Abstract
Introduction
Rotatory chair testing has been used to evaluate horizontal canal function. Frequently used tests include sinusoidal harmonic acceleration test (SHAT) and velocity step test (VST).
Objectives
Assessment of age effect on the SHAT and VST and assessment of test-retest reliability of the parameters of those two tests.
Methods
A prospective study was performed on 100 subjects with no ear or vestibular complaints and normal vestibular evaluation. They were divided into two groups; Group A: below 50 years of age and Group B: 50 years of age or above. SHAT was presented at frequencies 0.02, 0.04, 0.08, 0.16, 0.32, 0.64 Hz with a peak velocity of 60°/s. VST was performed using a maximum velocity of 100°/s with acceleration and deceleration of 200°/s2. Thirty subjects were tested twice to assess reliability.
Results
Study participants ranged in age from 20 to 67 years. Regarding group A, the mean age was 30.92 ± 7.31 and 55.36 ± 4.61 for group B. No significant differences were found in SHAT parameters between the two groups. As well, there was no significant difference in VST per-rotatory time constant, however, post-rotatory time constant was significantly longer for Group B (P value < 0.05). Intraclass correlation coefficient (ICC) values showed moderate to good reliability (ICC 0.580–0.818) for SHAT parameters for the lower frequencies and indicated moderate reliability for VST time constant (ICC 0.509–0.652).
Conclusions
Age has no significant effect on the parameters of SHAT and VST. Test-retest reliability is generally good for both tests.
Keywords: Rotatory chair testing, Sinusoidal harmonic acceleration test, Velocity step test, Age effect, Test-retest reliability
1. Introduction
Rotatory chair testing is a part of the vestibular test battery. It has primarily been used for analyzing the vestibulo-ocular reflex (VOR) through stimulation of the lateral semicircular canals (González and Kiderman, 2013).
There are several advantages to rotary chair testing. It allows vestibular system stimulation across multiple frequencies. It is natural, physiologic and well-tolerated (Barin, 2009). Moreover, the stimulus is computer-controlled and doesn't depend on the anatomy of the ear resulting in precise and repeatable evaluation of the vestibular function (Barin, 2009; Zapala and Brey, 2009).
Standard rotational testing involves Sinusoidal Harmonic Acceleration test (SHAT) and Velocity-step test (VST) for evaluation of the lateral semicircular canal function. SHAT exhibits stimuli at various sinusoidal frequencies where gain, phase and symmetry of eye movements are analyzed, while VST provides rapid acceleration till constant velocity is reached followed by rapid deceleration and time constant is recorded (González and Kiderman, 2013).
Rotatory chair has several clinical applications. It is considered the “gold standard” in the evaluation of bilateral vestibular lesions (Byerly, 2014). It can differentiate between true bilateral vestibular loss and false positive caloric weakness secondary to faulty irrigation and can determine the range of affected frequencies and the magnitude of bilateral vestibular lesions (Barin, 2009).
Additionally, it can assess vestibular central compensation following acute vestibular dysfunction (Maes et al., 2011) and can monitor the level of vestibulotoxicity (Barin, 2009). Rotational testing is also used in the evaluation of children who can't tolerate caloric testing (Valente, 2011) and may be used if caloric irrigation is impossible or not explicable, e.g. middle ear effusion (British Society of Audiology, 2010).
It is important to consider the effect of age on vestibular function tests before evaluating an elderly patient for a better evaluation of the patient's response. However, previous studies on the effect of age on the SHAT and VST showed contradictory results; with either marginal clinical significance or no measurable changes reported. Moreover, there is a paucity of studies on the rotatory chair tests; SHAT and VST, with variable normal ranges for the test parameters from lab to lab. Therefore, it is imperative to have age-related normative data related to the used laboratory equipment for rotatory chair tests. (Maes et al., 2008; Chan et al., 2016).
Phase and time constant are believed to be the most consistent parameters in rotatory chair tests (Jenkins and Goldberg, 1988; Li et al., 1991; Barin, 2009). However, gain is considered the least consistent parameter (Li et al., 1991) as it is influenced by many variables e.g. mental alertness, fatigue, habituation, antidizziness medications, light leaks, and calibration errors (Goulson et al., 2016; Zalewski et al., 2019).
Accordingly, this study aimed to assess age effect on the SHAT and VST for accurate interpretation of response parameters. As well, test-retest reliability was assessed for those two tests to standardize the rotational chair testing in our lab as an objective and reliable tool for evaluation of dizzy patients.
2. Materials and methods
2.1. Ethical considerations
This study was performed following the Code of Ethics of the World Medical Association (Declaration of Helsinki) for studies involving humans, and the protocol was evaluated and accepted by the ethics committee of the Faculty of Medicine Alexandria University on February 20, 2020. After protocol approval, all subjects signed an informed consent form to be involved in the study.
2.2. Subjects
The study was carried out during the year of 2021 at the Audio-Vestibular Medicine Clinic of Alexandria Main University Hospital. One hundred healthy subjects were enrolled in this study. Inclusion criteria were as follows: 1) Adult and elderly subjects with no gender limit. 2) Normal peripheral hearing or mild presbycusis (bilateral symmetrical sensorineural hearing loss at high frequencies with hearing thresholds ≤40 dBHL). 3)Normal vestibular bedside tests. 4) Normal videonystagmography test battery. Exclusion criteria were as follows: 1) History of ear disease, ear surgery, or hearing impairment (except for mild presbycusis). 2) Vestibular complaints and migraine.
Subjects were divided into two groups; Group A included 50 subjects below 50 years of age and Group B included 50 subjects 50 years of age or above. A randomly selected group of thirty subjects (30%) were tested twice, with a two- or three-week interval between the two test sessions, to evaluate the test-retest reliability.
2.3. Rotatory chair testing
It was performed using Nanotorque rotatory chair (Difra Instrumentation, Belgium). It is an earth vertical axis rotary equipment capable of measuring VOR with frequencies up to 1.28 Hz using velocities up to 200°/s.
Subjects were seated in the rotatory chair with the head flexed downward by approximately 30° to bring the lateral semicircular canals into the plane of rotation for maximum stimulation.
Video-oculography was used to record slow phase eye movements in total darkness with eyes open. Mental alerting tasks were performed to keep subjects alert during testing. Tests were done by an audiologist and results were interpreted by another one.
SHAT and VST were performed on all subjects. For SHAT, the subject was rotated alternately to the right and the left about the vertical axis at different frequencies from the lowest frequency 0.02 Hz (according to the limitation of the device) up to 0.64 Hz using a peak velocity of 60°/s (Ahmed, 2014; Chan et al., 2016; Kim et al., 2017). The eye velocity responses were averaged over multiple cycles. The number of cycles was as follows: 3 at 0.02, 0.04 Hz, 6 at 0.08, 0.16 Hz, and 9 at 0.32, 0.64 Hz. Gain, phase, and symmetry of eye movements were recorded at each frequency.
SHAT gain is the ratio of peak eye velocity to peak head (chair) velocity, phase is the timing difference between peak head and eye velocities, while symmetry/directional preponderance assesses whether the nystagmus is stronger to the right or the left, and is displayed as the percentage difference between the average maximum slow phase eye velocity for the respective rotation to the right and to the left. (Barin, 2009; Goulson et al., 2016; Zalewski et al., 2019).
For VST the subject was rapidly accelerated to reach a constant velocity and then was suddenly decelerated. The test was carried out for both right and left rotations. An acceleration of 200°/s2 was used, resulting in per-rotatory nystagmus, till a maximum velocity of 100°/s was reached. Subject was rotated at this velocity for 60 s then a deceleration of 200°/s2 was used resulting in post-rotatory nystagmus (Maes et al., 2007). Time constant is measured after the chair begins to rotate (per-rotatory time constant) and after it is stopped abruptly (post-rotatory time constant) for right and left rotations (Goulson et al., 2016).
VST time constant is the time needed for the nystagmus peak slow phase eye velocity to decrease to 37% of that velocity (Goulson et al., 2016; Zalewski et al., 2019).
2.4. Statistical analysis of the data
Data were introduced to the computer and interpreted using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). Qualitative data were demonstrated using numbers and percentages. The Kolmogorov-Smirnov test was used to confirm the normality of distribution. Quantitative data were demonstrated using range (minimum and maximum), mean, standard deviation, and 95% confidence interval. A p-value less than 0.05 was considered statistically significant.
The used tests were chi-square test, student t-test, and Mann Whitney test. ICC was used to evaluate the agreement between the test and re-test. It was classified as follows: Less than 0.5: poor agreement, 0.5 to less than 0.75: moderate agreement, 0.75 to less than 0.9: good agreement, greater than 0.9: high agreement.
3. Results
3.1. Demographic data
A total of 100 subjects were enrolled in the study, they were 56 males and 44 females with a male/female ratio of 1.3. Their age ranged from 20 to 67 years with the mean of 43.14 ± 13.7. For group A, the male/female ratio was 0.3 (12 males/38 females) with the mean age of 30.92 ± 7.31 (20–47 years). In group B, the male/female ratio was 7 (44 males/6 females) and the mean age was 55.36 ± 4.61 (50–67 years). The male/female ratio for the retested subjects was 0.8 (14 males/16 females) with the mean age of 39 ± 13 (24–61 years).
3.2. SHAT and VST data
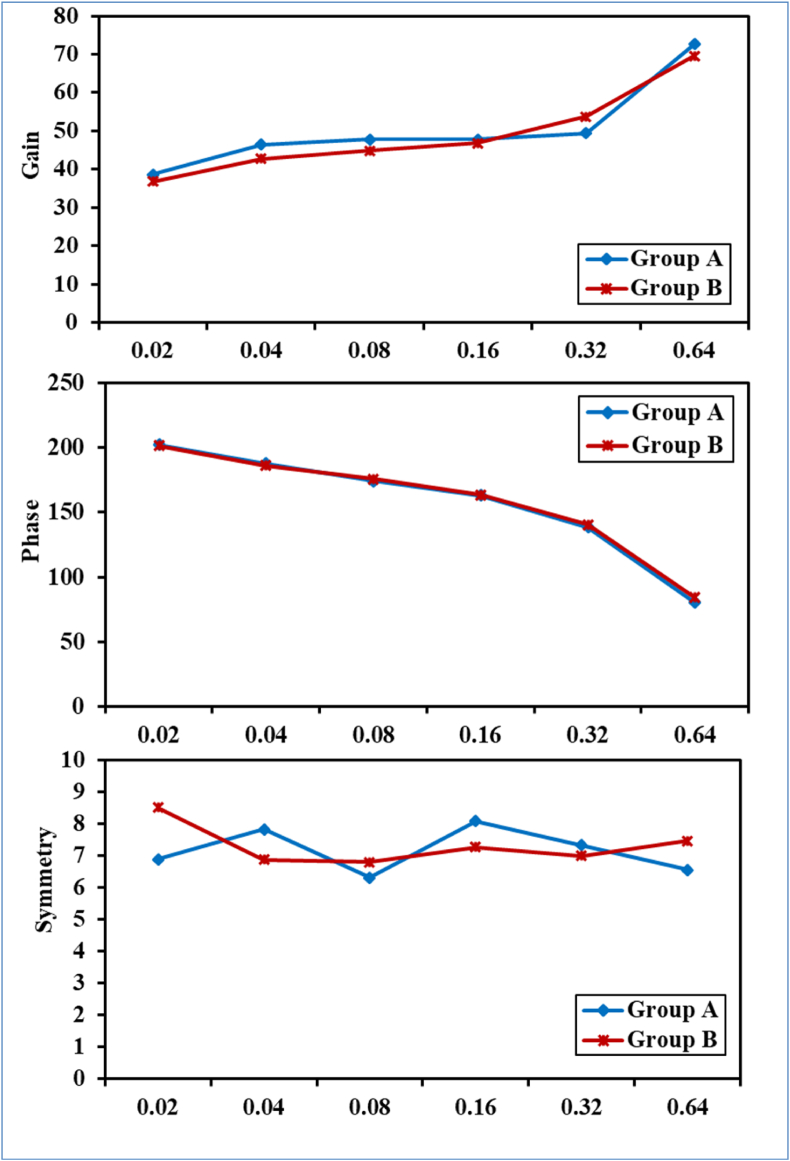
Total data of SHAT for all 100 participants were expressed for gain, phase, and symmetry at all tested frequencies (Table 1). The two studied groups were compared and there were no statistically significant differences in VOR gain, phase, and symmetry between the two groups. (Fig. 1).
Table 1.
SHAT data of the total sample for gain, phase and symmetry at all tested frequencies.
| Frequency | Gain | Phase | Symmetry |
|---|---|---|---|
| 0.02 Hz | |||
| Min. – Max. | 20.0–68.50 | 189.4–223.1 | 0.0–29.0 |
| Mean ± SD. | 37.74 ± 10.13 | 201.8 ± 6.82 | 7.71 ± 5.85 |
| 95% C.I. | 35.73–39.74 | 200.5–203.2 | 6.55–8.87 |
| 0.04 Hz | |||
| Min. – Max. | 25.0–73.0 | 173.7–201.0 | 0.0–30.0 |
| Mean ± SD. | 44.65 ± 10.44 | 186.9 ± 5.29 | 7.36 ± 5.67 |
| 95% C.I. | 42.58–46.72 | 185.8–187.9 | 6.24–8.48 |
| 0.08 Hz | |||
| Min. – Max. | 27.0–76.0 | 160.3–189.4 | 0.0–26.0 |
| Mean ± SD. | 46.39 ± 11.65 | 175.0 ± 5.07 | 6.56 ± 5.77 |
| 95% C.I. | 44.07–48.70 | 174.0–176.0 | 5.41–7.71 |
| 0.16 Hz | |||
| Min. – Max. | 26.50–69.0 | 149.1–175.0 | 0.0–26.0 |
| Mean ± SD. | 47.34 ± 10.86 | 163.3 ± 4.83 | 7.69 ± 5.67 |
| 95% C.I. | 45.18–49.50 | 162.4–164.3 | 6.56–8.82 |
| 0.32 Hz | |||
| Min. – Max. | 29.50–77.50 | 123.8–157.7 | 0.0–20.0 |
| Mean ± SD. | 51.62 ± 12.09 | 139.6 ± 6.15 | 7.17 ± 4.89 |
| 95% C.I. | 49.22–54.01 | 138.4–140.9 | 6.20–8.14 |
| 0.64 Hz | |||
| Min. – Max. | 40.50–99.50 | 47.70–111.1 | 0.0–27.0 |
| Mean ± SD. | 71.15 ± 13.49 | 82.24 ± 12.65 | 7.02 ± 6.08 |
| 95% C.I. | 68.47–73.82 | 79.73–84.75 | 5.81–8.23 |
SD: Standard deviation CI: confidence interval for the mean.
Figure (1).
Comparison between group A and group B according to SHAT gain, phase and symmetry represented as average, without significant difference between the two studied groups.
As well, VST time constant total data with comparison between the two studied groups were demonstrated in (Table 2). No statistically significant difference was found in per clockwise time constant (Per CW TC) and per counterclockwise time constant (Per CCW TC) between the two groups, while Post CW TC and Post CCW TC were significantly longer for group B (P < 0.005).
Table 2.
Total data and Comparison between the two studied groups according to VST time constant.
| VST | Total (n = 100) | Group A (n = 50) | Group B (n = 50) | P |
|---|---|---|---|---|
| Per CW TC | ||||
| Min. – Max. | 9.0–26.60 | 9.0–26.60 | 9.60–23.80 | 0.308 |
| Mean ± SD. | 15.38 ± 3.73 | 15.0 ± 4.04 | 15.76 ± 3.38 | |
| 95% C.I. | 14.64–16.12 | 13.85–16.15 | 14.80–16.72 | |
| Post CW TC | ||||
| Min. – Max. | 9.90–30.0 | 9.90–30.0 | 13.50–26.60 | 0.003∗ |
| Mean ± SD. | 18.11 ± 4.54 | 16.77 ± 4.87 | 19.46 ± 3.77 | |
| 95% C.I. | 17.21–19.01 | 15.39–18.15 | 18.39–20.53 | |
| Per CCW TC | ||||
| Min. – Max. | 8.0–27.0 | 8.10–23.60 | 8.0–27.0 | 0.178 |
| Mean ± SD. | 13.61 ± 3.49 | 13.14 ± 3.70 | 14.08 ± 3.23 | |
| 95% C.I. | 12.92–14.30 | 12.09–14.19 | 13.16–15.0 | |
| Post CCW TC | ||||
| Min. – Max. | 8.30–27.80 | 8.30–23.80 | 8.90–27.80 | 0.001∗ |
| Mean ± SD. | 17.75 ± 4.60 | 16.18 ± 3.84 | 19.31 ± 4.80 | |
| 95% C.I. | 16.83–18.66 | 15.09–17.27 | 17.94–20.67 | |
SD: Standard deviation CI: confidence interval for the mean.
p: p value for comparing between the studied groups ∗: Statistically significant at p ≤ 0.05.
Per CW TC: per clockwise time constant, Post CW TC: post clockwise time constant.
Per CCW TC: per counterclockwise time constant, Post CCW TC: post counterclockwise time constant.
3.3. Test-retest reliability
Test-retest reliability parameters for the SHAT and VST were presented in (Table 3, Table 4). No significant differences could be demonstrated between test and retest sessions for all response parameters of both tests (P0 value for comparing between Test and Re-Test >0.05). ICC values (0.580–0.818) showed moderate to good reliability for gain, phase, and symmetry except for the higher frequencies (0.32 Hz, 0.64 Hz), and indicated moderate reliability for the time constant (ICC 0.509–0.652).
Table 3.
Reliability statistics of SHAT parameters for the total sample (n = 30).
| Test | Re-Test | p0 | ICC | |
|---|---|---|---|---|
| Gain | ||||
| 0.02 | 39.40 ± 11.83 | 42.50 ± 12.19 | tp = 0.056 | 0.749∗ |
| 0.04 | 46.27 ± 12.36 | 46.20 ± 13.24 | tp = 0.963 | 0.818∗ |
| 0.08 | 49.97 ± 12.91 | 47.68 ± 12.76 | tp = 0.139 | 0.795∗ |
| 0.16 | 50.50 ± 12.27 | 48.50 ± 10.98 | tp = 0.260 | 0.665∗ |
| 0.32 | 55.65 ± 11.79 | 56.45 ± 10.77 | tp = 0.741 | 0.326∗ |
| 0.64 | 74.60 ± 13.91 | 74.78 ± 11.61 | tp = 0.952 | 0.182 |
| Phase | ||||
| 0.02 | 203.16 ± 5.97 | 202.80 ± 6.99 | tp = 0.672 | 0.758∗ |
| 0.04 | 189.25 ± 5.10 | 189.32 ± 6.12 | tp = 0.928 | 0.775∗ |
| 0.08 | 177.44 ± 4.54 | 176.45 ± 5.48 | tp = 0.170 | 0.710∗ |
| 0.16 | 164.47 ± 4.36 | 163.06 ± 5.52 | tp = 0.054 | 0.699∗ |
| 0.32 | 141.75 ± 5.18 | 140.35 ± 9.63 | tp = 0.435 | 0.224 |
| 0.64 | 81.31 ± 10.48 | 84.99 ± 7.97 | tp = 0.065 | 0.363∗ |
| Symmetry | ||||
| 0.02 | 7.87 ± 6.87 | 6.97 ± 5.99 | Zp = 0.425 | 0.762∗ |
| 0.04 | 6.80 ± 5.33 | 7.23 ± 6.10 | Zp = 0.650 | 0.602∗ |
| 0.08 | 6.33 ± 6.06 | 6.83 ± 6.28 | Zp = 0.650 | 0.599∗ |
| 0.16 | 6.50 ± 5.96 | 8.10 ± 6.80 | Zp = 0.165 | 0.580∗ |
| 0.32 | 7.03 ± 4.77 | 6.50 ± 5.04 | Zp = 0.476 | 0.181 |
| 0.64 | 9.10 ± 7.08 | 7.17 ± 5.18 | Zp = 0.289 | 0.294 |
Table 4.
Reliability statistics of VST time constant for the total sample (n = 30).
| Test | Re-Test | tp0 | ICC | |
|---|---|---|---|---|
| VST | ||||
| Per CW TC | 14.19 ± 3.13 | 15.18 ± 3.40 | 0.105 | 0.509∗ |
| Post CW TC | 17.31 ± 4.38 | 16.13 ± 3.73 | 0.067 | 0.652∗ |
| Per CCW TC | 12.20 ± 2.66 | 12.80 ± 3.22 | 0.225 | 0.592∗ |
| Post CCW TC | 16.18 ± 3.83 | 16.61 ± 3.83 | 0.495 | 0.603∗ |
Data were expressed using Mean ± SD. t: Paired t-test Z: Wilcoxon signed ranks test.
p0: p value for comparing between Test and Re-Test ∗: Statistically significant at p ≤ 0.05.
ICC: Intraclass correlation coefficient.
ICC; Below 0.5: Poor, 0.5 - <0.75: Moderate, 0.75–0.9: Good, Above 0.9: Excellent.
4. Discussion
4.1. SHAT and VST
The SHAT and VST were studied on 100 subjects with normal vestibular function. A comparison of the present normative data of SHAT and VST was done with other studies in the literature. For SHAT (Table 5, Table 6 and Table 7) comparisons were made between the most resembling frequencies, i.e. 0.04 Hz with 0.05 Hz, 0.08 Hz with 0.1 Hz, 0.16 Hz with 0.2 Hz, 0.32 Hz with 0.5 Hz and 0.64 Hz with 1 Hz. All studies used stimulus velocities ranging from 50 to 60°/s.
Table 5.
Total sample data of the present study and literature data (mean ± SD) for SHAT Gain (%) as a function of frequency (Hz).
| Parameter | Author | N | 0.02 | 0.04/0.05 | 0.08/0.1 | 0.16/0.2 | 0.32/0.5 | 0.64/1 |
|---|---|---|---|---|---|---|---|---|
| Gain | Present study | 100 | 37.74 ± 10.13 | 44.65 ± 10.44 | 46.39 ± 11.65 | 47.34 ± 10.86 | 51.62 ± 12.09 | 71.15 ± 13.49 |
| Durney (2016) | 31 | 43 ± 9 | 51 ± 13 | 50 ± 14 | 53 ± 14 | 57 ± 12 | ||
| Su et al. (2000) | 56 | 47 ± 16 | 50 ± 16 | 50 ± 16 | 53 ± 17 | |||
| Ahmed (2014) | 100 | 57 ± 7.9 | 60 ± 8 | 66 ± 7 | 63 ± 8.3 | 64 ± 8.2 | 66 ± 6.5 | |
| Maes et al. (2008) | 150 | 52.65 ± 13.36 | 59.73 ± 14.22 | 58.77 ± 13.98 | 51.24 ± 12.08 | |||
| Henry and Dibartolomeo (1993) | 20 | 55 ± 14 | 58 ± 11 | 65 ± 11 | 64 ± 14 | 69 ± 16 | ||
| Li et al. (1991) | 41 | 66 ± 14 | 72 ± 13 | 77 ± 16 | 71 ± 17 | |||
| Möller et al. (1990) | 15 | 65 ± 14 | 69 ± 11 | 73 ± 14 | 77 ± 21 | 69 ± 16 | ||
| Wall et al. (1984) | 50 | 50.36 ± 15.27 | 60.80 ± 20.04 | 62.92 ± 20.72 | 62.21 ± 21.95 | 60.26 ± 19.34 | 69.61 ± 24.60 |
Table 6.
Total sample data of the present study and literature data (mean ± SD) for SHAT Phase (◦) as a function of frequency (Hz).
| Parameter | Author | N | 0.02 | 0.04/0.05 | 0.08/0.1 | 0.16/0.2 | 0.32/0.5 | 0.64/1 |
|---|---|---|---|---|---|---|---|---|
| Phase | aPresent study | 100 | 21.8 ± 6.82 | 6.9 ± 5.29 | −5 ± 5.07 | −16.7 ±4.83 | −40.4 ± 6.15 | −97.76 ± 12.65 |
| Durney (2016) | 31 | 23.67±5.14 | 11.52±4.05 | 2.42 ± 3.66 | 1.57 ± 2.76 | 1.53 ± 3.12 | ||
| Ahmed (2014) | 100 | −24.68±5.65 | −12.37±2.88 | −4.78±1.84 | −5.77±2.33 | 0.91±3.46 | 5.99±3.84 | |
| Maes et al. (2008) | 150 | 25.8 ± 6.87 | 13 ±4.16 | 5.40 ±3.81 | −0.40 ±4.92 | |||
| Henry and Dibartolomeo (1993) | 20 | 25.8 ±5.2 | 15.10 ± 4.5 | 7.30 ±4.93 | 2.90 ±3.13 | 0.9 ±3.51 | ||
| Li et al. (1991) | 41 | 24.8 ± 5.3 | 15.1 ± 4.4 | 8.5 ± 3.8 | 2.4 ± 4.8 | |||
| Möller et al. (1990) | 15 | 24 ± 3 | 16 ± 3 | 7 ± 3 | 2 ± 3 | 4 ± 2 | ||
| Wall et al. (1984) | 50 | 26.5 ± 5.91 | 11.08 ± 6.5 | 3.23 ±4.53 | −4.84±5.56 | −12.10 ± 7.94 | −14.38 ± 11.71 |
180° was subtracted from phase values.
Table 7.
Total sample data of the present study and literature data (mean ± SD) for SHAT Symmetry (%) as a function of frequency (Hz).
| Parameter | Author | N | 0.02 | 0.04/0.05 | 0.08/0.1 | 0.16/0.2 | 0.32/0.5 | 0.64/1 |
|---|---|---|---|---|---|---|---|---|
| Symmetry | Present study | 100 | 7.71 ± 5.85 | 7.36 ± 5.67 | 6.56 ± 5.77 | 7.69 ± 5.67 | 7.17 ± 4.89 | 7.02 ± 6.08 |
| Durney (2016) | 31 | 3.3±9.27 |
3.98 ± 8.46 | −0.36 ± 11.71 | −1.41 ± 9.02 | 0.31 ± 7.81 | ||
| Ahmed (2014) | 100 | 4.99 ± 2.89 | 3.99 ± 3.08 | 5.02 ± 4.59 | 4.88 ± 3.95 | 5.55 ± 3.88 | 3.28 ± 2.87 | |
| Maes et al. (2008) | 150 | −4.02 ± 15.02 | −3.32 ± 14.38 | −2.69 ± 14.38 | −3.17 ± 14.83 | |||
| Henry and Dibartolomeo (1993) | 20 | −7.2 ± 10.7 | −3.5 ± 14 | −1.3 ± 7.9 | −1.1 ± 7.89 | −2.2 ± 6.20 | ||
| Li et al. (1991) | 41 | −0.2 ± 6.5 | −0.8 ± 6.2 | 0.5 ± 5.4 | 0.7 ± 6.1 |
In this study gain values for the SHAT increased with the increase in the test frequency, but were somewhat lower than most other studies (Wall et al., 1984; Möller et al., 1990; Li et al., 1991; Henry and Dibartolomeo, 1993; Su et al., 2000; Maes et al., 2008; Ahmed, 2014; Durney, 2016). The differences in gain values, despite the use of similar stimulus parameters, could be explained by the dependency of the gain on many subjective factors (Goulson et al., 2016; Zalewski et al., 2019).
In the current study, 180° was subtracted from the recorded phase values according to the manufacturer (Difra instrumentation) to be comparable to values of other rotatory chair equipments. It was observed that phase decreased as test frequency increased in agreement with previous studies, but with lower values especially for the higher frequencies which may be secondary to limitations of the device having no head restraint (Wall et al., 1984; Möller et al., 1990; Li et al., 1991; Henry and Dibartolomeo, 1993; Maes et al., 2008; Ahmed, 2014; Durney, 2016). In general, symmetry values of other studies varied from 0.2 to 7.2% (Li et al., 1991; Henry and Dibartolomeo, 1993; Maes et al., 2008; Ahmed, 2014; Durney, 2016), and in the current study ranged from 6.56 to 7.71%.
For the VST (Table 8), all studies used stimulus velocities ranging from 60 to 120°/s. Time constant values were comparable to literature data (Dizio and Lackner, 1990; Baloh et al., 1993; Su et al., 2000; Maes et al., 2008; Ahmed, 2014).
Table 8.
Total sample data of the present study and literature data (mean +SD) for VST Time constant (seconds).
| Author | N | Time constant |
|---|---|---|
| Present study | 100 | 16.21 ± 4.09 |
| Su et al. (2000) | 56 | 13.48 ± 3.61 |
| Ahmed (2014) | 100 | 16.39 ± 2.66 |
| Maes et al. (2008) | 150 | 13.38 ± 4.06 |
| Baloh et al. (1993) | 25 | 15.1 ± 6.7 (for the young group in this study aged 18–39 years) |
| Dizio and Lackner (1990) | 10 | 15.5 ± 3.18 (for younger individuals in this study aged 18–22 years) |
In the current study, subjects were divided into two groups (group A below 50 years and group B 50 years of age or above) to study the effect of age on SHAT and VST parameters. There were no statistically significant differences in SHAT gain, phase, and symmetry. However, the post-rotatory time constant for both CW and CCW directions was significantly longer for the older group.
All the body sensory systems, including the vestibular system, are affected by aging. Degeneration has been described in nearly all sensory components of the vestibular system, including vestibular end organs, hair cells, vestibular ganglion, nerve fibers, neurons of the vestibular nucleus, and even Purkinje cells of the cerebellum showed a significant decrease in number (Baloh et al., 1993; Rauch et al., 2001; Jang et al., 2006; Maes et al., 2010; Zalewski, 2015).
Generally, the onset of the marked decline of hair cells was described to be between 65 and 70 years of age by the majority of histologic reports evaluating degeneration of vestibular hair cells (Zalewski, 2015). It was reported that 65% of individuals above 60 years old complain of imbalance or dizziness (Hobeika, 1999). However, the age of onset and rate of degeneration can differ broadly regarding the specific sensory cells, neurons or organs (Zalewski, 2015).
Although significant degeneration associated with age has been detected in almost every vestibular cell and neuron (Maes et al., 2010), various studies have described the effect of age on vestibular tests often with contradictory results (Maes et al., 2010). It was hypothesized that as a result of central vestibular compensation those degenerative changes were not always reflected on tests of vestibular function especially when using non physiologic or modest stimuli that lack the intensity to overcome compensation, such as caloric tests or low-frequency rotational tests (Paige, 1991).
Generally, it is expected for SHAT gain values to decrease as a result of general vestibular deterioration resulting from age-related anatomical degeneration, while the phase increases due to reduction in the velocity storage function with age. The velocity storage system causes an increase in gain and decrease in phase thereby enhancing VOR performance at low frequency movements, age-related decline in this mechanism can lead to low gain and high phase, mainly at the low frequencies (Paige, 1991). Additionally, the VST time constant is expected to decrease as a result of deterioration in the velocity storage system with age.
For the SHAT, increasing age has been associated with a decrease in gain values, sometimes more marked in low frequencies (Peterka et al., 1990; Enrietto et al., 1999), in high frequencies (Li et al., 1991), or with using high velocities (Baloh et al., 1993). However, elderly patients wouldn't tolerate the use of stronger stimuli during routine clinical evaluation (Zalewski, 2015). Additionally, the increase in phase values was the most reported finding related to aging (Baloh et al., 1993), at low frequencies (Paige, 1991; Enrietto et al., 1999) or may occur at higher frequencies (Peterka et al., 1990).
On the other hand, other studies couldn't find age-dependent changes in gain (Maes et al., 2010; Chang et al., 2014) and phase (Wall et al., 1984; Maes et al., 2010; Chang et al., 2014). Moreover, literature has reported no significant symmetry differences related to age (Peterka et al., 1990; Li et al., 1991; Maes et al., 2010) which indicated the fact that aging affects the vestibular system bilaterally and symmetrically (Maes et al., 2010).
For VST and following the increase in phase values, Baloh et al. have described an age-related decrease in time constant values (Baloh et al., 1993). However, not all studies confirmed this finding. Maes et al. showed no significant age correlations for the VST response parameters (Maes et al., 2010). Additionally, Dizio and Lackner demonstrated a shorter time constant in the young subjects group and explained that the time constant of the cupula-endolymph complex may have become longer with age due to change in the effective elasticity of the cupula because of cupulolithiasis precipitated by degeneration of otoconia (Dizio and Lackner, 1990).
Absent age-related differences in the present study for SHAT parameters could be explained by the relatively young age of participants in group B with the mean age of 55.36 ± 4.61 years and most age-related vestibular changes start between 65 and 70 years of age (Zalewski, 2015). The significantly longer post rotatory time constant for the older group could be attributed mostly to limitations of the device lacking head restraint resulting in inaccurate measurements with the abrupt stop of the chair in VST, or to the age-related longer time constant of the cupula as previously explained by Dizio and Lackner (1990).
4.2. Test-retest reliability of rotatory chair tests
The results of test-retest reliability of the SHAT and VST showed no significant differences in the mean of all response parameters between test and retest conditions for SHAT at all frequencies and for VST.
Generally, the ICC values for SHAT and VST showed a moderate to high correlation between test and retest values, except for frequencies 0.32 and 0.64 Hz. Maes et al. studied test-retest reliability of SHAT (for frequencies 0.01, 0.02, 0.05, 0.1, 0.2 Hz) and VST, and obtained ICC values comparable to those obtained by the current study (Maes et al., 2008). Unfortunately, gain in SHAT is regarded to be the most inconsistent parameter within and between subjects, unlike SHAT phase and VST time constant values which were believed to be highly reliable parameters (Jenkins and Goldberg, 1988; Li et al., 1991). However, the current study and Maes et al. (2008) demonstrated that despite the subjective character of gain, it was highly reliable, especially for the lower frequencies (0.02–0.16 Hz). In the present study, poor reliability of the highest frequencies 0.32 and 0.64 Hz could be attributed to the lack of head restraint leading to inaccurate interpretation of response parameters at high frequencies.
Additionally, it was observed in the current study that ICC values for SHAT parameters at the lower frequencies (0.02–0.16 Hz) were higher than those of the VST time constant which indicated higher reliability of SHAT compared to VST, this was in agreement with Maes et al. (2008). The higher reliability of SHAT has resulted from averaging the slow component eye velocity through a number of cycles, unlike VST where the response was obtained from a single rotation in each direction without averaging scheme (Goulson et al., 2016).
There are some limitations to this study. First, the relatively young age of participants in group B, resulted from the common complaints of dizziness and vertigo among the elderly population which was against the inclusion criteria of the study. Therefore, further studies with a larger sample of elderly patients are needed to investigate the effect of age on rotatory chair tests. Secondly, a technical limitation due to the lack of head restraint which may have affected the study results.
5. Conclusions
Regarding SHAT parameters, age had no effect on VOR gain, phase, and symmetry. As for VST, the per-rotatory time constant was not affected by age, however, the post-rotatory time constant was significantly longer in the older group.
Generally, test-retest reliability for both tests was good. SHAT parameters for the lower frequencies (0.02–0.16 Hz) showed better test-retest reliability than the higher frequencies and VST time constant showed moderate reliability.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Ahmed M.F. Standardization of rotatory chair velocity step and sinusoidal harmonic acceleration tests in an adult population. Adv. Arab. Acad. Audio-Vestibul. J. 2014;1(2):80–86. doi: 10.4103/2314-8667.149016. [DOI] [Google Scholar]
- Baloh R., Jacobson K., Socotch T. The effect of aging on visual-vestibuloocular responses. Exp. Brain Res. 1993;95(3):509–516. doi: 10.1007/BF00227144. [DOI] [PubMed] [Google Scholar]
- Barin K. Rotational tests of vestibular function. Semin. Hear. 2009;30(4):253–266. doi: 10.1055/s-0029-1241126. [DOI] [Google Scholar]
- British Society of Audiology . 2010. Recommended Procedure: the Caloric Test.https://www.thebsa.org.uk/wp-content/uploads/2014/04/Recommended-procedure-for-the-Caloric-test.pdf [Google Scholar]
- Byerly A.M. The Ohio State University; 2014. A Comprehensive Review of the Vestibular System. Doctor.http://hdl.handle.net/1811/61612 [Google Scholar]
- Chan F.M., Galatioto J., Amato M., Kim A.H. Normative data for rotational chair stratified by age. Laryngoscope. 2016;126(2):460–463. doi: 10.1002/lary.25497. [DOI] [PubMed] [Google Scholar]
- Chang N.N., Hiss M.M., Sanders M.C., Olomu O.U., MacNeilage P.R., Uchanski R.M., Hullar T.E. Vestibular perception and the vestibulo-ocular reflex in young and older adults. Ear Hear. 2014;35(5):565–570. doi: 10.1097/AUD.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizio P., Lackner J.R. Age differences in oculomotor responses to step changes in body velocity and visual surround velocity. J. Gerontol. 1990;45(3):M89–M94. doi: 10.1093/geronj/45.3.m89. [DOI] [PubMed] [Google Scholar]
- Durney T. Towson University; Doctor: 2016. Normative Data for the Sinusoidal Harmonic Acceleration and Visual Suppression Subtests of Rotational Testing for the Towson University Hearing and Balance Center.https://mdsoar.org/bitstream/handle/11603/2033/TSP2015Durney_redacted.pdf?sequence=1&isAllowed=y [Google Scholar]
- Enrietto J.A., Jacobson K.M., Baloh R.W. Aging effects on auditory and vestibular responses: a longitudinal study. Am. J. Otolaryngol. 1999;20(6):371–378. doi: 10.1016/s0196-0709(99)90076-5. [DOI] [PubMed] [Google Scholar]
- González J.E., Kiderman A. In: Encyclopedia of Otolaryngology: Head and Neck Surgery. Kountakis S.E., editor. Springer-Verlag; Berlin Heidelberg: 2013. Rotary chair; pp. 2321–2330. [Google Scholar]
- Goulson A.M., McPherson J.H., Shepard N.T. In: Balance Function Assessment and Management. second ed. Jacobson G.P., Shepard N.T., editors. Plural Publishing; San Diego: 2016. Background and introduction to whole-body rotational testing; pp. 347–364. [Google Scholar]
- Henry D.F., Dibartolomeo J.D. Closed-loop caloric, harmonic acceleration and active head rotation tests: norms and reliability. Otolaryngol. Head Neck Surg. 1993;109(6):975–987. doi: 10.1177/019459989310900602. [DOI] [PubMed] [Google Scholar]
- Hobeika C.P. Equilibrium and balance in the elderly. Ear Nose Throat J. 1999;78(8):558–566. doi: 10.1177/014556139907800810. [DOI] [PubMed] [Google Scholar]
- Jang Y.S., Hwang C.H., Shin J.Y., Bae W.Y., Kim L.S. Age-related changes on the morphology of the otoconia. Laryngoscope. 2006;116(6):996–1001. doi: 10.1097/01.mlg.0000217238.84401.03. [DOI] [PubMed] [Google Scholar]
- Jenkins H.A., Goldberg J. Test-retest reliability of the rotatory test in normal subjects. Adv. Oto-Rhino-Laryngol. 1988;41:190–195. doi: 10.1159/000416055. [DOI] [PubMed] [Google Scholar]
- Kim B.J., Won Y.K., Hyun J., Na W.S., Jung J.Y., Suh M.W. Comparisons in outcome and subject comfort between rotation chair systems. J. Audiol. Otol. 2017;21(2):88–94. doi: 10.7874/jao.2017.21.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.W., Hooper R.E., Cousins V.C. Sinusoidal harmonic acceleration testing in normal humans. Laryngoscope. 1991;101(2):192–196. doi: 10.1288/00005537-199102000-00016. [DOI] [PubMed] [Google Scholar]
- Maes L., Dhooge I., D'haenens W., Bockstael A., Keppler H., Philips B., Swinnen F., Vinck B.M. The effect of age on the sinusoidal harmonic acceleration test, pseudorandom rotation test, velocity step test, caloric test, and vestibular-evoked myogenic potential test. Ear Hear. 2010;31(1):84–94. doi: 10.1097/AUD.0b013e3181b9640e. [DOI] [PubMed] [Google Scholar]
- Maes L., Dhooge I., De Vel E., D'haenens W., Bockstael A., Keppler H., Philips B., Swinnen F., Vinck B.M. Normative data and test-retest reliability of the sinusoidal harmonic acceleration test, pseudorandom rotation test and velocity step test. J. Vestib. Res. 2008;18(4):197–208. doi: 10.3233/VES-2008-18403. [DOI] [PubMed] [Google Scholar]
- Maes L., Vinck B., De Vel E., D'haenens W., Bockstael A., Dhooge I. Evaluation of the rotatory vestibular test: exploration of stimulus parameters. B-ENT. 2007;3(3):119–126. [PubMed] [Google Scholar]
- Maes L., Vinck B.M., Wuyts F., D'Haenens W., Bockstael A., Keppler H., Philips B., Swinnen F., Dhooge I. Clinical usefulness of the rotatory, caloric, and vestibular evoked myogenic potential test in unilateral peripheral vestibular pathologies. Int. J. Audiol. 2011;50(8):566–576. doi: 10.3109/14992027.2011.576706. [DOI] [PubMed] [Google Scholar]
- Möller C., Ödkvist L., White V., Cyr D. The plasticity of compensatory eye movements in rotatory tests: 1. The effect of alertness and eye closure. Acta Otolaryngol. 1990;109(1–2):15–24. doi: 10.3109/00016489009107410. [DOI] [PubMed] [Google Scholar]
- Paige G.D. The aging vestibulo-ocular reflex (VOR) and adaptive plasticity. Acta Otolaryngol. 1991;111(Suppl. 481):297–300. doi: 10.3109/00016489109131406. [DOI] [PubMed] [Google Scholar]
- Peterka R., Black F., Schoenhoff M. Age-related changes in human vestibulo-ocular reflexes: sinusoidal rotation and caloric tests. J. Vestib. Res. 1990;1(1):49–59. doi: 10.3233/ves-1990-1106. [DOI] [PubMed] [Google Scholar]
- Rauch S.D., Velazquez-VillaseÑor L., Dimitri P.S., Merchant S.N. Decreasing hair cell counts in aging humans. Ann. N. Y. Acad. Sci. 2001;942(1):220–227. doi: 10.1111/j.1749-6632.2001.tb03748.x. [DOI] [PubMed] [Google Scholar]
- Su Y.Y., Chiou W.Y., Weng P.K., Wang H.W. Computerized rotational vestibular testing in normal subjects. Zhonghua yi xue za zhi= Chin. Med. J.; Free China ed. 2000;63(5):377–383. [PubMed] [Google Scholar]
- Valente L.M. Assessment techniques for vestibular evaluation in pediatric patients. Otolaryngol. Clin. 2011;44(2):273–290. doi: 10.1016/j.otc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Wall C., Hunt A., Black F. Effects of age, sex and stimulus parameters upon vestibulo-ocular responses to sinusoidal rotation. Acta Otolaryngol. 1984;98(3–4):270–278. doi: 10.3109/00016488409107563. [DOI] [PubMed] [Google Scholar]
- Zalewski C.K. Aging of the human vestibular system. Semin. Hear. 2015;36(3):175–196. doi: 10.1055/s-0035-1555120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski C.K., McCaslin D.L., Carlson M.L. In: Diagnosis and Treatment of Vestibular Disorders. Babu S., Schutt C.A., Bojrab D.I., editors. Springer; Swizerland: 2019. Rotary chair testing; pp. 75–98. [Google Scholar]
- Zapala D.A., Brey R.H. In: Clinical Neurophysiology. Daube J.R., Rubin D.I., editors. Oxford University Press; New York: 2009. Vertigo and balance; pp. 594–598. [Google Scholar]