−2, respectively, and the concentration of VS in ME and CVD samples was 1.2 × 1013 cm−2. More importantly, antisite MS and MS2 of MX2 have the single-atom structure with individual under-coordinated metal atoms (Figure 10D) and can be spontaneously formed during PVD, rather than by creating vacancies to capture single heteroatoms (Figure 10E). Hence, they hold great promise to be advanced SACs. Yao et al. theoretically explored the activities of WS and WS2 toward NRR.133 The density of these defects in Figure 10D was about 6 × 1013 cm−2, which is a practically viable value because an average density of 3 × 1013 cm−2 for antisite MoS2 has already been reported for MoS2.132 With such a practically viable density, the loadings of W SAs in WS and WS2 are ∼4.5 wt %, significantly larger than those of reported SACs with non-carbon supports (usually <2 wt %). Strong N2 adsorption on WS can be proved by the partial density of states (PDOS) (Figure 10F), and such a strong interaction activated the adsorbed N2 by elongating the N-N bond length from 1.108 Å for free N2 molecule to 1.190 Å. Consequently, WS can catalyze NRR at a limiting potential of −0.24 V through the enzymatic mechanism, superior than other MX2-based NRR catalysts, including MX2-based SACs with external SAs.103,134,135 The high activity of WS was ascribed to the movement of the active W atom (W0) during the reaction, where the movement can be confirmed the PDOS of N2∗ on WS (Figure 10G). Besides high activity, WS and WS2 also exhibited high selectivity, where both H∗ adsorption (key step for HER) and N2H4 formation (side product) are inhibited.
Figure 10.
Antisite defect-based SACs with intrinsic Mo SAs
(A and B) STEM images for antisite defects in a PVD sample (A) and vacancy defects in an ME sample (B). The case of CVD samples is similar to (B).
(C) Counts of various point defects in PVD, CVD, and ME samples.
(A–C) Reproduced with permission.132 Copyright 2015, Nature Publishing Group.
(D) Optimized structures of antisite WS and WS2.
(D) Reproduced with permission.133 Copyright 2019, The Royal Society of Chemistry.
(E) Scheme of the vacancy-and-capture formation of MX2-based SACs with external SAs and the spontaneous formation of the single-atom structure of antisite MS in MX2.
(F and G) PDOS of side-on-adsorbed N2∗ (F) and end-on-adsorbed N2∗ (G) on WS. (F–G) Reproduced with permission.133 Copyright 2019, The Royal Society of Chemistry.
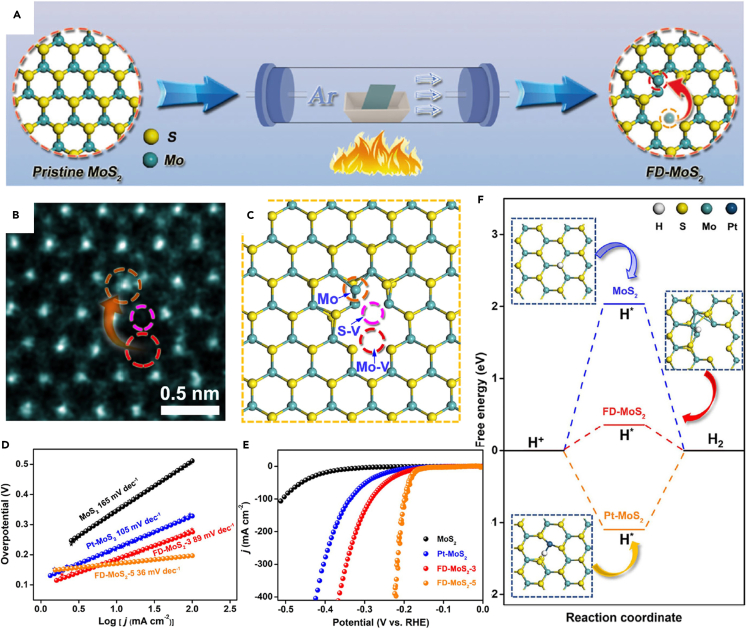
Besides the antisite defects MS and MS2, the Frenkel defects with interstitial metal atoms of MX2 can be also regarded as intrinsic SAs.136 Xu et al. first synthesized pristine MoS2 monolayer by CVD and then annealed the as-prepared MoS2 materials in Ar atmosphere at 400°C to fabricate Frenkel-defected MoS2 (FD-MoS2, Figure 11A), where FD-MoS2-3 and FD-MoS2-5 denote the annealing time of 3 and 5 min, respectively. With increasing annealing time from 3 to 5 min, the average concentration of Frenkel defects increased from 0.50% to 0.85%. The HAADF-STEM image and the atomic model of FD-MoS2-3 with highlighted Frenkel defected sites are shown in Figures 11B and 11C. It can be seen that Mo atom leaves their own lattice sites and becomes an interstitial anchored by nearby locations, which is consistent with the definition of Frenkel defects. The Frenkel defect also has the single-atom structure with the SAs of the interstitial Mo atoms. FD-MoS2-3 and FD-MoS2-5 exhibited superior HER performances compared to pristine MoS2 and Pt-MoS2 (a SAC with external Pt SAs on MoS2) (Figures 11B and 11C), highlighting the Frenkel-defect engineering strategy on 2D materials. Figure 11F is the free energy profile of HER on pristine MoS2, Pt-MoS2, and FD-MoS2, and the Frenkel defect had the optimal free energy of H∗ adsorption (close to 0 eV). This experimental work strongly supports the feasibility of metal compound-based SACs with intrinsic SAs.
Figure 11.
Frenkel defect-based SACs with intrinsic Mo SAs
(A) Schematic synthetic procedure for FD-MoS2.
(B and C) Atomic-resolution HAADF-STEM image (B) and atomic structure (C) of FD-MoS2.
(D and E) Polarization curves (D) and Tafel plots (E) of pristine MoS2, Pt-MoS2, FD-MoS2-3, and FD-MoS2-5 in 0.5 M H2SO4.
(F) Free energy profile of HER on pristine MoS2, Pt-MoS2, and FD-MoS2, including the top views of H∗.
(A–F) Reproduced with permission.136 Copyright 2022, Nature Publishing Group.
Perspectives and challenges
SACs have evolved to be a research frontier in thermocatalysis, electrocatalysis, and photocatalysis. The unique atomic structure enables every active site works efficiently, making SACs highly promising. Moreover, the active sites of SACs are well-defined, a tremendous advance in the field of heterogeneous catalysts, and the structure-activity relationship thus can be comprehensively understood and subsequently exploited in the rational design of high-performance SACs. Though numerous progress have been made in recent years, the breakthrough or the commercialization of SACs is still limited by the relatively low SAs loading because of the favorable aggregation of external SAs. To circumvent this inartificial limitation to obtain high-loading SACs, fabricating SACs with intrinsic SAs is of great worth and necessity. However, further development of SACs with high-loading intrinsic SAs for advancing the practical implementation is still challenging.
Currently the number of experimental studies regarding SACs with intrinsic SAs is negligible compared to that of SACs with external SAs, and future theoretical studies regarding intrinsic SAs thus should focus on (1) higher experimental feasibility: High-loading intrinsic SAs are indeed naturally owned by some systems, such as 2D MTP sheets,107,117,118,119,120 but the corresponding experimental synthesis is of great difficulty. Predicted performances based on SACs systems with higher experimental feasibility, such as 2D M-HAB sheet with the single-metal nodes and the ligand of hexaaminobenzene,137 will be more attractive for experimentalists, providing them practically meaningful evidence and guidance; (2) higher catalytic performance: The predicted performances of the aforementioned SACs with intrinsic SAs are not excellent enough.107,117,118,119,120,133 High-throughput techniques and ML should be utilized to make highly efficient screening for thousands of candidates and thereby achieve higher performance; (3) higher stability: A common concern for SACs is the stability under harsh reaction conditions, where SAs, the ligand of SAs, and even the substrate might greatly vary, leading to activity decay. Though the thermodynamic and kinetic stabilities of the aforementioned SACs with intrinsic SAs have been theoretically confirmed,107,117,118,119,120,133 their stabilities under harsh reaction conditions are still doubtful. More complex theoretical simulations, such as the diverse implicit/explicit solvation models, the computational Pourbaix diagrams, and even molecule dynamics, should be conducted to provide stronger evidence for SACs durability.
On the other hand, future experimental studies regarding intrinsic SAs should focus on: (1) higher SAs loading/density: The loading/density of intrinsic SAs can indeed exceed that of external SAs, but is still low compared to the whole surface. More advances in experiments are required. For example, increasing the density of antisite defects by optimizing the chemical potential of the corresponding transition metal; increasing the fraction of exchanged metal nodes in MOFs by optimizing the experimental conditions; (2) advanced characterization and detection techniques: In situ and operando spectroscopies should be well used to explore (i) the real active sites, (ii) the reaction intermediates, (iii) the variation in the coordination environment/valence state of intrinsic SAs, and (iv) the activity decay behavior of SACs, which could in turn provide guidance for theoretical research.
Acknowledgments
The authors acknowledge financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC), and the University of Toronto.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Li Xin Chen, Email: lixin.chen@utoronto.ca.
Chandra Veer Singh, Email: chandraveer.singh@utoronto.ca.
References
- 1.Le Page A.J. Editions Technip; 1987. Applied Heterogeneous Catalysis. [Google Scholar]
- 2.Schlögl R. Heterogeneous Catalysis. Angew. Chen. Int. Ed. 2015;54:3465–3520. doi: 10.1002/anie.201410738. [DOI] [PubMed] [Google Scholar]
- 3.MacFarlane D.R., Cherepanov P.V., Choi J., Suryanto B.H., Hodgetts R.Y., Bakker J.M., Ferrero Vallana F.M., Simonov A.N. A Roadmap to the Ammonia Economy. Joule. 2020;4:1186–1205. doi: 10.1016/j.joule.2020.04.004. [DOI] [Google Scholar]
- 4.Resasco J., Abild-Pedersen F., Hahn C., Bao Z., Koper M.T.M., Jaramillo T.F. Enhancing the Connection between Computation and Experiments in Electrocatalysis. Nat. Catal. 2022;5:374–381. doi: 10.1038/s41929-022-00789-0. [DOI] [Google Scholar]
- 5.Lee J.S., Tai Kim S., Cao R., Choi N.S., Liu M., Lee K.T., Cho J. Metal–Air Batteries with High Energy Density: Li–Air Versus Zn–Air. Adv. Energy Mater. 2011;1:34–50. doi: 10.1002/aenm.201000010. [DOI] [Google Scholar]
- 6.Munnik P., de Jongh P.E., de Jong K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015;115:6687–6718. doi: 10.1021/cr500486u. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J., Wang L., Zhang B., Zhao H., Kolb U., Zhu Y., Liu L., Han Y., Wang G., Wang C., et al. Sinter-Resistant Metal Nanoparticle Catalysts Achieved by Immobilization within Zeolite Crystals Via Seed-Directed Growth. Nat. Catal. 2018;1:540–546. doi: 10.1038/s41929-018-0098-1. [DOI] [Google Scholar]
- 8.Campelo J.M., Luna D., Luque R., Marinas J.M., Romero A.A. Sustainable Preparation of Supported Metal Nanoparticles and Their Applications in Catalysis. ChemSusChem. 2009;2:18–45. doi: 10.1002/cssc.200800227. [DOI] [PubMed] [Google Scholar]
- 9.Herzing A.A., Kiely C.J., Carley A.F., Landon P., Hutchings G.J. Identification of Active Gold Nanoclusters on Iron Oxide Supports for CO Oxidation. Science. 2008;321:1331–1335. doi: 10.1126/science.1159639. [DOI] [PubMed] [Google Scholar]
- 10.Xu Z., Xiao F.S., Purnell S.K., Alexeev O., Kawi S., Deutsch S.E., Gates B.C. Size-Dependent Catalytic Activity of Supported Metal Clusters. Nature. 1994;372:346–348. doi: 10.1038/372346a0. [DOI] [Google Scholar]
- 11.Corma A., Concepción P., Boronat M., Sabater M.J., Navas J., Yacaman M.J., Larios E., Posadas A., López-Quintela M.A., Buceta D., et al. Exceptional Oxidation Activity with Size-Controlled Supported Gold Clusters of Low Atomicity. Nat. Chem. 2013;5:775–781. doi: 10.1038/nchem.1721. [DOI] [PubMed] [Google Scholar]
- 12.Yang X.F., Wang A., Qiao B., Li J., Liu J., Zhang T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013;46:1740–1748. doi: 10.1021/ar300361m. [DOI] [PubMed] [Google Scholar]
- 13.Wu G., More K.L., Johnston C.M., Zelenay P. High-Performance Electrocatalysts for Oxygen Reduction Derived from Polyaniline, Iron, and Cobalt. Science. 2011;332:443–447. doi: 10.1126/science.1200832. [DOI] [PubMed] [Google Scholar]
- 14.Qiao B., Wang A., Yang X., Allard L.F., Jiang Z., Cui Y., Liu J., Li J., Zhang T. Single-Atom Catalysis of CO Oxidation Using Pt1/FeOx. Nat. Chem. 2011;3:634–641. doi: 10.1038/nchem.1095. [DOI] [PubMed] [Google Scholar]
- 15.Xie F., Cui X., Zhi X., Yao D., Johannessen B., Lin T., Tang J., Woodfield T.B.F., Gu L., Qiao S.Z. A General Approach to 3D-Printed Single-Atom Catalysts. Nat. Synth. 2023;2:129–139. doi: 10.1038/s44160-022-00193-3. [DOI] [Google Scholar]
- 16.Han G.F., Li F., Rykov A.I., Im Y.K., Yu S.Y., Jeon J.P., Kim S.J., Zhou W., Ge R., Ao Z., et al. Abrading Bulk Metal into Single Atoms. Nat. Nanotechnol. 2022;17:403–407. doi: 10.1038/s41565-022-01075-7. [DOI] [PubMed] [Google Scholar]
- 17.Daelman N., Capdevila-Cortada M., López N. Dynamic Charge and Oxidation State of Pt/CeO2 Single-Atom Catalysts. Nat. Mater. 2019;18:1215–1221. doi: 10.1038/s41563-019-0444-y. [DOI] [PubMed] [Google Scholar]
- 18.Xu H., Cheng D., Cao D., Zeng X.C. A Universal Principle for a Rational Design of Single-Atom Electrocatalysts. Nat. Catal. 2018;1:339–348. doi: 10.1038/s41929-018-0063-z. [DOI] [Google Scholar]
- 19.Xu H., Liu E., Xie J., Smyth R.L., Zhou Q., Zhao R., Zang N., Long X., Tang Y., Estill J., et al. Highly Selective Electrocatalytic CO2 Reduction to Ethanol by Metallic Clusters Dynamically Formed from Atomically Dispersed Copper. Nat. Energy. 2020;8:623–632. doi: 10.1038/s41560-020-0666-x. [DOI] [Google Scholar]
- 20.Chen Z.W., Lu Z., Chen L.X., Jiang M., Chen D., Singh C.V. Machine-Learning-Accelerated Discovery of Single-Atom Catalysts Based on Bidirectional Activation Mechanism. Chem Catal. 2021;1:183–195. doi: 10.1016/j.checat.2021.03.003. [DOI] [Google Scholar]
- 21.Lee B.H., Park S., Kim M., Sinha A.K., Lee S.C., Jung E., Chang W.J., Lee K.S., Kim J.H., Cho S.P., et al. Reversible and Cooperative Photoactivation of Single-Atom Cu/TiO2 Photocatalysts. Nat. Mater. 2019;18:620–626. doi: 10.1038/s41563-019-0344-1. [DOI] [PubMed] [Google Scholar]
- 22.Han L., Song S., Liu M., Yao S., Liang Z., Cheng H., Ren Z., Liu W., Lin R., Qi G., et al. Stable and Efficient Single-Atom Zn Catalyst for CO2 Reduction to CH4. J. Am. Chem. Soc. 2020;142:12563–12567. doi: 10.1021/jacs.9b12111. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z.W., Yan J.M., Jiang Q. Single or Double: Which Is the Altar of Atomic Catalysts for Nitrogen Reduction Reaction? Small Methods. 2019;3:1800291. doi: 10.1002/smtd.201800291. [DOI] [Google Scholar]
- 24.Cao L., Liu W., Luo Q., Yin R., Wang B., Weissenrieder J., Soldemo M., Yan H., Lin Y., Sun Z., et al. Atomically Dispersed Iron Hydroxide Anchored on Pt for Preferential Oxidation of CO in H2. Nature. 2019;565:631–635. doi: 10.1038/s41586-018-0869-5. [DOI] [PubMed] [Google Scholar]
- 25.Kong N., Fan X., Liu F., Wang L., Lin H., Li Y., Lee S.T. Single Vanadium Atoms Anchored on Graphitic Carbon Nitride as a High-Performance Catalyst for Non-Oxidative Propane Dehydrogenation. ACS Nano. 2020;14:5772–5779. doi: 10.1021/acsnano.0c00659. [DOI] [PubMed] [Google Scholar]
- 26.Xiong Y., Dong J., Huang Z.Q., Xin P., Chen W., Wang Y., Li Z., Jin Z., Xing W., Zhuang Z., et al. Single-Atom Rh/N-Doped Carbon Electrocatalyst for Formic Acid Oxidation. Nat. Nanotechnol. 2020;15:390–397. doi: 10.1038/s41565-020-0665-x. [DOI] [PubMed] [Google Scholar]
- 27.Maurer F., Jelic J., Wang J., Gänzler A., Dolcet P., Wöll C., Wang Y., Studt F., Casapu M., Grunwaldt J.D. Tracking the Formation, Fate and Consequence for Catalytic Activity of Pt Single Sites on CeO2. Nat. Catal. 2020;3:824–833. doi: 10.1038/s41929-020-00508-7. [DOI] [Google Scholar]
- 28.Wei S., Li A., Liu J.C., Li Z., Chen W., Gong Y., Zhang Q., Cheong W.C., Wang Y., Zheng L., et al. Direct Observation of Noble Metal Nanoparticles Transforming to Thermally Stable Single Atoms. Nat. Nanotechnol. 2018;13:856–861. doi: 10.1038/s41565-018-0197-9. [DOI] [PubMed] [Google Scholar]
- 29.Hannagan R.T., Giannakakis G., Réocreux R., Schumann J., Finzel J., Wang Y., Michaelides A., Deshlahra P., Christopher P., Flytzani-Stephanopoulos M., et al. First-Principles Design of a Single-Atom–Alloy Propane Dehydrogenation Catalyst. Science. 2021;372:1444–1447. doi: 10.1126/science.abg838. [DOI] [Google Scholar]
- 30.Vijay S., Ju W., Brückner S., Tsang S.C., Strasser P., Chan K. Unified Mechanistic Understanding of CO2 Reduction to CO on Transition Metal and Single Atom Catalysts. Nat. Catal. 2021;4:1024–1031. doi: 10.1038/s41929-021-00705-y. [DOI] [Google Scholar]
- 31.Li H., Wang L., Dai Y., Pu Z., Lao Z., Chen Y., Wang M., Zheng X., Zhu J., Zhang W., et al. Synergetic Interaction between Neighbouring Platinum Monomers in CO2 Hydrogenation. Nat. Nanotechnol. 2018;13:411–417. doi: 10.1038/s41565-018-0089-z. [DOI] [PubMed] [Google Scholar]
- 32.Yao Y., Huang Z., Xie P., Wu L., Ma L., Li T., Pang Z., Jiao M., Liang Z., Gao J., et al. High Temperature Shockwave Stabilized Single Atoms. Nat. Nanotechnol. 2019;14:851–857. doi: 10.1038/s41565-019-0518-7. [DOI] [PubMed] [Google Scholar]
- 33.Yin P., Yao T., Wu Y., Zheng L., Lin Y., Liu W., Ju H., Zhu J., Hong X., Deng Z., et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. Int. Ed. 2016;128:10958–10963. doi: 10.1002/ange.201604802. [DOI] [PubMed] [Google Scholar]
- 34.Zhao C., Dai X., Yao T., Chen W., Wang X., Wang J., Yang J., Wei S., Wu Y., Li Y. Ionic Exchange of Metal–Organic Frameworks to Access Single Nickel Sites for Efficient Electroreduction of CO2. J. Am. Chem. Soc. 2017;139:8078–8081. doi: 10.1021/jacs.7b02736. [DOI] [PubMed] [Google Scholar]
- 35.Song Z., Zhang L., Doyle-Davis K., Fu X., Luo J.L., Sun X. Recent Advances in MOF-Derived Single Atom Catalysts for Electrochemical Applications. Adv. Energy Mater. 2020;10:2001561. doi: 10.1002/aenm.202001561. [DOI] [Google Scholar]
- 36.Chen Y., Ji S., Wang Y., Dong J., Chen W., Li Z., Shen R., Zheng L., Zhuang Z., Wang D., Li Y. Isolated Single Iron Atoms Anchored on N-Doped Porous Carbon as an Efficient Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2017;56:6937–6941. doi: 10.1002/anie.201702473. [DOI] [PubMed] [Google Scholar]
- 37.Geng Z., Liu Y., Kong X., Li P., Li K., Liu Z., Du J., Shu M., Si R., Zeng J. Achieving a Record-High Yield Rate of 120.9 Mugnh3 Mgcat.-1 H-1 for N2 Electrochemical Reduction over Ru Single-Atom Catalysts. Adv. Mater. 2018;30:1803498. doi: 10.1002/adma.201803498. [DOI] [PubMed] [Google Scholar]
- 38.Gu J., Hsu C.S., Bai L., Chen H.M., Hu X. Atomically Dispersed Fe(3+) Sites Catalyze Efficient CO2 Electroreduction to CO. Science. 2019;364:1091–1094. doi: 10.1126/science.aaw751. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L., Zhou H., Yang X., Zhang S., Zhang H., Yang X., Su X., Zhang J., Lin Z. Boosting Electroreduction Kinetics of Nitrogen to Ammonia Via Atomically Dispersed Sn Protuberance. Angew. Chem. Int. Ed. 2023;62 doi: 10.1002/anie.202217473. [DOI] [PubMed] [Google Scholar]
- 40.Zhuo H.Y., Zhang X., Liang J.X., Yu Q., Xiao H., Li J. Theoretical Understandings of Graphene-Based Metal Single-Atom Catalysts: Stability and Catalytic Performance. Chem. Rev. 2020;120:12315–12341. doi: 10.1021/acs.chemrev.0c00818. [DOI] [PubMed] [Google Scholar]
- 41.Wang A., Li J., Zhang T. Heterogeneous Single-Atom Catalysis. Nat. Rev. Chem. 2018;2:65–81. doi: 10.1038/s41570-018-0010-1. [DOI] [Google Scholar]
- 42.Wang Y., Mao J., Meng X., Yu L., Deng D., Bao X. Catalysis with Two-Dimensional Materials Confining Single Atoms: Concept, Design, and Applications. Chem. Rev. 2019;119:1806–1854. doi: 10.1021/acs.chemrev.8b00501. [DOI] [PubMed] [Google Scholar]
- 43.Li L., Chang X., Lin X., Zhao Z.J., Gong J. Theoretical Insights into Single-Atom Catalysts. Chem. Soc. Rev. 2020;49:8156–8178. doi: 10.1039/D0CS00795A. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y., Ji S., Chen C., Peng Q., Wang D., Li Y. Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications. Joule. 2018;2:1242–1264. doi: 10.1016/j.joule.2018.06.019. [DOI] [Google Scholar]
- 45.Lang R., Du X., Huang Y., Jiang X., Zhang Q., Guo Y., Liu K., Qiao B., Wang A., Zhang T. Single-Atom Catalysts Based on the Metal–Oxide Interaction. Chem. Rev. 2020;120:11986–12043. doi: 10.1021/acs.chemrev.0c00797. [DOI] [PubMed] [Google Scholar]
- 46.Kaiser S.K., Chen Z., Faust Akl D., Mitchell S., Pérez-Ramírez J. Single-Atom Catalysts across the Periodic Table. Chem. Rev. 2020;120:11703–11809. doi: 10.1021/acs.chemrev.0c00576. [DOI] [PubMed] [Google Scholar]
- 47.Ding S., Hülsey M.J., Pérez-Ramírez J., Yan N. Transforming Energy with Single-Atom Catalysts. Joule. 2019;3:2897–2929. doi: 10.1016/j.joule.2019.09.015. [DOI] [Google Scholar]
- 48.Beniya A., Higashi S. Towards Dense Single-Atom Catalysts for Future Automotive Applications. Nat. Catal. 2019;2:590–602. doi: 10.1038/s41929-019-0282-y. [DOI] [Google Scholar]
- 49.Lin L., Zhou W., Gao R., Yao S., Zhang X., Xu W., Zheng S., Jiang Z., Yu Q., Li Y.W., et al. Low-Temperature Hydrogen Production from Water and Methanol Using Pt/Alpha-MoC Catalysts. Nature. 2017;544:80–83. doi: 10.1038/nature21672. [DOI] [PubMed] [Google Scholar]
- 50.Cheng N., Stambula S., Wang D., Banis M.N., Liu J., Riese A., Xiao B., Li R., Sham T.K., Liu L.M., et al. Platinum Single-Atom and Cluster Catalysis of the Hydrogen Evolution Reaction. Nat. Commun. 2016;7:13638. doi: 10.1038/ncomms13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu G., Robertson A.W., Li M.M.J., Kuo W.C.H., Darby M.T., Muhieddine M.H., Lin Y.C., Suenaga K., Stamatakis M., Warner J.H., Tsang S.C.E. MoS2 Monolayer Catalyst Doped with Isolated Co Atoms for the Hydrodeoxygenation Reaction. Nat. Chem. 2017;9:810–816. doi: 10.1038/nchem.2740. [DOI] [PubMed] [Google Scholar]
- 52.Li M., Zhao Z., Cheng T., Fortunelli A., Chen C.Y., Yu R., Zhang Q., Gu L., Merinov B.V., Lin Z., et al. Ultrafine Jagged Platinum Nanowires Enable Ultrahigh Mass Activity for the Oxygen Reduction Reaction. Science. 2016;354:1414–1419. doi: 10.1126/science.aaf9050. [DOI] [PubMed] [Google Scholar]
- 53.Yang N., Cheng H., Liu X., Yun Q., Chen Y., Li B., Chen B., Zhang Z., Chen X., Lu Q., et al. Amorphous/Crystalline Hetero-Phase Pd Nanosheets: One-Pot Synthesis and Highly Selective Hydrogenation Reaction. Adv. Mater. 2018;30:1803234. doi: 10.1002/adma.201803234. [DOI] [PubMed] [Google Scholar]
- 54.Zhang F., Zhu Y., Lin Q., Zhang L., Zhang X., Wang H. Noble-Metal Single-Atoms in Thermocatalysis, Electrocatalysis, and Photocatalysis. Energy Environ. Sci. 2021;14:2954–3009. doi: 10.1039/D1EE00247C. [DOI] [Google Scholar]
- 55.Li M., Duanmu K., Wan C., Cheng T., Zhang L., Dai S., Chen W., Zhao Z., Li P., Fei H., et al. Single-Atom Tailoring of Platinum Nanocatalysts for High-Performance Multifunctional Electrocatalysis. Nat. Catal. 2019;2:495–503. doi: 10.1038/s41929-019-0279-6. [DOI] [Google Scholar]
- 56.Lin J., Wang A., Qiao B., Liu X., Yang X., Wang X., Liang J., Li J., Liu J., Zhang T. Remarkable Performance of Ir1/FeOx Single-Atom Catalyst in Water Gas Shift Reaction. J. Am. Chem. Soc. 2013;135:15314–15317. doi: 10.1021/ja408574m. [DOI] [PubMed] [Google Scholar]
- 57.Wei H., Liu X., Wang A., Zhang L., Qiao B., Yang X., Huang Y., Miao S., Liu J., Zhang T. FeOx-Supported Platinum Single-Atom and Pseudo-Single-Atom Catalysts for Chemoselective Hydrogenation of Functionalized Nitroarenes. Nat. Commun. 2014;5:5634. doi: 10.1038/ncomms6634. [DOI] [PubMed] [Google Scholar]
- 58.Du P., Qi R., Zhang Y., Gu Q., Xu X., Tan Y., Liu X., Wang A., Zhu B., Yang B., Zhang T. Single-Atom-Driven Dynamic Carburization over Pd1–FeOx Catalyst Boosting CO2 Conversion. Chem. 2022;8:3252–3262. doi: 10.1016/j.chempr.2022.08.012. [DOI] [Google Scholar]
- 59.Li L., Wang A., Qiao B., Lin J., Huang Y., Wang X., Zhang T. Origin of the High Activity of Au/Feox for Low-Temperature Co Oxidation: Direct Evidence for a Redox Mechanism. J. Catal. 2013;299:90–100. doi: 10.1016/j.jcat.2012.11.019. [DOI] [Google Scholar]
- 60.Sun L., Cao L., Su Y., Wang C., Lin J., Wang X. Ru1/FeOx Single-Atom Catalyst with Dual Active Sites for Water Gas Shift Reaction without Methanation. Appl. Catal., B. 2022;318:121841. doi: 10.1016/j.apcatb.2022.121841. [DOI] [Google Scholar]
- 61.Lin J., Qiao B., Li N., Li L., Sun X., Liu J., Wang X., Zhang T. Little Do More: A Highly Effective Pt1/FeOx Single-Atom Catalyst for the Reduction of NO by H2. Chem. Commun. 2015;51:7911–7914. doi: 10.1039/C5CC00714C. [DOI] [PubMed] [Google Scholar]
- 62.Wei H., Ren Y., Wang A., Liu X., Liu X., Zhang L., Miao S., Li L., Liu J., Wang J., et al. Remarkable Effect of Alkalis on the Chemoselective Hydrogenation of Functionalized Nitroarenes over High-Loading Pt/FeOx Catalysts. Chem. Sci. 2017;8:5126–5131. doi: 10.1039/C7SC00568G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sadykov I.I., Zabilskiy M., Clark A.H., Krumeich F., Sushkevich V., van Bokhoven J.A., Nachtegaal M., Safonova O.V. Time-Resolved Xas Provides Direct Evidence for Oxygen Activation on Cationic Iron in a Bimetallic Pt-FeOx/Al2O3 Catalyst. ACS Catal. 2021;11:11793–11805. doi: 10.1021/acscatal.1c02795. [DOI] [Google Scholar]
- 64.Ding K., Gulec A., Johnson A.M., Schweitzer N.M., Stucky G.D., Marks L.D., Stair P.C. Identification of Active Sites in CO Oxidation and Water-Gas Shift over Supported Pt Catalysts. Science. 2015;350:189–192. doi: 10.1126/science.aac6368. [DOI] [PubMed] [Google Scholar]
- 65.Yang M., Li S., Wang Y., Herron J.A., Xu Y., Allard L.F., Lee S., Huang J., Mavrikakis M., Flytzani-Stephanopoulos M. Catalytically Active Au-O (Oh) X-Species Stabilized by Alkali Ions on Zeolites and Mesoporous Oxides. Science. 2014;346:1498–1501. doi: 10.1126/science.1260526. [DOI] [PubMed] [Google Scholar]
- 66.Vilé G., Albani D., Nachtegaal M., Chen Z., Dontsova D., Antonietti M., López N., Pérez-Ramírez J. A Stable Single-Site Palladium Catalyst for Hydrogenations. Angew. Chem. Int. Ed. 2015;54:11265–11269. doi: 10.1002/anie.201505073. [DOI] [PubMed] [Google Scholar]
- 67.Peterson E.J., DeLaRiva A.T., Lin S., Johnson R.S., Guo H., Miller J.T., Hun Kwak J., Peden C.H.F., Kiefer B., Allard L.F., et al. Low-Temperature Carbon Monoxide Oxidation Catalysed by Regenerable Atomically Dispersed Palladium on Alumina. Nat. Commun. 2014;5:4885–4911. doi: 10.1038/ncomms5885. [DOI] [PubMed] [Google Scholar]
- 68.Thomas J.M. Tens of Thousands of Atoms Replaced by One. Nature. 2015;525:325–326. doi: 10.1038/525325a. [DOI] [PubMed] [Google Scholar]
- 69.Liu P., Zhao Y., Qin R., Mo S., Chen G., Gu L., Chevrier D.M., Zhang P., Guo Q., Zang D., et al. Photochemical Route for Synthesizing Atomically Dispersed Palladium Catalysts. Science. 2016;352:797–801. doi: 10.1126/science.aaf5251. [DOI] [PubMed] [Google Scholar]
- 70.Han B., Guo Y., Huang Y., Xi W., Xu J., Luo J., Qi H., Ren Y., Liu X., Qiao B., Zhang T. Strong Metal–Support Interactions between Pt Single Atoms and TiO2. Angew. Chem. Int. Ed. 2020;59:11824–11829. doi: 10.1002/anie.202003208. [DOI] [PubMed] [Google Scholar]
- 71.Guo Y., Huang Y., Zeng B., Han B., Akri M., Shi M., Zhao Y., Li Q., Su Y., Li L., et al. Photo-Thermo Semi-Hydrogenation of Acetylene on Pd1/TiO2 Single-Atom Catalyst. Nat. Commun. 2022;13:2648. doi: 10.1038/s41467-022-30291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang Y., Asokan C., Xu M., Graham G.W., Pan X., Christopher P., Li J., Sautet P. Rh Single Atoms on TiO2 Dynamically Respond to Reaction Conditions by Adapting Their Site. Nat. Commun. 2019;10:4488. doi: 10.1038/s41467-019-12461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thang H.V., Pacchioni G., DeRita L., Christopher P. Nature of Stable Single Atom Pt Catalysts Dispersed on Anatase TiO2. J. Catal. 2018;367:104–114. doi: 10.1016/j.jcat.2018.08.025. [DOI] [Google Scholar]
- 74.Humphreys J., Lan R., Tao S. Development and Recent Progress on Ammonia Synthesis Catalysts for Haber–Bosch Process. Adv. Energy Sustain. Res. 2021;2:2000043. doi: 10.1002/aesr.202000043. [DOI] [Google Scholar]
- 75.Erisman J.W., Sutton M.A., Galloway J., Klimont Z., Winiwarter W. How a Century of Ammonia Synthesis Changed the World. Nat. Geosci. 2008;1:636–639. doi: 10.1038/ngeo325. [DOI] [Google Scholar]
- 76.Foster S.L., Bakovic S.I.P., Duda R.D., Maheshwari S., Milton R.D., Minteer S.D., Janik M.J., Renner J.N., Greenlee L.F. Catalysts for Nitrogen Reduction to Ammonia. Nat. Catal. 2018;1:490–500. doi: 10.1038/s41929-018-0092-7. [DOI] [Google Scholar]
- 77.Appl M. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH; 2012. Ammonia, 2. Production Processes; pp. 140–225. [DOI] [Google Scholar]
- 78.Wu L., Guo T., Li T. Rational Design of Transition Metal Single-Atom Electrocatalysts: A Simulation-Based, Machine Learning-Accelerated Study. J. Mater. Chem. 2020;8:19290–19299. doi: 10.1039/D0TA06207C. [DOI] [Google Scholar]
- 79.Wu L., Guo T., Li T. Machine Learning-Accelerated Prediction of Overpotential of Oxygen Evolution Reaction of Single-Atom Catalysts. iScience. 2021;24:102398. doi: 10.1016/j.isci.2021.102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu L., Guo T., Li T. Data-Driven High-Throughput Rational Design of Double-Atom Catalysts for Oxygen Evolution and Reduction. Adv. Funct. Mater. 2022;32:2203439. doi: 10.1002/adfm.202203439. [DOI] [Google Scholar]
- 81.Fei H., Dong J., Arellano-Jiménez M.J., Ye G., Dong Kim N., Samuel E.L., Peng Z., Zhu Z., Qin F., Bao J. Atomic Cobalt on Nitrogen-Doped Graphene for Hydrogen Generation. Nat. Commun. 2015;6:1–8. doi: 10.1038/ncomms9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang W., Lee C.C., Jasniewski A.J., Ribbe M.W., Hu Y. Structural Evidence for a Dynamic Metallocofactor During N2 Reduction by Mo-Nitrogenase. Science. 2020;368:1381–1385. doi: 10.1126/science.aaz674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee C.C., Kang W., Jasniewski A.J., Stiebritz M.T., Tanifuji K., Ribbe M.W., Hu Y. Evidence of Substrate Binding and Product Release Via Belt-Sulfur Mobilization of the Nitrogenase Cofactor. Nat. Catal. 2022;5:443–454. doi: 10.1038/s41929-022-00782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Milton R.D., Minteer S.D. Nitrogenase Bioelectrochemistry for Synthesis Applications. Acc. Chem. Res. 2019;52:3351–3360. doi: 10.1021/acs.accounts.9b00494. [DOI] [PubMed] [Google Scholar]
- 85.Zheng Y., Jiao Y., Zhu Y., Li L.H., Han Y., Chen Y., Du A., Jaroniec M., Qiao S.Z. Hydrogen Evolution by a Metal-Free Electrocatalyst. Nat. Commun. 2014;5:3783–3788. doi: 10.1038/ncomms4783. [DOI] [PubMed] [Google Scholar]
- 86.Jiao Y., Zheng Y., Davey K., Qiao S.Z. Activity Origin and Catalyst Design Principles for Electrocatalytic Hydrogen Evolution on Heteroatom-Doped Graphene. Nat. Energy. 2016;1:16130–16139. doi: 10.1038/nenergy.2016.130. [DOI] [Google Scholar]
- 87.Ji S., Wang Z., Zhao J. A Boron-Interstitial Doped C2N Layer as a Metal-Free Electrocatalyst for N2 Fixation: A Computational Study. J. Mater. Chem. 2019;7:2392–2399. doi: 10.1039/C8TA10497B. [DOI] [Google Scholar]
- 88.Liu X., Dai L. Carbon-Based Metal-Free Catalysts. Nat. Rev. Mater. 2016;1:16064–16112. doi: 10.1038/natrevmats.2016.64. [DOI] [Google Scholar]
- 89.Rao C.N.R., Chhetri M. Borocarbonitrides as Metal-Free Catalysts for the Hydrogen Evolution Reaction. Adv. Mater. 2019;31:1803668. doi: 10.1002/adma.201803668. [DOI] [PubMed] [Google Scholar]
- 90.Navalon S., Dhakshinamoorthy A., Alvaro M., Antonietti M., García H. Active Sites on Graphene-Based Materials as Metal-Free Catalysts. Chem. Soc. Rev. 2017;46:4501–4529. doi: 10.1039/C7CS00156H. [DOI] [PubMed] [Google Scholar]
- 91.Légaré M.A., Bélanger-Chabot G., Dewhurst R.D., Welz E., Krummenacher I., Engels B., Braunschweig H. Nitrogen Fixation and Reduction at Boron. Science. 2018;359:896–900. doi: 10.1126/science.aaq1684. [DOI] [PubMed] [Google Scholar]
- 92.Yu X., Han P., Wei Z., Huang L., Gu Z., Peng S., Ma J., Zheng G. Boron-Doped Graphene for Electrocatalytic N2 Reduction. Joule. 2018;2:1610–1622. doi: 10.1016/j.joule.2018.06.007. [DOI] [Google Scholar]
- 93.Shi M.M., Bao D., Wulan B.R., Li Y.H., Zhang Y.F., Yan J.M., Jiang Q. Au Sub-Nanoclusters on TiO2 toward Highly Efficient and Selective Electrocatalyst for N2 Conversion to NH3 at Ambient Conditions. Adv. Mater. 2017;29:1606550. doi: 10.1002/adma.201606550. [DOI] [PubMed] [Google Scholar]
- 94.Lv C., Yan C., Chen G., Ding Y., Sun J., Zhou Y., Yu G. An Amorphous Noble-Metal-Free Electrocatalyst That Enables Nitrogen Fixation under Ambient Conditions. Angew. Chem. Int. Ed. 2018;130:6181–6184. doi: 10.1002/ange.201801538. [DOI] [PubMed] [Google Scholar]
- 95.Chen S., Perathoner S., Ampelli C., Mebrahtu C., Su D., Centi G. Electrocatalytic Synthesis of Ammonia at Room Temperature and Atmospheric Pressure from Water and Nitrogen on a Carbon-Nanotube-Based Electrocatalyst. Angew. Chem. Int. Ed. 2017;56:2699–2703. doi: 10.1002/anie.201609533. [DOI] [PubMed] [Google Scholar]
- 96.Bao D., Zhang Q., Meng F.L., Zhong H.X., Shi M.M., Zhang Y., Yan J.M., Jiang Q., Zhang X.B. Electrochemical Reduction of N2 under Ambient Conditions for Artificial N2 Fixation and Renewable Energy Storage Using N2/NH3 Cycle. Adv. Mater. 2017;29:1604799. doi: 10.1002/adma.201604799. [DOI] [PubMed] [Google Scholar]
- 97.Yao Y., Zhu S., Wang H., Li H., Shao M. A Spectroscopic Study on the Nitrogen Electrochemical Reduction Reaction on Gold and Platinum Surfaces. J. Am. Chem. Soc. 2018;140:1496–1501. doi: 10.1021/jacs.7b12101. [DOI] [PubMed] [Google Scholar]
- 98.Ling C., Niu X., Li Q., Du A., Wang J. Metal-Free Single Atom Catalyst for N2 Fixation Driven by Visible Light. J. Am. Chem. Soc. 2018;140:14161–14168. doi: 10.1021/jacs.8b07472. [DOI] [PubMed] [Google Scholar]
- 99.Liu J., Liu Y., Liu N., Han Y., Zhang X., Huang H., Lifshitz Y., Lee S.T., Zhong J., Kang Z. Metal-Free Efficient Photocatalyst for Stable Visible Water Splitting Via a Two-Electron Pathway. Science. 2015;347:970–974. doi: 10.1126/science.aaa3145. [DOI] [PubMed] [Google Scholar]
- 100.Wang X., Maeda K., Thomas A., Takanabe K., Xin G., Carlsson J.M., Domen K., Antonietti M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009;8:76–80. doi: 10.1038/nmat2317. [DOI] [PubMed] [Google Scholar]
- 101.Zhu J., Wei Y., Chen W., Zhao Z., Thomas A. Graphitic Carbon Nitride as a Metal-Free Catalyst for No Decomposition. Chem. Commun. 2010;46:6965–6967. doi: 10.1039/C0CC01432J. [DOI] [PubMed] [Google Scholar]
- 102.Lv X., Wei W., Li F., Huang B., Dai Y. Metal-Free B@g-CN: Visible/Infrared Light-Driven Single Atom Photocatalyst Enables Spontaneous Dinitrogen Reduction to Ammonia. Nano Lett. 2019;19:6391–6399. doi: 10.1021/acs.nanolett.9b02572. [DOI] [PubMed] [Google Scholar]
- 103.Liu C., Li Q., Wu C., Zhang J., Jin Y., MacFarlane D.R., Sun C. Single-Boron Catalysts for Nitrogen Reduction Reaction. J. Am. Chem. Soc. 2019;141:2884–2888. doi: 10.1021/jacs.8b13165. [DOI] [PubMed] [Google Scholar]
- 104.Wang M., Liu S., Qian T., Liu J., Zhou J., Ji H., Xiong J., Zhong J., Yan C. Over 56.55% Faradaic Efficiency of Ambient Ammonia Synthesis Enabled by Positively Shifting the Reaction Potential. Nat. Commun. 2019;10:341. doi: 10.1038/s41467-018-08120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zang W., Yang T., Zou H., Xi S., Zhang H., Liu X., Kou Z., Du Y., Feng Y.P., Shen L., et al. Copper Single Atoms Anchored in Porous Nitrogen-Doped Carbon as Efficient Ph-Universal Catalysts for the Nitrogen Reduction Reaction. ACS Catal. 2019;9:10166–10173. doi: 10.1021/acscatal.9b02944. [DOI] [Google Scholar]
- 106.Zhao W., Zhang L., Luo Q., Hu Z., Zhang W., Smith S., Yang J. Single Mo1(Cr1) Atom on Nitrogen-Doped Graphene Enables Highly Selective Electroreduction of Nitrogen into Ammonia. ACS Catal. 2019;9:3419–3425. doi: 10.1021/acscatal.8b05061. [DOI] [Google Scholar]
- 107.Liu J.H., Yang L.M., Ganz E. Electrocatalytic Reduction of CO2 by Two-Dimensional Transition Metal Porphyrin Sheets. J. Mater. Chem. 2019;7:11944–11952. doi: 10.1039/C9TA01188A. [DOI] [Google Scholar]
- 108.Wang H.Y., Weng C.C., Yuan Z.Y. Insights into Efficient Transition Metal-Nitrogen/Carbon Oxygen Reduction Electrocatalysts. J. Energy Chem. 2021;56:470–485. doi: 10.1016/j.jechem.2020.08.030. [DOI] [Google Scholar]
- 109.Tylus U., Jia Q., Strickland K., Ramaswamy N., Serov A., Atanassov P., Mukerjee S. Elucidating Oxygen Reduction Active Sites in Pyrolyzed Metal–Nitrogen Coordinated Non-Precious-Metal Electrocatalyst Systems. J. Phys. Chem. C. 2014;118:8999–9008. doi: 10.1021/jp500781v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Varela A.S., Ju W., Bagger A., Franco P., Rossmeisl J., Strasser P. Electrochemical Reduction of CO2 on Metal-Nitrogen-Doped Carbon Catalysts. ACS Catal. 2019;9:7270–7284. doi: 10.1021/acscatal.9b01405. [DOI] [Google Scholar]
- 111.Zagal J.H., Specchia S., Atanassov P. Mapping Transition Metal-Mn4 Macrocyclic Complex Catalysts Performance for the Critical Reactivity Descriptors. Curr. Opin. Electrochem. 2021;27:100683. doi: 10.1016/j.coelec.2020.100683. [DOI] [Google Scholar]
- 112.He T., Chen S., Ni B., Gong Y., Wu Z., Song L., Gu L., Hu W., Wang X. Zirconium–Porphyrin-Based Metal–Organic Framework Hollow Nanotubes for Immobilization of Noble-Metal Single Atoms. Angew. Chem. Int. Ed. 2018;130:3551–3556. doi: 10.1002/ange.201800817. [DOI] [PubMed] [Google Scholar]
- 113.Wang X., Chen W., Zhang L., Yao T., Liu W., Lin Y., Ju H., Dong J., Zheng L., Yan W., et al. Uncoordinated Amine Groups of Metal–Organic Frameworks to Anchor Single Ru Sites as Chemoselective Catalysts toward the Hydrogenation of Quinoline. J. Am. Chem. Soc. 2017;139:9419–9422. doi: 10.1021/jacs.7b01686. [DOI] [PubMed] [Google Scholar]
- 114.He X., He Q., Deng Y., Peng M., Chen H., Zhang Y., Yao S., Zhang M., Xiao D., Ma D., et al. A Versatile Route to Fabricate Single Atom Catalysts with High Chemoselectivity and Regioselectivity in Hydrogenation. Nat. Commun. 2019;10:3663. doi: 10.1038/s41467-019-11619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nakamura Y., Aratani N., Shinokubo H., Takagi A., Kawai T., Matsumoto T., Yoon Z.S., Kim D.Y., Ahn T.K., Kim D., et al. A Directly Fused Tetrameric Porphyrin Sheet and Its Anomalous Electronic Properties That Arise from the Planar Cyclooctatetraene Core. J. Am. Chem. Soc. 2006;128:4119–4127. doi: 10.1021/ja057812l. [DOI] [PubMed] [Google Scholar]
- 116.Tanaka T., Osuka A. Conjugated Porphyrin Arrays: Synthesis, Properties and Applications for Functional Materials. Chem. Soc. Rev. 2015;44:943–969. doi: 10.1039/C3CS60443H. [DOI] [PubMed] [Google Scholar]
- 117.Yao X., Chen Z.W., Wang Y.R., Lang X.Y., Zhu Y.F., Gao W., Jiang Q. High-Loading Intrinsic Active Sites for Ammonia Synthesis Using Efficient Single-Atom Catalyst: 2D Tungsten-Porphyrin Sheet. Appl. Surf. Sci. 2020;529:147183. doi: 10.1016/j.apsusc.2020.147183. [DOI] [Google Scholar]
- 118.Luo G., Wang Y., Li Y. Two-Dimensional Iron-Porphyrin Sheet as a Promising Catalyst for Oxygen Reduction Reaction: A Computational Study. Sci. Bull. 2017;62:1337–1343. doi: 10.1016/j.scib.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 119.Liu S., Dhar P., Wu J.D., Tan Y., Cheng Z., Shen Z., Fan M. Two-Dimensional Transition Metal Porphyrin Sheets as a Promising Single-Atom-Catalyst for Dinitrogen Electrochemical Reduction to Ammonia: A Theoretical Study. J. Clin. Med. 2019;8:1492–1499. doi: 10.1021/acs.jpcc.9b10294. [DOI] [Google Scholar]
- 120.Impeng S., Roongcharoen T., Maitarad P., Wu H., Chitpakdee C., Promarak V., Shi L., Namuangruk S. High Selective Catalyst for Ethylene Epoxidation to Ethylene Oxide: A DFT Investigation. Appl. Surf. Sci. 2020;513:145799. doi: 10.1016/j.apsusc.2020.145799. [DOI] [Google Scholar]
- 121.Klet R.C., Wang T.C., Fernandez L.E., Truhlar D.G., Hupp J.T., Farha O.K. Synthetic Access to Atomically Dispersed Metals in Metal–Organic Frameworks Via a Combined Atomic-Layer-Deposition-in-Mof and Metal-Exchange Approach. Chem. Mater. 2016;28:1213–1219. doi: 10.1021/acs.chemmater.5b04887. [DOI] [Google Scholar]
- 122.Zhang H., Hwang S., Wang M., Feng Z., Karakalos S., Luo L., Qiao Z., Xie X., Wang C., Su D., et al. Single Atomic Iron Catalysts for Oxygen Reduction in Acidic Media: Particle Size Control and Thermal Activation. J. Am. Chem. Soc. 2017;139:14143–14149. doi: 10.1021/jacs.7b06514. [DOI] [PubMed] [Google Scholar]
- 123.Manzeli S., Ovchinnikov D., Pasquier D., Yazyev O.V., Kis A. 2D Transition Metal Dichalcogenides. Nat. Rev. Mater. 2017;2:17033–17115. doi: 10.1038/natrevmats.2017.33. [DOI] [Google Scholar]
- 124.Wang Q.H., Kalantar-Zadeh K., Kis A., Coleman J.N., Strano M.S. Electronics and Optoelectronics of Two-Dimensional Transition Metal Dichalcogenides. Nat. Nanotechnol. 2012;7:699–712. doi: 10.1038/nnano.2012.193. [DOI] [PubMed] [Google Scholar]
- 125.Fu Q., Han J., Wang X., Xu P., Yao T., Zhong J., Zhong W., Liu S., Gao T., Zhang Z., et al. 2D Transition Metal Dichalcogenides: Design, Modulation, and Challenges in Electrocatalysis. Adv. Mater. 2021;33 doi: 10.1002/adma.201907818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yao X., Wang Z., Zhang H., Zhu Y., Jiang Q. Thickness-Dependent Bandgap of Transition Metal Dichalcogenides Dominated by Interlayer Van Der Waals Interaction. Physica E. 2019;113:11–19. doi: 10.1016/j.physe.2018.12.037. [DOI] [Google Scholar]
- 127.Choi W., Choudhary N., Han G.H., Park J., Akinwande D., Lee Y.H. Recent Development of Two-Dimensional Transition Metal Dichalcogenides and Their Applications. Mater. Today. 2017;20:116–130. doi: 10.1016/j.mattod.2016.10.002. [DOI] [Google Scholar]
- 128.Mak K.F., Shan J. Photonics and Optoelectronics of 2D Semiconductor Transition Metal Dichalcogenides. Nat. Photonics. 2016;10:216–226. doi: 10.1038/nphoton.2015.282. [DOI] [Google Scholar]
- 129.Yao X., Wang Y.R., Lang X.Y., Zhu Y.F., Jiang Q. Composition-and Layer-Dependent Bandgap of Two-Dimensional Transition Metal Dichalcogenides Alloys. Physica E. 2020;124 doi: 10.1016/j.physe.2020.114243. [DOI] [Google Scholar]
- 130.Yu Z., Pan Y., Shen Y., Wang Z., Ong Z.Y., Xu T., Xin R., Pan L., Wang B., Sun L., et al. Towards Intrinsic Charge Transport in Monolayer Molybdenum Disulfide by Defect and Interface Engineering. Nat. Commun. 2014;5:5290. doi: 10.1038/ncomms6290. [DOI] [PubMed] [Google Scholar]
- 131.Radisavljevic B., Radenovic A., Brivio J., Giacometti V., Kis A. Single-Layer MoS2 Transistors. Nat. Nanotechnol. 2011;6:147–150. doi: 10.1038/nnano.2010.279. [DOI] [PubMed] [Google Scholar]
- 132.Hong J., Hu Z., Probert M., Li K., Lv D., Yang X., Gu L., Mao N., Feng Q., Xie L., et al. Exploring Atomic Defects in Molybdenum Disulphide Monolayers. Nat. Commun. 2015;6:6293. doi: 10.1038/ncomms7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yao X., Chen Z., Wang Y., Lang X., Gao W., Zhu Y., Jiang Q. Activated Basal Planes of WS2 by Intrinsic Defects as Catalysts for the Electrocatalytic Nitrogen Reduction Reaction. J. Mater. Chem. 2019;7:25961–25968. doi: 10.1039/C9TA10050D. [DOI] [Google Scholar]
- 134.Li L., Li B., Guo Q., Li B. Theoretical Screening of Single-Atom-Embedded Mosse Nanosheets for Electrocatalytic N2 Fixation. J. Phys. Chem. C. 2019;123:14501–14507. doi: 10.1021/acs.jpcc.9b02657. [DOI] [Google Scholar]
- 135.Ma X., Zhang H.M., Liu Y.Q., Li D., Lv H., He H., Huang C. N2 Reduction Using Single Transition-Metal Atom Supported on Defective WS2 Monolayer as Promising Catalysts: A DFT Study. Appl. Surf. Sci. 2019;41:684–685. doi: 10.1016/j.apsusc.2019.05.022. [DOI] [Google Scholar]
- 136.Xu J., Shao G., Tang X., Lv F., Xiang H., Jing C., Liu S., Dai S., Li Y., Luo J., Zhou Z. Frenkel-Defected Monolayer MoS2 Catalysts for Efficient Hydrogen Evolution. Nat. Commun. 2022;13:2193. doi: 10.1038/s41467-022-29929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lahiri N., Lotfizadeh N., Tsuchikawa R., Deshpande V.V., Louie J. Hexaaminobenzene as a Building Block for a Family of 2D Coordination Polymers. J. Am. Chem. Soc. 2017;139:19–22. doi: 10.1021/jacs.6b09889. [DOI] [PubMed] [Google Scholar]