Abstract
Background
Access to left atrial appendage closure (LAAC) in Canada is limited, due to funding restrictions. This work aimed to assess Canadian clinical practice on patient selection, postprocedural antithrombotic therapy, and safety and/or efficacy with WATCHMAN device implantation.
Methods
Seven Canadian centres implanting the WATCHMAN device participated in this prospective multicentre, observational registry. All procedures were done under general anesthesia with transesophageal echocardiography guidance. Patients were prospectively followed for 2years. The long-term stroke rate was compared with the expected rate based on the CHA2DS2-VASc score.
Results
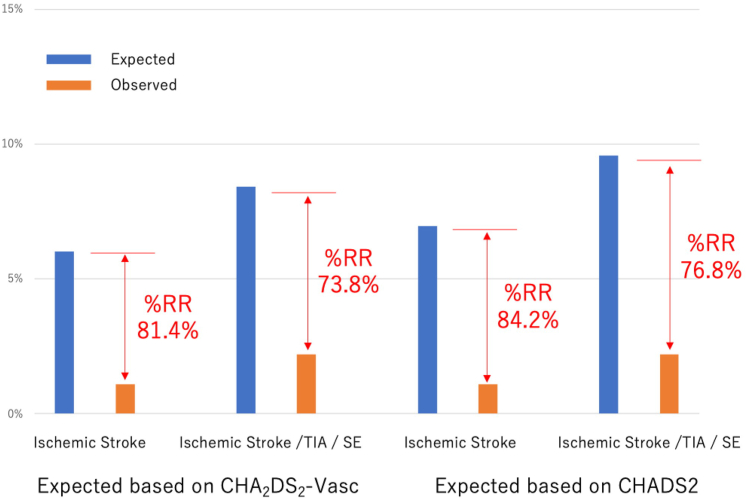
A total of 272 patients who underwent LAAC with the WATCHMAN device between December 2013 and August 2019 (mean age: 75.4 years [standard deviation {SD}: 8.75]; male, 63.2%; CHA2DS2-VASc score: 4.35 [SD: 1.64]; HAS-BLED score: 3.55 [SD: 0.94]) were included. Most patients (90.4%) had prior history of bleeding (major, 80.5%; minor, 21.7%). The WATCHMAN device was successfully implanted in 269 patients (98.9%), with a few procedure-related complications, including 5 pericardial effusions requiring drainage (1.8%), and 1 death (0.4%; 22 days post-LAAC from respiratory failure). Post-LAAC antithrombotic therapy included dual antiplatelet therapy in 70.6%, single antiplatelet therapy in 18.4%, and oral anticoagulation in 13.6%. During the follow-up period (mean: 709.7 days [SD: 467.2]), an 81.4% reduction of the ischemic stroke rate occurred, based on the expected rate from the CHA2DS2-VASc score (6.0% expected vs 1.1% observed). Device-related thrombus was detected in 1.8%.
Conclusions
The majority of Canadian patients who underwent LAAC had oral anticoagulation contraindication due to prior bleeding, and most were safely treated with antiplatelet therapy post-LAAC, with a low device-related thrombus incidence. Long-term follow-up demonstrated that LAAC achieved a significant reduction in ischemic stroke rate.
RÉsumÉ
Contexte
Au Canada, l’accès à la fermeture de l’appendice auriculaire gauche (FAAG) est limité en raison de restrictions quant au financement de cette intervention. Le présent rapport visait à évaluer les pratiques cliniques canadiennes sur la sélection des patients, le traitement antithrombotique après l’intervention et l’innocuité ou l’efficacité par l’implantation d’un dispositif WATCHMAN.
Méthodologie
Sept centres canadiens procédant à l’implantation du dispositif WATCHMAN ont participé à ce registre observationnel, prospectif et multicentrique. Toutes les interventions ont été réalisées sous anesthésie générale avec guidage par échocardiographie transœsophagienne. Les patients ont par la suite été suivis de manière prospective pendant deux ans. Le taux d’AVC à long terme a été comparé au taux attendu, selon le score CHA2DS2-VASc.
Résultats
Ont été inclus à l’étude 272 patients ayant subi une FAAG avec implantation d’un dispositif WATCHMAN entre décembre 2013 et août 2019 (âge moyen : 75,4 ans [écart-type {É.-T.} : 8,75]; hommes : 63,2 %; score CHA2DS2-VASc : 4,35 [É.-T. : 1,64]; score HAS-BLED : 3,55 [É.-T. : 0,94]). La plupart des patients (90,4 %) avaient des antécédents de saignements (majeurs : 80,5 %; mineurs : 21,7 %). Le dispositif WATCHMAN a bien été implanté chez 269 patients (98,9 %), avec quelques complications associées à l’intervention, dont cinq effusions péricardiques nécessitant un drainage (1,8 %) et un décès (0,4 %; 22 jours après la FAAG, en raison d’une insuffisance respiratoire). Le traitement antithrombotique après la FAAG comprenait une bithérapie antiplaquettaire dans 70,6 % des cas, une monothérapie antiplaquettaire dans 18,4 % des cas et une anticoagulothérapie orale dans 13,6 % des cas. Pendant la période de suivi (durée moyenne : 709,7 jours [É.-T. : 467,2]), on a noté une réduction de 81,4 % du taux d’AVC ischémique observé par rapport au taux attendu selon le score CHA2DS2-VASc (taux attendu : 6,0 %; taux observé : 1,1 %). Un thrombus associé au dispositif a été détecté dans 1,8 % des cas.
Conclusions
La majorité des patients canadiens qui ont subi une FAAG présentaient des contre-indications à l’anticoagulothérapie orale en raison de leurs antécédents de saignements, et la plupart ont été traités de manière sécuritaire par des thérapies antiplaquettaires après la chirurgie, avec un faible taux d’incidence de thrombus associé au dispositif. Le suivi à long terme a montré que la FAAG permet d’obtenir une réduction importante du taux d’AVC ischémique.
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is a major cause of stroke, responsible for 15% of all strokes and 30% of strokes in patients aged > 80 years.1 Strokes associated with AF are more severe; AF-related stroke victims have a 50% greater likelihood of becoming disabled, and a > 50% likelihood of dying.2,3 Accordingly, stroke prevention is one of the main pillars of AF management. The Canadian Cardiovascular Society recommends anticoagulation for most patients who are either aged 65 years or older or have a CHADS2 (Congestive Heart Failure, Hypertension, Age ≥ 75, Diabetes, and Prior Stroke/Transient Ischemic Attack [doubled]) score ≥ 1; the European Society of Cardiology recommends anticoagulation for those with a CHAD2DS2-VASc (Congestive Heart Failure, Hypertension, Age [≥ 75 Years] [doubled], Diabetes Mellitus, Stroke [doubled], Vascular Disease, Age [65-74] Years, Sex Category [Female]) scores ≥ 1.4,5
The benefit of oral anticoagulation (OAC) treatment in stroke prevention has to be balanced with the risk of major bleeding. Despite the safety profile of non-vitamin K oral anticoagulation (NOAC), the annual incidence of major bleeding ranges from 2.13% to 3.6%, with an annual incidence of intracranial hemorrhage ranging from 0.1% to 0.5%.6, 7, 8 Therefore, even though OAC is effective for thromboembolic prevention, a large proportion of eligible patients are not on therapy, for multiple reasons. These challenges have led to investigations of device-based therapies for nonvalvular AF, including percutaneous left atrial appendage closure (LAAC) to prevent stroke, which is a major source of thrombus related to AF. Randomized controlled trials (WATCHMAN LAA Closure Technology for Embolic Protection in Patients With Atrial Fibrillation [PROTECT-AF] and Prospective Randomized Evaluation of the WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation vs Long-Term Warfarin Therapy [PREVAIL]) have shown safety and efficacy of the WATCHMAN device (Boston Scientific, Marlborough, MA), in comparison to warfarin, in patients eligible for OAC.9, 10, 11 Among patients with contraindications to OAC, randomized trials comparing LAAC to antiplatelet/no therapy are ongoing, but enrollment rates have been very slow, and the Assessment of the WATCHMAN FLXTM Device in Patients Unsuitable for Oral Anticoagulation (ASAP-TOO) trial was stopped prematurely due to recruitment challenges.12 Nevertheless, based on cumulative evidence, LAAC is given a class IIB recommendation for patients with high stroke risk and contraindications to long-term OAC treatment.5
Despite accumulating data, access to LAAC in Canada is limited due to funding restrictions. We aimed to assess the early Canadian clinical experience with LAAC, assessing patient selection, procedural outcomes, postprocedural antithrombotic therapy, and the safety and long-term efficacy of WATCHMAN implantation.
Methods
The Canadian WATCHMAN Registry is a multicentre, prospective, nonrandomized, observational registry enrolling consecutive patients undergoing LAAC with the WATCHMAN legacy device in Canadian cardiac catheterization or electrophysiological laboratories. All Canadian centres implanting WATCHMAN devices were invited to participate in this multicentre registry; 7 of 11 sites participated. Institutional research ethics board approvals were obtained, and patients gave informed consent for prospective follow-up.
Patient population
Patients with nonvalvular AF (paroxysmal, persistent, or permanent) who met the inclusion and exclusion criteria were included. Inclusion criteria included the following: (i) age ≥ 18 years; (ii) CHADS2 score ≥ 1 and/or CHA2DS2-VASc score ≥ 2; and (iii) prior major bleeding (intracranial, gastrointestinal, intraocular, respiratory, genitourinary, retroperitoneal, pericardial, anemia requiring transfusions, etc.), or contraindications to long-term OAC (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs/Alcohol Concomitantly [HAS-BLED] score ≥ 3, high fall risk, cerebral aneurysm, blood dyscrasias, aortic dissection, renal failure, etc.), or failure of OAC (stroke/transient ischemic attack [TIA] while on OAC), or patients deemed not suitable for long-term anticoagulation. Patients with the presence of left atrial appendage (LAA) thrombus or severe untreated mitral stenosis were excluded.
Pre-procedure imaging
Pre-procedural baseline transesophageal echocardiography (TEE) was routinely recommended, or alternatively, cardiac computed tomography angiography (CCTA) was performed instead of TEE pre-procedure, to rule out pre-existing LAA thrombus and to evaluate LAA anatomy and dimensions for accurate measurement of the widest LAA ostium (usually at the level of the circumflex artery) at 0, 45, 90, and 135 degrees, and also the available depth of the LAA (from ostium to apex of the LAA).
Procedural details
All procedures were done under general anesthesia with TEE guidance. Intravenous heparin was administered pre- or immediately following transseptal puncture, to maintain an activated clotting time > 250-300 seconds during the procedure. To ensure adequate mean left atrial pressure (> 12 mm Hg) for more accurate LAA measurements, saline bolus was administered if necessary. Device sizing selection was based upon the maximum LAA ostium diameter. Oversizing was recommended, by 9%-25%, based on the widest LAA measurement. Before device release, fulfillment of the following 4 criteria (PASS) was confirmed: (i) position (device distal or at the ostium of the LAA; protrusion of shoulder by < 5-7 mm was acceptable); (ii) anchor (testing stability by retracting the deployment knob and letting go, to assess return to original position); (iii) size (device shoulder compressed 8%-20% of original size on TEE); and (iv) seal (assess TEE for any residual flow; must be < 5 mm before release). When all criteria were met, the device was released. Final angiography and TEE assessment were then performed.
Postprocedure dual antiplatelet therapy (DAPT) with aspirin 81 mg/d and clopidogrel 75 mg/d is commonly recommended for 3 months and followed by life-long aspirin alone; however, the post-LAAC antithrombotic regimen was at physician discretion. Postprocedural imaging with TEE or CCTA was recommended at 1-6 months post-LAA closure.
Clinical follow-up
Long-term events (stroke, TIA, systemic embolism [SE], cardiovascular death, noncardiovascular death, major bleeding, and minor bleeding) were collected prospectively. Clinical or telephone follow-up were obtained at 3, 12, and 24 months post-LAAC.
Definitions
Procedural major adverse events (MAEs) were defined as a composite of death, device embolization, stroke, SE, myocardial infarction, cardiac tamponade, and major bleeding. Other procedural complications (pericardial effusion not requiring drainage; minor bleeding) were also assessed. For long-term follow-up, major adverse cardiovascular events (MACEs), defined as a composite of death, stroke, TIA, SE, and myocardial infarction, were assessed. In addition, major and minor bleeding was also evaluated. For patients who underwent device surveillance (CCTA or TEE), the incidence of device-related thrombus (DRT) and peri-device leak (PDL) was assessed. The definition of DRT and PDL was in accordance with previous studies.13
Statistical analysis
For continuous variables, means and standard deviations were calculated and compared using the Wilcoxon test. Binary variables are reported as counts and percentages, and between-group differences were assessed using the χ2 test. The efficacy of the WATCHMAN in preventing stroke, TIA, and SE was tested by comparing the observed event-rate at follow-up with the predicted event-rate, using the CHADS2 and CHA2DS2-VASc scores.1 The average annual risk for the whole study population was calculated from the predicted individual patient annual risk. The observed annualized ischemic stroke rate and thromboembolic event-rate (stroke, TIA, and SE) were subtracted from the predicted event-rates, and divided by the predicted event-rate x100, to obtain the % relative risk reduction (% relative reduction).
Data were analyzed using R software, version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). All P values were 2-sided, and significance was defined as P < 0.05 for all analyses.
Results
Baseline characteristics
A total of 272 patients (mean age: 75.4 years [standard deviation {SD} 8.8]; male, 63.2%; CHA2DS2-VASc: 4.4 [SD: 1.6]; HASBLED score: 3.6 [SD: 0.9]) were enrolled in the Canadian WATCHMAN Registry between December 2013 and August 2019, from 7 Canadian centres (Table 1): 140 cases were performed in Vancouver, 41 in Laval, 37 in Saskatoon, 32 in Regina, 11 in Montreal, 9 in Winnipeg, and 2 in Calgary. About one third of patients had a history of stroke (29.4%), TIA (14.7%), or SE (4.0%). Only 5.1% of patients had undergone prior catheter ablation for AF. Most patients (90.4%) had prior history of bleeding (major, 80.5%; minor, 21.7%).
Table 1.
Baseline characteristics
| Baseline characteristic | N = 272 |
|---|---|
| Age, y | 75.36 (± 8.75) |
| Male | 172 (63.2) |
| Body mass weight, kg/m2 | 28.19 (± 6.02) |
| Atrial fibrillation type | |
| Permanent or persistent | 153 (56.2) |
| Paroxysmal | 118 (43.4) |
| Unknown | 1 (0.4) |
| History of LAA thrombus | 13 (4.8) |
| Hypertension | 203 (74.6) |
| Dyslipidemia | 176 (64.7) |
| Diabetes | 90 (33.1) |
| Smoking history | |
| Current smoker | 22 (8.1) |
| Former smoker | 120 (44.1) |
| Coronary artery disease | 104 (38.2) |
| Prior percutaneous coronary intervention | 54 (19.9) |
| Coronary artery bypass grafting | 40 (14.7) |
| Chronic heart failure | 70 (25.7) |
| Left ventricular ejection fraction < 40% | 53 (19.5) |
| Prior valve surgery | 21 (7.7) |
| Prior stroke | 80 (29.4) |
| Prior transient ischemic attack | 40 (14.7) |
| Prior systemic embolization | 11 (4.0) |
| Prior atrial fibrillation ablation | 14 (5.1) |
| Peripheral artery disease | 19 (7.0) |
| Pacemaker | 55 (20.2) |
| Intracardiac defibrillator | 11 (4.0) |
| Creatinine, μmol/L | 106.76 (± 46.59) |
| Laboratory data | |
| Estimated GFR, mL/min per 1.73 m2 | 59.37 (± 20.06) |
| Hemoglobin baseline, g/dL | 125.26 (± 19.94) |
| Indications for LAA closure | |
| CHA2DS2-VASc | 4.35 (± 1.64) |
| HAS-BLED score | 3.55 (± 0.94) |
| Previous minor bleeding | 59 (21.7) |
| Previous major bleeding | 219 (80.5) |
| Intracranial | 99 (36.4) |
| Gastrointestinal | 106 (39.0) |
| Retroperitoneal | 4 (1.5) |
| Intraocular | 7 (2.6) |
| Respiratory | 6 (2.2) |
| Urogenital | 8 (2.9) |
Values are n (%), or mean (± standard deviation).
CHA2DS2-VASc, Congestive Heart Failure, Hypertension, Age [≥ 75 Years] [doubled], Diabetes Mellitus, Stroke [doubled], Vascular Disease, Age [65-74] Years, Sex Category [Female]; GFR, glomerular filtration rate; HAS-BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly [> 65 Years], Drugs/Alcohol Concomitantly; LAA, left atrial appendage.
Procedural details and in-hospital outcomes
Almost all patients (95.2%) were evaluated by pre-procedural TEE, and computed tomography angiography was performed for 58.8% of patients prior to LAAC. The WATCHMAN device was successfully implanted in 269 patients (98.9%; Table 2). The mean total procedure time was 89.1 minutes (SD: 31.9), and the mean length of hospital stay was 1.6 days (SD: 3.3). An MAE was confirmed in 3.3% of patients, including 5 pericardial effusions requiring drainage (1.8%) and 1 death (0.4%; 22 days post-LAAC from respiratory failure; Table 3). A total of 6 mild pericardial effusions (2.2%) that did not require intervention occurred. Post-LAAC antithrombotic agents included DAPT in 70.6%, single antiplatelet therapy (SAPT) in 18.4%, and OAC in 13.6% (Table 4).
Table 2.
Procedural details
| Procedural detail | N = 272 |
|---|---|
| Technical success | 269 (98.9) |
| Implanted WATCHMAN size, mm | |
| 21 | 28 (10.3) |
| 24 | 48 (17.6) |
| 27 | 80 (29.4) |
| 30 | 63 (23.2) |
| 33 | 50 (18.4) |
| Failed implantation | 3 (1.1) |
| Total procedural time, min | 89.12 (± 31.86) |
| Fluoroscopy time, min | 13.66 (± 8.39) |
| Total contrast, mL | 84.66 (± 52.30) |
| Length of hospital stay, d | 1.64 (± 3.32) |
| Number of devices attempted | 1.30 (± 0.60) |
| Baseline TEE performed | 259 (95.2) |
| LAA dimension by TEE, mm | 22.35 (± 3.96) |
| LAA depth by TEE, mm | 30.37 (± 6.43) |
| Baseline CTA performed | 160 (58.8) |
| LAA dimension by CCTA, mm | 25.09 (± 4.65) |
| LAA depth by CTA, mm | 30.47 (± 6.80) |
Values are n (%), or mean (± standard deviation). WATCHMAN (Boston Scientific, Marlborough, MA).
CCTA, cardiac CTA; CTA, computed tomography angiography; LAA, left atrial appendage; SD, standardized difference; TEE, transesophageal echocardiography.
Table 3.
Procedural complications
| Procedural complication | N = 272 |
|---|---|
| Major adverse event | 9 (3.3) |
| Death | 1 (0.4) |
| Stroke/TIA/systemic embolization | 0 (0) |
| Myocardial infarction | 1 (0.4) |
| Pericardial tamponade requiring drainage | 5 (1.8) |
| Device embolization | 0 (0) |
| Major bleeding | 2 (0.7) |
| Other in-hospital complications | |
| Pericardial effusion (small, no drainage) | 6 (2.2) |
| Minor bleed (eg, hematoma) | 9 (3.3) |
TIA, transient ischemic attack.
Table 4.
Discharge antithrombotic agents
| Discharge antithrombotic agents | N = 272 |
|---|---|
| Aspirin | 230 (84.6) |
| Clopidogrel | 203 (74.6) |
| Ticagrelor | 1 (0.4) |
| Warfarin | 5 (1.8) |
| Direct oral anticoagulant | 32 (11.8) |
| Antithrombotic regimen | |
| Dual antiplatelet therapy | 192 (70.6) |
| Single antiplatelet therapy | 50 (18.4) |
| Oral anticoagulant | 37 (13.6) |
| None | 6 (2.2) |
Long-term clinical outcomes
During the follow-up period (mean 709.7 days [SD: 467.2]), a MACE was confirmed in 16.5% of patients (including 3.7% who had stroke, and 1.5% who had a TIA; Table 5). A total of 31 patients died during follow-up, and 67.7% of these deaths were due to noncardiovascular reasons. Bleeding events occurred in 19.1% of patients who underwent LAAC during follow-up (major bleeding, 8.8%; minor bleeding, 10.3%); 9 cases of major bleeding (1.9%) occurred within 3 months postprocedure. Follow-up device surveillance (either TEE or computed tomography angiography; at a mean of 103 days [SD: 115] postprocedure) was performed in most patients (84.2%), and DRT was detected in 5 patients (1.8%) and severe PDL (> 5 mm) was confirmed in 3 cases (1.1%).
Table 5.
Adverse events during follow-up
| Overall events | N = 272 |
|---|---|
| Follow-up, d, mean (SD) | 709.7 (467.2) |
| Composite death/stroke/TIA/MI | 45 (16.5) |
| Cardiovascular death | 10 (3.7) |
| Noncardiovascular death | 21 (7.7) |
| Stroke | 10 (3.7) |
| TIA | 4 (1.5) |
| Systemic embolization | 0 (0) |
| MI | 2 (0.7) |
| Major bleeding | 24 (8.8) |
| Minor bleeding | 28 (10.3) |
| Device surveillance (TEE or CTA) | 229 (84.2) |
| Device-related thrombus | 5 (1.8) |
| Peri-device leak (on TEE; N = 216), mm | |
| Any leak | 76 (35.2) |
| Minimal, < 1 | 27 (12.5) |
| Minor, 1–3 | 24 (11.1) |
| Moderate, 3–5 | 20 (9.3) |
| Severe, > 5 | 3 (1.4) |
Values are n (%), unless otherwise indicated.
CTA, computed tomography angiography; MI, myocardial infarction; SD, standardized difference; TEE, transesophageal echocardiography; TIA, transient ischemic attack.
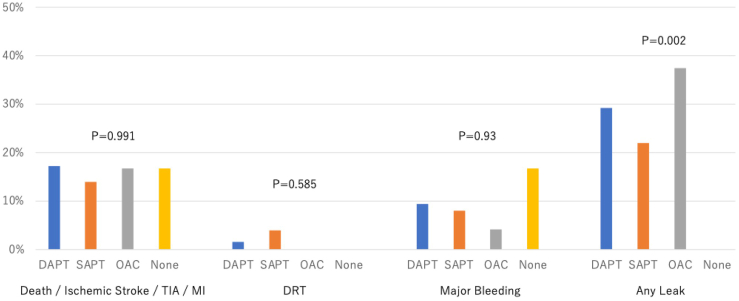
A reduction of 81.4% and 73.8%, respectively, occurred in the ischemic stroke rate, and the composite ischemic stroke, TIA, and SE rate, based on the expected rate from the CHA2DS2-VASc score (ischemic stroke: 6.0% expected vs 1.1% observed; composite of ischemic stroke, TIA, and SE: 8.4% vs 2.2%; Fig. 1). When stratified by the antithrombotic strategy at the time of discharge, the rates of MACEs, DRT, a bleeding event, and any PDL were not different (Fig. 2).
Figure 1.
Observed/expected ratio for thromboembolic events based on CHAD2DSs-VASc (Congestive Heart Failure, Hypertension, Age [≥ 75 Years] [doubled], Diabetes Mellitus, Stroke [doubled], Vascular Disease, Age [65-74] Years, Sex Category [Female]) and CHADS2 (Congestive Heart Failure, Hypertension, Age ≥ 75, Diabetes, and Prior Stroke/Transient Ischemic Attack [doubled]) scores. RR, relative reduction; SE, systemic embolization; TIA, transient ischemic attack.
Figure 2.
Adverse events during follow-up, by antithrombotic strategy. DAPT, dual antiplatelet therapy; DRT, device-related thrombus; MI, myocardial infarction; OAC, oral anticoagulant; SAPT, single antiplatelet therapy; TIA, transient ischemic attack.
Discussion
In this largest to-date Canadian prospective registry of patients undergoing LAAC with the WATCHMAN device, we found the following: (i) more than one third of patients had a history of cerebrovascular disease and > 90% had a prior history of bleeding events; (ii) the WATCHMAN device was successfully implanted in the majority of cases (98.9%), with 3.3% having a peri-procedural MAE, including 5 pericardial effusions requiring drainage (1.8%), and 1 death due to respiratory failure (0.4%); (iii) DAPT was the predominant antithrombotic regimen after LAAC (70.6%), and OAC was used in only 18.4%; and (iv) the follow-up data showed an 81.4% reduction of ischemic stroke rate based on the expected rate from the CHA2DS2-VASc score (6.0% expected vs 1.1% observed).
This study cohort comprises the early Canadian experience with the WATCHMAN legacy device. Health Canada approved use of the WATCHMAN device in January 2016, and prior to this time period, implantation of the WATCHMAN was performed under the jurisdiction of the special access program. Thus, patient selection for this procedure was rigorous and restricted, and a high proportion of our patients had had prior bleeding events (90.4%), with most having had major bleeding (more than one third had had prior intracranial bleeding). The National Cardiovascular Data Registry LAAO Registry, a prospective, nationwide registry designed to function as the formal postmarket surveillance entity in the US, enrolled patients undergoing LAAC with the WATCHMAN device during a similar timeframe (between January 2016 and December 2018), and it showed lower proportions of prior major bleeding and intracranial bleeding (69.5% vs 80.5%, and 11.9% vs 36.4%, respectively), compared to those in our Canadian cohort.14 This disparity is due largely to the stricter indication for LAAC used in Canada, due to funding restrictions, where percutaneous LAAC is generally performed in patients with high stroke risk and absolute/relative contraindications to OAC or failure on OAC therapy. Randomized trial data on LAAC in patients contraindicated to OAC have yet to be published. The ASAP-TOO study was stopped prematurely,15 whereas the STROKE-CLOSE (NCT02830152) and CLOSURE-AF (NCT03463317) studies are ongoing, but enrollment rates are slow. Thus, real-world registries evaluating the effectiveness and safety of LAAC in patients contraindicated to OAC treatment remain highly relevant in the current era. Also large, randomized trials are ongoing comparing use of LAAC vs NOACs in AF patients suitable for OAC treatment (eg, the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients [CHAMPION-AF; NCT04394546] and CATALYST [NCT04226547] trials), which may expand the indications for LAAC in Canada in the near future.
The technical success rate with this device in our study was 98.9%, which was higher than that of pivotal trials, such as PROTECT-AF and PREVAIL,9,10 and is compatible with the rate seen in recent registries.16,17 The rate of in-hospital adverse events in our registry was also compatible with that in pivotal trials and other registries,9,10,17,18 with the most frequent peri-procedure complication being cardiac tamponade requiring drainage. The improved implant success rate of recent registries, including our study, is attributed to increasing operator experience and is likely complimented by the use of pre-procedure computed tomography (CT) assessment. A learning curve phenomenon was previously described with LAAC, and 30 cases was proposed as a threshold in order to both reach proficiency and optimize clinical outcomes.19 Similar to other structural heart disease interventions, CT assessment has been used widely as a pre-procedure evaluation and can provide accurate LAA morphology and sizing.20 The next-generation WATCHMAN FLX device (Boston Scientific, Marlborough, MA) is now commercially available, and early studies have shown this new device to be associated with a lower incidence of adverse events and a high incidence of effective appendage closure.21
In the Canadian WATCHMAN Registry, the majority of patients were managed with antiplatelet therapy, rather than anticoagulation therapy. In most patients (> 70%), DAPT was prescribed and then was de-escalated to SAPT at a point1-6 months after the procedure. In selected patients with a very high risk for bleeding (∼10%), SAPT was the initial antithrombotic regimen post-LAAC. In contrast, in the early US experience with the WATCHMAN legacy device (Boston Scientific, Marlborough, MA), > 90% of patients undergoing LAAC were discharged home on OACs with either warfarin or an NOAC.14 Despite the significant difference in the antithrombotic regimen, the incidence of ischemic stroke in our cohort was 3.7% during the mean 2-year follow-up, which was comparable to that in the US cohort (1.4% during 1-year follow-up). Important to note is that the rate of major bleeding in our study cohort was lower than that in the US cohort (8.8% for mean 2-year follow-up vs 7.9% for 1-year follow-up).14 In addition, > 80% of patients underwent post-implant surveillance, using either CT or TEE, and DRT was detected in only 1.8%, a level that appeared lower than that previously reported (3.8%).22 Given the lower incidence of bleeding events, along with similar efficacy to prevent ischemic events and DRT, our data suggest that an antiplatelet-dominant antithrombotic strategy post-LAAC is safe. Further investigation is warranted to determine the optimal antithrombotic regimen.
Limitations
Our study has several limitations. Our Canadian WATCHMAN Registry included 7 of 11 sites performing LAAC in Canada; thus, we did not enroll all WATCHMAN cases performed in Canada during the assessed time period. Nevertheless, we believe our registry was representative of the clinical practice, patient selection, and outcomes of real-world LAAC in Canada. This registry is observational; thus, the use of pre-procedural imaging, device surveillance postprocedure, and use of an antithrombotic regimen were at the discretion of the physicians. Although the clinical data were prospectively collected by each centre, the events and imaging results were not adjudicated. The new-generation WATCHMAN FLX device has now largely replaced the WATCHMAN legacy device. Given the excellent performance of the WATCHMAN FLX device, as reported in the Protection Against Embolism for Nonvalvular AF Patients: Investigational Device Evaluation of the Watchman FLX LAA Closure Technology (PINNACLE) Registry21, its implementation may influence safety and effectiveness, but this new device was not evaluated in our cohort.
Conclusions
The majority of Canadian patients who underwent WATCHMAN LAAC had OAC contraindication due to prior bleeding, and most were safely treated with antiplatelet therapy post-LAAC with low incidence of DRT. The incidence of LAAC procedural complications was low, and the follow-up observed ischemic stroke rate was lower than the predicted rate based on the CHADS2 and CHA2DS2-VASc scores. In the absence of published randomized trial data, our registry findings support the use of WATCHMAN LAAC in patients with contraindications to OAC. Further investigations are warranted to investigate new device iterations and the optimal antithrombotic regimen post-LAAC for patients contraindicated to OAC.
Acknowledgments
Ethics Statement
Institutional research ethics board approvals were obtained and patients gave informed consents for prospective follow-up.
Patient Consent
The authors confirm that a patient consent form(s) has been obtained for this article.
Funding Sources
This study is supported by an unrestricted research grant from Boston Scientific.
Disclosures
This study is supported by an unrestricted research grant from Boston Scientific. J.S. has received unrestricted research grant supports from the Canadian Institutes for Health Research; the Heart & Stroke Foundation of Canada; the National Institutes of Health; the University of British Columbia Division of Cardiology; AstraZeneca; Abbott; St Jude Medical; Boston Scientific; and Servier); salary support from Michael Smith Foundation of Health Research; speaker honoraria from AstraZeneca, Abbott, Boston Scientific, and Sunovion; consultancy and advisory board honoraria from AstraZeneca; St Jude Medical; Abbott; Boston Scientific; Baylis; Gore; and FEops); and proctorship honoraria from Abbott, St Jude Medical, and Boston Scientific). T.I. has received speaker honoraria from Boston Scientific and Edwards Lifesciences. C.M. has received consultancy, advisory board, and speaker honoraria from Abbott; Boston Scientific; Medtronic; Servier; Novartis; and BMS. R.I. received research support from Biomodex, Edwards, Medtronic, and Opsens; has received speaker honoraria from Boston Scientific; Edwards; Medtronic; and Opsens; and has been a proctor for Abbott and Edwards. C.P. has received speaker honoraria from BMS; Servier; Pfizer; Boston Scientific; Bayer; and Boehringer. The other authors have no conflicts of interest to disclose.
Footnotes
See page 528 for disclosure information.
References
- 1.Rosamond W., Flegal K., Furie K., et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Lamassa M., Di Carlo A., Pracucci G., et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (The European Community Stroke Project) Stroke. 2001;32:392–398. doi: 10.1161/01.str.32.2.392. [DOI] [PubMed] [Google Scholar]
- 3.Skanes A.C., Healey J.S., Cairns J.A., et al. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol. 2012;28:125–136. doi: 10.1016/j.cjca.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Andrade J.G., Aguilar M., Atzema C., et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36:1847–1948. doi: 10.1016/j.cjca.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 6.Connolly S.J., Ezekowitz M.D., Yusuf S., et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 7.Patel M.R., Mahaffey K.W., Garg J., et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 8.Granger C.B., Alexander J.H., McMurray J.J., et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 9.Holmes D.R., Reddy V.Y., Turi Z.G., et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 10.Holmes D.R., Jr., Kar S., Price M.J., et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Reddy V.Y., Doshi S.K., Kar S., et al. 5-year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–2975. doi: 10.1016/j.jacc.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Holmes DR, Reddy VY, Buchbinder M, et al. The Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation (ASAP-TOO) trial. Am Heart J 2017;189:68-74. [DOI] [PubMed]
- 13.Saw J., Tzikas A., Shakir S., et al. Incidence and clinical impact of device-associated thrombus and peri-device leak following left atrial appendage closure with the Amplatzer Cardiac Plug. JACC Cardiovasc Interv. 2017;10:391–399. doi: 10.1016/j.jcin.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 14.Price M.J., Slotwiner D., Du C., et al. Clinical outcomes at 1 year following transcatheter left atrial appendage occlusion in the United States. JACC Cardiovasc Interv. 2022;15:741–750. doi: 10.1016/j.jcin.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks D.S., Mensah G.A., Kennard E.D., Detre K., Holmes D.R., Jr. Race, baseline characteristics, and clinical outcomes after coronary intervention: the New Approaches in Coronary Interventions (NACI) registry. Am Heart J. 2000;140:162–169. doi: 10.1067/mhj.2000.106645. [DOI] [PubMed] [Google Scholar]
- 16.Boersma L.V., Schmidt B., Betts T.R., et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37:2465–2474. doi: 10.1093/eurheartj/ehv730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman J.V., Varosy P., Price M.J., et al. The NCDR Left Atrial Appendage Occlusion Registry. J Am Coll Cardiol. 2020;75:1503–1518. doi: 10.1016/j.jacc.2019.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes D.R., Jr., Reddy V.Y., Gordon N.T., et al. Long-term safety and efficacy in continued access left atrial appendage closure registries. J Am Coll Cardiol. 2019;74:2878–2889. doi: 10.1016/j.jacc.2019.09.064. [DOI] [PubMed] [Google Scholar]
- 19.Jung R.G., Simard T., Killu A., et al. Learning curve and outcomes of left atrial appendage closure. JACC Cardiovasc Interv. 2021;14:2750–2752. doi: 10.1016/j.jcin.2021.08.067. [DOI] [PubMed] [Google Scholar]
- 20.Saw J., Fahmy P., Spencer R., et al. Comparing measurements of CT angiography, TEE, and fluoroscopy of the left atrial appendage for percutaneous closure. J Cardiovasc Electrophysiol. 2016;27:414–422. doi: 10.1111/jce.12909. [DOI] [PubMed] [Google Scholar]
- 21.Kar S., Doshi S.K., Sadhu A., et al. Primary outcome evaluation of a next-generation left atrial appendage closure device: results from the PINNACLE FLX trial. Circulation. 2021;143:1754–1762. doi: 10.1161/CIRCULATIONAHA.120.050117. [DOI] [PubMed] [Google Scholar]
- 22.Saw J., Nielsen-Kudsk J.E., Bergmann M., et al. Antithrombotic therapy and device-related thrombosis following endovascular left atrial appendage closure. JACC Cardiovasc Interv. 2019;12:1067–1076. doi: 10.1016/j.jcin.2018.11.001. [DOI] [PubMed] [Google Scholar]