Abstract
Objective
Contribute to clarifying the existence of subclinical hearing deficits associated with aging.
Design
In this work, we study and compare the auditory perceptual and electrophysiological performance of normal-hearing young and adult subjects (tonal audiometry, high-frequency tone threshold, a triplet of digits in noise, and click-evoked auditory brainstem response).
Study sample
45 normal hearing volunteers were evaluated and divided into two groups according to age. 27 subjects were included in the “young group” (mean 22.1 years), and 18 subjects (mean 42.22 years) were included in the “adult group.”
Results
In the perceptual tests, the adult group presented significantly worse tonal thresholds in the high frequencies (12 and 16 kHz) and worse performance in the digit triplet tests in noise. In the electrophysiological test using the auditory brainstem response technique, the adult group presented significantly lower I and V wave amplitudes and higher V wave latencies at the supra-threshold level. At the threshold level, we observed a significantly higher latency in wave V in the adult group. In addition, in the partial correlation analysis, controlling for the hearing level, we observed a relationship (negative) between age and speech in noise performance and high-frequency thresholds. No significant association was observed between age and the auditory brainstem response.
Conclusion
The results are compatible with subclinical hearing loss associated with aging.
Keywords: Age-related hearing loss, Cochlear synaptopathy, Auditory electrophysiology, Speech perception, Aging
1. Introduction
Our auditory system undergoes progressive functional and structural deterioration as we age, manifested mainly by decreased audiometric thresholds. This phenomenon is known as age-related hearing loss or presbycusis, and clinically it manifests itself around the sixth decade of life. The way that presbycusis affects individuals depends on extrinsic and intrinsic factors such as occupational or recreational exposure to noise and genetic or otological diseases (Howarth and Shone, 2006; Jafari et al., 2020).
Age-related hearing loss affects our hearing capacity progressively as we age, but it does not mean that all perceptual hearing properties are affected similarly over time. In fact, in middle-aged people, audiometric thresholds are generally observed within normal limits, but some processes could deteriorate the performance of our auditory system (Peelle, 2018). In this line, it has been reported that they would have alterations in the processing of the fine temporal structure of sound, which is most likely due to the hypofunction of the inhibitory system responsible for the coding of the rapid sound changes (Šuta et al., 2011; Ruggles et al., 2012; Erb et al., 2020).
Postmortem human studies have shown a sustained decrease in ganglion cells of the auditory nerve (Otte et al., 1978; Makary et al., 2011). Thus, animal models suggest that normal aging leads to a deterioration of postsynaptic cochlear structures, even before the decline of cochlear functionality. Sergeyenko et al. (2013) observed in long-lived mice (CBA/CaJ) that have not been exposed to noise, diffuse and steady degeneration of inner hair cells (IHCs), ribbons, and ganglion cells in the absence of hair cell damage or loss. Functionally, this deterioration was evidenced by a decrease in the amplitude of the early waves (I-III) of the auditory brainstem response (ABR) (Sergeyenko et al., 2013). In humans, Johannesen et al. (2019) observed a relationship between the wave I amplitude growth ratio from click-evoked ABR whit the age. The authors identified these findings as positive evidence for cochlear synaptopathy due to aging in humans (Johannesen et al., 2019).
Independent of neurobiological mechanisms, we know that aging progressively affects our hearing capacity, and some manifestations could appear even before the decline of the audiometric thresholds. The main manifestation reported is the speech-in-noise test auditory spatial abilities and auditory processing in general (Schneider and Pichora-Fuller, 2001; Banh et al., 2012; Kathleen Pichora-Fuller and Singh, 2006; Peters and Sethares, 2002; Uchida et al., 2003; Ruggles et al., 2012).
Here, we hypothesize that it is possible to observe a subclinical hearing loss in perceptual and electrophysiological auditory tasks associated with aging, before the decrease in audiometric thresholds, even in conventional tests in the audiological clinic. To test this, we measured perceptual and electrophysiological tests with easy access to the audiological clinic, measured at the threshold and supra-threshold levels. This will allow us to contribute to clarifying the existence of a subclinical condition and give clues about its eventual evaluation in the clinic.
2. Methods
2.1. Data collection
This study presents data from 45 individuals with normal auditions, ranging from 20 to 60 years old. These data were obtained in two independent studies, with the same measurement protocol in the tests reported here (electrophysiological and psychoacoustic). All volunteers were recruited mainly from the university environment.
2.2. Subjects
To demonstrate possible age-related subclinical hearing damage, we compared the performance in auditory and electrophysiological tests in two age-differentiated groups. The 45 hearing-impaired volunteers recruited had to meet the criterion of having audiometric thresholds equal to or lower than 20 dB HL (Anon and ANSI,) between the frequencies of 0.125 and 8 kHz (convenient sample). 27 subjects were included in the “young group” (YG), ranging from 20 to 24 years old (mean 22.1 years), where 13 were women, and 14 were men. 18 participants were included in the group of “adult group” (AG) ranging from 34 to 60 years old (mean 42.22 years), where 12 were women, and 6 were men. All smokers were excluded from this study.
The subjects in this study were volunteers who were not paid for their participation All participants agreed to be part of the research and signed an informed consent approved by the Ethics Committee of the Faculty of Medicine of the University of Chile.
2.3. Perceptual tests
The measurements were performed in a Single-walled soundproof, located inside an acoustically attenuating room in the Audiology and Auditory Perception Laboratory, Medical Technology Department, Universidad de Chile.
2.3.1. Hearing threshold
The hearing threshold was obtained using a calibrated audiometer (AC40e, Interacoustics ®) for each ear at 0.125, 0.250, 0.5, 1, 2, 3, 4, 6, 8, 12, and 16 kHz frequencies. To measure frequencies 0.125 Hz - 8 kHz, a TDH-39 headphone was used, and Koss R/80 for 12 and 16 kHz (Anon and ANSI,).
2.3.2. Speech-in-noise-test
A speech-in-noise test was specially customized for this study. For this, a triplet digit test in noise was set up, emulating Pérez-González et al. (2013). The stimuli were configured using Adobe Audition ® software to generate two lists of 25 triplets of digits with different levels of signal-to-noise ratio (SNR). The numbers included in the lists were from 1 to 9, pre-recorded by a male native speaker of Chilean Spanish in a single-wall sound-attenuating booth.
Both lists were created with the numbers randomly ordered and containing the same number of repetitions for each digit. The noise consisted of 32 talkers babble-noise played in reverse. The noise sounded uninterrupted ipsilaterally during the time the triplets were presented. Before the list of triplets was measured, 3 training triplets were added to the test, which was [1,2,3] - [4,5,6] and [7,8,9]. The two lists, A and B, have a signal-to-noise ratio (SNR) of −10 and −15 dB, respectively, and were stored digitally in a computer and connected to the AC40 audiometer to generate the sound. Given a possible asymmetry in performance between the ears, the test was performed only in the right ear (Kimura, 2011; Bidelman and Bhagat, 2015) at a comfortable level between 50- and 55-dB HL. The subjects had to write down the triplets they had heard to be reviewed later; a response was considered correct when all three digits were correct and presented in the same order. A total of 25 correct answers corresponds to 100% of the test score.
2.4. Electrophysiological test
2.4.1. Auditory brainstem response
Auditory brainstem response was recorded in the right ear using Eclipse EP- 25 (Interacoustics Eclipse® equipment) and inserted earphones supplied with the system (Kimura, 2011; Bidelman and Bhagat, 2015). The stimulus used was a 100 μs click at a rate of 21.1 Hz. It began by presenting a stimulation at a supra-threshold level (80 dB nHL), and subsequently, the intensity was lowered by 20 dB until reaching the intensity of 20 dB nHL. The record was filtered using a 100–3000 Hz band-pass, 2000 repetitions, and alternating polarity. Measurements were performed with surface electrodes: the positive electrode in Cz, the reference electrode in the right mastoid, and the ground electrode in front. The amplitudes, latencies of waves I, III, and V, and their intervals were determined from the recordings by an expert audiologist.
In the statistical analysis, we used a parametric test (t-student test) to compare the means between the two groups (young v/s adult). On the other hand, to determine how audiometric thresholds could influence the possible associations between all the variables studied, we used a partial correlation analysis (Pearson's correlation coefficient).
3. Results
3.1. Perceptual measurements
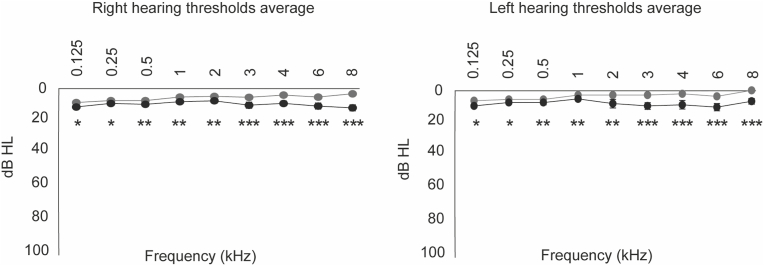
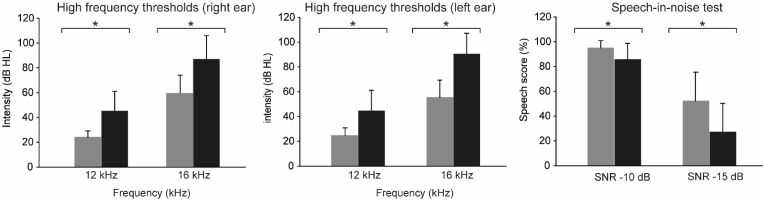
4.1.1 All subjects had hearing thresholds below 20 dB HL at conventional audiometric thresholds. However, as shown in Fig. 1, the thresholds are higher in the GA at all frequencies in both ears. This difference is statistically significant (one-tailed, unpaired, t-test, p < 0.05). The high-frequency hearing thresholds were evaluated at 12 and 16 kHz (right and left ear). Fig. 2 and Table 1 show that the AG presents increased high-frequency thresholds compared to the YG. The average for the 12 kHz in YG was 24.4 dB HL ± 6.41 in the right ear and 23.9 ± 5.06 for the left ear, while the average for the 16 kHz frequency in the right ear the average was 55.4 dB HL ± 14.0, and in the left ear was 56.3 dB HL ± 19.3. In the AG, the average for 12 kHz in the right ear was 44.4 dB HL ± 16.7 and 45 dB HL ± 16.1 for the left ear, while the average for the frequency of 16 kHz in the right ear was 90.3 ± 16.9 dB HL and in the left ear 86.8 dB HL ± 19.3. When comparing the average of the absolute thresholds of both frequencies between the groups, it is observed that the AG has higher hearing thresholds than the YG in both ears' 12 kHz and 16 kHz frequencies. There is a difference of 20 dB HL in the frequency of 12 kHz in the right ear and 22.1 dB HL in the left ear. On the other hand, at the frequency of 16 kHz in the right ear, there is a difference of 34.9 dB HL and in the left ear, 30.5 dB HL. All the differences found between the thresholds are significant (one-tailed, unpaired, t-test, p < 0.01) (Table 1).
Fig. 1.
Hearing threshold average. The figure shows the average hearing thresholds between 125 and 8000 Hz for the young (gray) and adult (black) groups in the right and left ear.
Fig. 2.
High-frequency auditory thresholds. (Left and middle) Average high-frequency thresholds for 12 and 16 kHz (dB HL) are gray for the young and black for middle-aged adults. The error bars represent the standard error. (Right) Average speech score (%) of the young group (gray) and middle age group (black). Error bars represent the standard error.
Table 1.
This table shows the averages of ABR (amplitude and latency for waves I, III, and V), high-frequency hearing thresholds (12 kHz and 16 kHz), and SNR at −10 dB and −15 dB for the young (YG) and adult (AG) groups. The significant differences obtained in these comparisons are also shown (p-value).
| High frequency thresholds average |
SNR percentage average |
|||||
|---|---|---|---|---|---|---|
| Groups | 12 kHz RE | 12 kHz LE | 16 kHz RE | 16 kHz LE | SNR -10 dB | SNR -15 dB |
| YG | 24.4 dB HL | 23.9 dB HL | 55.4 dB HL | 56.3 dB HL | 94.9% | 52.4% |
| AG | 44.4 dB HL | 45 dB HL | 90.3 dB HL | 86.8 dB HL | 85.5% | 27.1% |
| p-value | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| High frequency thresholds average | SNR percentage average | |||||
|---|---|---|---|---|---|---|
| Groups | 12 kHz RE | 12 kHz LE | 16 kHz RE | 16 kHz LE | SNR -10 dB | SNR -15 dB |
| YG | 24.4 dB HL | 23.9 dB HL | 55.4 dB HL | 56.3 dB HL | 94.9% | 52.4% |
| AG | 44.4 dB HL | 45 dB HL | 90.3 dB HL | 86.8 dB HL | 85.5% | 27.1% |
| p-value | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| Groups | 12 kHz RE | 12 kHz LE | 16 kHz RE | 16 kHz LE | SNR -10 dB | SNR -15 dB |
| YG | 1.40 ms | 3.56 ms | 5.29 ms | 0.28 μV | 0.41 μV | 0.49 μV |
| YG | 1.40 ms | 3.56 ms | 5.29 ms | 0.28 μV | 0.41 μV | 0.49 μV |
| AG | 1.44 ms | 3.57 ms | 5.42 ms | 0.20 μV | 0.34 μV | 0.39 μV |
| p-value | p > 0.05 | p > 0.05 | p > 0.05 | p < 0.01 | p > 0.05 | p < 0.01 |
| High frequency thresholds average | SNR percentage average | |||||
|---|---|---|---|---|---|---|
| Groups | 12 kHz RE | 12 kHz LE | 16 kHz RE | 16 kHz LE | SNR -10 dB | SNR -15 dB |
| YG | 24.4 dB HL | 23.9 dB HL | 55.4 dB HL | 56.3 dB HL | 94.9% | 52.4% |
| AG | 44.4 dB HL | 45 dB HL | 90.3 dB HL | 86.8 dB HL | 85.5% | 27.1% |
| p-value | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| ABR latency average | ABR amplitude average | |||||
| Groups | Wave I ABR latency | Wave III ABR latency | Wave V ABR latency | Wave I ABR amplitude | Wave III ABR amplitude | Wave V ABR amplitude |
3.1.1. Speech-in-noise performance
Here, we compared the results obtained in both groups in the digit triplet discrimination test in the presence of background noise (Fig. 2). As can be seen, the YG shows better performance in the speech-in-noise test. This difference was significant (one-tailed, unpaired, t-test, p < 0.01) in both lists. In the SNR -10 list, the average percentage performance of YG was 94.9% ± 6.03, while AG obtained 85.5% ± 13.1. On the other hand, in the SNR -15 list, the average YG was 52.4% ± 23.2, and the average AG was 27.1% ± 23.0 (Table 1).
3.2. Electrophysiological recordings
3.2.1. 2.1 ABR
The main result observed was a reduction of the auditory evoked response in the AG, characterized by a slight increase in latencies and a decrease in amplitudes. In Fig. 3, an increase in the latencies of waves I (non-significant), III (non-significant), and V (significant, one-tailed, unpaired, t-test, p = 0.015) was observed in the AG. The latency in the I ABR wave of YG was 1.40 ± 0.11 (ms), while in the AG, it was 1.44 ± 0.13 (ms). For the V ABR wave, the YG had an average latency of 5.29 ± 0.12 (ms), while in the AG, it was 5.42 ± 0.24 (ms). Finally, there were no significant differences between the groups in the III ABR wave; the YG average had a latency of 3.53 ± 0.11 (ms) and the AG 3.57 ± 0.16 (ms). (Fig. 3 and Table 1).
Fig. 3.
ABR amplitude and latencies. (Top row). Average of I, III, and V ABR amplitude and latencies (ms) at 80 dB nHL for the young (gray) and middle-aged adult (black) groups. (Bottom row). Threshold intensity of ABR wave V amplitude and ratio I/V wave amplitude for the young (blue) and middle-aged adult (cyan) groups. Error bars represent standard error. Error bars represent the standard error.
On the other hand, the amplitude of I, III, and V ABR waves were compared between the YG and AG. In these three cases, the amplitudes obtained in the YG were greater than in the AG and were statistically significant. The amplitude of wave I in YG was 0.28 ± 0.11 (μV), and in the AG, it was 0.20 ± 0.09 (μV.) This difference was significant (one-tailed, unpaired, t-test, p = 0.008). Regarding ABR wave III amplitude, in the YG, it reaches 0.41 ± 0.13 (μV), while in the AG, this value was 0.34 ± 0.11 (μV) observing a significant difference between the two groups (one-tailed, unpaired, t-test, p = 0.03). At last, it should be noted that the amplitude of ABR wave V in YG was 0.49 ± 0.16 (μV), and in the AG, it was 0.39 ± 0.15 (μV). This difference was significant (one-tailed, unpaired, t-test, p = 0.01). (Fig. 3 and Table 1).
Finally, in all subjects except one, wave V was observed at 20 dB NHL (S34 AG), in which case wave V was recorded at 30 dB. At the near-threshold level, we found a higher latency in AG than YG (8,0 ± 0.65 (ms) vs. 7,64 ± 0.27 (ms)respectively). This difference was significant (one-tailed, unpaired, t-test, p = 0.008).
Once it was determined that the adult group presented a lower performance in the electrophysiological and perceptual tests, both at the threshold and suprathreshold levels, it becomes relevant to know which variables are more strongly related to age. A critical issue is that the hearing thresholds influence the possible associations between the other variables studied. To statistically control this potential bias, we performed a partial correlation analysis (Pearson's correlation coefficient), controlling for the hearing level (average of the thresholds of 500, 1000, and 2000 Hz), for the AG, between, age, speech-in-noise performance; ABR latency and amplitude (latencies and amplitudes of waves I, III, and IV; I/V amplitude ratio; wave V latency at threshold level) and high-frequency thresholds.
The analysis showed a significant (bilateral) negative correlation between the age of the speech in noise performance: SNR -10 (r = −0.724, p = 0.002) and SNR-15 (r = −0.516, p = 0.041), and the age with the 12.5Khz (r = −0.688, p = 0.003) and 16Khz (r = −0.526, p = 0.036) high-frequency thresholds (see Fig. 4). Unlike what was observed in the performance in the perceptual tests, no significant association was observed between age and the auditory brainstem response at threshold or supra-threshold level (latencies and amplitudes of waves I, III, and IV; I/V amplitude ratio; V wave latency at threshold level). The analysis also reflects that speech in noise is related to high-frequency thresholds. Is noted a significant (bilateral) negative correlation between the SNR -10 test with the 12.5 kHz threshold (r = −0.752, p < 0.001) and the SNR-15 test with the 12.5 kHz threshold (r = −0.598, p = 0.014). These results reveal an association between high-frequency tonal thresholds and speech in noise performance, as seen in the simple visual inspection of Fig. 4 (bottom row, SNR -10 vs. 12khz in the adult group).
Fig. 4.
Correlation between age, 12 kHz threshold, and speech performance test. The top and middle rows show the correlation between age with speech performance in noise (SNR -10 and SNR-15) with age (12 kHz) in the adult group. The bottom row shows the correlation between SNR-10 and SNR-15 with the 12 kHz threshold in the adult group.
All these results reveal an association between age, high-frequency tonal thresholds, and speech performance in noise. The young group had no significant correlation or association between these factors.
4. Discussion
Here, we aim to study if there is any evidence of subclinical hearing loss associated with aging. To test this, we compared and analyzed the performance in perceptual and physiological tests of two groups (young and adults) of normal hearing volunteers. The main results suggest a decrease in the auditory function in the older group, manifested in perceptive (high-frequency tonal threshold; speech-in-noise discrimination) and electrophysiological tests (auditory brainstem response). Our central hypothesis explaining the results is that there would be a loss of auditory nerve fibers in the adult subjects, resulting in a lower response in both the ABR and the speech-in-noise tests. The main findings are analyzed below.
4.1. Electrophysiological and perceptual measurements. Affected by the loss of auditory fibers?
4.1.1. Auditory electrophysiological findings
When comparing the auditory brainstem response between the group of young people and adults, a decrease in the amplitude of waves I, III, and V at the suprathreshold level and an increase in wave V latency at wave V at the supra-threshold and threshold level. These three findings are compatible with a reduction of the auditory brainstem response (Konrad-Martin et al., 2012).
These results may be explained by the constant loss of auditory pathway fibers or function during a lifetime. The loss of auditory nerve fibers generates a functional disconnection between the auditory system's peripheral transducers, unrelated to lowering the audiometric threshold. This could explain why message coding is complicated in background noise, leading to various perceptual manifestations. Lopez-Poveda. (2014) analyzed this situation using a ''stochastic under sampling'' model. The model assumes that the auditory fibers would respond by stochastically discharging to a sound stimulus so that the sound representation would depend on the probability of discharge and the number of fibers available. Therefore, age-induced auditory deafferentation would cause a degradation in the quality of the sound wave representation at the neural level, like an undersampling of a signal. Pichora-Fuller et al. (2007) argue that aging probably reduces the temporal synchrony of neural discharges in the auditory system, leading to a loss in temporal resolution through jittering. These authors suggest that this lack of synchrony explains the poor performance in speech-in-noise tests in elderly subjects (Pichora-Fuller et al., 2007).
Buran et al. (2022) report through computational modeling that age (and the associated loss of auditory nerve fibers) can lead to a decrease in the ABR response, particularly in the wave I amplitude (Buran et al., 2022). In this line, Sergeyenko et al. (2013) have shown in animal models that there is damage to the synapse between ganglion cells and inner hair cells (ribbon synapses) before spiral ganglion neurons body and nucleus degeneration. This cochlear synaptopathy is mainly caused by the aging (Sergeyenko et al., 2013). Therefore, counting spiral ganglion neurons are not the most accurate way to quantify functional damage to the auditory nerve since it could count cells that do not synapse. The results obtained in this work are compatible with this approach.
4.1.2. Perceptive electrophysiological findings
In the case of perceptive auditory results, many studies have shown that high frequencies are the first to deteriorate in human and animal models in acoustic trauma and aging. This damage has been related to the loss of outer hair cells, mainly at the base of the cochlea (Liberman, 1978; Wang et al., 2002). This deterioration of the perceptual response is also evidenced by worse performance in the speech-in-noise tests. For this reason, we decided to study high-frequency auditory thresholds (12,5 and 16 kHz). The results show a marked and significant increment of the absolute high-frequency thresholds in the oldest group of volunteers.
On the other hand, we studied the performance comparison between YG and AG and whether this was related to high-frequency hearing thresholds. We were interested in knowing which variables of the measures are best associated with the perception of speech in noise, which is the evaluation that most closely resembles the auditory demand in everyday listening. In this context, we observed that the speech perception in noise was strongly related to the high-frequency thresholds studied. Our results are consistent with the findings reported by Johannesen et al. (2019). Like us, they observe an association between speech intelligibility and age in normal hearing (Johannesen et al., 2019). As age increases, speech performance in noise decreases, and we did not observe an association between the auditory evoked response and the perception of speech, unlike what was reported by Johannesen et al. (2019). Megarbane and Fuente (2020) reported that in normal-hearing listeners, the wave V/I ratio was associated with speech-in-noise performance (hearing-in-noise test or HINT), specifically in the left ear (Megarbane and Fuente, 2020). In our data, although we found a decrease in the V/I ratio of the adult group (relative to the young group), these were neither significant nor strongly correlated with speech-in-noise performance. This discrepancy could be because Megarbane and Fuente (2020) observed an association in the left ear (not evaluated in this study) and because, in our correlational analysis, we controlled for the auditory threshold variable.
4.2. Subclinical hearing damage
As mentioned above, the present results provide evidence of age-related subclinical hearing damage. Once the differences in the perceptual and electrophysiological performances between the groups had been established, it was necessary to evaluate which variables were more directly related to age. Along this line, we observed that the high-frequency thresholds and the speech-in-noise performance were strongly correlated, not so the auditory evoked response. This subclinical hearing damage has been described in animal models of noise exposure (Kujawa and Liberman, 2009; Furman et al., 2013; Valero et al., 2017), aging (Sergeyenko et al., 2013) in demyelinating diseases (Wan and Corfas, 2017) or ototoxic drugs (Ruan et al., 2014). On the other hand, some works in humans have also been described where the idea of subclinical noise- or age-induced hearing damage that electrophysiological techniques can measure is raised (Skoe and Tufts, 2018; Bramhall et al., 2019).
But it remains to be answered if they provide evidence of age-related synaptopathy in humans. Although our study was not designed to answer such a question, if we differentiate the expected findings between noise-induced cochlear synaptopathy and age-related synaptopathy, we can contrast our results against these theoretical models.
The model of subclinical damage caused by exposure to noise supposes damage mainly on fibers with a low-spontaneous discharge rate so that the main manifestations would be at the suprathreshold level (Bharadwaj et al., 2014). In contrast, eventual damage due to aging, in addition to cochlear synapsis, could affect all types of nerve fibers, which is why it could manifest itself at the threshold and suprathreshold levels (Sergeyenko et al., 2013). From that perspective, our results are compatible with the theoretical model since we observed manifestations at the threshold (increase in the latency of wave V of the ABR; increase in the threshold of the frequency of 12 and 16 kHz, associated with age) and at the supra-threshold level (increase in latencies and decrease in the amplitudes of the waves I and V, associated with age).
4.3. Limitations
One of the limitations of our study is that we did not use a noise exposure survey among all participants. In this regard, although all volunteers had normal hearing, the history of noise exposure in the 34–60 age group may partly explain the differences found in our data. On the other hand, we only excluded smokers in this study. In this sense, other chronic pathologies could have influenced some deterioration in the obtained electrophysiological or auditory perceptual results.
4.4. Conclusions
In this work, we evidenced significant differences in perceptual and electrophysiological test performance between the young (20–24 years old) and adults (34–60 years old). At the perceptual level, the main differences observed were lower performance in both high-frequency threshold and speech-in-noise test performance in the oldest age group. On the other hand, in the electrophysiological tests, the auditory evoked response reduction was generally observed in the oldest age group, characterized by a lower amplitude of waves I and III of the ABR. Additionally, the correlational study showed a strong (negative) association between age and speech in noise performance and high-frequency thresholds (12.5 and 16 kHz). All these findings provide evidence in favor of subclinical hearing damage associated with aging, with manifestations at the threshold and suprathreshold levels. This subclinical damage may be caused mainly by a loss of the auditory fibers related to aging.
This research contributes to supporting the idea that it is necessary to advance in the development of hearing tests that provide greater sensitivity than classical tonal audiometry in the audiological clinic to be able to evidence this condition of subclinical hearing damage, especially in middle-aged subjects with normal hearing, but who manifest the sensation of suboptimal hearing.
Acknowledgements
Work supported by a grant of the University of Chile (UI-10/16) to EA.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Anon ANSI S3.6-1996 - specification for audiometers. https://webstore.ansi.org/standards/asa/ansis31996?gclid=Cj0KCQjw16KFBhCgARIsALB0g8LUp9GvGasg8leGQQ6NSl_GvU5wSTiTLgrtM9Z7vhGhPP7nhvk-rxkaAuevEALw_wcB Available at:
- Banh J., Singh G., Pichora-Fuller M.K. Age affects responses on the speech, spatial, and qualities of hearing scale (SSQ) by adults with minimal audiometric loss. J. Am. Acad. Audiol. 2012;23(2):81–91. doi: 10.3766/jaaa.23.2.2. http://www.thieme-connect.de/DOI/DOI?10.3766/jaaa.23.2.2 Available at: [DOI] [PubMed] [Google Scholar]
- Bharadwaj H.M., Verhulst S., Shaheen L., Charles Liberman M., Shinn-Cunningham B.G. Cochlear neuropathy and the coding of supra-threshold sound. Front. Syst. Neurosci. 2014;8(FEB):26. doi: 10.3389/fnsys.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman G.M., Bhagat S.P. Right-ear advantage drives the link between olivocochlear efferent “antimasking” and speech-in-noise listening benefits. Neuroreport. 2015;26(8):483–487. doi: 10.1097/WNR.0000000000000376. https://pubmed.ncbi.nlm.nih.gov/25919996/ Available at: [DOI] [PubMed] [Google Scholar]
- Bramhall N., Beach E.F., Epp B., Le Prell C.G., Lopez-Poveda E.A., et al. The search for noise-induced cochlear synaptopathy in humans: mission impossible? Hear. Res. 2019;377:88–103. doi: 10.1016/j.heares.2019.02.016. [DOI] [PubMed] [Google Scholar]
- Buran B.N., McMillan G.P., Keshishzadeh S., Verhulst S., Bramhall N.F. Predicting synapse counts in living humans by combining computational models with auditory physiology. J. Acoust. Soc. Am. 2022;151(1):561. doi: 10.1121/10.0009238. https://asa.scitation.org/doi/abs/10.1121/10.0009238 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb J., Schmitt L.M., Obleser J. Temporal selectivity declines in the aging human auditory cortex. Elife. 2020;9:1–21. doi: 10.7554/eLife.55300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman A.C., Kujawa S.G., Charles Liberman M. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J. Neurophysiol. 2013;110(3):577–586. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth A., Shone G.R. Ageing and the auditory system. Postgrad. Med. 2006;82(965):166–171. doi: 10.1136/pgmj.2005.039388. https://pmj.bmj.com/content/82/965/166 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari Z., Copps T., Hole G., Kolb B.E., Mohajerani M.H. Noise damage accelerates auditory aging and tinnitus: a Canadian population-based study. Otol. Neurotol. 2020;41(10):1316–1326. doi: 10.1097/MAO.0000000000002848. https://journals.lww.com/otology-neurotology/Fulltext/2020/12000/Noise_Damage_Accelerates_Auditory_Aging_and.2.aspx Available at: [DOI] [PubMed] [Google Scholar]
- Johannesen P.T., Buzo B.C., Lopez-Poveda E.A. Evidence for age-related cochlear synaptopathy in humans unconnected to speech-in-noise intelligibility deficits. Hear. 2019;374:35–48. doi: 10.1016/j.heares.2019.01.017. [DOI] [PubMed] [Google Scholar]
- Kathleen Pichora-Fuller M., Singh G. Effects of age on auditory and cognitive processing: implications for hearing aid fitting and audiologic rehabilitation. Trends Amplif. 2006;10(1) doi: 10.1177/108471380601000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D. From ear to brain. Brain Cognit. 2011;76(2):214–217. doi: 10.1016/j.bandc.2010.11.009. https://pubmed.ncbi.nlm.nih.gov/21236541/ Available at: [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D., Dille M.F., McMillan G., Griest S., McDermott D., et al. Age-related changes in the auditory brainstem response. J. Am. Acad. Audiol. 2012;23(1):18–35. doi: 10.3766/jaaa.23.1.3. http://www.thieme-connect.de/DOI/DOI?10.3766/jaaa.23.1.3 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa S.G., Liberman M.C. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 2009;29(45):14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman M.C. Auditory-nerve response from cats raised in a low-noise chamber. J. Acoust. Soc. Am. 1978;63(2):442–455. doi: 10.1121/1.381736. https://asa.scitation.org/doi/abs/10.1121/1.381736 Available at: [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda E.A. Why do I hear but not understand? Stochastic undersampling as a model of degraded neural encoding of speech. Frontiers in Neuroscience. 2014;8:348. doi: 10.3389/fnins.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary C.A., Shin J., Kujawa S.G., Liberman M.C., Merchant S.N. Age-related primary cochlear neuronal degeneration in human temporal bones. JARO J. Assoc. Res. Otolaryngol. 2011;12(6):711–717. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megarbane L., Fuente A. Association between speech perception in noise and electrophysiological measures: an exploratory study of possible techniques to evaluate cochlear synaptopathy in humans. Int. J. Audiol. 2020;59(6):427–433. doi: 10.1080/14992027.2020.1718783. https://www.tandfonline.com/doi/abs/10.1080/14992027.2020.1718783 Available at: [DOI] [PubMed] [Google Scholar]
- Otte J., Schuknecht H.F., Kerr A.G. Laryngoscope; 1978. Ganglion Cell Populations in Normal and Pathological Human Cochleae Implications for Cochlear Implantation. [DOI] [PubMed] [Google Scholar]
- Peelle J.E. Listening effort: how the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear Hear. 2018;39(2):204–214. doi: 10.1097/AUD.0000000000000494. https://journals.lww.com/ear-hearing/Fulltext/2018/03000/Listening_Effort__How_the_Cognitive_Consequences.4.aspx Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-González P., Arocena J.M.G., Poveda E.A.L. 2013. A Castilian Spanish Digit Triplet Identification Test for Assessing Speech Intelligibility in Quiet and in Noise. [Google Scholar]
- Peters A., Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J. Comp. Neurol. 2002;442(3):277–291. doi: 10.1002/cne.10099. https://pubmed.ncbi.nlm.nih.gov/11774342/ Available at: [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller M.K., Schneider B.A., MacDonald E., Pass H.E., Brown S. Temporal jitter disrupts speech intelligibility: a simulation of auditory aging. Hear. Res. 2007;223(1–2):114–121. doi: 10.1016/j.heares.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Ruan Q., Ao H., He J., Chen Z., Yu Z., et al. Topographic and quantitative evaluation of gentamicin-induced damage to peripheral innervation of mouse cochleae. Neurotoxicology. 2014;40:86–96. doi: 10.1016/j.neuro.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Ruggles D., Bharadwaj H., Shinn-Cunningham B.G. Why middle-aged listeners have trouble hearing in everyday settings. Curr. Biol. 2012;22(15):1417–1422. doi: 10.1016/j.cub.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider B.A., Pichora-Fuller M.K. Age-related changes in temporal processing: implications for speech perception. Semin. Hear. 2001;22(3):227–238. http://www.thieme-connect.com/products/ejournals/html/10.1055/s-2001-15628 Available at: [Google Scholar]
- Sergeyenko Y., Lall K., Charles Liberman M., Kujawa S.G. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J. Neurosci. 2013;33(34):13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoe E., Tufts J. Evidence of noise-induced subclinical hearing loss using auditory brainstem responses and objective measures of noise exposure in humans. Hear. Res. 2018;361:80–91. doi: 10.1016/j.heares.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Šuta D., Rybalko N., Pelánová J., Popelář J., Syka J. Age-related changes in auditory temporal processing in the rat. Exp. Gerontol. 2011;46(9):739–746. doi: 10.1016/j.exger.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Uchida Y., Nakashima T., Ando F., Niino N., Shimokata H. Prevalence of self-perceived auditory problems and their relation to audiometric thresholds in a middle-aged to elderly population. Acta Otolaryngol. 2003;123(5):618–626. doi: 10.1080/00016480310001448. [DOI] [PubMed] [Google Scholar]
- Valero M.D., Burton J.A., Hauser S.N., Hackett T.A., Ramachandran R., et al. Noise-induced cochlear synaptopathy in rhesus monkeys (Macaca mulatta) Hear. Res. 2017;353:213–223. doi: 10.1016/j.heares.2017.07.003. http://www.ncbi.nlm.nih.gov/pubmed/28712672 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan G., Corfas G. Transient auditory nerve demyelination as a new mechanism for hidden hearing loss. Nat. Commun. 2017;8(1):1–13. doi: 10.1038/ncomms14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hirose K., Liberman M.C. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. JARO J. Assoc. Res. Otolaryngol. 2002;3(3):248–268. doi: 10.1007/s101620020028. https://link.springer.com/article/10.1007/s101620020028 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]