Abstract
Background
In the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease trial, finerenone reduced the risk of cardiovascular events in patients with chronic kidney disease (CKD) and type 2 diabetes, while in the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease trial, it improved renal and cardiovascular outcomes in patients with advanced CKD. However, no previous studies have assessed patients with CKD and type 2 diabetes with an estimated glomerular filtration rate (eGFR) below 25 mL/min/1.73 m2
Methods
Nine patients with CKD and type 2 diabetes who received finerenone 10 mg/day were analyzed retrospectively. Changes in eGFR, urinary protein, and serum potassium levels were studied from 1 year before administration of finerenone until 6 months after administration.
Results
The mean baseline eGFR slope was −7.63 ± 9.84 (mL/min/1.73 m2/year). After finerenone treatment, the mean eGFR slope significantly improved −1.44 ± 3.17 (mL/min/1.73 m2/6 months, P=0.038). However, finerenone treatment did not significantly reduce proteinuria. Furthermore, finerenone did not increase serum potassium levels.
Conclusions
Patients treated with finerenone showed a significantly slower decline in eGFR. Furthermore, aside from the present study, no reports have indicated the effectiveness of finerenone in patients with advanced CKD with an eGFR below 25 mL/min/1.73 m2. As confirmed in our clinical trials, the finding that finerenone is effective in a wide range of renal functions can be generalized to clinical practice. However, sample size in this study was small. Thus, further large-scale investigations will be needed.
Keywords: Finerenone, Type 2 diabetes, Chronic kidney disease, Diabetic kidney disease, Estimate glomerular filtration rate slope, Proteinuria
1. Introduction
Diabetic kidney disease (DKD) is a major microvascular complication of diabetes that increases the global disease burden and leads to chronic kidney disease (CKD) and end-stage renal disease (ESRD) [1,2].
Good metabolic control is necessary to prevent the onset and progression of DKD. Treatment of DKD focuses on decreasing albuminuria and improving renal function. In addition to strict glycemic control, renin-angiotensin system (RAS) inhibitors, sodium-glucose cotransporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 receptor agonists, and dipeptidyl peptidase-4 (DPP-4) inhibitors are used in patients with DKD [[3], [4], [5]]. Incretin-related drugs elicit vasotropic effects and decrease diabetes-induced inflammation and oxidative stress, ameliorating DKD [6,7]. Recent large-scale clinical trials have indicated that SGLT2 inhibitors delayed DKD progression [8]. Despite notable advances, there has been limited headway in preventing and treating DKD, and residual risk remains. Mineralocorticoid receptor antagonists (MRAs), alone or in combination with angiotensin-converting enzyme inhibitors (ACEIs), reduce albuminuria and delay renal function decline in patients with DKD. Despite their renoprotective effects, MRAs have not garnered significant attention as a viable treatment option for DKD owing to the potential risks of hyperkalemia and acute renal failure. Finerenone, a potent selective non-steroidal MRA, has demonstrated efficacy in decreasing albuminuria and preventing the progression from acute kidney injury to CKD [9,10]. Furthermore, finerenone has been shown to reduce albuminuria and delay the decline in estimated glomerular filtration rate (eGFR); the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) trial demonstrated that finerenone decreased the incidence of cardiovascular events in patients with DKD, while in the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) trial, it improved renal and cardiovascular outcomes in patients with advanced CKD [11,12]. Thus, we propose that the main therapeutic agents for DKD are RAS inhibitors, SGLT2 inhibitors, incretin-based therapies, and non-steroidal MRAs, in other words, the DKD “fantastic four” [13,14].
However, the efficacy and safety of finerenone in CKD and type 2 diabetes with an eGFR below 25 mL/min/1.73 m2 remain unclear. The aim of this study was to determine whether the renoprotective effect of finerenone was also observed in advanced DKD with eGFR below 25 mL/min/1.73 m2. To the best of our knowledge, this study represents the first investigation into the efficacy and safety of finerenone, specifically in patients with DKD and eGFR below 25 mL/min/1.73 m2.
2. Patients and methods
2.1. Study design and collection of medical data
This study retrospectively reviewed and analyzed the medical records of nine patients. The patients underwent outpatient care at the Department of Nephrology, Osaka Medical and Pharmaceutical University (Osaka, Japan). Eligible participants were patients with CKD aged 20 years or older who were clinically diagnosed with type 2 diabetes and had an eGFR <25 mL/min/1.73 m2. Potential participants were required to have serum potassium levels <4.9 mEq/L at the screening visit, preferably without adjustments for dose, drug selection, or other antihypertensive or antidiabetic treatments. All procedures involving human participants performed in this study were in accordance with the ethical standards of the national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The requirement for informed consent was waived, and the institutional review board rules did not perform an ethical review because the number of patients in this study was nine. Data were collected and analyzed retrospectively using electronic medical records maintained by Osaka Medical and Pharmaceutical University. Blood and urine biochemical analyses were performed using a Labospect 008 autoanalyzer (Hitachi, Tokyo, Japan). Data on serum creatinine, serum potassium, total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, fasting blood glucose, urine protein-to-creatinine ratio (UPCR), and patient characteristics (e.g., medication, age, sex, blood pressure, and body mass index [BMI]) were obtained from electronic medical records. UPCR was calculated by dividing the level of protein (mg/dL) in a spot urine test by the creatine level (mg/dL). The eGFR of each patient was calculated using the formula by isotope dilution mass spectrometry (IDMS) traceable 4-variable MDRD (Modification of Diet in Renal Disease) (IDMS-MDRD Study formula) with the 3-variable Japanese equation: 194 × serum creatinine - 1.094 × age - 0.287 × 0.739 (if female) [[15], [16], [17], [18]].
2.2. Data analysis
The eGFR declining slope, changes in proteinuria, and changes in serum potassium were compared between the values 1 year before and at the time of finerenone treatment and the values 6 months after finerenone treatment. The statistical significance of the differences was determined using Wilcoxon test. All analyses were performed using StatView (SAS Institute) and Excel. Statistical significance was set at P<0.05.
3. Results
Table 1 shows the baseline characteristics of the nine patients (three males and six females). The average age at onset was 71.3 ± 11.9 years. The median duration of diabetes was 12 years. The average baseline systolic blood pressure and BMI were 130 ± 17.7 mmHg and 26.0 ± 4.8 kg/m2, respectively. Baseline glycosylated hemoglobin was 6.9 ± 0.8%, serum potassium was 4.3 ± 0.4 mEq/L, total cholesterol was 194 ± 33 mg/dL, median triglyceride was 169 mg/dL (range, 96–345 mg/dL), and HDL cholesterol was 44 mg/dL (range, 73–75 mg/dL). The average baseline of renal function was as follows; eGFR (18.6 ± 3.2 mL/min/1.73 m2) and UPCR (4.6 ± 3.5).
Table 1.
Clinical baseline characteristics of the patients.
| Variables | |
|---|---|
| Number of patients, n | 9 |
| Men/women, n | 3/6 |
| Age, years | 71.3 (11.9) |
| BMI, kg/m2 | 26.0 (4.8) |
| Duration of diabetes, years | 12 [8–41] |
| HbA1c, % | 6.9 (0.8) |
| Serum potassium, mEq/L | 4.3 (0.4) |
| eGFR, mL/min/1.73m2 | 18.6 (3.2) |
| UPCR, g/gCr | 4.6 (3.5) |
| Systolic BP, mmHg | 130 (17.7) |
| TC, mg/dL | 194 (33.0) |
| TG, mg/dL | 165 [96–345] |
| HDL-C, mg/dL | 44 [73–75] |
| Medications | |
| Glinide, n (%) | 1 (11.1) |
| DPP4 inhibitor, n (%) | 3 (27.3) |
| SGLT2 inhibitor, n (%) | 6 (66.7) |
| Statin, n (%) | 1 (55.6) |
| EPA, n (%) | 1 (11.1) |
| ARB, n (%) | 1 (11.1) |
| ARNI, n (%) | 1 (11.1) |
| Ca-blocker, n (%) | 4 (44.4) |
| α-blocker, n (%) | 1 (11.1) |
| αβ-blocker, n (%) | 3 (27.3) |
| Thiazide, n (%) | 3 (27.3) |
| Loop diuretics, n (%) | 2 (22.2) |
BMI, body mass index; HbA1c, glycated hemoglobin; eGFR, estimated glomerular filtration rate; UPCR, urine protein-creatinine ratio; BP, blood pressure; TC, total cholesterol; TG, triglyceride, HDL-C, high-density lipoprotein cholesterol; DPP4, dipeptidyl peptidase 4; SGLT2, sodium-glucose cotransporter 2; EPA, eicosapentaenoic acid; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; Ca, calcium.
Age, BMI, HbA1c, serum potassium, eGFR, UPCR, systolic BP, TC were expressed as mean±standard deviation. Duration of diabetes, TG, HDL-C were expressed as median.
All the patients were treated with finerenone (10 mg/day). Concomitant medications were as follows: glinide (11.1%), DPP-4 inhibitors (27.3%), SGLT2 inhibitors (66.7%), statins (55.6%), eicosapentaenoic acid (11.1%), angiotensin receptor blockers (ARBs) (11.1%), angiotensin receptor neprilysin inhibitors (11.1%), calcium channel blockers (44.4%), α-blockers (11.1%), αβ-blockers (27.3%), thiazide (27.3%), and loop diuretics (22.2%).
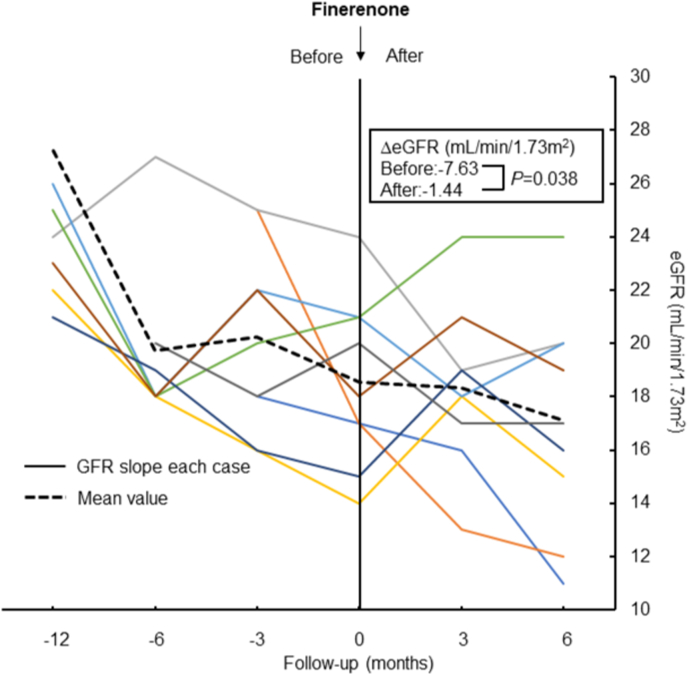
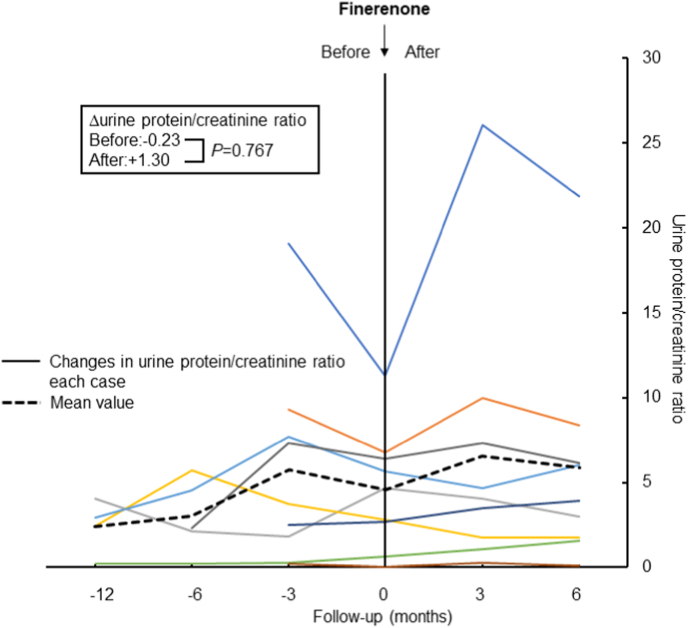
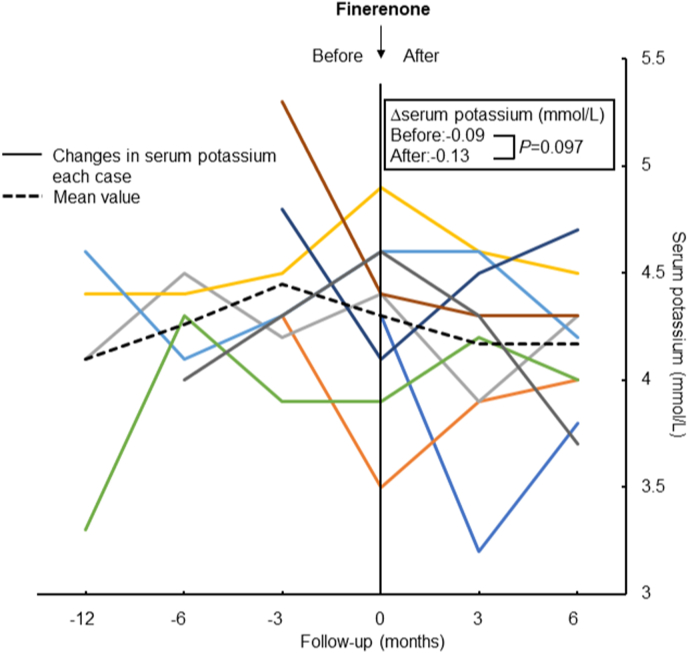
Compared with pretreatment value, eGFR declining slope was significantly decreased after the administration of finerenone (−7.63 vs. −1.44 mL/min/1.73 m2; P=0.038, Fig. 1). However, there was no reduction in proteinuria with finerenone treatment (−0.23 vs. 1.30 UPCR; P=0.767, Fig. 2). Systolic blood pressure did not change with finerenone administration (130 ± 17.7 vs. 142 ± 24.8 mmHg; P=0.255). Lastly, no difference was observed in serum potassium before and after finerenone administration from baseline (−0.09 vs. −0.13 mEq/L; P=0.097, Fig. 3).
Fig. 1.
Slopes of eGFR before and after administration of finerenone. The same color in the left and right panels shows the same patients and the black dashed line shows the mean value for all patients. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Change in urine protein/creatinine ratio before and after administration of finerenone. The same color in the left and right panels shows the same patients, and the black dashed line shows the mean value for all patients. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Change in serum potassium before and after administration of finerenone. The same color in the left and right panels shows the same patients, and the black dashed line shows the mean value for all patients. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
This study shows for the first time the renal effects of finerenone in patients with CKD and type 2 diabetes with an eGFR below 25 mL/min/1.73 m2; our results showed that administration of finerenone significantly decreased the declining slope in patients with advanced DKD. The objective of the FIDELIO-DKD trial was to evaluate whether finerenone could decelerate the progression of CKD or lower the incidence of cardiovascular complications and mortality among patients with type 2 diabetes receiving the highest recommended dosage of ACEIs or ARBs. The main objectives of the study were to assess renal failure, which was defined as ESRD or eGFR below 15 mL/min/1.73 m2, a decrease in eGFR greater than 40% from the baseline, and death resulting from renal causes. The primary outcome was a statistically significant decrease in the finerenone group [11]. In the FIGARO-DKD trial, the effectiveness and safety of finerenone in improving cardiovascular and renal outcomes were examined in patients with CKD and type 2 diabetes. Participants in this study were divided into two groups: those with a urinary albumin-to-creatinine ratio greater than 30–300 mg/g creatinine and an eGFR greater than 60 mL/min/1.73 m2. This indicates that the FIGARO-DKD trial included a larger number of patients with early-stage DKD compared to the FIDELIO-DKD trial, which was 9.5% in the finerenone group and 10.8% in the placebo group (hazard ratio [HR]: 0.87; 95% confidence interval [CI]: 0.76–1.01) [12]. These findings demonstrate that finerenone exerts protective effects on the kidneys of patients with DKD. However, both trials included patients with DKD who had relatively preserved renal function. Thus, it is highly significant that our study is the first to demonstrate that finerenone has renoprotective effects in patients with DKD and progressive renal impairment.
The Finerenone in chronic kidney disease and type 2 diabetes (FIDELITY) conducted a pooled analysis of the FIDELIO-DKD and FIGARO-DKD trials and combined them to assess the impact of finerenone on the kidneys and heart. The analysis included 13,026 participants with a median duration of 3 years. In this study, finerenone exhibited a significant risk reduction compared to the placebo for all composite cardiovascular and renal outcomes. The composite cardiovascular event showed a 14% risk reduction (HR: 0.86; 95% CI: 0.78–0.95), while the composite renal outcome showed a 23% risk reduction (HR: 0.77; 95% CI: 0.67–0.88). Notably, ESRD risk requiring dialysis decreased by 20% (HR: 0.80; 95% CI: 0.64–0.99) [19]. The changes in blood pressure observed among the study participants were modest, suggesting that the cardiovascular and renal protective effects of finerenone may not be attributable to changes in blood pressure.
In DKD, hyperglycemia and insulin resistance activate protein kinase C (PKC), which modifies cell signaling in glomerular endothelial cells, mesangial cells, and podocytes [[20], [21], [22]]. This activation affects inflammatory cytokines such as nuclear factor κB, interleukin-6, tumor necrosis-α, and plasminogen activator-1 [23]. Furthermore, the activation of PKC in glomeruli is associated with transforming growth factor-β signaling, which regulates extracellular matrix, such as type 4 collagen and alpha-smooth muscle cell, resulting in mesangial expansion [24,25].
Our previous reports indicated that insulin/insulin substrate 1 signaling regulates endothelial nitric oxide synthase, inhibited by diabetes-induced PKC activation. Furthermore, this inhibited signaling is partially reversed by exogenous incretin-related drugs [3,6,7]. Interestingly, finerenone reduces albuminuria and positively affects endothelial function and arterial elasticity by increasing nitric oxide bioavailability [26]. Inflammation and oxidative stress play essential roles in DKD initiation, development, and progression [27]. In addition, they can promote renal fibrosis [17,28]. Finerenone has stronger anti-fibrotic activity than eplerenone. It has been proposed that finerenone-specific improvement of the mineralocorticoid receptor (MR) target gene tenascin X, an important regulator of fibrosis, is related to the differential regulation of MR cofactors [29]. The anti-inflammatory effects of incretin-based drugs or hypoxia-inducible factor-prolyl hydroxylase domain inhibitors have been shown to be insufficient for the complete remission of DKD [30]. Therefore, the residual risk of DKD remains problematic. Finerenone also inhibits the progression of acute kidney injury to CKD by exerting anti-inflammatory and antioxidant effects.
Thus, Kidney Disease Improving Global Outcomes (KDIGO) 2022 clinical practice guideline for management in DKD states that the use of non-steroidal MRA should be considered if the urine albumin creatinine ratio is more than 30 and serum potassium is normal [31]. American Diabetes Association (ADA) clinical guideline 2023 recommends administration of finerenone in patients with DKD presenting with albuminuria who are being treated with a maximum dose of ACEIs or ARBs to improve cardiovascular outcomes and reduce the risk of DKD progression [32].
In summary, our study showed that finerenone caused a significantly slower decline in the eGFR in patients with advanced DKD. However, this study had several limitations. We retrospectively analyzed a cohort from a single center. This study included only a small number of patients. Furthermore, short follow-up period of this study and the fact that it is not a randomized controlled trial are also limitations of our study. Although our study has many limitations, this is the first report on the effect of finerenone in advanced CKD with an eGFR below 25 mL/min/1.73 m2 in the real world. Thus, finerenone is effective against a wide range of renal disorders, as confirmed in this study.
Funding
This work was supported by JSPS KAKENHI, Japan Grant Number 22K08368 (to A. Mima).
Data availability statement
Data are available from the author upon reasonable request.
CRediT authorship contribution statement
Akira Mima: Conceptualization, Methodology, Software, Data curation, Writing – original draft, preparation, Writing – review & editing. Rina Lee, Ami Murakami, Hidemasa Gotoda, Ryosuke Akai, Sayumi Kidooka: Visualization, Investigation, Writing – review & editing. Rina Lee, Takahiro Nakamoto, Suguru Kido: Visualization, Investigation, Software, Validation. Shinji Lee: Supervision.
Declaration of competing interest
A. Mima received a speaker's honorarium from Novartis, Kyowa Kirin, Torii, Kowa, Bayer, Eli Lilly, Mochida, Viatris, and Boehringer Ingelheim. A. Mima received research grants from Kyowa Kirin, Sumitomo Pharma, Chugai, Otsuka, Torii, Kowa, and Boehringer Ingelheim.
Acknowledgments
The authors would also like to thank Ms. Ayumi Noguchi for her contribution in preparing the clinical data and for her insights on statistical analysis. In addition, we extend our appreciation to Honyaku Center Inc. for their assistance with English language editing.
References
- 1.Diabetes Control and Complications Trial Research Group. Nathan D.M., Genuth S., Lachin J., Cleary P., Crofford O., et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Bailey C.J., Grant P.J. The UK prospective diabetes study. Lancet. 1998;352:1932. doi: 10.1016/S0140-6736(98)00090-7. [DOI] [PubMed] [Google Scholar]
- 3.Mima A., Qi W., King G.L. Implications of treatment that target protective mechanisms against diabetic nephropathy. Semin Nephrol. 2012;32:471–478. doi: 10.1016/j.semnephrol.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mima A. Renal protection by sodium-glucose cotransporter 2 inhibitors and its underlying mechanisms in diabetic kidney disease. J Diabet Complicat. 2018;32:720–725. doi: 10.1016/j.jdiacomp.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Mima A. Sodium-glucose cotransporter 2 inhibitors in patients with non-diabetic chronic kidney disease. Adv Ther. 2021;38:2201–2212. doi: 10.1007/s12325-021-01735-5. [DOI] [PubMed] [Google Scholar]
- 6.Mima A., Hiraoka-Yamomoto J., Li Q., Kitada M., Li C., Geraldes P., et al. Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCβ activation in diabetes. Diabetes. 2012;61:2967–2979. doi: 10.2337/db11-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mima A., Yasuzawa T., Nakamura T., Ueshima S. Linagliptin affects IRS1/Akt signaling and prevents high glucose-induced apoptosis in podocytes. Sci Rep. 2020;10:5775. doi: 10.1038/s41598-020-62579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanner C., Inzucchi S.E., Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:1801–1802. doi: 10.1056/NEJMc1611290. [DOI] [PubMed] [Google Scholar]
- 9.Bolignano D., Palmer S.C., Navaneethan S.D., Strippoli G.F. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014:CD007004. doi: 10.1002/14651858.CD007004.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Goenka L., Padmanaban R., George M. The ascent of mineralocorticoid receptor antagonists in diabetic nephropathy. Curr Clin Pharmacol. 2019;14:78–83. doi: 10.2174/1574884713666181116100946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakris G.L., Agarwal R., Anker S.D., Pitt B., Ruilope L.M., Rossing P., et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B., Filippatos G., Agarwal R., Anker S.D., Bakris G.L., Rossing P., et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385:2252–2263. doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 13.Mima A. A narrative review of diabetic kidney disease: previous and current evidence-based therapeutic approaches. Adv Ther. 2022;39:3488–3500. doi: 10.1007/s12325-022-02223-0. [DOI] [PubMed] [Google Scholar]
- 14.Mima A., Nomura A., Fujii T. Current findings on the efficacy of incretin-based drugs for diabetic kidney disease: a narrative review. Biomed Pharmacother. 2023;165 doi: 10.1016/j.biopha.2023.115032. [DOI] [PubMed] [Google Scholar]
- 15.Imai E., Horio M., Nitta K., Yamagata K., Iseki K., Tsukamoto Y., Ito S., Makino H., Hishida A., Matsuo S. Modification of the modification of Diet in renal disease (MDRD) study equation for Japan. Am J Kidney Dis. 2007;50:927–937. doi: 10.1053/j.ajkd.2007.09.004. https://doi: 10.1053/j.ajkd.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 16.Matsuo S., Imai E., Horio M., Yasuda Y., Tomita K., Nitta K., Yamagata K., Tomino Y., Yokoyama H., Hishida A. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. https://doi: 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 17.Mima A. Prediction of decreased estimated glomerular filtration rate using liver fibrosis markers: a renal biopsy-based study. Sci Rep. 2022;12 doi: 10.1038/s41598-022-22636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mima A., Murakami A., Lee R., Lee S. Predictive significance of glomerular insulin receptor substrate-1 in patients with diabetic kidney disease. Metabol Open. 2023;18 doi: 10.1016/j.metop.2023.100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal R., Filippatos G., Pitt B., Anker S.D., Rossing P., Joseph A., et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43:474–484. doi: 10.1093/eurheartj/ehab777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mima A., Ohshiro Y., Kitada M., Matsumoto M., Geraldes P., Li C., et al. Glomerular-specific protein kinase C-β-induced insulin receptor substrate-1 dysfunction and insulin resistance in rat models of diabetes and obesity. Kidney Int. 2011;79:883–896. doi: 10.1038/ki.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mima A., Kitada M., Geraldes P., Li Q., Matsumoto M., Mizutani K., et al. Glomerular VEGF resistance induced by PKCδ/SHP-1 activation and contribution to diabetic nephropathy. Faseb J. 2012;26:2963–2974. doi: 10.1096/fj.11-202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koya D., Haneda M., Nakagawa H., Isshiki K., Sato H., Maeda S., et al. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC β inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. Faseb J. 2000;14:439–447. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- 23.Mima A., Yasuzawa T., King G.L., Ueshima S. Obesity-associated glomerular inflammation increases albuminuria without renal histological changes. FEBS Open Bio. 2018;8:664–670. doi: 10.1002/2211-5463.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mima A., Matsubara T., Arai H., Abe H., Nagai K., Kanamori H., et al. Angiotensin II-dependent Src and Smad1 signaling pathway is crucial for the development of diabetic nephropathy. Lab Invest. 2006;86:927–939. doi: 10.1038/labinvest.3700445. [DOI] [PubMed] [Google Scholar]
- 25.Mima A., Abe H., Nagai K., Arai H., Matsubara T., Araki M., et al. Activation of Src mediates PDGF-induced Smad1 phosphorylation and contributes to the progression of glomerulosclerosis in glomerulonephritis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gil-Ortega M., Vega-Martín E., Martín-Ramos M., González-Blázquez R., Pulido-Olmo H., Ruiz-Hurtado G., et al. Finerenone reduces intrinsic arterial stiffness in Munich Wistar Frömter rats, a genetic model of chronic kidney disease. Am J Nephrol. 2020;51:294–303. doi: 10.1159/000506275. [DOI] [PubMed] [Google Scholar]
- 27.Mima A. Inflammation and oxidative stress in diabetic nephropathy: new insights on its inhibition as new therapeutic targets. J Diabetes Res. 2013;2013 doi: 10.1155/2013/248563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasuzawa T., Nakamura T., Ueshima S., Mima A. Protective effects of eicosapentaenoic acid on the glomerular endothelium via inhibition of EndMT in diabetes. J Diabetes Res. 2021;2021 doi: 10.1155/2021/2182225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latouche C., Sainte-Marie Y., Steenman M., Castro Chaves P., Naray-Fejes-Toth A., Fejes-Toth G., et al. Molecular signature of mineralocorticoid receptor signaling in cardiomyocytes: from cultured cells to mouse heart. Endocrinology. 2010;151:4467–4476. doi: 10.1210/en.2010-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mima A. Hypoxia-inducible factor-prolyl hydroxylase inhibitors for renal anemia in chronic kidney disease: advantages and disadvantages. Eur J Pharmacol. 2021;912 doi: 10.1016/j.ejphar.2021.174583. [DOI] [PubMed] [Google Scholar]
- 31.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102:S1–S127. doi: 10.1016/j.kint.2022.06.008. https://doi: 10.1016/j.kint.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 32.ElSayed N.A., Aleppo G., Aroda V.R., Bannuru R.R., Brown F.M., Bruemmer D., et al. Cardiovascular disease and risk management: standards of care in diabetes-2023. Diabetes Care. 2023;46:S158–S190. doi: 10.2337/dc23-S010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the author upon reasonable request.